Abstract
Robotic pancreaticoduodenectomy (PD) remains one of the most advanced robotic procedures. Improved ergonomics and stable 3D vision with robotic platform helped overcome the technical challenges of pancreatic reconstruction in minimally invasive PD. However, inadequate understanding of the complex vascular anatomy of the pancreatic head and uncinate process often results in intra-operative bleeding and prolongs the learning curve. The technique of precise identification and systematic control of the vessels supplying the head and the uncinate process is described in this report. A good understanding of the common vascular anatomy and variations along with stepwise precise vascular control described in this report could minimise intra-operative bleeding and shorten the learning curve associated with robotic PD.
Keywords: Laparoscopic, pancreatic cancer, pancreaticoduodenectomy, periampullary tumour, robotic
INTRODUCTION
Minimally invasive pancreaticoduodenectomy (PD) using the laparoscopic approach was first reported by Gagner and Pomp in 1994.[1] However, due to the complexity and technical challenges during resection and reconstruction, laparoscopic PD has not become a standard procedure.[2] The superior dexterity of the endowristed instruments, stable high definition 3D vision, and tremor filtration available with the robotic platform can potentially overcome some of the limitations of laparoscopic PD, especially during reconstruction. However, an incomplete understanding of the complex vascular anatomy of the head and uncinate process of the pancreas can pose technical difficulties and bleeding during the resection phase in minimally invasive PD. The technique of precise and systematic vascular control of the branches of the superior mesenteric artery (SMA) and vein (SMV) during robotic PD is described in this report.
PRE-OPERATIVE PREPARATION
Pancreatic protocol computed tomography (CT) is the preferred staging investigation in patients with periampullary and pancreatic tumours who do not have any metastasis on clinical evaluation. Pre-operative endoscopic biliary drainage is done in patients with cholangitis, hypoalbuminaemia requiring nutritional optimisation and serum bilirubin level >15 mg/dL. Pancreatic and periampullary tumours without evidence of vascular involvement were considered for robotic PD. Staging laparoscopy is done to rule out metastasis before docking of robotic arms.
POSITIONING OF PATIENT AND PORTS
The procedure was performed with the patient in supine with a split leg position. The Xi robotic system is used, and the patient cart is docked from the right side of the patient. The bedside assistant surgeon stands between the patient's legs. Four 8-mm robotic trocars are placed in a straight horizontal line at the level of the umbilicus with at least 6 cm distance between trocars [Figure 1]. A 12 mm assistant trocar is placed in the infraumbilical region. During uncinate dissection, the camera is moved to R2, and the assistant trocar is converted to R3 using trocar in the trocar technique. In this technique, the robotic trocar is placed inside the assistant 12 mm trocar and docked to the robotic arm without having to change the trocar [Figure 1].
Figure 1.
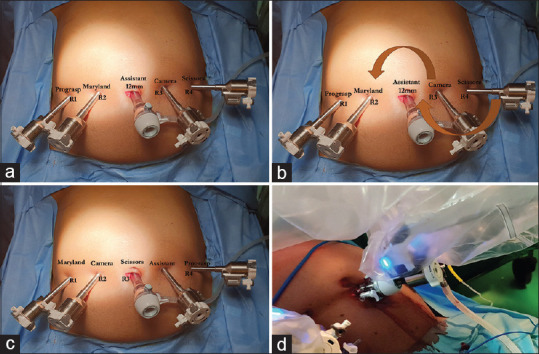
(a) The trocar position and robotic instruments used for control of gastrocolic trunk and dissection of the hepatoduodenal ligament. (b) Change in position of the camera and robotic instruments (arrows) for uncinate dissection. (c) Robotic instruments used during uncinate dissection and pancreatic transection. (d) Trocar in trocar technique. The robotic trocar is placed inside the assistant 12 mm trocar and docked to the robotic arm
OPERATIVE STEPS
Sequence of vascular control
The arterial supply of the head and uncinate process of the pancreas is from the superior and inferior pancreaticoduodenal arteries that form an arcade along the medial border of the duodenum [Figure 2]. The anterosuperior (ASPDA) and posterosuperior (PSPDA) pancreaticoduodenal arteries originate from the gastroduodenal artery (GDA). The anteroinferior (AIPDA) and posteroinferior pancreaticoduodenal (PIPDA) arteries often arise as a common trunk along with the first jejunal artery from the SMA. Four veins drain the head and the uncinate process of the pancreas [Figure 2]. Anterosuperior pancreaticoduodenal vein (ASPDV) joins with right gastroepiploic and right colic vein to form a gastrocolic trunk that drains to SMV. Posterosuperior pancreaticoduodenal vein (PSPDV) drains directly to the portal vein. Posteroinferior pancreaticoduodenal vein (PIPDV) flows to the first jejunal vein, which travels posterior to SMA and joins the right posterior wall of SMV. The drainage of the anteroinferior pancreaticoduodenal vein (AIPDV) is variable either directly to SMV or to the first jejunal vein. In the present technique, ASPDV is controlled first, followed by ASPDA and PSPDA (by ligating GDA). This is followed by the control of AIPDV and PIPDV. Before pancreatic neck transection, AIPDA and PIPDA are controlled by ligating IPDA. PSPDV is ligated at the end to prevent vascular congestion of the uncinate region [Figure 2].
