Abstract
Factors associated with the severity with which different challenge models (CMs) compromise growth performance in pigs were investigated using hierarchical clustering on principal components (HCPC) analysis. One hundred seventy-eight studies reporting growth performance variables (average daily gain [ADG], average daily feed intake [ADFI], gain:feed [GF], and final body weight [FBW]) of a Control (Ct) vs. a Challenged (Ch) group of pigs using different CMs (enteric [ENT], environmental [ENV], lipopolysaccharide [LPS], respiratory [RES], or sanitary condition [SAN] challenges) were included. Studies were grouped by similarity in performance in three clusters (C1, C2, and C3) by HCPC. The effects of CM, cluster, and sex (males [M], females [F], mixed [Mi]) were investigated. Linear (LRP) and quadratic (QRP) response plateau models were fitted to assess the interrelationships between the change in ADG (∆ADG) and ADFI (∆ADFI) and the duration of challenge. All variables increased from C1 through C3, except for GF, which decreased (P < 0.05). LPS was more detrimental to ADG than ENV, RES, and SAN models (P < 0.05). Furthermore, LPS also lowered GF more than all the other CMs (P < 0.05). The ∆ADG independent of ∆ADFI was significant in LPS and SAN (P < 0.05), showed a trend toward the significance in ENT and RES (P < 0.10), and was not significant in ENV (P > 0.10), while the ∆ADG dependent on ∆ADFI was significant in ENT, ENV, and LPS only (P < 0.05). The critical value of ∆ADFI influencing the ∆ADG was significant in pigs belonging to C1 (P < 0.05) but not C2 or C3 (P > 0.10). The ∆ADG independent of duration post-Ch (irreparable portion of growth) was significant in C1 and C2 pigs, whereas the ∆ADFI independent of duration post-Ch (irreparable portion of feed intake) was significant in C1 pigs only (P < 0.05). Moreover, the time for recovery of ADG and ADFI after Ch was significant in pigs belonging to C1 and C2 (P < 0.05). Control F showed reduced ADG compared with Ct-M, and Ch-F showed reduced ADFI compared with Ch-M (P < 0.05). Moreover, the irreparable portion of ΔADG was 4.8 higher in F (−187.7; P < 0.05) compared with M (−39.1; P < 0.05). There are significant differences in growth performance response to CM based on cluster and sex. Furthermore, bacterial lipopolysaccharide appears to be an appropriate noninfectious model for immune stimulation and growth impairment in pigs.
Keywords: challenge models, feed intake, growth, meta-analysis, swine
Introduction
Feed intake and feed efficiency interactively influence body weight (BW) gain, which is a simple and indirect measure that impacts financial return in pork production. These three indices are used to reflect good management strategies, the success of an operation, and as a guide to decision-making. However, there are a multitude of factors hampering growth performance of pigs in commercial herds, creating a gap between potential and actual performance (Patience et al., 2015). Among the factors contributing to this variability, immune status likely plays a pivotal role (Laanen et al., 2013). Interestingly, even when not exhibiting clinical signs of disease, there is a marked reduction in both voluntary feed intake and nutrient utilization efficiency in disease-challenged compared with non-challenged animals (Le Floc′h et al., 2004).
A number of challenge models (CMs) have been utilized in research to investigate the mechanisms of growth impairment in disease-challenged pigs, including enteric (ENT) (Wellington et al., 2019, 2020) and respiratory (RES) pathogen challenge (Schweer et al., 2017; Dee et al., 2018), environmental stressors (ENV) (e.g., heat stress and high stocking densities) (Rauw et al., 2017; Laskoski et al., 2019), systemic immune stimulation using bacterial lipopolysaccharide (LPS) (Lara et al., 2018; Wellington et al., 2018), and sanitary conditions (SAN) (Jayaraman et al., 2015; van der Meer et al., 2016). For example, weaned pigs showed a 21% and 20% decrease in average daily gain (ADG) and feed intake (ADFI), respectively, post-challenge with enterotoxigenic Escherichia coli K88 compared with a non-challenged group (Pan et al., 2017). Likewise, Schweer et al. (2016) reported a 30% and 25% reduction in ADG and ADFI, respectively, when comparing pigs infected or not with porcine reproductive and respiratory syndrome virus (PRRSV). ADG and ADFI were reduced by 2% and 3% and by 7% and 4% in growing pigs housed in crowder pens (Wastell et al., 2018) or in hot environments (Lan and Kim, 2018) compared with those housed in adequate space allowance or ambient temperature, respectively. Lipopolysaccharide injection reduced ADG (−14%) and ADFI (−15%) in weanling pigs compared with a group injected with saline (Kang et al., 2014). Finally, deterioration of SAN (e.g., no hygiene protocol applied, lack of medication or preventive treatment, and uncleaned pens after a previous batch of pigs) has been shown to impair ADG and ADFI by 19% and 23%, respectively, when compared with pigs housed in clean pens (Jayaraman et al., 2015, 2017).
Since there is no accurate predictor of pig performance under this multifactorial scenario, investigating the factors that influence the reduction in growth performance assists in the development of strategies to mitigate the effects of disease challenge in pigs. For example, it has been shown that the SAN at weaning is critical for immune development, suggesting that timing of exposure to challenge agents may differently impact the ability of the animal to respond (Brown et al., 2006). Also, more recently, notable chronological immunological development differences were observed between healthy female and male piglets, which may indicate sex-specific responsiveness to disease and dietary interventions (Christoforidou et al., 2019). In this sense, a meta-analytical approach is able to set the basis for 1) gathering a greater amount of information, 2) reducing the effects of the particularities of single studies, 3) minimizing the influence of a reviewer’s personal opinion, and thus 4) leading to unbiased conclusions (Leandro, 2008; Vesterinen et al., 2014).
The objective of the present study was to investigate, using a multivariate approach, whether and to which extent inherent factors, including sex, BW, and feed intake at the beginning of the experiments, would influence growth performance response of pigs to different CMs.
Materials and Methods
Literature search, selection criteria, and database
Academic databases and search engines including Google Scholar, PubMed, CAB Abstracts, SciELO, and Science Direct were systematically searched. Additionally, reference lists in selected papers were screened for identification of related studies. Finally, results from unpublished theses were included if meeting the selection criteria. The search results were imported into a citation manager. Duplicates were removed. After title and abstract screening, full-text examination was conducted by two independent researchers. The specific keywords used for the systematic search are provided in Supplementary Table 1.
The following criteria were used to include data from a given study to the database: dissertation, thesis, or full article from peer-reviewed journals; published between 1985 and 2019 inclusive; evaluation of results from a control (Ct) vs. a challenged (Ch) group; description of the randomization process; evaluation of ENT, ENV, LPS, RES, or SAN as a CM; and animals studied were postweaned pigs. Enteric pathogen challenges corresponded to infection of pigs with E. coli, Lawsonia intracellularis, porcine deltacoronavirus, porcine rotavirus, Salmonella Typhimurium, and porcine epidemic diarrhea virus. Pigs were inoculated orally or intragastrically. Infection was conducted with one to four inoculations and doses varied from 1.0 × 105 to 1.0 × 1012 CFU/mL for bacterial infections and from 1.0 × 103 to 5.0 × 107 TCID50/mL for viral infections. Environmental stressors corresponded to pigs housed in high ambient temperatures (i.e., above thermoneutral zone for that age of pig) or in pens with limited space allowance (i.e., increased density for that age of pig). For studies evaluating the effects of high ambient temperature, pigs were housed at 14 to 28 vs. 28 to 38 °C, and, for studies evaluating space allowance, pigs were housed at 0.16 to 1.18 vs. 0.08 to 0.81 m2/pig. LPS corresponded to stimulate an immune response using bacterial LPS from E. coli. Pigs were injected intramuscularly, intraperitoneally, or intravenously. Immune stimulation was induced with one to seven inoculations, and doses varied from 1 to 200 μg/kg BW. RES corresponded to infection of pigs with Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae, Pasteurella multocida, porcine circovirus type 2, PRRSV, porcine respiratory coronavirus, pseudorabies live vaccine, and Salmonella choleraesuis. Pigs were inoculated intramuscularly, intranasally, or intratracheally. Infection was conducted with one to two inoculations and doses varied from 1.0 × 105 to 3.0 × 1010 CFU/mL for bacterial infections, from 13 to 200 μg of P. multocida toxin (Pm-T)/mL for P. multocida infection, and from 1.0 × 105 to 1.1 × 1011 TCID50/mL for viral infections. SAN corresponded to pigs housed in pens with poor hygiene conditions. The protocols generally consisted of keeping pigs in pens that were not cleaned or disinfected after a previous occupation by a different batch of pigs or in pens in which manure from a different batch of pigs had been added resulting in a mild, continuous stimulation of the immune system (Le Floc′h et al., 2006, 2009; van der Meer et al., 2016). When multiple challenges were evaluated in a given study, but separately, data were included separately for each challenge. In three studies evaluating RES, additional inoculations were performed (e.g., PRRSV and M. hyopneumoniae, Roberts and Almond, 2003; M. hyopneumoniae and porcine circovirus type 2, Roberts et al., 2004; Kim et al., 2011; and PRRSV and porcine circovirus type 2, Park et al., 2014). As they did not produce discrepant response in performance variables compared with the other RES studies that they were maintained in the database.
Design criteria included CMs (ENT, ENV, LPS, RES, or SAN), sex (males [M], females [F], or mixed sex [Mi]), duration of challenge, group (Ct vs. Ch), and initial BW (IBW). The database comprised a total of 178 studies. Table 1 presents the general characteristics of studies within each CM, and Supplementary Table 2 presents the main experimental details of each study included in the analysis.
