Abstract
Purpose
With improved technology, more patients with nasopharyngeal cancer (NPC) are receiving definitive treatment with proton therapy, which allows greater sparing of dose to normal tissues without compromising efficacy. As there is no randomized data, the purpose of this study was to systematically review the available literature on proton therapy in this setting, focusing on the toxicity endpoints.
Materials and Methods
A systematic search using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines was conducted in 5 databases: PubMed, Embase, SCOPUS, Web of Science, and the Cochrane Central Register of Controlled Trials. A total of 491 studies were found on the topic of NPC and proton therapy. Following independent study selection by 2 investigators, 9 studies were found to have sufficient focus and relevance to be incorporated into the systematic review.
Results
All 9 studies were retrospective and examined only NPC patients except for one that also included paranasal sinus cancer. One study was a reirradiation study. Four studies used 3D or double scatter technique, while all others used intensity-modulated proton therapy. Oncologic outcomes were similar to intensity-modulated radiation therapy (IMRT) rates, with 2-year local and regional progression-free survival (LRFS) ranging from 84% to 100%, 2-year progression-free survival (PFS) ranging from 75% to 88.9%, and 2-year overall survival (OS) ranging from 88% to 95% in the up-front setting. Four comparison studies with IMRT found significantly lower feeding tube rates (20% versus 65%, P = .015; and 14% versus 85%, P < .001) with proton therapy as well as lower mucositis (G2 46% versus 70%, P = .019; and G3 11% versus 76%, P = .0002). All other acute and late effects were largely improved with proton therapy but not statistically significant.
Conclusions
NPC patients receiving proton therapy maintain good outcomes with improved toxicity profile, likely due to sparing of dose to normal structures. Prospective studies are ongoing to better quantify the magnitude.
Keywords: nasopharyngeal cancer, proton therapy, systematic review
Introduction
Nasopharyngeal carcinoma (NPC) comprises 0.6% of all cancers worldwide and is an endemic entity in Asia [1]. Owing to its anatomic location, the mainstay of treatment is a nonsurgical approach with radiation therapy alone for stage I and definitive chemoradiation for stage II and higher. Local control remains excellent at 90% [2–4], thus the focus has been on improving the toxicity profile from treatment as patients are living longer.
Prior to technologic advances, a survey study on the quality of life of patients with head and neck cancer found that NPC patients had the highest morbidity [5] likely due to the treatment fields encompassing salivary glands, pterygoid muscles, and temporomandibular joints. Another survey of NPC survivors found that xerostomia, hearing impairment, dysphagia, and trismus were the most common late sequelae following conventional radiation [6, 7]. Moreover, as compared to other patients with head and neck cancer, NPC patients are more likely to develop neurocognitive decline [8, 9]. Since then, intensity-modulated radiation therapy (IMRT) has been widely adopted following results from the Radiation Therapy Oncology Group (RTOG) phase II trial 0225 [2], which showed minimal toxicity yielding improved compliance rates throughout treatment, conferring benefit to local control.
While the increased conformality with IMRT allows full therapeutic dose to tumors near critical structures, there is still significant toxicity due to the entrance and exit dose in the beam paths [10]. Fortunately, proton therapy can further improve the therapeutic ratio as the radiation is able to penetrate to the depth of the target with little to no exit dose beyond it [11]. The technology with proton therapy has evolved where intensity-modulated proton therapy (IMPT) further reduces toxicity without compromising efficacy [12]. IMPT can now be delivered with spot-scanning, which scans the target without introducing scatter outside the beam path and further conforms the dose to target volumes by using different energies within the spots.
Planning comparison studies have shown dosimetric advantages of IMPT over IMRT with respect to delivering lower doses to organs at risk in NPC [13, 14]. Whether this translates into clinically meaningful endpoints showing reduction in acute and late toxicity is still unclear. To our knowledge, there are no prospective studies or published randomized trials evaluating the efficacy and toxicity of proton therapy in NPC even though proton therapy is rapidly increasing in use for this subsite. Our aim was therefore to perform a systematic review of the available literature to address the utility of proton therapy for adult patients with NPC, focusing on the toxicity profile.
Materials and Methods
Literature Search Strategy
This systematic review was conducted by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. Literature searches were conducted on August 6, 2020, in 5 bibliographic databases by a medical librarian: PubMed, Embase (Embase.com), SCOPUS, Web of Science, and the Cochrane Central Register of Controlled Trials. The search strategy included components related to NPC and proton therapy. The inclusion criteria were broad initially to allow screening at the individual level. The search terms used were subject headings (MeSH, Emtree) and/or keywords in the title, abstract, and author keywords fields. Boolean operators ‘OR' and ‘AND' were used to combine the search terms and the search strategy components. Details of the search strategy are presented separately (see Appendix). The searches in all databases except for Cochrane CENTRAL were limited to the English language. Deduplication of search results from different databases was done with Covidence systematic review management software (Veritas Health Innovation, Melbourne, Australia).
Study Selection
Based on the initial search, 491 studies were identified after duplicates were removed. Two reviewers (A.L. and S.K.) independently screened all studies, and 48 full-text studies were assessed for eligibility. The exclusion criteria were single case studies, review articles, abstracts, animal or phantom studies, pediatric studies, adenoid cystic carcinoma of the nasopharynx studies, and those that did not include toxicity endpoints. Studies including other heavy ion particles such as carbon and multiple head and neck subsites were also excluded, as endpoints were not reported separately for NPC patients receiving proton therapy. Comparison and reirradiation studies were included as well as those examining proton boost following photon therapy. No limit was imposed for the size of the cohort. All disagreements were resolved through discussion until consensus was reached. Thus, 9 original studies were incorporated into the systematic review. The selection process and inclusion of references in the systematic review is shown in the Figure.
Figure.