Figure 2.
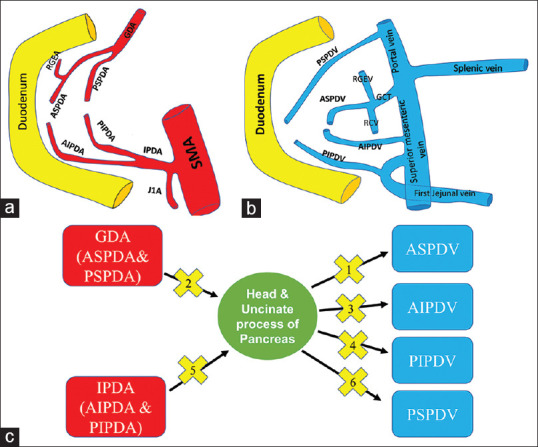
(a) The arterial supply of the head and uncinate process of the pancreas. The anterosuperior (ASPDA), posterosuperior (PSPDA), anteroinferior (AIPDA) and posteroinferior pancreaticoduodenal arteries (b) venous drainage of the pancreas. Anterosuperior pancreaticoduodenal vein, anteroinferior pancreaticoduodenal vein, posterosuperior pancreaticoduodenal vein, posteroinferior pancreaticoduodenal vein (anterosuperior pancreaticoduodenal vein). (c) Sequence of vascular control in the present technique. The numbers within the cross symbol indicate the sequence of vascular control
Control of anterosuperior pancreaticoduodenal vein
The gastrocolic omentum is divided to enter the lesser sac and visualise the anterior surface of the pancreas and posterior gastric wall. The fusion fascia of two posterior layers of the greater omentum and transverse mesocolon close to the antropyloric region is opened to identify the right gastroepiploic vein and right colic vein [Figure 3]. The right gastroepiploic vein is further dissected to identify its confluence with the ASPDV and the formation of a gastrocolic trunk. Control of ASPDV is achieved by ligation and division of the gastrocolic trunk close to SMV [Figure 3]. Rarely when ASPDV directly joins SMV, it needs to be identified and separately divided.
Figure 3.