Table 1.
General characteristics of studies included in the meta-analysis
| Amount2 | |||||
|---|---|---|---|---|---|
| Item | N 1 | Control | Challenge | Unit | ROA3 |
| ENT | |||||
| Enterotoxigenic Escherichia coli Lawsonia intracellularis Salmonella Typhimurium |
1 to 4 | 0 | 1.0 × 105 to 1.0 × 1012 | CFU/mL | Oral Intragastric |
| Porcine deltacoronavirus Porcine rotavirus Porcine epidemic diarrhea virus |
1 | 0 | 1.0 × 103 to 5.0 × 107 | TCID50/mL | Oral Intragastric |
| ENV | |||||
| Thermal stress | — | 14 to 28 | 28 to 38 | °C | — |
| Stocking density | — | 0.16 to 1.18 | 0.08 to 0.81 | m2/pig | — |
| LPS | |||||
| Escherichia coli lipopolysaccharide | 1 to 7 | 0 | 1 to 200 | μg/kg BW | Intramuscular Intraperitoneal Intravenous |
| RES | |||||
| Actinobacillus pleuropneumoniae Mycoplasma hyopneumoniae Salmonella choleraesuis |
1 | 0 | 1.0 × 105 to 3.0 × 1010 | CFU/mL | Intranasal Intratracheal Intramuscular |
| Pasteurella multocida | 1 | 0 | 13 to 200 | μg of Pm-T/mL | Intranasal Intramuscular |
| Porcine circovirus type 2 Porcine respiratory coronavirus Pseudorabies live vaccine PRRSV |
1 to 2 | 0 | 1.0 × 105 to 1.1 × 1011 | TCID50/mL | Intranasal Intratracheal Intramuscular |
1N, number of times of challenge administration (where applicable).
2Amount, amount administered in Ct and Ch pigs with respective units; CFU, colony-forming unit; TCID50, median tissue culture infectious dose; Pm-T, Pasteurella multocida toxin.
3Route of administration (where applicable).
Response information
The dependent variables extracted for meta-analysis were final BW (FBW), ADG, ADFI, and gain:feed (GF). The ADG, ADFI, and GF were reported after (LPS, ENT, and RES) or during (ENV and SAN) exposure to the CM.
General model and clustering
We carried out a hierarchical clustering on principal components (HCPC) analysis (Lê et al., 2008), which grouped studies in three clusters (C1, C2, and C3). This approach is a type of exploratory analysis capable of organizing observed similarities in homogeneous studies using Ward’s method, applying squared Euclidean distance as the similarity measure. The statistical technique starts with building a hierarchical tree; then, the sum of within-cluster inertia is calculated for each partition, with the suggested partition being the one with the higher relative loss of inertia. In the present study, we used only the performance variables (IBW, FBW, ADG, ADFI, and GF) from the Ct group of each study to perform the cluster analysis as the differences between experimental designs could be reflected in different responses to CM. For this purpose, the HCPC function of the FactoMineR package (Lê et al., 2008) was used.
Table 2 presents the summary of the output parameters by cluster. Figure 1a and b shows the three groups (clusters) formed by cluster analysis and the dendrogram of hierarchical clustering, respectively. The data were analyzed using a linear mixed model (St-Pierre, 2001), with the random effect of studies and the fixed effects of cluster (C1, C2, and C3), group (Ct vs. Ch), CM (ENT, ENV, LPS, RES, and SAN), and sex (M, F, and Mi). Thus, the general model used was:
Table 2.
Descriptive statistics of traits reported by the studies used in the meta-analysis split by clusters1
| Item | C1 | C2 | C3 |
|---|---|---|---|
| IBW, kg | 7.62 ± 2.53 (3.80; 21.90) | 19.01 ± 7.38 (4.63; 38.00) | 46.22 ± 21.04 (8.00; 104.00) |
| FBW, kg | 15.96 ± 6.50 (5.02; 35.10) | 36.92 ± 10.03 (14.61; 65.93) | 76.61 ± 23.67 (26.43; 144.48) |
| ADG, kg/d | 0.38 ± 0.12 (0.09; 0.64) | 0.68 ± 0.13 (0.46; 1.10) | 0.95 ± 0.14 (0.68; 1.29) |
| ADFI, kg/d | 0.60 ± 0.19 (0.20; 1.13) | 1.32 ± 0.29 (0.93; 1.99) | 2.50 ± 0.56 (1.74; 3.99) |
| GF, kg/kg | 0.68 ± 0.11 (0.33; 1.08) | 0.56 ± 0.08 (0.30; 0.73) | 0.37 ± 0.11 (0.00; 0.52) |
1Data are expressed as mean ± SEM. The interval presented in parentheses corresponds to the data range.
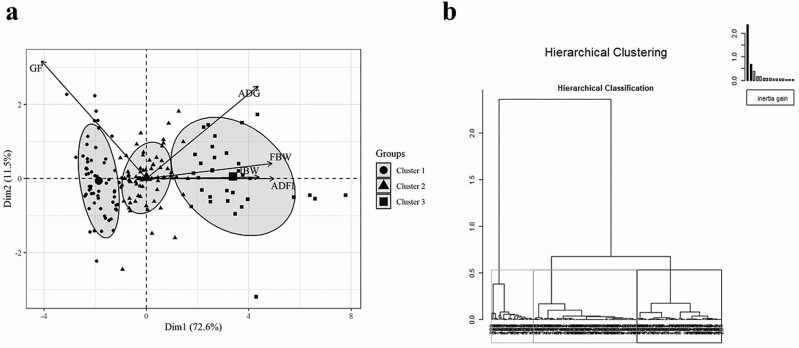
Figure 1.
Biplot (a) of variables and studies used in the meta-analysis represented for the first (Dim1) and second dimensions (Dim2). Each data plot represents a study, and each cluster is depicted by a given shape. Ellipses represent the concentration of each cluster in normal probability, whereas its centroid is represented by a bigger symbol with the same shape as the studies in the same cluster. Dendrogram (b) obtained by hierarchical classification showing the relatedness of studies belonging to C1 (light grey box), C2 (dark grey box), and C3 (black box). Cluster formation was determined according to the loss of inertia (top right), which became stable after C3.
where Ri = random effect of studies (i = 1, ..., 152 studies), Gj = group (j = Ct vs. Ch), Dk = CM (k = ENT, ENV, LPS, RES, and SAN), Cl = clusters formed (l = C1, C2, and C3), Sn = sex (M, F, and Mi), and eijklmn = the unexplained residual error N(0,σ2). The cluster and sex interactions with groups (Ct and Ch) were evaluated.
Estimates of fixed and random effects were obtained using the restricted maximum likelihood method, and parameter estimates were obtained using the lmer function of the lme4 package (Bates et al., 2015). The need to model error heteroscedasticity was evaluated based on Akaike’s information criterion and Schwarz’s Bayesian information criterion, and the need to include covariates in the model was tested using the same criteria. The significance level was set at P ≤ 0.05 and a trend toward significance considered at 0.05 < P < 0.10.
Reduction in growth rate as a function of feed intake and temporal evaluation of ADG and ADFI
Linear (LRP; 1) and quadratic (QRP; 2) response plateau regression models were applied to investigate the effects of CM, cluster, and sex on the relationship between the change in ADG (∆ADG) and the change in ADFI (∆ADFI), and the analysis of temporal ∆ADG and ∆ADFI post-Ch according to the methodology adapted from Pastorelli et al. (2012):
| (1) |
| (2) |
where Y is the response variable estimated (ΔADG for evaluation of relation with ΔADFI, or ΔADG and ΔADFI for temporal changes analysis), α is the plateau value, β is the slope (difference between intercept and plateau), and γ is the time necessary to reach the plateau for temporal change analysis or the maximum ΔADFI above which there is no ΔADG. The maximum values of Y in equation 1 were equal to α and in equation 2 were calculated as α + − β2 / (4 * γ). The critical points X in equation 1 were equal to γ and in equation 2 were calculated as −0.5 * β / γ. The intersection between Y and X values is the breakpoint of the models. The significance of the parameters was tested using an F-test and were set at P < 0.05 and a trend toward significance when 0.05 < P < 0.10. The normality of residuals was tested using Shapiro–Wilk test, and outliers were verified based on the evaluation of Studentized residuals in which absolute values exceed three leverage values, and cook’s distance (Sauvant et al., 2008). All statistical analyses were conducted using R software program (R Core Team, 2020), and graphics were generated with the ggplot2 package (Wickham, 2016). Finally, the partitioning of the ∆ADG independent of ADFI or dependent on ADFI was also calculated for each CM, cluster, and sex (Figure 2). In this approach, it is possible to identify the reduction in ADG at the same ADFI, indicating an increase in maintenance requirement, and the reduction in ADG dependent on ADFI, indicating a decrease in feed efficiency (Pastorelli et al., 2012). Regression parameters for plateau models provided in Supplementary File 2 describing the interrelationship between ΔADG and ΔADFI within each cluster, sex, and CM were used for calculation. Briefly, the intercept of LRP (α) and QRP (α + − β2 / (4 * γ)) reflects the reduction in ADG not related to the reduction in ADFI, which can be interpreted as indicator for maintenance and the remaining portion of the model is regarded as associated to the change in feed efficiency (ADFI dependent). All the codes used in the present meta-analysis are included in Supplementary File 1.