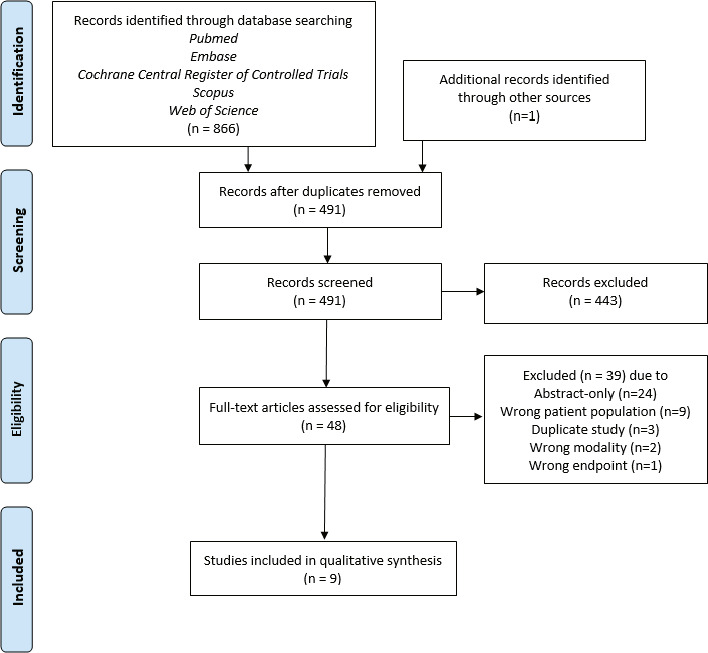
PRISMA diagram of the selection process and inclusion in the systematic review. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data Extraction
The following details were extracted from the included papers: authors, year of publication, type of study, number of patients, type of proton therapy, prescribed dose, number of fractions, systemic therapy (neoadjuvant, concurrent, adjuvant), stage, and follow-up period. The variations found in units of dose were kept as reported. Acute and late toxicity rates were the primary outcomes of interest, while local and regional progression-free survival (LRFS), progression-free survival (PFS), and overall survival (OS) were secondary endpoints. Any missing data were indicated as such.
Results
As shown in the Figure, 9 total studies met criteria for data extraction, which included a total of 369 patients with a median follow-up of 25 months (range, 10–98 months). Details of the studies included in this review are provided in Table 1. All studies were of NPC patients except for one that also included patients treated for paranasal sinus primaries [16]. Four studies originated from the United States, 2 from Italy, 1 from France, 1 from the Czech Republic, and 1 from South Korea. All had at least 10 patients in the cohort and were entirely retrospective. Four studies were matched where patients receiving proton therapy were compared to patients receiving photon therapy [16, 17, 21, 23]. Three used a mixed-beam approach with photons followed by a proton therapy boost [19, 21, 23,]. Four of the 9 studies used 3D or double scatter while all others used IMPT. Prescribed doses ranged from 68.4 to 78 GyE except for 1 study from Italy examining reirradiation outcomes for recurrent NPC where prescribed dose was 60 GyE [20]. Concurrent chemotherapy was given in all 9 studies; 7 administered neoadjuvant systemic treatment and 4 gave adjuvant chemotherapy as well. Median follow-up for up-front radiation studies was 25.8 months and ranged from 21 to 98 months. With respect to oncologic endpoints, all studies except 1 reported either LRFS, PFS, OS, or a combination. As expected, outcomes were similar to IMRT rates with 2-year LRFS ranging from 84% to 100%, 2-year PFS ranging from 75% to 88.9%, and 2-year OS ranging from 88% to 95%. For the reirradiation study, the reported rate for 2-year LRFS was 72.9% and for OS, 59.3% [20].
Table 1.
NPC and proton therapy study characteristics ordered by year published.
|
Study (year; country) |
Type |
N |
Modality |
Prescribed dose |
No. fraction |
Systemic therapy |
Stage |
Median FU, mo |
Outcomes at 2 y |
|||||||
| % NACT |
% Con |
% Adj |
T1-2 |
T3-4 |
N0-1 |
N2-3 |
LRFS |
PFS |
OS |
|||||||
| Holliday et al (2015; USA) [17] | Retrospective, matched | 10 20 | IMPT IMRT | 70 GyE 70 Gy | 33–35 33–35 | 80 85 | 100 90 | 10 0 | 60 55 | 40 45 | 40 35 | 60 65 | 21.6 25.8 | N = 10a N = 19a | N = 9a N = 19a | |
| Lewis et al (2016; USA) [18] | Retrospective | 10 | IMPT | 70 GyE | 33–35 | 77.8 | 100 | 22.2 | 66.6 | 33.3 | 44.4 | 55.6 | 24.5 | 100 | 88.9 | 88.9 |
| McDonald et al (2016; USA) [16]b | Retrospective, matched | 26 (15 NPC) 14 (2 NPC) | IMRT 3D-PT | 71.4 Gy 71.8 GyE | 0 21.4 | 88.5 50 | 0 14.2 | 19.2 7.1 | 80.8 92.9 | 57.7 78.6 | 42.3 21.4 | |||||
| Beddok et al (2019; France) [19] | Retrospective | 17 | Mixed-beam EBRTc/ DS-PT | 70–78 GyE | 35–39 | 65 | 100 | 0 | 100 | 100 | 0 | 98 | 94 | 88 | 88 | |
| Dionisi et al (2019; Italy) [20] | Retrospective | 17 rNPC | 3D-PT | 60 GyE (30.6–66) | 1.8–2 Gy/Fx | 11.8 | 47 | 0 | 5.9 | 88.2 | 0 | 0 | 10 | 72.9 | 59.3 | |
| Park et al (2019; Korea) [21] | Retrospective | 63 35 | IMRT Mixed-beam IMRT/IMPT | 68.4 GyE 68.4 GyE | 30 30 | 0 0 | 100 100 | 0 0 | Stage: II 12.7%, III 36.5%, IV 50.8% Stage: II 45.7%, III 22.9%, IV 31.4% | 81 (1 y) 87.1 (1 y) | ||||||
| Sanford et al (2019; USA) [22] | Retrospective | 12 61 | IMRT DS-PT | 70 Gy 70 GyE | 29 | 92 | 38 | 28 | 72 | 90 90 | 96 | 85 | 92 | |||
| Alterio et al (2020; Italy) [23] | Retrospective | 17 27 | IMRT Mixed-beam IMRT/IMPT | 69.96–70 Gy 70–74 GyE | 33–35 35–37 | 15 (88.2) 16 (59.3) | 16 (94.1) 27 (100) | 0 0 | 17 (100) 27 (100) | 8 (47.1) 12 (44.4) | 9 (52.9) 15 (55.6) | 51 25 | 89 94 | 69 76 | N = 15a N = 22a | |
| Jiri et al (2020; Czech Republic) [24] | Retrospective | 40 | IMPT | 70–76 GyE | 35–38 | 34 (85) | 19 (44.5) | 24 (56.5) | 14 (32.5) | 29 (67.5) | 24 | 84 | 75 | 80 | ||
Abbreviations: NPC, nasopharyngeal cancer; GyE, Gray equivalent; FU, follow-up; NACT, neoadjuvant chemotherapy; Con, concurrent chemotherapy; Adj, adjuvant chemotherapy; LRFS, local and regional progression-free survival; PFS, progression-free survival; OS, overall survival; IMPT, intensity-modulated proton therapy; -, no information; IMRT, intensity-modulated radiation therapy; 3D-PT, 3-dimensional conformal proton therapy; EBRT, external beam radiation therapy; DS-PT, double-scattering proton therapy; rNPC, recurrent nasopharyngeal cancer; 3DCRT, 3-dimensional conformal radiation therapy.