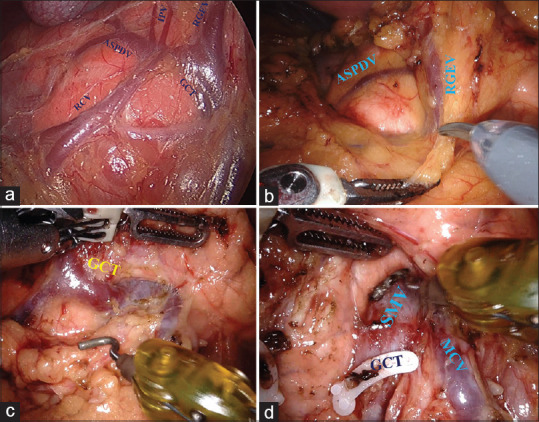
(a) Typical anatomy of anterosuperior pancreaticoduodenal vein. Anterosuperior pancreaticoduodenal vein joins with right gastroepiploic (RGEV) and right colic vein to form a gastrocolic trunk. Occasionally infrapyloric veins joins at the confluence of RGEV and anterosuperior pancreaticoduodenal vein. (b) The fusion fascia (pointed by scissors) of two posterior layers of the greater omentum and transverse mesocolon is opened to identify RGEV, right colic vein and gastrocolic trunk. (c) Gastrocolic trunk is draining into the superior mesenteric vein. (d) Gastrocolic trunk divided and infrapancreatic superior mesenteric vein exposed. Middle colic vein joining SMV could be visualised
Control of anterosuperior pancreaticoduodenal artery and posterosuperior pancreaticoduodenal artery
The control of ASPDA and PSPDA is achieved by ligation and division of GDA. The dissection of the hepatic artery node facilitates the identification of the common hepatic artery and GDA. Small supraduodenal vessels arising close to the origin of GDA needs to be controlled with energy device to avoid bleeding during looping of the GDA. As PSPDA arises from the proximal portion of GDA, it should be divided proximal to the origin of PSPDA [Figure 4]. ASPDA is the terminal branch of the GDA after the origin of the right gastroepiploic artery. Suprapancreatic portal vein lies in the floor of the triangle formed by the upper border of the pancreas, common hepatic artery and GDA [Figure 4].
Figure 4.
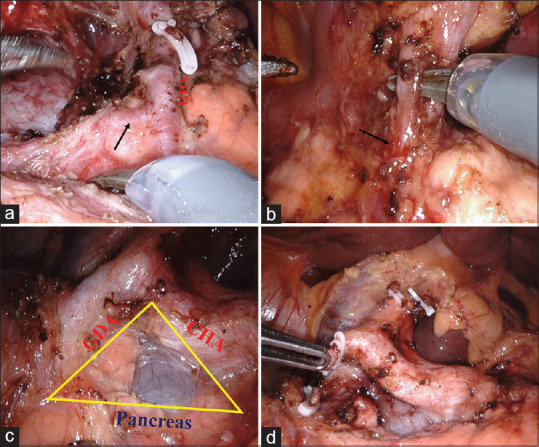
(a) Posterosuperior pancreaticoduodenal artery arises from the proximal portion (arrow) of the gastroduodenal artery. (b) Anterosuperior pancreaticoduodenal artery is the terminal branch (arrow) of gastroduodenal artery along with the right gastroepiploic artery (dissected with scissors). (c) Suprapancreatic portal vein lies in the triangle formed by gastroduodenal artery, common hepatic artery and upper border of the pancreas. (d) Gastroduodenal artery ligated and divided to control PSPDA and ASPDA
Control of anteroinferior pancreaticoduodenal vein
The AIPDV is controlled after mobilisation of duodenojejunal flexure and delivery of proximal jejunum to the supracolic department. This step straightens the embryological mesenteric twist that forms because of small bowel rotation and allows proximal jejunal mesentery and the uncinate process of the pancreas (along with mesopancreas) to lie in the same plane [Figure 5]. The AIPDV, if draining directly to SMV, can be controlled at this stage [Figure 5].
Figure 5.
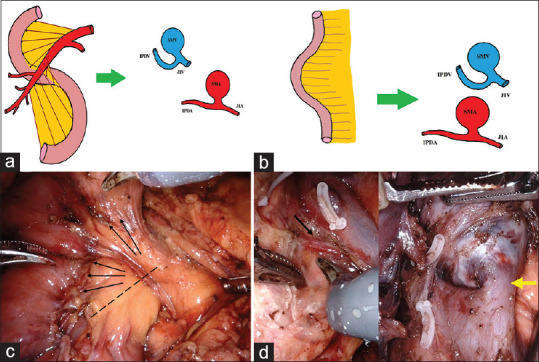
(a) Mesenteric twist resulting from small bowel rotation places the uncinate process and proximal jejunal mesentery in a different direction. (b) Mobilisation and delivery of proximal jejunum to the supracolic department allows jejunal mesentery and uncinate process to lie in the same plane. (c) Both lie in the same plane (marked by arrows). The dotted line indicates the line of transection to facilitate complete uncinate and mesopancreas dissection (d) normal-sized AIPDV (black arrow in the left picture) and large AIPDV draining to superior mesenteric vein (yellow arrow in the right picture)
Control of posteroinferior pancreaticoduodenal vein
The jejunal mesentery is divided on a diagonal line towards the first jejunal vein and not close to the bowel wall. This facilitates complete dissection of station 17 nodes, which are the first group of involved lymph nodes in periampullary and pancreatic cancer.[3] Once the mesenteric division reaches the first jejunal vein, further dissection is done along the wall of the first jejunal vein. PIPDV joining the first jejunal vein close to its confluence with SMV is ligated and divided [Figure 6]. Rarely, PIPDV drains directly to SMV [Figure 6].