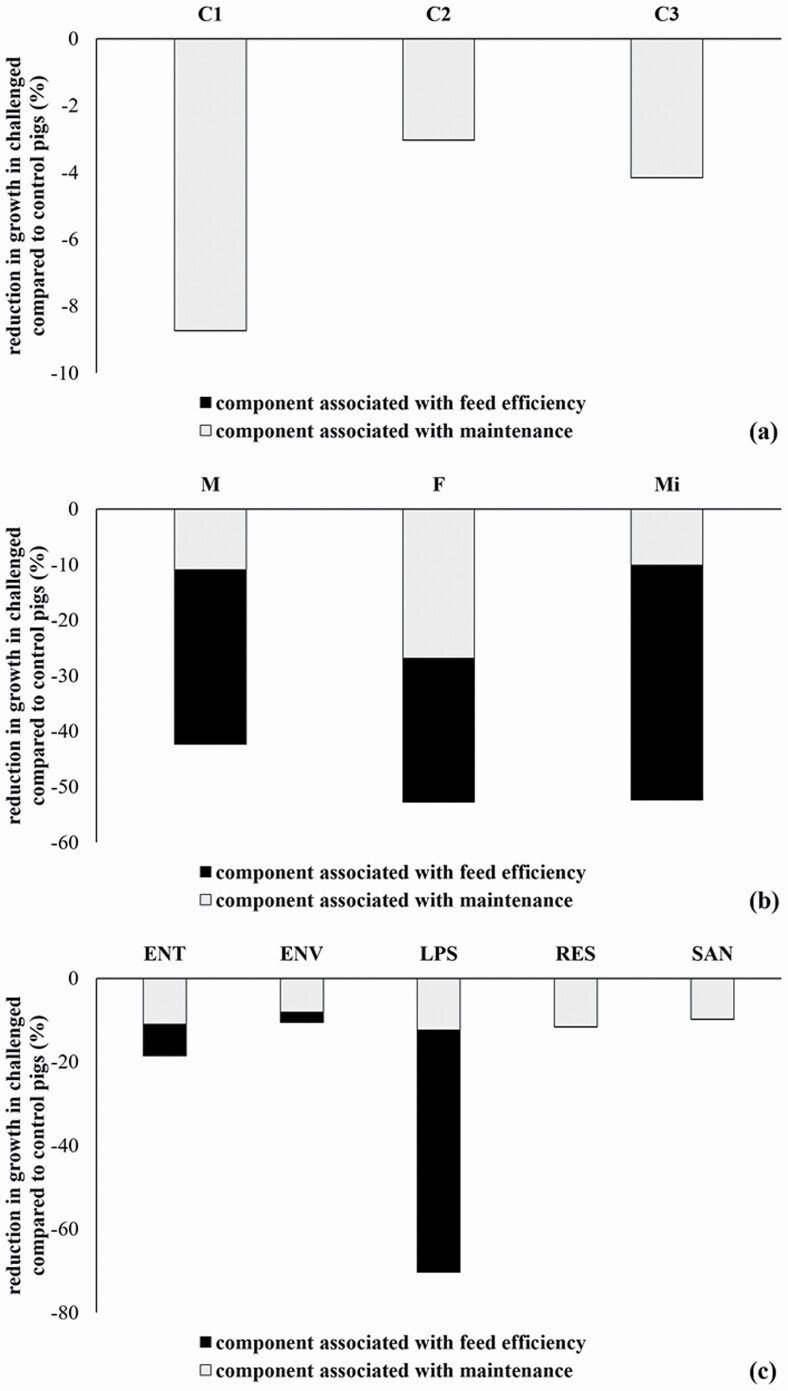
Figure 2.
Partitioning of the reduction in growth in Ch compared with Ct pigs by clusters (C1, C2, and C3) (a); sex (M, F, and Mi) (b); and CMs (ENT, ENV, LPS, RES, or SAN) (c), stratified in component associated with maintenance requirement or/and component associated with maintenance requirement.
Results
General model effects
Table 3 presents the estimated parameters of linear mixed models for performance variables of pigs used in the meta-analysis. The intercept values were significant for ADG, ADFI, GF, and FBW (P < 0.05). Regardless of CM, there was a reduction in ADG and GF, and a trend for reduction of FBW in Ch pigs compared with Ct pigs (P < 0.05). There was no difference in ADFI between Ch and Ct pigs (P > 0.10).
Table 3.
Estimated parameters of linear mixed models for performance variables of pigs used in the meta-analysis
| Variable1 | ||||
|---|---|---|---|---|
| ADG, kg/d | ADFI, kg/d | GF, kg/kg | FBW, kg | |
| Parameter2 | ||||
| Intercept | 0.35 ± 0.03*** | 0.50 ± 0.10*** | 0.76 ± 0.04*** | 15.10 ± 3.34*** |
| Challenge | −0.08 ± 0.03** | −0.05 ± 0.05 | −0.15 ± 0.05** | −1.01 ± 0.57† |
| C2 | 0.35 ± 0.03*** | 0.85 ± 0.09*** | −0.16 ± 0.4*** | 20.66 ± 3.44*** |
| C3 | 0.59 ± 0.04*** | 2.03 ± 0.11*** | −0.29 ± 0.05*** | 62.36 ± 3.93*** |
| F | −0.12 ± 0.05* | −0.20 ± 0.13 | −0.07 ± 0.06 | 0.63 ± 4.79 |
| Mi | 0.01 ± 0.03 | −0.03 ± 0.09 | −0.03 ± 0.04 | −0.55 ± 3.21 |
| Challenge:C2 | −0.06 ± 0.03† | −0.08 ± 0.06 | 0.11 ± 0.05* | −1.81 ± 1.63** |
| Challenge:C3 | −0.11 ± 0.04** | −0.42 ± 0.06*** | 0.13 ± 0.06* | −3.58 ± 0.69*** |
| Challenge:F | −0.06 ± 0.05 | −0.21 ± 0.08* | −0.03 ± 0.07 | −0.51 ± 0.85 |
| Challenge:Mi | −0.02 ± 0.03 | 0.01 ± 0.05 | −0.01 ± 0.05 | −0.17 ± 0.60 |
| Summary of fit | ||||
| No. of observations | 218 | 208 | 202 | 218 |
| No. of studies | 103 | 98 | 95 | 102 |
| RMSE3 | 0.08 | 0.13 | 0.13 | 1.44 |
| R | 0.75 | 0.79 | 0.33 | 0.73 |
| r2 | 0.86 | 0.95 | 0.49 | 0.99 |
| AIC | −237.06 | 108.01 | −98.97 | 1,429.97 |
| BIC | −182.91 | 161.41 | −46.04 | 1,484.12 |
1Symbols (†, *,**,***) represent significant differences with, P < 0.10, P < 0.05, P < 0.01, and P < 0.001, respectively.
2Main (Intercept, Challenge, C2, C3, Gilts, and Mi) and interaction (Challenge:C2, Challenge:C3, Challenge:Gilts, and Challenge:Mi) units correspond to the same units as their respective variable units. Values are least square mean ± SE.
3AIC, Akaike information criterion; BIC, Bayesian information criterion; R, coefficient of correlation; r2, coefficient of determination; RMSE, root mean square error.
Cluster effects
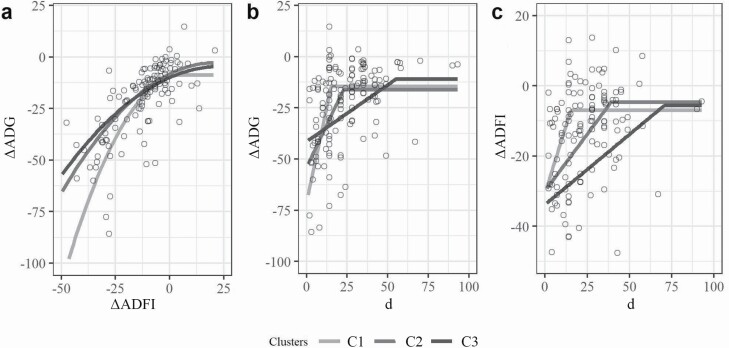
In general, IBW, FBW, ADG, and ADFI increased and GF decreased from C1 to C3 (P < 0.05; Table 3) as confirmed by means reported in Table 2. Pigs from C2 had greater reduction in FBW (P < 0.05) and tended to have greater reduction in ADG (P < 0.10) while showing lower reduction in GF (P < 0.05) compared with C1 pigs. Pigs from C3 had greater reduction in ADG, ADFI, GF, and FBW (P < 0.05) compared with C1 pigs (Table 3). Figure 3a shows the effects of clusters on the relationship between ΔADG and ΔADFI in pigs, with regression parameters reported in Supplementary File 2. All clusters were best adjusted by QRP. The plateau in ΔADG was significant for all the clusters (C1: −9.74, C2: −8.22, and C3: 10.00; P < 0.05), whereas the slope (ΔADG × ΔADFI) was not significant for any cluster (C1: 0.36, C2: 0.52, and C3: 0.45; P > 0.10). The critical value in ΔADFI was significant only in C1 pigs (−0.03; P < 0.05). Figure 3b and c shows the effect of cluster on the relationship between ΔADG or ΔADFI and the duration of challenge in pigs, with regression parameters reported in Supplementary File 2. All clusters were best adjusted by LRP for ADG and ADFI. The plateau in ΔADG was significant only for C1 and C2 (C1: −14.49 and C2: −16.26; P < 0.05), whereas the slope (3.75; ΔADG × duration) and the critical value in duration (15.15) were significant only for C1 (P < 0.05). Moreover, the plateau in ΔADFI was significant only for C1 (−6.93; P < 0.05), whereas the slope (ΔADFI × duration) was significant only for C1 (1.54) and C2 (0.64) (P < 0.05).
Figure 3.