At the time of last follow-up.
The study included nasopharyngeal and paranasal sinus sites. No separate outcome for each site was reported. The values shown in the table therefore represent the entire cohort.
EBRT = 3DCRT or IMRT.
Toxicities
All studies reported various acute toxicities but there was heterogeneity in the reporting of the data. For example, 1 study did not clearly correlate the acute toxicity with the treatment modality (proton versus IMRT) [22]. The Common Terminology Criteria for Adverse Events was used for grading of side effects except for 1 study [24], which used the RTOG toxicity grading system. Of the 4 matched studies, 2 compared feeding tube rates and found significantly improved rates in the proton cohort (20% versus 65%, P = .015 [17] and 14% versus 85%, P < .001 [16]). Two other matched studies found that of the reported toxicities, higher-grade mucositis was significantly improved in the proton cohort (G2 46% versus 70%, P = .019 [21] and G3 11% versus 76%, P = .0002 [23]). All other acute toxicities were not significantly different between modalities in these 2 studies. Details on reported acute toxicities can be found in Table 2.
Table 2.
NPC and proton therapy acute toxicities ordered by year published.
|
Study (year) |
Proton technique |
IMRT, N (%) |
Proton, N (%) |
P
value |
|||||||||
|
Dermatitis |
Mucositis |
Wt loss |
Feeding tube/ swallowing |
Xerostomia |
Dermatitis |
Mucositis |
Wt loss |
Feeding tube/ swallowing |
Xerostomia |
Others |
|||
| Holliday et al (2015) [17] | IMPT | G1+ 30 (100) | %wt loss = 5.7 | Overall 13 (65)a,b | G1 1 (10) G2 4 (40) G3 4 (40) | %wt loss = 7.6 | Overall 2 (20)a,b | aFeeding tube .015 | |||||
| Lewis et al (2016) [18] | IMPT | G2 5 (50) G3 4 (40) | G2 8 (80) G3 1 (10) | %wt loss = 6 G1 5 (50) G2 3 (30) | Overall 3 (30)b | ||||||||
| McDonald et al (2016) [16] | 3D-PT | Overall 22 (84.6)a | Overall 2 (14.3)a | aFeeding tube <.001 | |||||||||
| Beddok et al (2019) [19] | Mixed-beam EBRTc/ DS-PT | G3 1 (5.9) | Middle ear inflammation 6 (35) | ||||||||||
| Dionisi et al (2019) [20] | 3D-PT | G1 3 (17.6) | G1 4 (23.5), G2 3 (17.6) | G1 hearing 1 (5.9) G1 fatigue 2 (11.7) G1 CN disorder 1 (5.9) G1 pain 3 (17.6) G2 pain 1 (5.9) | |||||||||
| Park et al (2019) [21] | Mixed-beam IMRT/ IMPT | G3+ 2 (3.2) | G2+ 44 (69.8)a | G3+ 5 (14.3) | G2+ 16 (45.7)a | Analgesic use: G2 54 (IMRT) vs 37.1% (mixed beam) | aMucositis .019 | ||||||
| Alterio et al (2020) [23] | Mixed-beam IMRT/IMPT | G1 8 (47.1) G2 9 (52.9) | G1 2 (11.8) G2 2 (11.8) G3 13 (76.4)a | G1 9 (52.9) G2 6 (35.3) | G1 7 (41.2) G2 5 (29.4) G3 4 (23.5) | G1 10 (58.8) G2 6 (35.3) | G1 9 (33.3) G2 15 (55.6) | G1 11 (40.8) G2 13 (48.1) G3 3 (11.1)a | G1 14 (51.8) G2 5 (18.5) | G1 11 (40.8) G2 8 (29.6) G3 4 (14.8) | G1 19 (70.4) G2 2 (7.4) | aMucositis .0002 | |
| Jiri et al (2020) [24]d | IMPT | G1 8 (18.6) G2 29 (67.4) G3 6 (14) | G1 11 (25.6) G2 28 (65.1) G3 3 (7) | Deviation from baseline: >15%: 13 (30.2), 5%–15%: 26 (60.5), <5%: 4 (9.3) | G1 12 (27.9) G2 18 (41.9) G3 4 (9.3) | ||||||||
Abbreviations: NPC, nasopharyngeal cancer; IMRT, intensity-modulated radiation therapy; Wt, weight; IMPT, intensity-modulated proton therapy; G, toxicity grade; -, no information; 3D-PT, 3-dimensional conformal proton therapy; EBRT, external beam radiation therapy; DS-PT, double-scattering proton therapy; CN, cranial nerve; RTOG, Radiation Therapy Oncology Group.