Figure 6.
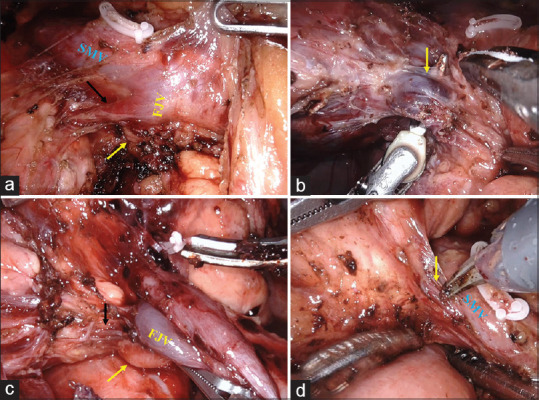
(a) Posteroinferior pancreaticoduodenal vein joining first jejunal vein close to its confluence with superior mesenteric vein. The first jejunal artery lies posterior to posteroinferior pancreaticoduodenal vein (arrow). (b) Enlarged posteroinferior pancreaticoduodenal vein (arrow) in a patient with a large lesion in the head of the pancreas. (c) Small posteroinferior pancreaticoduodenal vein (black arrow) joining first jejunal vein that can be easily controlled with energy devices. Inferior pancreaticoduodenal artery lies posterior to first jejunal vein (yellow arrow). (d) posteroinferior pancreaticoduodenal vein (arrow) draining directly to superior mesenteric vein
Control of anteroinferior pancreaticoduodenal artery and posteroinferior pancreaticoduodenal artery
The division of PIPDV provides access to the SMA plane. As mentioned previously, AIPDA and PIPDA often originate as a common trunk along with the first jejunal artery from the SMA [Figure 7]. The main trunk of IPDA is ligated and divided after dissecting it off the first jejunal artery. Rarely, AIPDA and PIPDA originate separately from the SMA [Figure 7]. Control of IPDA facilitates the dissection of the remaining portion of the uncinate process from SMA.
Figure 7.
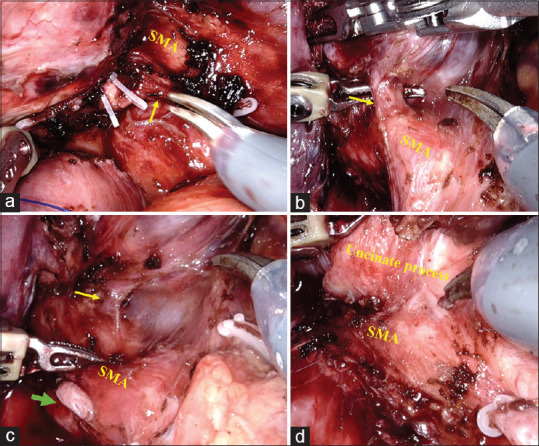
(a) Clipping of the main trunk of the inferior pancreaticoduodenal artery arising from the superior mesenteric artery along with the first jejunal artery (arrow). (b) Inferior pancreaticoduodenal artery (arrow) originate directly from superior mesenteric artery. (c) Posteroinferior pancreaticoduodenal artery (clipped and marked with the green arrow) and anteroinferior pancreaticoduodenal artery, marked with yellow arrow) originate separately from the superior mesenteric artery. (d) Dissection of the remaining portion of the uncinate process from superior mesenteric artery is completed after the control of inferior pancreaticoduodenal artery
Control of posterosuperior pancreaticoduodenal vein
The pancreatic neck is transected after the control of the IPDA. The remaining portion of the head and uncinate process of the pancreas is dissected off the portal vein and SMA. At the superior part of the pancreas, PSPDV is ligated and divided [Figure 8]. Occasionally, more than one PSPDV might be draining to the portal vein.