Effect of clusters (C1, C2, and C3) on (a) the relationship between ΔADG and ΔADFI, (b) the relationship between the change in ΔADG, or (c) feed intake (ΔADFI) and the duration of challenge, in pigs. Responses are expressed as the difference between Ch and Ct pigs. Lines represent the LRP or the QRP adjustments. Refer to Supplementary File 2 for parameters of model adjustment and significances.
Sex effects
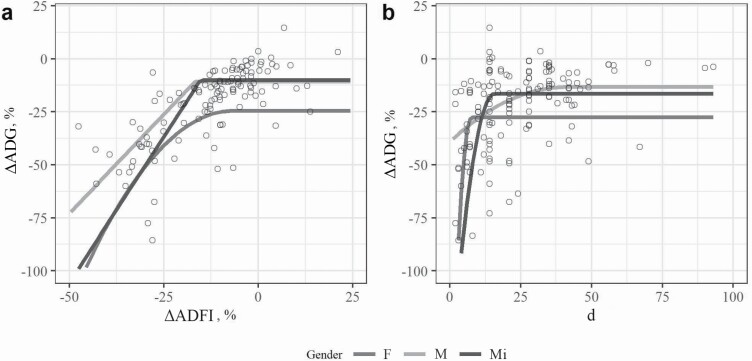
Unchallenged F had lower ADG compared with Ct-M (P < 0.05; Table 3). Challenged F showed reduced ADFI compared with Ch-M, regardless of the CM (P < 0.05; Table 3). Figure 4a shows the effects of sex on the relationship between ΔADG and ΔADFI in pigs, with regression parameters reported in Supplementary File 2. Male and Mi were best adjusted by LRP, and F was best adjusted by QRP. The plateau in ΔADG (M: −10.90 and Mi: −10.11), the slope (M: 1.86 and Mi: 2.75; ΔADG × ΔADFI), and the critical value in ΔADFI (M: −16.82 and Mi: −15.34) were significant for M and Mi (P < 0.05), and the critical value in ΔADFI (plateau in ΔADG) was significant only for M (−16.8232) and Mi (−15.349) (P < 0.05). Figure 4b shows the effects of sex on the relationship between ΔADG and duration of challenge in pigs, with regression parameters reported in Supplementary File 2. All sexes were best adjusted by QRP. The plateau in ΔADG was significant for all the sex (M: −39.15, F: −187.78, and Mi: −151.69; P < 0.05), whereas the slope (17.15; ΔADG × duration) and the critical value in duration (−0.54; plateau in ΔADG) were significant only for Mi (P < 0.05).
Figure 4.
Effect of sex (M, F, and Mi) on (a) the relationship between ΔADG and ΔADFI and (b) the relationship between ΔADG and the duration of challenge, in pigs. Responses are expressed as the difference between Ch and Ct pigs. Lines represent the LRP or the QRP adjustments. Refer to Supplementary File 2 for parameters of model adjustment and significances.
CM effects
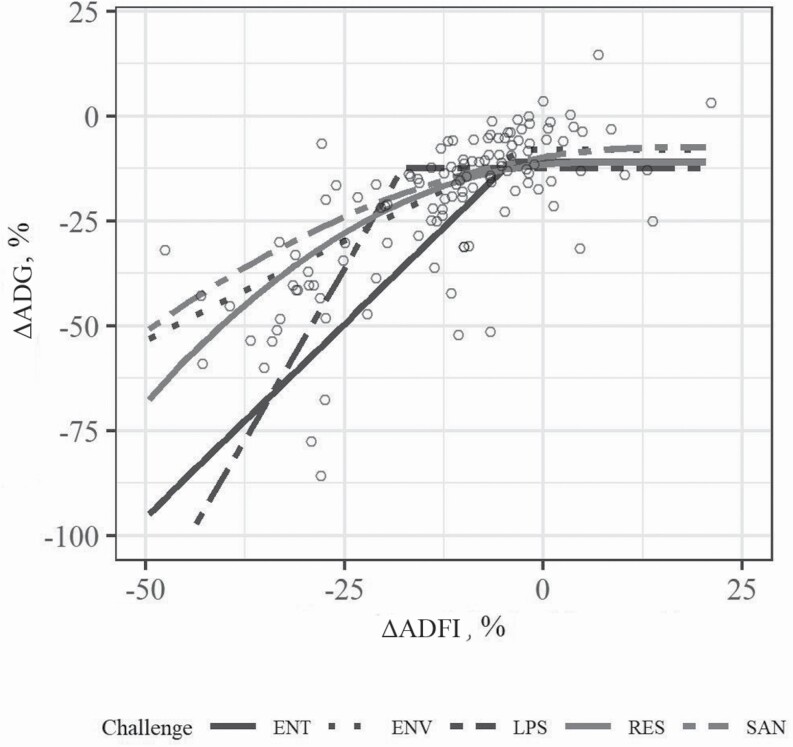
Table 4 presents the comparison between CM on the changes in growth performance variables of pigs. LPS reduced ADG to a higher extent than ENV, RES, and SAN (P < 0.05). Furthermore, LPS resulted in a higher degree of reduction in GF compared with all the other CMs (P < 0.05). Figure 5 shows the effects of CM on the relationship between ΔADG and ΔADFI in pigs, with regression parameters reported in Supplementary File 2. All CMs were best adjusted by LRP, except for RES, which was adjusted by QRP. The plateau in ΔADG was negative for all CM and significant for LPS (−12.37) and SAN (−9.78) (P < 0.05), showed a trend toward the significance for ENT (−11.00) and RES (−11.56) (P < 0.10), and was not significant for ENV (−8.06) (P > 0.10). The slopes (ΔADG × ΔADFI) were significant for ENT (1.84), ENV (0.95), and LPS (3.27) (P < 0.05) and were not significant for RES (0.16) and SAN (0.30) (P > 0.10).
Table 4.
Comparison between CMs on the changes in growth performance parameters of pigs
| CMs1 | |||||
|---|---|---|---|---|---|
| Item | ENT | ENV | LPS | RES | SAN |
| ADG, kg/d | |||||
| ENT | — | ||||
| ENV | −0.03 ± 0.04 | — | |||
| LPS | 0.06 ± 0.04 | 0.09 ± 0.04* | — | ||
| RES | −0.06 ± 0.04 | −0.03 ± 0.03 | −0.12 ± 0.04** | — | |
| SAN | −0.04 ± 0.04 | 0.01 ± 0.04 | −0.10 ± 0.04* | 0.02 ± 0.03 | — |
| ADFI, kg/d | |||||
| ENT | — | ||||
| ENV | −0.01 ± 0.11 | — | |||
| LPS | −0.06 ± 0.12 | −0.05 ± 0.13 | — | ||
| RES | −0.01 ± 0.09 | −0.01 ± 0.08 | 0.05 ± 0.13 | — | |
| SAN | 0.02 ± 0.11 | 0.02 ± 0.11 | 0.08 ± 0.13 | 0.02 ± 0.11 | — |
| GF, kg/kg | |||||
| ENT | — | ||||
| ENV | 0.07 ± 0.05 | — | |||
| LPS | 0.19 ± 0.05** | 0.11 ± 0.06* | — | ||
| RES | 0.02 ± 0.05 | −0.05 ± 0.04 | −0.17 ± 0.06* | — | |
| SAN | 0.02 ± 0.04 | −0.06 ± 0.05 | −0.17 ± 0.05* | −0.01 ± 0.05 | |
| FBW, kg | |||||
| ENT | — | ||||
| ENV | −0.94 ± 2.06 | — | |||
| LPS | 3.14 ± 4.30 | 4.09 ± 4.33 | — | ||
| RES | −0.88 ± 1.80 | 0.07 ± 1.11 | −4.02 ± 4.23 | — | |
| SAN | −3.58 ± 3.92 | −2.63 ± 3.92 | −6.72 ± 2.70 | −2.70 ± 3.88 | — |
1Symbols (*,**) represent significant differences with 0.05 < P < 0.10, P < 0.05, and P < 0.01, respectively. Values represent the change (Ct − Ch) in each parameter caused by the CM in the left column subtracted from the change caused by the CM in the upper row ± SE.
Figure 5.
Effect of CMs (ENT, ENV, LPS, RES, or SAN) on the relationship between ΔADG and ΔADFI in pigs. Responses are expressed as results of the challenged pigs relative to that of a control group. Lines represent the LRP or the QRP adjustments. Refer to Supplementary File 2 for parameters of model adjustment and significances.
Discussion
The objective of this meta-analysis was to investigate the effects of different challenges on the change in growth performance variables in postweaned pigs and to identify factors that affect the response to CM. To achieve this, a total of 178 studies published between 1985 and 2019 assessing the effects of one of five CMs (ENT, ENV, LPS, RES, or SAN) were gathered. Using HCPC, studies were grouped by similarity in three clusters with increasing IBW, FBW, ADG, and ADFI and decreasing GF from C1 through C3. Linear regression plateau and QRP models were fitted to investigate the effects of CM, cluster, sex, and duration of challenge on the reduction of growth performance variables. To our knowledge, this is the first attempt to use the HCPC model to quantify and explore growth suppression caused by multiple immune CMs in pigs. The benefit of using principal component analysis (PCA) for cluster formation is that correlations can be removed in a variable set, thereby reducing collinearity. The PCA performed produces orthogonal, completely uncorrelated axes as outputs, which can be used subsequently for clustering, in the place of their original variables. In this sense, the PCA is applied to the correlation and not the covariance matrix, which prevents distortion by the different variables (Dormann et al., 2013). More recently, a very similar HCPC approach was used to cluster pig studies according to performance variables and investigate the effects of dietary intervention (Pompeu et al., 2017).