Statistically significant.
Placement during or after treatment.
EBRT=3DCRT or IMRT.
Toxicity evaluation using RTOG scale.
With respect to late effects, the most frequently reported were xerostomia and hearing loss. One study comparing a mixed-beam approach with IMRT/IMPT compared to IMRT alone found the proton group trended toward less soft tissue fibrosis (P = .07) [23]. One matched study where gastrostomy tube insertion was the primary endpoint found 2 cases of G1 temporal lobe necrosis (TLN) in the IMRT group and 1 case each of G1 TLN and G3 TLN in the proton group [17]. One study using a mixed-beam approach reported 1 grade-5 necrosis–induced nasopharyngeal bleed and upon further review, the patient had T4 disease with significant comorbidities including human immunodeficiency virus and hepatitis B and C coinfections [19]. Furthermore this study prescribed doses up to 78 GyE. The reirradiation study reported overall grade 3 to 5 toxicities of 23.5% [20]. Details on reported late toxicities can be found in Table 3.
Table 3.
NPC and proton therapy late toxicities ordered by year published.
|
Study (year) |
IMRT, N (%) |
Proton, N (%) |
|||||||||
|
Proton technique |
Xerostomia |
Feeding tube/ swallowing |
Hearing |
TLN |
Others |
Xerostomia |
Feeding tube/ swallowing |
Hearing |
TLN |
Others |
|
| Holliday et al (2015) [17] | IMPT | 3 (15)a | G3 2 (10) | G1 2 (10) | 3 (30) | 0a | G1 1(10) G3 1(10) | ||||
| Lewis et al (2016) [18] | IMPT | G1 6 (60) G2 2 (20) | |||||||||
| Beddok et al (2019) [19] | Mixed-beam EBRTb/ DS-PT | 12 (70.6) | Overall 9 (52.9) G3 4 (23.5) | Overall 6 (35.3) G2+ 1 (5.9) | Gr5 necrosis-induced nasopharyngeal bleeding 1 (5.9) | ||||||
| Dionisi et al (2019) [20] | 3D-PT | G2 1 (5.9) G3 1 (5.9) | G3 3 (17.6) | G2 trismus 1 (5.9), G2 soft tissue necrosis 2 (11.8), G2 osteonecrosis 1 (5.9) | |||||||
| Sanford et al (2019) [22] | DS-PT | G3 1 (8.3) | G3 4 (7) | G3 1 (1.6) | G3 CN deficit 1 (1.6), no G2 xerostomia in both modalities | ||||||
| Alterio et al (2020) [23] | Mixed-beam IMRT /IMPT | G0-1 11 (68.8) G2 5 (31.3) | G0-1 16 (100) | G0-1 15 (93.8) G2 1 (6.3) | G0-1 16 (100) | G0-1 16 (61.5) G2 10 (38.5) | G0-1 26 (100) | G0-1 25 (96.2) G2 1 (3.8) | G0-1 26 (100) | Trend to less soft tissue fibrosis (P = .07) | |
| Jiri et al (2020) [24]c | IMPT | G2 3 (7) | G2 2 (5) | G2 3 (7) G3 0 | G3+ 1 (2) | ||||||
Abbreviations: NPC, nasopharyngeal cancer; IMRT, intensity-modulated radiation therapy; TLN, temporal lobe necrosis; IMPT, intensity-modulated proton therapy; -, no information; G, toxicity grade; EBRT, external beam radiation therapy; DS-PT, double scattering proton therapy; 3D-PT, 3-dimensional conformal proton therapy; CN, cranial nerve; 3DCRT, 3-dimensional conformal radiation therapy; RTOG, Radiation Therapy Oncology Group.
Swallowing dysfunction evaluated by a speech pathologist of a modified barium swallow.
EBRT = 3DCRT or IMRT.
Toxicity evaluation using RTOG scale.
Discussion
This study provides a current and systematic review focusing on outcomes for NPC patients treated with proton therapy. We found planning studies were common but those focusing on actual toxicity rates are relatively rare, thus 9 studies were formally included in this review. Encouragingly, the studies were diverse in country of origin, indicating that the use of proton therapy in this subsite is increasingly adopted worldwide.
The benefit of proton therapy with respect to minimizing dose to normal structures has been clearly elucidated in dosimetric studies. Namely, Jacobi et al [25] used modern normal tissue complication probability (NTCP) modeling for acute toxicities to compare IMRT to IMPT. They found IMPT was able to maintain target coverage while reducing NTCP values and identified patients with upper head and neck tumors as gaining the most benefit with IMPT, particularly for swallowing-related side effects. Another planning comparison study of NPC patients found that the volume of mucosa and esophagus receiving ≥20 Gy and ≥30 Gy was significantly lower with IMPT than with helical tomotherapy and this was the case for most other organs at risk assessed [14]. This appeared to translate into clinically meaningful endpoints as shown in 2 comparison studies [16, 17], resulting in significantly lower feeding tube rates with proton therapy, even when using 2D conformal techniques. McDonald et al [16] also found a significantly lower opioid pain medication requirement with proton therapy in its cohort of patients with NPC and paranasal sinus cancer (odds ratio 0.09; 95% confidence interval, 0.01–0.57; P = .006).
Another acute toxicity that was notably improved was higher-grade mucositis. In the IMRT era, RTOG 0225 reported 29.4% for mucositis inhibiting oral intake [2], Cao et al [26] reported 21% for grade 3 mucositis in T4 NPC patients, and Songthong et al [27] reported 15% for grade 3 or higher mucositis. In the current series of proton studies, grade 3 or higher mucositis ranged from 7% to 11.1%. Two of these studies compared a mixed-beam approach to IMRT and still found significantly lower rates for higher-grade oral mucositis [21, 23]. The reirradiation study from our Italian colleagues [20] reported a rate of 17.6% for grade 2 mucositis among their 17 patients, of which half received concurrent chemotherapy. As supported by Lewis et al [18], proton therapy minimizes dose to the anterior cavity, which translates into lower rates of higher-grade oral mucositis toward the end of treatment, even offering substantial benefit when used as a boost over IMRT alone.