Figure 8.
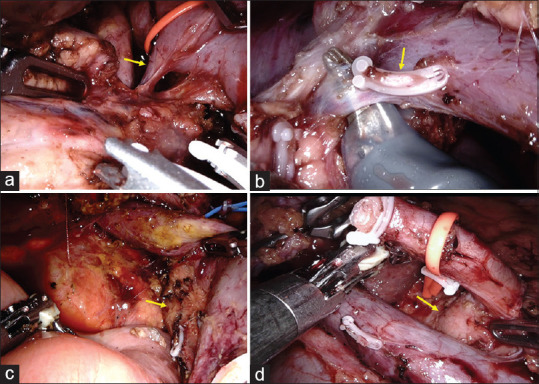
(a) Posterosuperior pancreaticoduodenal vein (arrow) draining to the portal vein. (b) Two posterosuperior pancreaticoduodenal veins draining to the portal vein. The first one clipped (arrow), and the second one dissected with scissors. (c) Post-resection with complete clearance of the superior mesenteric artery (arrow). (d) Post-resection and lymphadenectomy with skeletonisation of the portal vein and hepatic artery till the right side of celiac trunk (arrow)
Reconstruction
Pancreaticojejunostomy was performed using the modified Blumgart technique. Hepaticojejunostomy was performed in a single layer using 4/0 polydioxanone suture. Antecolic stapled gastrojejunostomy was completed, and the stapler entry site was closed in two layers using 3/0 polydioxanone and polypropylene suture. Feeding jejunostomy was routinely performed in all patients. Two closed suction drains are placed close to pancreaticojejunostomy and hepaticojejunostomy.
POST-OPERATIVE CARE
On the 1st post-operative day, enteral feeds are given through feeding jejunostomy. Oral feeds are started on the 3rd or 4th post-operative day after removal of the nasogastric tube. Drains are removed on the 3rd post-operative day if the drain fluid amylase is within the normal limits, and drain volume is <100 mL. Of the 43 patients who underwent minimally invasive PD at our centre, the technique of precise vascular control during robotic PD was used in 20 patients. The clinical details and the operative parameters of 20 patients who underwent PD utilising this technique are summarised in Table 1.
Table 1.
Demographic and operative parameters of patients (n=20) who underwent robotic pancreaticoduodenectomy with precise vascular control technique
| Parameter | Value |
|---|---|
| Age, median (range) | 47 (40-67) |
| Gender (male/female) | 9/11 |
| Disease, n (%) | |
| Ampullary carcinoma | 9 (45.0) |
| Bile duct carcinoma | 5 (25.0) |
| Pancreatic carcinoma | 5 (25.0) |
| Pancreatic neuroendocrine tumor | 1 (5.0) |
| Operative time (min), median (range) | 400 (360-510) |
| Resection time (min), median (range) | 235 (210-320) |
| Blood loss (mL), median (range) | 225 (125-650) |
| Conversion, n (%) | 0 |
| Post-operative complications (≥ Clavien Dindo IIIa),n (%) | 5 (25.0) |
| Post-operative hospital stay (days), median (range) | 8 (6-16) |
DISCUSSION
The precise vascular control technique described in this report allows standardisation of resection part of minimally invasive PD. The long learning curve associated with laparoscopic PD is due to difficult reconstruction and inadequate understanding of the complex vascular anatomy of the pancreatic head and uncinate process. The superior ergonomics with robotic surgery helped to overcome some of the technical challenges associated with reconstruction, especially pancreaticojejunostomy. However, bleeding-related complications, particularly from the pancreaticoduodenal veins during the uncinate dissection, remain common intra-operative complications. Studies have reported that surgical proficiency in robotic PD is achieved after 80 cases.[4] The stepwise control of vessels described in this report could minimise the bleeding complications and shorten the learning curve associated with robotic PD. Furthermore, the systematic approach, along with a dual console, facilitates the training of surgical residents in robotic PD.