The reduction in ADG, GF, and FBW revealed by our analysis is consistent with the literature, as both stressful conditions and immune system stimulation generally reduce the efficiency of nutrient utilization (Le Floc′h et al., 2004). In general, subclinical levels of disease have been shown to reduce lean tissue growth by 20% to 35% and feed efficiency by 10% to 20% in growing pigs (Williams et al., 1997a, 1997b; Le Floc′h et al., 2009). This is partly explained by a redistribution of nutrients from diet and body reserves toward the support of immune system after exposure to pathogens, toxins, and virulence factors among other stressors (Reeds et al., 1994). The lack of effect of Ch on ADFI suggests that the major component of reduced growth in challenged pigs is due to the changes in nutrient utilization efficiency rather than decreased feed intake (Rakhshandeh et al., 2012). This is in contrast to recent results from a meta-analysis investigating the growth response to disease challenge in pigs, which showed both an effect on nutrient utilization and feed intake on the reduction in growth (Pastorelli et al., 2012). Due to an imbalance between studies within each factor, we could not test for significant interactions between CM, cluster, and sex and, therefore, only main effects are discussed here.
CM effects
The greater reduction in ADG and GF observed in LPS is consistent with the reported substantial cytokine production and consequent sepsis and septic shock associated with this CM (Opal et al., 1999; Alfieri et al., 2012; Wu et al., 2016). Circulating levels of interleukin (IL)-6 are known to drastically increase after LPS injection, which has a direct relationship with the febrile changes of body temperature (LeMay et al., 1990; Roth et al., 1993). In pigs, the model has been used to trigger inflammation (Johnson and von Borell, 1994) leading to BW loss (Jiang et al., 2009; Kang et al., 2014). Recent studies showed altered requirements of various amino acids during LPS challenge in pigs for growth functions (Rakhshandeh et al., 2010; de Ridder et al., 2012; Litvak et al., 2013; Wellington et al., 2018), suggesting that the negative effects of LPS challenge are more related to a change in maintenance requirements/nutrient utilization efficiency than a reduction in feed intake. It is possible that the reduced response in ADG with ENV compared with LPS is due to lack of response in nutrient utilization, with pigs largely responding to high environmental temperature through reduction in feed intake and altering feeding behavior (e.g., reducing meal frequency and duration; Rauw et al., 2020). The severity of LPS compared with RES as a CM is supported by previous meta-analytical findings from Pastorelli et al. (2012) who reported total recovery in RES-challenged pigs, possibly through compensatory growth (Kyriazakis and Emmans, 1992; Lovatto et al., 2000). Furthermore, in response to LPS challenge, pigs experience direct injection of a toxin, whereas, in experimental models of RES, the main findings are usually associated with neutrophilia and damage to cilia (e.g., Blanchard et al., 1992; Gauger et al., 2011). Also, it has been shown that the response associated with infection of pigs with pathogens, such as M. hyopneumoniae, leads to a more tissue-specific response (Thanawongnuwech and Thacker, 2003; Leal Zimmer et al., 2019). Furthermore, SAN is generally used to simulate low-level environmental pathogen exposure and is, therefore, characterized by a continuous and mild stimulation of the immune system. As a result, there is evidence that housing pigs in poor sanitary conditions (e.g., dirty pens) does not always result in reduction in ADG (Gentry, 2001). The efficacy of SAN as a CM may be dependent on a number of factors, such as the pathogens present and pathogen load, source of manure, and other health protocols used. Likewise, differences in response to LPS may be expected depending on the period after which LPS is administered (e.g., short- vs. long-term studies), as LPS tolerance has been observed in pigs following multiple injections, limiting the impact of LPS on performance parameters. Interestingly, no differences between CM were observed for the changes in ADFI (P > 0.10) suggesting that all models were equally detrimental to feed intake. This contrasts with the findings from Pastorelli et al. (2012) showing the strongest response in ADFI for respiratory diseases and digestive bacterial infections in pigs; however, numerical differences were not tested statistically in that study. It should be highlighted that the present meta-analysis contrasted an acute CM (LPS) with more chronic stressors (ENT, ENV, RES, and SAN). Lipopolysaccharide is a one-time injection, while pathogen and environmental challenges occur over time (e.g., after exposure, it takes hours–days for clinical signs to develop in a cascade of action–reactions). We were unable to fit reasonable models (e.g., LRP or QRP) to the relationship between ΔADG or ΔADFI and the duration of experiments for the different CMs, which would give an insight into the adaptation post-challenge initiation and consequently detect differences between acute and chronic models. Moreover, the present meta-analysis presents an inherent inability to test for the interaction between CM and clusters. These investigations merit further attention in future approaches.
The plateau in ΔADG may be interpreted as the reduction in ADG independent of the reduction in ADFI, indicating alterations in maintenance requirements. The slope indicates the reduction in ADG dependent on the reduction in ADFI, indicating alterations in feed efficiency. It can be inferred from our analysis that LPS and ENT have equal components of alterations in maintenance requirements and feed efficiency. Interestingly, Pastorelli et al. (2012) reported no contribution of increased maintenance requirements to reduced growth performance with LPS challenge in a meta-analytical approach. The increased protein degradation rate and decreased protein utilization for body protein retention triggered by LPS may further depress feed efficiency in pigs (Daiwen et al., 2008). There is strong evidence of altered requirements of multiple amino acids for growth in LPS-challenged pigs (Rakhshandeh et al., 2010; de Ridder et al., 2012; Litvak et al., 2013; Wellington et al., 2018), which highlights that maintenance requirements and/or nutrient utilization efficiency may be affected by this CM. It is well-documented that there is reduced barrier function and impaired digestive function with gastrointestinal disease, which likely decreases nutrient utilization efficiency with ENT models (Kim et al., 2012). Also, for RES and SAN, changes in maintenance requirements seem to play a larger role than alterations in feed efficiency on the performance response. Indeed, there is evidence for increased amino acid requirement in both RES- and SAN-challenged pigs (Le Floc′h et al., 2009; Jayaraman et al., 2015; Schweer et al., 2019). Finally, in ENV pigs, the major component was related to changes in feed efficiency rather than maintenance requirements, which agrees with meta-analytical approach by Renaudeau et al. (2011), showing that the primary cause of decreased ADG associated with heat stress is due to a decrease in feed intake. Likewise, Laskoski et al. (2019) reported a positive quadratic effect of decreased number of pigs per feeder hole on feed efficiency, and this was consistent with an increased in ADFI as stocking density decreased.
Cluster effects
HCPC analysis clustered studies into groups (i.e., C1, C2, and C3) based on similarity in IBW, FBW, ADG, ADFI, and GF. The average IBW of pigs in C1, C2, and C3 was 7.62, 19.01, and 46.22 kg, respectively, which is representative of postweaned, nursery, and grower pigs, respectively. Figure 1 shows the variable axis and the spatial distribution of each study related to the first and second principal components with a cumulative inertia of 84.1%. The centroid position of each cluster in these two components allows for comparison between them. Moreover, the angle between variable axes reveals how they are related to each other. The smaller the angle between two variables is, the more positively correlated they are, which may be concluded from the relation between IBW, FBW, and ADFI. Also, it can be inferred from this database that the heavier the pig is at the beginning of the experiment (i.e., higher IBW), the smaller the GF is, as revealed by their opposite directions (negatively correlated). These are expected findings as pigs have increased growth rate and voluntary feed intake and become less efficient over time (Quiniou et al., 2000; Lawlor et al., 2002). Of note, the only data manipulation during the clustering process was selecting data from the Ct group within each study as explained above. The statistical software is allowed to identify where the loss of inertia becomes stable, that is, where there is no detectable difference between clusters (Figure 1b). We detected a clear difference (loss of inertia) between the first two clusters (need for clustering) and a narrower, but still present, difference between the second and third clusters. From the fourth cluster onwards, the difference was not significant (loss of inertia became stable), which explains why three clusters were formed from our database. Interestingly, the clusters automatically generated were representative of weaned, nursery, and grower pigs despite no interference in the clustering process.
Our analysis showed that, regardless of the stage of production (cluster), the major component of reduction in ADG was due to changes in nutrient requirements (Figure 2). Interestingly, the critical value in ΔADFI was only significant for pigs belonging to C1, which means that feed intake level around weaning may be an important component for recovery of detrimental effects of stressors in lighter, postweaned pigs (Spreeuwenberg et al., 2001; Verdonk et al., 2007). This is in agreement with evidence that diminished feed intake is a major contributor to poor performance after weaning and that nutrient supply (e.g., increased intake) may be pivotal for restoring performance (Spreeuwenberg et al., 2001). Furthermore, this is possibly a result of the limited feed intake capacity in newly weaned pigs (i.e., impact of gut fill) and inability to meet growth potential due to limits in feed intake (Dong and Pluske, 2007). Several concurrent stressors are known to impair voluntary feed intake around weaning, including changing from a milk-based to a cereal-based diet (Williams, 2003), depletion of passive immunity from the sows’ secretions (King and Pluske, 2003, Gallois et al., 2009), and mixing with unfamiliar littermates (Moeser et al., 2007).