Another advantage of proton therapy is the decreased integral dose [28], which offers the potential advantage of lowering the incidence of radiation-induced tumors and allows room for reirradiation as patients are living longer with improved outcomes. Similarly, late effects also become a concern. The median follow-up for the up-front radiation studies was 25.8 months, which is not considerably long in a disease site with 90% local control.
In the studies reporting xerostomia, the mixed-beam approaches generally had higher rates of grade 2 (20%–38.5%) versus IMPT alone (7%), but grade 1 rates in IMPT studies (60%–61.5%) were comparable to those of IMRT studies [29, 30]. Planning studies have shown lower mean doses to the parotids and submandibular glands [13, 14], which should theoretically translate into lower xerostomia rates. Thus, the variation in rates may be related to physician grading, heterogeneity of the patients in the cohorts, and quality of the treatment planning at different centers.
Other notable late toxicities included hearing loss, which ranged widely from grade 2 at 3.8% [23] to grade 4 at 23.5% [19]. These were from mixed-beam studies and were similar to published IMRT studies at 25% from a large retrospective investigation of 869 patients and 13% for grade 2+ auditory toxicity from RTOG 0225 [2]. Even though the mixed-beam studies were published contemporaneously, some of the patients included were treated more commonly with 3D conformal techniques in the early 2000s, which may account for higher than expected rates. A purely IMPT study with median follow-up of 2 years reported lower rates with grade 2 hearing impairment at 7% and no grade 3 hearing changes [24].
Temporal lobe necrosis rates are comparable for patients treated with mixed beam and with pure IMRT and IMPT. In the mixed-beam studies, Alterio et al [23] reported no clinically significant central nervous system (CNS) necrosis (100% grade 0-1), and Beddok et al [19] found only 1 case of grade 2 TLN with all others having grade 0 to 1 necrosis. The TLN rates were also low among patients receiving only proton therapy with 1 case of grade 3 TLN in each reported study by Holliday et al [17], Jiri et al [24], and Sanford et al [22]. Of note, IMRT overall TLN rates of up to 15% have been reported with longer follow-up (beyond 5 years) [31, 32]; however, these were not graded, thus highlighting the lack of consistency in the reporting of rates. Literature suggests earlier onset of TLN following treatment with proton therapy compared to IMRT [33], which may be due to lack of conformality at the high-dose region, particularly with older proton techniques as used in the study by Beddok and colleagues [19]. Furthermore, the dose delivered may be higher at the distal edge, as the RBE can be higher than 1.1. Finally, MRI is a common modality to assess NPC patients, thus its use in monitoring tumor response and treatment-related CNS changes may inevitably detect changes that are subtle.
It is notable that all the studies in this review are retrospective in nature. Currently, a phase II trial from the Shanghai Proton and Heavy Ion Center (NCT04528394) is recruiting NPC patients to be randomly assigned to photon followed by carbon boost versus proton followed by carbon boost with grade 2 xerostomia at 6 months as the primary endpoint [34]. A completed phase II trial (NCT00592501) evaluating proton boost for NPC patients with concurrent chemotherapy evaluated acute toxicities, quality of life, and treatment compliance [35]. Long-term results are pending. Finally, HN001 (NCT02135042) evaluating postchemoradiation adjuvant therapy based on plasma Epstein-Barr virus DNA levels allows proton therapy, thus subgroup analysis is anticipated to assess the benefit in this cohort [36] (Table 4).
Table 4.
Ongoing clinical studies focusing on NPC population registered on www.clinicaltrials.gov as of October 2020.
|
ClinicalTrials.gov identifier |
Phase |
Study |
Institute |
Arm 1 |
Arm 2 |
Primary endpoint |
Estimated primary completion |
| NCT04528394 [34] | II, recruiting | A Randomized Phase II Trial Evaluating Toxicity and Efficacy Between Proton and Photon for Nasopharyngeal Carcinoma | Shanghai Proton and Heavy Ion Center Shanghai, Shanghai, China | Photon + carbon | Proton + carbon | Grade 2 xerostomia at 6 mo | June 2021 |
| NCT00592501 [35] | II, completed | A Phase II Study of Proton Radiotherapy With Chemotherapy for Nasopharyngeal Carcinoma | MGH, Boston, Massachusetts, USA | Photon + proton with cisplatin/5-fluorouracil | Acute toxicities, QOLs, treatment compliance | Completed | |
| NCT02135042a [36] | II/III, recruiting | Randomized Phase II and Phase III Studies of Individualized Treatment for Nasopharyngeal Carcinoma Based on Biomarker Epstein Barr Virus (EBV) Deoxyribonucleic Acid (DNA) | Multi-institutional study, USA | Cisplatin/5-fluorouracil after chemoradiationa, b Cisplatin/5-fluorouracil after chemoradiationa, c | Gemcitabine/paclitaxel after chemoradiationa, b Observation after chemoradiationa, c | Progression-free survival Overall survival | July 2021 |
Abbreviations: NPC, nasopharyngeal cancer; MGH, Massachusetts General Hospital; QOLs, quality-of-life outcomes; EBV, Epstein-Barr virus.
The trial allows both IMRT and IMPT during chemoradiation.
Phase II, detectable plasma EBV DNA.
Phase III, undetectable plasma EBV DNA.
The limitations of this review include the small number of studies, which is not unexpected as the implementation of this technology is still on the rise. Furthermore, the heterogeneity of the included studies with respect to proton technique, utilization of systemic therapy, and inclusion of proton boost following photon-based radiation makes aggregated interpretation of the data difficult. Missing data also limited our ability to perform quantitative pooled analyses. The relatively short follow-up also limited our understanding of all late effects that could manifest. Still, this review provides a snapshot of the toxicity profile, which will be further elucidated with longer follow-up.