The technique described in this report is based on the standard vascular anatomy of the pancreaticoduodenal vessels. While a complete discussion of the variations of vascular anatomy is beyond the scope of this report, it is important to understand common anatomical variations. Variations in the branching pattern of GDA to PSPDA and ASPDA is less common compared to the origin of IPDA. As the first jejunal vein is preserved in the present technique, IPDA is divided distal to the origin of the first jejunal artery when it originates as a common trunk from SMA. Studies have shown that ligation of IPDA before pancreatic transection reduces congestion and blood loss during PD.[5,6] In the open PD with IPDA first approach, it is easier to identify the root of the IPDA on the left side of the SMA.[6] However, the caudal view in a minimally invasive approach provides better visualisation of the right side of SMA. A direct approach to the right side of the SMA and IPDA can result in bleeding from inferior pancreaticoduodenal veins. Hence, in this technique, control of AIPDV and PIPDV is recommended before approaching the IPDA on the right side of SMA. The drainage pattern of AIPDV and PIPDV is more variable compared to ASPDV and PSPDV. Studies based on multidetector-row CT with multiplanar reformation have reported that AIPDV and PIPDV often join to form a common trunk and then drain to the first jejunal vein as a single inferior pancreaticoduodenal vein.[7,8] However, in cadaveric studies, separate drainage of AIPDV and PIPDV was identified as a common pattern.[9,10] AIPDV can be easily recognised as a distinct vein when it drains to SMV. However, when it drains separately to the first jejunal vein, it is not easy to differentiate it from PIPDV. In such a scenario, it is often interpreted as more than one PIPDV draining to the first jejunal vein. The sequence of vascular control described in this study is applicable to both laparoscopic and robotic PD.
CONCLUSION
A good understanding of the common vascular anatomy and variations along with stepwise precise vascular control described in this report could minimise intra-operative bleeding and shorten the learning curve associated with robotic PD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–10. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 2.van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): A multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4:199–207. doi: 10.1016/S2468-1253(19)30004-4. [DOI] [PubMed] [Google Scholar]
- 3.Tashiro K, Kuroki N, Einama T, Iwasaki O, Miyata Y, Aosasa S, et al. Prognostic significance of regional lymph node metastasis according to station in ampullary carcinoma. J Hepatobiliary Pancreat Sci. 2020;27:712–20. doi: 10.1002/jhbp.791. [DOI] [PubMed] [Google Scholar]
- 4.Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: Identification of the learning curve. JAMA Surg. 2015;150:416–22. doi: 10.1001/jamasurg.2015.17. [DOI] [PubMed] [Google Scholar]
- 5.Horiguchi A, Ishihara S, Ito M, Nagata H, Shimizu T, Furusawa K, et al. Pancreatoduodenectomy in which dissection of the efferent arteries of the head of the pancreas is performed first. J Hepatobiliary Pancreat Surg. 2007;14:575–8. doi: 10.1007/s00534-006-1198-x. [DOI] [PubMed] [Google Scholar]
- 6.Ohigashi H, Ishikawa O, Eguchi H, Yamada T, Sasaki Y, Noura S, et al. Early ligation of the inferior pancreaticoduodenal artery to reduce blood loss during pancreaticoduodenectomy. Hepatogastroenterology. 2004;51:4–5. [PubMed] [Google Scholar]
- 7.Hongo N, Mori H, Matsumoto S, Okino Y, Ueda S, Shuto R. Anatomical variations of peripancreatic veins and their intrapancreatic tributaries: Multidetector-row CT scanning. Abdom Imaging. 2010;35:143–53. doi: 10.1007/s00261-007-9195-3. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Mori H, Kiyosue H, Matsumoto S, Hori Y, Maeda T. CT assessment of the inferior peripancreatic veins: Clinical significance. AJR Am J Roentgenol. 2000;174:677–84. doi: 10.2214/ajr.174.3.1740677. [DOI] [PubMed] [Google Scholar]
- 9.Ibukuro K. Vascular anatomy of the pancreas and clinical applications. Int J Gastrointest Cancer. 2001;30:87–104. doi: 10.1385/IJGC:30:1-2:087. [DOI] [PubMed] [Google Scholar]
- 10.Kimura W. Surgical anatomy of the pancreas for limited resection. J Hepatobiliary Pancreat Surg. 2000;7:473–9. doi: 10.1007/s005340070017. [DOI] [PubMed] [Google Scholar]