The relationships between ΔADG, ΔADFI, and the duration of challenge revealed important differences between clusters on the responsiveness to challenge. The plateau in ΔADG or ΔADFI indicates the immediate reduction in each parameter; the slope indicates the significance of the relationship between each parameter and the duration postchallenge (i.e., ΔADG × duration; ΔADFI × duration); and the critical value in duration indicates the time required after which no further attenuation in ΔADFI or ΔADG is achieved. Our database shows that an irreparable portion of growth and feed intake depression was observed only in lighter pigs (i.e., C1 and C2). Moreover, the significance in ΔADG × duration reveals a more immediate decrease in growth postchallenge in C1 pigs, which was not observed in C2 or C3. Likewise, for ADFI, the significance in ΔADFI × duration shows a more immediate reduction in feed intake postchallenge in C1 and C2 pigs but not in C3 pigs. Interestingly, the critical value in duration (plateau in ΔADG) was significant for pigs belonging to C1 and C2 only. Our model showed 15.1, 22.7, and 55.2 as the days required after which no further improvement in ADG was observed. Moreover, the critical value in duration (plateau in ΔADFI) was significant for pigs belonging to C1 and C2, and not in C3 pigs. Taken together, our results suggest that younger pigs (i.e., C1 and C2) experience a more sudden and dramatic decrease in ADG and ADFI, whereas older pigs (i.e., C3) require a longer period for full recovery of ADG and ADFI.
Sex effects
The lower ADG in Ct-F compared with Ct-M confirms previous evidence of 6% to 7% lower growth rate of gilts vs. barrows from weaning until finishing phase (Comstock et al., 1944; Boler et al., 2014; Puls et al., 2014). Interestingly, our analysis revealed that Ch-F showed reduced ADFI compared with Ch-M, regardless of the CM, which corroborates earlier evidence showing that systemic immune stimulation in barrows and gilts led to increased serum tumor necrosis factor-α, IL-6, and haptoglobin levels in the latter compared with the former, which may have a direct effect on reducing feed intake (Williams et al., 2009). Furthermore, inflammatory cytokines are known to compromise animal growth through the stimulation of skeletal muscle proteolysis and adipose tissue lipolysis (Janeway et al., 2001). Furthermore, despite being not significant in the model, F experienced a 2-fold greater decrease in ADG independent of ADFI compared with M, and the change in ΔADFI beyond which there was no improvement in ΔADG (i.e., critical value in Δ ADFI) was 340-fold narrower in F than in M, which is consistent with a greater overall reduction in ADFI of Ch-F compared with Ch-M.
We reported a plateau in ΔADG 4.8 higher in F compared with M, meaning that a greater proportion of the irreparable decrease in growth may be expected in challenged gilts. Our findings suggest that M may be more efficient in recovering ADFI, shortening the ADG recovery time, and enabling a higher plateau in ADG than in females. Recently, Christoforidou et al. (2019) reported that 28-d-old female pigs showed greater potential for local immune regulation (e.g., less antigen-presenting cells and greater regulatory T-cell numbers) compared with males. Moreover, females produced a greater systemic antibody response to injected ovalbumin and dietary soy and synthesized more immunoglobulin A in mesenteric lymph nodes. Likewise, Williams et al. (2009) inoculated barrows and gilts with LPS and reported that the magnitude of pro-inflammatory cytokine response, the magnitude of the norepinephrine response, and the production of serum amyloid A were sex dependent. Despite being speculative, the evidence showing that female pigs have a more robust local immune system and a stronger systemic immune response could reflect increased nutrient requirements, meriting further investigation. Future studies using CM in pigs should carefully consider including sex as a factor of dimorphism in immune development, considering that sex-specific differences in innate immune- and stress-related hormones may be associated with different growth responses to challenge. It is known that differences in growth performance and nutrient requirements between boars, barrows, and gilts generally become more pronounced as they reach heavier weights (Quiniou et al., 2010). Thus, it may be expected that a marked interactive effect of clusters (production stage) and sex would occur on the changes in performance variables between Ch and Ct pigs, even though our database and model did not allow for the analysis of this interaction.
Conclusions and Implications
In the present meta-analysis, we were able to further characterize the differences in performance parameters of immune-challenged pigs as influenced by CM, sex, and production stage (cluster). The HCPC approach represents a valuable tool for the swine industry where representative variables are identified and explored in large, highly correlated datasets of production system. Moreover, clustering techniques are useful for decision-making in the industry, where the ability for daily monitoring of a large number of datasets is limited. Finally, in the future, the determination of nutrient requirements for diseased pigs will largely rely on the estimations of the changes in ADG and ADFI, as well as the time required to reach the plateau in these variables after exposure to challenge, which brings further importance to the present meta-analysis.
In summary, regardless of CM, pigs exposed to immune stimulation had reduced growth performance over the entire BW range of 7 to 77 kg. LPS and ENT seem to result in the most dramatic response in growth, which is most likely the result of the systemic stimulation of the immune system by the former and the direct damage to the gastrointestinal tract by the latter. Female pigs showed greater negative response in performance when compared with male pigs, which corroborates earlier findings of sexual dimorphism regarding immune system development. Further research on CMs should focus on including sex as a major factor of influence when evaluating pig response. Finally, our multivariate approach reveals that postweaned piglets until 15 kg BW were more affected by a challenge than pigs from 19 to 36 and 46 to 76 kg stage of production. Specifically, younger pigs experienced a more sudden and dramatic decrease in ADG and ADFI, whereas older pigs appeared to have a longer period for recovery of these variables reaching a higher plateau. Although we could not test for the interaction between CM, sex, and cluster, the individual effects clearly show that the factors captured in this meta-analysis influence response of growth in challenged pigs.
Supplementary Material
Acknowledgment
The Prairie Swine Centre receives general program funding from the Saskatchewan Ministry of Agriculture, Saskatchewan Pork Development Board, Alberta Pork, Manitoba Pork, and Ontario Pork.
Glossary
Abbreviations
- ∆ADFI
change in ADFI
- ∆ADG
change in ADG
- ADFI
average daily feed intake
- ADG
average daily gain
- AIC
Akaike’s information criterion
- BIC
Schwarz’s Bayesian information criterion
- BW
body weight
- C (1–3)
cluster (1–3)
- Ch
challenged group
- CM
challenge models
- Ct
control group
- ENT
enteric pathogen challenge
- ENV
environmental stressors
- F
female pigs
- FBW
final BW
- GF
gain:feed
- HCPC
hierarchical clustering on principal components
- IBW
initial BW
- LPS
bacterial lipopolysaccharide challenge
- LRP
linear response plateau
- M
male pigs
- Mi
mixed-sex pigs
- PCA
principal component analysis
- PRRSV
porcine reproductive and respiratory syndrome virus
- QRP
quadratic response plateau
- RES
respiratory pathogen challenge
- RMSE
root mean square error
- SAN
sanitary condition challenge
Authors’ Contributions
D.A.C., L.A.R., F.N.A.F., and M.O.C. designed the study; L.A.R. and F.N.A.F. performed the statistical analysis of the data. D.A.C., L.A.R., M.O.C., M.O.W., and D.A.C. wrote the manuscript. The manuscript was read and approved by all authors.
Conflict of interest statement
All the authors declare no conflicts of interest, financial, or otherwise.
Data Availability
The datasets used and analyzed are available from the corresponding author on reasonable request.