In conclusion, this systematic review confirms that NPC patients receiving proton therapy have outcomes comparable to those of photon-based radiation with a largely improved toxicity profile, likely due to sparing of dose to normal structures. We await results from prospective studies to better quantify the magnitude of benefit.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Anna Lee, MD, MPH, receives funding and salary support unrelated to this project from the National Institutes of Health (NIH) National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787-01). Johannes A. Langendijk, MD, PhD, receives consultancy fees from IBA paid to the UMCG Research BV and the Department of Radiation Oncology, University of Groningen; University Medical Center Groningen receives research funding with IBA, RaySearch, Elekta, VisionRT, and Siemens. Jiade Lu, MD, MBA, receives royalties from Springer-Nature for published books. Nancy Y. Lee, MD, FASTRO, is a consultant for Merck, Merck Serono, and Pfizer, and reports research funding from Astra Zeneca, Merck, and Pfizer. The authors have no additional conflicts of interest to disclose.
Funding: The authors have no funding to disclose.
Ethical Approval: This study did not involve human subjects/participants and was exempt from IRB approval.
Appendix
Literature Search Strategies
PubMed
(“Nasopharyngeal Neoplasms”[Mesh] OR “Pharyngeal Neoplasms”[Mesh:NoExp] OR “Nasopharynx”[Mesh] OR “Pharynx”[Mesh:NoExp] OR nasopharynx[tiab] OR naso-pharynx[tiab] OR nasopharyngeal[tiab] OR naso-pharyngeal[tiab] OR rhinopharynx[tiab] OR rhino-pharynx[tiab] OR rhinopharyngeal[tiab] OR rhino-pharyngeal[tiab] OR choana[tiab] OR choanae[tiab] OR choanal[tiab]) AND ((“Proton Therapy”[Mesh] OR “Heavy Ion Radiotherapy”[Mesh:NoExp] OR proton therapy[tiab] OR proton treatment[tiab] OR IMPT[tiab] OR PBT[tiab] OR particle therapy[tiab] OR particle treatment[tiab]) OR ((“Protons”[Mesh] OR proton[tiab] OR protons[tiab] OR particle[tiab]) AND (“Radiotherapy”[Mesh] OR “Radiotherapy Planning, Computer-Assisted”[Mesh] OR “Radiometry”[Mesh] OR “radiotherapy”[Subheading] OR radiotherapy[tiab] OR radio-therapy[tiab] OR chemoradiation[tiab] OR chemoradiotherapy[tiab] OR radio-chemotherapy[tiab] OR radiochemotherapy[tiab] OR radiation[tiab] OR reirradiation[tiab] OR irradiation[tiab] OR irradiate[tiab] OR irradiated[tiab] OR irradiating[tiab] OR dosimetry[tiab] OR dosimetric[tiab] OR beam[tiab] OR beams[tiab]))) AND “English”[la]
Embase
(‘nasopharynx tumor'/exp OR ‘nasopharynx'/exp OR ‘pharynx tumor'/de OR ‘nasopharynx':ti,ab,kw OR ‘naso pharynx':ti,ab,kw OR ‘nasopharyngeal':ti,ab,kw OR ‘naso pharyngeal':ti,ab,kw OR ‘rhinopharynx':ti,ab,kw OR ‘rhino-pharynx':ti,ab,kw OR ‘rhinopharyngeal':ti,ab,kw OR ‘rhino-pharyngeal':ti,ab,kw OR ‘choana':ti,ab,kw OR ‘choanae':ti,ab,kw OR ‘choanal':ti,ab,kw) AND (‘proton therapy'/exp OR ‘particle therapy'/de OR ‘proton therapy system'/exp OR ‘proton radiation'/exp OR ‘proton therapy':ti,ab,kw OR ‘proton treatment':ti,ab,kw OR ‘impt':ti,ab,kw OR ‘pbt':ti,ab,kw OR ‘particle therapy':ti,ab,kw OR ‘particle treatment':ti,ab,kw OR ((‘proton'/exp OR ‘proton':ti,ab,kw OR ‘protons':ti,ab,kw OR ‘particle':ti,ab,kw) AND (‘radiotherapy'/exp OR ‘radiotherapy planning'/exp OR ‘radiotherapy planning system'/exp OR ‘radiation measurement'/exp OR ‘radiotherapy':lnk OR ‘radiotherapy':ti,ab,kw OR ‘radio-therapy':ti,ab,kw OR ‘chemoradiation':ti,ab,kw OR ‘chemoradiotherapy':ti,ab,kw OR ‘radio-chemotherapy':ti,ab,kw OR ‘radiochemotherapy':ti,ab,kw OR ‘radiation':ti,ab,kw OR ‘reirradiation':ti,ab,kw OR ‘irradiation':ti,ab,kw OR ‘irradiate':ti,ab,kw OR ‘irradiated':ti,ab,kw OR ‘irradiating':ti,ab,kw OR ‘dosimetry':ti,ab,kw OR ‘dosimetrical':ti,ab,kw OR ‘dosimetrically':ti,ab,kw OR ‘dosimetric':ti,ab,kw OR ‘beam':ti,ab,kw OR ‘beams':ti,ab,kw))) AND [english]/lim
Cochrane Central Register of Controlled Trials
Title Abstract Keyword
( “nasopharynx” OR “naso-pharynx” OR “nasopharyngeal” OR “naso-pharyngeal” OR “rhinopharynx” OR “rhino-pharynx” OR “rhinopharyngeal” OR “rhino-pharyngeal” OR “choana” OR “choanae” OR “choanal” ) AND ( ( “proton therapy” OR “proton treatment” OR “IMPT” OR “PBT” OR “particle therapy” OR “particle treatment” ) OR ( ( “proton” OR “protons” OR “particle” ) AND ( “radiotherapy” OR “radio-therapy” OR “chemoradiation” OR “chemoradiotherapy” OR “radio-chemotherapy” OR “radiochemotherapy” OR “radiation” OR “reirradiation” OR “irradiation” OR “irradiate” OR “irradiated” OR “irradiating” OR “dosimetry” OR “dosimetrical” OR “dosimetrically” OR “dosimetric” OR “beam” OR “beams” ) ) )
Scopus