Literature Cited
- Alfieri, A., Watson J. J., Kammerer R. A., Tasab M., Progias P., Reeves K., Brown N. J., and Brookes Z. L.. . 2012. Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis. Crit. Care 16:R182. doi: 10.1186/cc11666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D., Mächler M., Bolker B., and Walker S.. . 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67:1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Blanchard, B., Vena M. M., Cavalier A., Le Lannic J., Gouranton J., and Kobisch M.. . 1992. Electron microscopic observation of the respiratory tract of SPF piglets inoculated with Mycoplasma hyopneumoniae. Vet. Microbiol. 30:329–341. doi: 10.1016/0378-1135(92)90020-t [DOI] [PubMed] [Google Scholar]
- Boler, D. D., Puls C. L., Clark D. L., Ellis M., Schroeder A. L., Matzat P. D., Killefer J., McKeith F. K., and Dilger A. C.. . 2014. Effects of immunological castration (Improvest) on changes in dressing percentage and carcass characteristics of finishing pigs. J. Anim. Sci. 92:359–368. doi: 10.2527/jas.2013-6863 [DOI] [PubMed] [Google Scholar]
- Brown, D. C., Maxwell C. V., Erf G. F., Davis M. E., Singh S., and Johnson Z. B.. . 2006. The influence of different management systems and age on intestinal morphology, immune cell numbers and mucin production from goblet cells in post-weaning pigs. Vet. Immunol. Immunopathol. 111:187–198. doi: 10.1016/j.vetimm.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Christoforidou, Z., Mora Ortiz M., Poveda C., Abbas M., Walton G., Bailey M., and Lewis M. C.. . 2019. Sexual dimorphism in immune development and in response to nutritional intervention in neonatal piglets. Front. Immunol. 10:2705. doi: 10.3389/fimmu.2019.02705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock, R. E., Winters L. M., and Cummings J. N.. . 1944. The effect of sex on the development of the pig III. Differences in growth rate between gilts and barrows by lines of breeding. J. Anim. Sci. 3:120–128. doi: 10.2527/jas1944.32120x [DOI] [Google Scholar]
- Daiwen, C., Keying Z., and Chunyan W.. . 2008. Influences of lipopolysaccharide-induced immune challenge on performance and whole-body protein turnover in weanling pigs. Livest. Sci. 113:291–295. doi: 10.1016/j.livsci.2007.06.011 [DOI] [Google Scholar]
- Dee, S., Guzman J. E., Hanson D., Garbes N., Morrison R., Amodie D., and Galina Pantoja L.. . 2018. A randomized controlled trial to evaluate performance of pigs raised in antibiotic-free or conventional production systems following challenge with porcine reproductive and respiratory syndrome virus. PLoS One 13:e0208430. doi: 10.1371/journal.pone.0208430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. Z., and Pluske J. R.. . 2007. The low feed intake in newly-weaned pigs: problems and possible solutions. Asian-Australas. J. Anim. Sci. 20:440–452. doi: 10.5713/ajas.2007.440 [DOI] [Google Scholar]
- Dormann, C. F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., Marquéz J. R. G., Gruber B., Lafourcade B., Leitão P. J., . et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 36:27–46. doi: 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Gallois, M., Rothkötter H. J., Bailey M., Stokes C. R., and Oswald I. P.. . 2009. Natural alternatives to in-feed antibiotics in pig production: can immunomodulators play a role? Animal 3:1644–1661. doi: 10.1017/S1751731109004236 [DOI] [PubMed] [Google Scholar]
- Gauger, P. C., Lager K. M., Vincent A. L., Opriessnig T., Cheung A. K., Butler J. E., and Kehrli M. E. Jr. 2011. Leukogram abnormalities in gnotobiotic pigs infected with porcine circovirus type 2. Vet. Microbiol. 154:185–190. doi: 10.1016/j.vetmic.2011.06.016 [DOI] [PubMed] [Google Scholar]
- Gentry, J. G. 2001. Alternative and outdoor housing systems for pigs: effects on growth, meat quality, and muscle characteristics [doctoral dissertation]. Lubbock (TX): Texas Tech University. [Google Scholar]
- Janeway, C. A., Travers P., Walport M., and Shlomchik M. J.. . 2001. Immunobiology. 5th rev. ed. New York (NY): Garland Science. [Google Scholar]
- Jayaraman, B., Htoo J., and Nyachoti C. M.. . 2015. Effects of dietary threonine:lysine ratios and sanitary conditions on performance, plasma urea nitrogen, plasma-free threonine and lysine of weaned pigs. Anim. Nutr. 1:283–288. doi: 10.1016/j.aninu.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, B., Htoo J. K., and Nyachoti C. M.. . 2017. Effects of different dietary tryptophan: lysine ratios and sanitary conditions on growth performance, plasma urea nitrogen, serum haptoglobin and ileal histomorphology of weaned pigs. Anim. Sci. J. 88:763–771. doi: 10.1111/asj.12695 [DOI] [PubMed] [Google Scholar]
- Jiang, Z. Y., Sun L. H., Lin Y. C., Ma X. Y., Zheng C. T., Zhou G. L., Chen F., and Zou S. T.. . 2009. Effects of dietary glycyl–glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J. Anim. Sci. 87:4050–4056. doi: 10.2527/jas.2008-1120 [DOI] [PubMed] [Google Scholar]
- Johnson, R. W., and von Borell E.. . 1994. Lipopolysaccharide-induced sickness behavior in pigs is inhibited by pretreatment with indomethacin. J. Anim. Sci. 72:309–314. doi: 10.2527/1994.722309x [DOI] [PubMed] [Google Scholar]
- Kang, P., Zhang L., Hou Y., Ding B., Yi D., Wang L., Zhu H., Liu Y., Yin Y., and Wu G.. . 2014. Effects of l-proline on the growth performance, and blood parameters in weaned lipopolysaccharide (LPS)-challenged pigs. Asian-Australas. J. Anim. Sci. 27:1150–1156. doi: 10.5713/ajas.2013.13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. C., Hansen C. F., Mullan B. P., and Pluske J. R.. . 2012. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 173:3–16. doi: 10.1016/j.anifeedsci.2011.12.022 [DOI] [Google Scholar]
- Kim, D., Kim C. H., Han K., Seo H. W., Oh Y., Park C., Kang I., and Chae C.. . 2011. Comparative efficacy of commercial Mycoplasma hyopneumoniae and porcine circovirus 2 (PCV2) vaccines in pigs experimentally infected with M. hyopneumoniae and PCV2. Vaccine 29:3206–3212. doi: 10.1016/j.vaccine.2011.02.034 [DOI] [PubMed] [Google Scholar]
- King, R. H., and Pluske J. R.. . 2003. Nutritional management of the pig in preparation for weaning. In: Pluske, J. R., Le Dividich J., and Verstegen M. W. A., editors. Weaning the pig: concepts and consequences. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 37–51. [Google Scholar]
- Kyriazakis, I., and Emmans G. C.. . 1992. The growth of mammals following a period of nutritional limitation. J. Theor. Biol. 156:485–498. doi: 10.1016/s0022-5193(05)80639-3 [DOI] [PubMed] [Google Scholar]
- Laanen, M., Persoons D., Ribbens S., de Jong E., Callens B., Strubbe M., Maes D., and Dewulf J.. . 2013. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 198:508–512. doi: 10.1016/j.tvjl.2013.08.029 [DOI] [PubMed] [Google Scholar]
- Lan, R., and Kim I.. . 2018. Effects of feeding diets containing essential oils and betaine to heat-stressed growing-finishing pigs. Arch. Anim. Nutr. 72:368–378. doi: 10.1080/1745039X.2018.1492806 [DOI] [PubMed] [Google Scholar]
- Lara, I. S., Carter S. D., Cooper C. V., Aparachita P., Perryman K. R., and Usry J. L.. . 2018. Effects of a chronic lipopolysaccharide challenge on growth performance and immune response of nursery pigs fed differing sources and concentrations of copper, manganese, and Zinc. J. Anim. Sci. 96(Suppl. 2):136. (Abstr.) doi: 10.1093/jas/sky073.251 [DOI] [Google Scholar]
- Laskoski, F., Faccin J. E. G., De Conti E. R., Mellagi A. P., Ulguim R., and Bortolozzo F.. . 2019. Effects of proportion of pigs per feeder hole and stocking density on growth performance and tail and ear biting in the nursery. J. Anim. Sci. 97(Suppl. 2):97. (Abstr.) doi: 10.1093/jas/skz122.174 [DOI] [Google Scholar]
- Lawlor, P. G., Lynch P. B., Caffrey P. J., and Doherty J. V. O.. . 2002. Effect of pre- and post-weaning management on subsequent pig performance to slaughter and carcass quality. Anim. Sci. 75:245–256. doi: 10.1017/S1357729800053005 [DOI] [Google Scholar]
- Lê, S., Josse J., and Husson F.. . 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25:1–18. doi: 10.18637/jss.v025.i01 [DOI] [Google Scholar]
- Le Floc′h, N., Jondreville C., Matte J. J., and Seve B.. . 2006. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the post-weaning period in piglets. Arch. Anim. Nutr. 60:23–34. doi: 10.1080/17450390500467810 [DOI] [PubMed] [Google Scholar]
- Le Floc′h, N., Lebellego L., Matte J. J., Melchior D., and Sève B.. . 2009. The effect of sanitary status degradation and dietary tryptophan content on growth rate and tryptophan metabolism in weaning pigs. J. Anim. Sci. 87:1686–1694. doi: 10.2527/jas.2008-1348 [DOI] [PubMed] [Google Scholar]
- Le Floc′h, N., Melchior D., and Obled C.. . 2004. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livest. Prod. Sci. 87:37–45. doi: 10.1016/j.livprodsci.2003.09.005 [DOI] [Google Scholar]
- Leal Zimmer, F. M. A., Moura H., Barr J. R., and Ferreira H. B.. . 2019. Intracellular changes of a swine tracheal cell line infected with a Mycoplasma hyopneumoniae pathogenic strain. Microb. Pathog. 137:103717. doi: 10.1016/j.micpath.2019.103717 [DOI] [PubMed] [Google Scholar]
- Leandro, G. 2008. Meta-analysis in medical research: the handbook for the understanding and practice of meta-analysis. 2nd rev. ed. Hoboken (NJ): Blackwell Publishing. [Google Scholar]
- LeMay, L. G., Vander A. J., and Kluger M. J.. . 1990. Role of interleukin 6 in fever in rats. Am. J. Physiol. 258(3 Pt 2):R798–R803. doi: 10.1152/ajpregu.1990.258.3.R798 [DOI] [PubMed] [Google Scholar]
- Litvak, N., Rakhshandeh A., Htoo J. K., and de Lange C. F.. . 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi: 10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Lovatto, P. A., Sauvant D., and Milgen J. V.. . 2000. Étude et modélisation du phénomène de croissance compensatrice chez le porc. Journ. Rech. Porc. 32:241–246. [Google Scholar]
- van der Meer, Y., Lammers A., Jansman A. J., Rijnen M. M., Hendriks W. H., and Gerrits W. J.. . 2016. Performance of pigs kept under different sanitary conditions affected by protein intake and amino acid supplementation. J. Anim. Sci. 94:4704–4719. doi: 10.2527/jas.2016-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser, A. J., Klok C. V., Ryan K. A., Wooten J. G., Little D., Cook V. L., and Blikslager A. T.. . 2007. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G173–G181. doi: 10.1152/ajpgi.00197.2006 [DOI] [PubMed] [Google Scholar]
- Opal, S. M., Scannon P. J., Vincent J. L., White M., Carroll S. F., Palardy J. E., Parejo N. A., Pribble J. P., and Lemke J. H.. . 1999. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 180:1584–1589. doi: 10.1086/315093 [DOI] [PubMed] [Google Scholar]
- Pan, L., Zhao P. F., Ma X. K., Shang Q. H., Xu Y. T., Long S. F., Wu Y., Yuan F. M., and Piao X. S.. . 2017. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 95:2627–2639. doi: 10.2527/jas.2016.1243 [DOI] [PubMed] [Google Scholar]
- Park, C., Seo H. W., Han K., and Chae C.. . 2014. Comparison of four commercial one-dose porcine circovirus type 2 (PCV2) vaccines administered to pigs challenged with PCV2 and porcine reproductive and respiratory syndrome virus at 17 weeks postvaccination to control porcine respiratory disease complex under Korean field conditions. Clin. Vaccine Immunol. 21:399–406. doi: 10.1128/CVI.00768-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli, H., van Milgen J., Lovatto P., and Montagne L.. . 2012. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952–961. doi: 10.1017/S175173111100228X [DOI] [PubMed] [Google Scholar]
- Patience, J. F., Rossoni-Serão M. C., and Gutiérrez N. A.. . 2015. A review of feed efficiency in swine: biology and application. J. Anim. Sci. Biotechnol. 6:33. doi: 10.1186/s40104-015-0031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeu, M. A., Rodrigues L. A., Cavalcanti L. F. L., Fontes D. O., and Toral F. L. B.. . 2017. A multivariate approach to determine the factors affecting response level of growth, carcass, and meat quality traits in finishing pigs fed ractopamine. J. Anim. Sci. 95:1644–1659. doi: 10.2527/jas.2016.1181 [DOI] [PubMed] [Google Scholar]
- Puls, C. L., Rojo A., Ellis M., Boler D. D., McKeith F. K., Killefer J., Gaines A. M., Matzat P. D., and Schroeder A. L.. . 2014. Growth performance of immunologically castrated (with Improvest) barrows (with or without ractopamine) compared to gilt, physically castrated barrow, and intact male pigs. J. Anim. Sci. 92:2289–2295. doi: 10.2527/jas.2013-6861 [DOI] [PubMed] [Google Scholar]
- Quiniou, N., Courboulay V., Salaün Y., and Chevillon P.. . 2010. Impact of the non castration of male pigs on growth performance and behaviour- comparison with barrows and gilts. A. M. EAAP. 61:1–7. [Google Scholar]
- Quiniou, N., Dubois S., and Noblet J.. . 2000. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 63:245–253. doi: 10.1016/S0301-6226(99)00135-9 [DOI] [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Rakhshandeh, A., Dekkers J. C., Kerr B. J., Weber T. E., English J., and Gabler N. K.. . 2012. Effect of immune system stimulation and divergent selection for residual feed intake on digestive capacity of the small intestine in growing pigs. J. Anim. Sci. 90(Suppl 4):233–235. doi: 10.2527/jas.53976 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh, A., Htoo J. K., and de Lange C. F. M.. . 2010. Immune system stimulation of growing pigs does not alter apparent ileal amino acid digestibility but reduces the ratio between whole body nitrogen and sulfur retention. Livest. Sci. 134:21–23. doi: 10.1016/j.livsci.2010.06.085 [DOI] [Google Scholar]
- Rauw, W. M., Mayorga E. J., Lei S. M., Dekkers J. C. M., Patience J. F., Gabler N. K., Lonergan S. M., and Baumgard L. H.. . 2017. Effects of diet and genetics on growth performance of pigs in response to repeated exposure to heat stress. Front. Genet. 8:155. doi: 10.3389/fgene.2017.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw, W. M., de Mercado de la Peña E., Gomez-Raya L., García Cortés L. A., Ciruelos J. J., and Gómez Izquierdo E.. . 2020. Impact of environmental temperature on production traits in pigs. Sci. Rep. 10:2106. doi: 10.1038/s41598-020-58981-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds, P. J., Fjeld C. R., and Jahoor F.. . 1994. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J. Nutr. 124:906–910. doi: 10.1093/jn/124.6.906 [DOI] [PubMed] [Google Scholar]
- Renaudeau, D., Gourdine J. L., and St-Pierre N. R.. . 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- de Ridder, K., Levesque C. L., Htoo J. K., and de Lange C. F.. . 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 90:3485–3491. doi: 10.2527/jas.2011-4830 [DOI] [PubMed] [Google Scholar]
- Roberts, N. E., and Almond G. W.. . 2003. Infection of growing swine with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae — effects on growth, serum metabolites, and insulin-like growth factor-I. Can. Vet. J. 44:31–37. [PMC free article] [PubMed] [Google Scholar]
- Roberts, E. S., van Heugten E., Spears J. W., Routh P. A., Lloyd K. L., and Almond G. W.. . 2004. Effects of dietary zinc on performance and immune response of growing pigs inoculated with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Asian-Australas. J. Anim. Sci. 17:1438–1446. doi:2004.17.10.1438 [Google Scholar]
- Roth, J., Conn C. A., Kluger M. J., and Zeisberger E.. . 1993. Kinetics of systemic and intrahypothalamic IL-6 and tumor necrosis factor during endotoxin fever in guinea pigs. Am. J. Physiol. 265(3 Pt 2):R653–R658. doi: 10.1152/ajpregu.1993.265.3.R653 [DOI] [PubMed] [Google Scholar]
- Sauvant, D., Schmidely P., Daudin J. J., and St-Pierre N. R.. . 2008. Meta-analyses of experimental data in animal nutrition. Animal 2:1203–1214. doi: 10.1017/S1751731108002280 [DOI] [PubMed] [Google Scholar]
- Schweer, W. P., Mendoza O. F., Shull C. M., Lehman J., Gaines A. M., Schwartz K. J., and Gabler N. K.. . 2019. Increased lysine: metabolizable energy ratio improves grower pig performance during a porcine reproductive and respiratory syndrome virus challenge. Transl. Anim. Sci. 3:393–407. doi: 10.1093/tas/txy108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweer, W. P., Schwartz K., Burrough E. R., Yoon K. J., Sparks J. C., and Gabler N. K.. . 2016. The effect of porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus challenge on growing pigs I: growth performance and digestibility. J. Anim. Sci. 94:514–522. doi: 10.2527/jas.2015-9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweer, W., Schwartz K., Patience J. F., Karriker L., Sparks C., Weaver M., Fitzsimmons M., Burkey T. E., and Gabler N. K.. . 2017. Porcine reproductive and respiratory syndrome virus reduces feed efficiency, digestibility, and lean tissue accretion in grow-finish pigs. Transl. Anim. Sci. 1:480–488. doi: 10.2527/tas2017.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreeuwenberg, M. A., Verdonk J. M., Gaskins H. R., and Verstegen M. W.. . 2001. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 131:1520–1527. doi: 10.1093/jn/131.5.1520 [DOI] [PubMed] [Google Scholar]
- St-Pierre, N. R. 2001. Invited Review: Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 84:741–755. doi: 10.3168/jds.S0022-0302(01)74530-4 [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech, R., and Thacker E. L.. . 2003. Interleukin-10, interleukin-12, and interferon-gamma levels in the respiratory tract following Mycoplasma hyopneumoniae and PRRSV infection in pigs. Viral Immunol. 16:357–367. doi: 10.1089/088282403322396154 [DOI] [PubMed] [Google Scholar]
- Verdonk, J. M. A. J., Bruininx E. M. A. M., van der Meulen J., and Verstegen M. W. A.. . 2007. Post-weaning feed intake level modulates gut morphology but not gut permeability in weaned piglets. Livest. Sci. 108:146–149. doi: 10.1016/j.livsci.2007.01.093 [DOI] [Google Scholar]
- Vesterinen, H. M., Sena E. S., Egan K. J., Hirst T. C., Churolov L., Currie G. L., Antonic A., Howells D. W., and Macleod M. R.. . 2014. Meta-analysis of data from animal studies: a practical guide. J. Neurosci. Methods 221:92–102. doi: 10.1016/j.jneumeth.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Wastell, M. E., Garbossa C. A. P., and Schinckel A. P.. . 2018. Effects of wet/dry feeder and pen stocking density on grow-finish pig performance. Transl. Anim. Sci. 2:358–364. doi: 10.1093/tas/txy073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, M. O., Agyekum A. K., Hamonic K., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2019. Effect of supplemental threonine above requirement on growth performance of Salmonella Typhimurium challenged pigs fed high-fiber diets. J. Anim. Sci. 97:3636–3647. doi: 10.1093/jas/skz225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, M. O., Hamonic K., Krone J. E. C., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2020. Effect of dietary fiber and threonine content on intestinal barrier function in pigs challenged with either systemic E. coli lipopolysaccharide or enteric Salmonella typhimurium. J. Anim. Sci. Biotechnol. 11:38. doi: 10.1186/s40104-020-00444-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, M. O., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2018. Impact of dietary fiber and immune system stimulation on threonine requirement for protein deposition in growing pigs. J. Anim. Sci. 96:5222–5232. doi: 10.1093/jas/sky381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. 2016. ggplot2: elegant graphics for data analysis. 2nd rev. ed. Houston (TX): Springer. [Google Scholar]
- Williams, I. H. 2003. Growth of the weaned pig. In: Pluske, J. R., Le Dividich J., and Verstegen M. W. A., editors. Weaning the pig: concepts and consequences. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 17–37. [Google Scholar]
- Williams, P. N., Collier C. T., Carroll J. A., Welsh T. H. Jr, and Laurenz J. C.. . 2009. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest. Anim. Endocrinol. 37:139–147. doi: 10.1016/j.domaniend.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Williams, N. H., Stahly T. S., and Zimmerman D. R.. . 1997a. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J. Anim. Sci. 75:2481–2496. doi: 10.2527/1997.7592481x [DOI] [PubMed] [Google Scholar]
- Williams, N. H., Stahly T. S., and Zimmerman D. R.. . 1997b. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J. Anim. Sci. 75:2472–2480. doi: 10.2527/1997.7592472x [DOI] [PubMed] [Google Scholar]
- Wu, H., Liu J., Li W., Liu G., and Li Z.. . 2016. LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway. Biochem. Biophys. Res. Commun. 471:240–246. doi: 10.1016/j.bbrc.2016.01.117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed are available from the corresponding author on reasonable request.