( TITLE-ABS-KEY ( “nasopharynx” OR “naso-pharynx” OR “nasopharyngeal” OR “naso-pharyngeal” OR “rhinopharynx” OR “rhino-pharynx” OR “rhinopharyngeal” OR “rhino-pharyngeal” OR “choana” OR “choanae” OR “choanal” ) ) AND ( ( TITLE-ABS-KEY ( “proton therapy” OR “proton treatment” OR “IMPT” OR “PBT” OR “particle therapy” OR “particle treatment” ) ) OR ( TITLE-ABS-KEY ( “proton” OR “protons” OR “particle” ) AND TITLE-ABS-KEY ( “radiotherapy” OR “radio-therapy” OR “chemoradiation” OR “chemoradiotherapy” OR “radio-chemotherapy” OR “radiochemotherapy” OR “radiation” OR “reirradiation” OR “irradiation” OR “irradiate” OR “irradiated” OR “irradiating” OR “dosimetry” OR “dosimetrical” OR “dosimetrically” OR “dosimetric” OR “beam” OR “beams” ) ) ) AND ( LIMIT-TO ( LANGUAGE , “English” ) )
Web of Science
Topic Search
( “nasopharynx” OR “naso-pharynx” OR “nasopharyngeal” OR “naso-pharyngeal” OR “rhinopharynx” OR “rhino-pharynx” OR “rhinopharyngeal” OR “rhino-pharyngeal” OR “choana” OR “choanae” OR “choanal” ) AND ( ( “proton therapy” OR “proton treatment” OR “IMPT” OR “PBT” OR “particle therapy” OR “particle treatment” ) OR ( ( “proton” OR “protons” OR “particle” ) AND ( “radiotherapy” OR “radio-therapy” OR “chemoradiation” OR “chemoradiotherapy” OR “radio-chemotherapy” OR “radiochemotherapy” OR “radiation” OR “reirradiation” OR “irradiation” OR “irradiate” OR “irradiated” OR “irradiating” OR “dosimetry” OR “dosimetrical” OR “dosimetrically” OR “dosimetric” OR “beam” OR “beams” ) ) )
Refined by: LANGUAGES: ( ENGLISH )
Timespan: All years. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, Jones C, Ang KK. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684–90. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, Hu K, Le QT, Colevas AD, Glisson BS, Chan AT, Ang KK. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–80. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Chen X, Lin S, Rong J, Yang M, Wen Q, Shang C, He L, Ren P, Xu S, Zhang J, Liu Q, Pang H, Shi X, Fan J, Sun X, Ma D, Tan B, Zhang T, Zhang L, Hu D, Du X, Zhang Y, Wen S, Zhang X, Wu J. Treatment outcomes after reduction of the target volume of intensity-modulated radiotherapy following induction chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a prospective, multi-center, randomized clinical trial. Radiother Oncol. 2018;126:37–42. doi: 10.1016/j.radonc.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Huguenin PU, Taussky D, Moe K, Meister A, Baumert B, Lutolf UM, Glanzmann C. Quality of life in patients cured from a carcinoma of the head and neck by radiotherapy: the importance of the target volume. Int J Radiat Oncol Biol Phys. 1999;45:47–52. doi: 10.1016/s0360-3016(99)00128-5. [DOI] [PubMed] [Google Scholar]
- 6.Fang FM, Chiu HC, Kuo WR, Wang CJ, Leung SW, Chen HC, Sun LM, Hsu HC. Health-related quality of life for nasopharyngeal carcinoma patients with cancer-free survival after treatment. Int J Radiat Oncol Biol Phys. 2002;53:959–68. doi: 10.1016/s0360-3016(02)02838-9. [DOI] [PubMed] [Google Scholar]
- 7.Cengiz M, Ozyar E, Esassolak M, Altun M, Akmansu M, Sen M, Uzel O, Yavuz A, Dalmaz G, Uzal C, Hicsonmez A, Sarihan S, Kaplan B, Atasoy BM, Ulutin C, Abacioglu U, Demiral AN, Hayran M. Assessment of quality of life of nasopharyngeal carcinoma patients with EORTC QLQ-C30 and H&N-35 modules. Int J Radiat Oncol Biol Phys. 2005;63:1347–53. doi: 10.1016/j.ijrobp.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 8.McDowell LJ, Ringash J, Xu W, Chan B, Lu L, Waldron J, Rock K, So N, Huang SH, Giuliani M, Hope A, O'Sullivan B, Bratman SV, Cho J, Kim J, Jang R, Bayley A, Bernstein LJ. A cross sectional study in cognitive and neurobehavioral impairment in long-term nasopharyngeal cancer survivors treated with intensity-modulated radiotherapy. Radiother Oncol. 2019;131:179–85. doi: 10.1016/j.radonc.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Kiang A, Weinberg VK, Cheung KH, Shugard E, Chen J, Quivey JM, Yom SS. Long-term disease-specific and cognitive quality of life after intensity-modulated radiation therapy: a cross-sectional survey of nasopharyngeal carcinoma survivors. Radiat Oncol. 2016;11:127. doi: 10.1186/s13014-016-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, Morrison WH, Ang KK, Garden AS. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Water TA, Bijl HP, Schilstra C, Pijls-Johannesma M, Langendijk JA. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist. 2011;16:366–77. doi: 10.1634/theoncologist.2010-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, Munsell MF, Palmer MB, Sahoo N, Zhang X, Liu W, Zhu XR. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–53. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taheri-Kadkhoda Z, Bjork-Eriksson T, Nill S, Wilkens JJ, Oelfke U, Johansson KA, Huber PE, Munter MW. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3:4. doi: 10.1186/1748-717X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widesott L, Pierelli A, Fiorino C, Dell'oca I, Broggi S, Cattaneo GM, Di Muzio N, Fazio F, Calandrino R, Schwarz M. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–96. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32. doi: 10.1186/s13014-016-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holliday EB, Garden AS, Rosenthal DI, Fuller CD, Morrison WH, Gunn GB, Phan J, Beadle BM, Zhu XR, Zhang X, Hanna E, Glisson BS, Hutcheson KA, El-Naggar AK, Hong J-H, Hung T-M, Uzel EK, Lewis G, Frank SJ. Proton therapy reduces treatment-related toxicities for patients with nasopharyngeal cancer: a case-match control study of intensity-modulated proton therapy and intensity-modulated photon therapy. Int J Particle Ther. 2015;2:19–28. [Google Scholar]
- 18.Lewis GD, Holliday EB, Kocak-Uzel E, Hernandez M, Garden AS, Rosenthal DI, Frank SJ. Intensity-modulated proton therapy for nasopharyngeal carcinoma: decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck. 2016;38(suppl 1):E1886–95. doi: 10.1002/hed.24341. [DOI] [PubMed] [Google Scholar]
- 19.Beddok A, Feuvret L, Noel G, Bolle S, Deberne M, Mammar H, Chaze A, Le Tourneau C, Goudjil F, Zefkili S, Herman P, Dendale R, Calugaru V. Efficacy and toxicity of proton with photon radiation for locally advanced nasopharyngeal carcinoma. Acta Oncol. 2019;58:472–4. doi: 10.1080/0284186X.2018.1543948. [DOI] [PubMed] [Google Scholar]
- 20.Dionisi F, Croci S, Giacomelli I, Cianchetti M, Caldara A, Bertolin M, Vanoni V, Pertile R, Widesott L, Farace P, Schwarz M, Amichetti M. Clinical results of proton therapy reirradiation for recurrent nasopharyngeal carcinoma. Acta Oncol. 2019;58:1238–45. doi: 10.1080/0284186X.2019.1622772. [DOI] [PubMed] [Google Scholar]
- 21.Park SG, Ahn YC, Oh D, Noh JM, Ju SG, Kwon D, Jo K, Chung K, Chung E, Lee W, Park S. Early clinical outcomes of helical tomotherapy/intensity-modulated proton therapy combination in nasopharynx cancer. Cancer Sci. 2019;110:2867–74. doi: 10.1111/cas.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanford NN, Lau J, Lam MB, Juliano AF, Adams JA, Goldberg SI, Lu HM, Lu YC, Liebsch NJ, Curtin HD, Chan AW. Individualization of clinical target volume delineation based on stepwise spread of nasopharyngeal carcinoma: outcome of more than a decade of clinical experience. Int J Radiat Oncol Biol Phys. 2019;103:654–68. doi: 10.1016/j.ijrobp.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Alterio D, D'Ippolito E, Vischioni B, Fossati P, Gandini S, Bonora M, Ronchi S, Vitolo V, Mastella E, Magro G, Franco P, Ricardi U, Krengli M, Ivaldi G, Ferrari A, Fanetti G, Comi S, Tagliabue M, Verri E, Ricotti R, Ciardo D, Jereczek-Fossa BA, Valvo F, Orecchia R. Mixed-beam approach in locally advanced nasopharyngeal carcinoma: IMRT followed by proton therapy boost versus IMRT-only: evaluation of toxicity and efficacy. Acta Oncol. 2020;59:541–8. doi: 10.1080/0284186X.2020.1730001. [DOI] [PubMed] [Google Scholar]
- 24.Jiri K, Vladimir V, Michal A, Matej N, Silvia S, Pavel V, Katerina D, Jana P, Barbora O, Eliska R, Petr L, Matej P, Alexander G, Jozef R. Proton pencil-beam scanning radiotherapy in the treatment of nasopharyngeal cancer: dosimetric parameters and 2-year results. Eur Arch Otorhinolaryngol. 2020] doi: 10.1007/s00405-020-06175-5. [published online ahead of print July 4. [DOI] [PubMed]
- 25.Jakobi A, Bandurska-Luque A, Stutzer K, Haase R, Lock S, Wack LJ, Monnich D, Thorwarth D, Perez D, Luhr A, Zips D, Krause M, Baumann M, Perrin R, Richter C. Identification of patient benefit from proton therapy for advanced head and neck cancer patients based on individual and subgroup normal tissue complication probability analysis. Int J Radiat Oncol Biol Phys. 2015;92:1165–74. doi: 10.1016/j.ijrobp.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 26.Cao CN, Luo JW, Gao L, Yi JL, Huang XD, Wang K, Zhang SP, Qu Y, Li SY, Xiao JP, Zhang Z, Xu GZ. Concurrent chemotherapy for T4 classification nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. PLoS One. 2015;10:e0119101. doi: 10.1371/journal.pone.0119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Songthong AP, Kannarunimit D, Chakkabat C, Lertbutsayanukul C. A randomized phase II/III study of adverse events between sequential (SEQ) versus simultaneous integrated boost (SIB) intensity modulated radiation therapy (IMRT) in nasopharyngeal carcinoma; preliminary result on acute adverse events. Radiat Oncol. 2015;10:166. doi: 10.1186/s13014-015-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol. 2013;23:88–96. doi: 10.1016/j.semradonc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Wolden SL, Chen WC, Pfister DG, Kraus DH, Berry SL, Zelefsky MJ. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64:57–62. doi: 10.1016/j.ijrobp.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 30.Wu LR, Liu YT, Jiang N, Fan YX, Wen J, Huang SF, Guo WJ, Bian XH, Wang FJ, Li F, Song D, Wu JF, Jiang XS, Liu JY, He X. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol. 2017;69:26–32. doi: 10.1016/j.oraloncology.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Su SF, Huang SM, Han F, Huang Y, Chen CY, Xiao WW, Sun XM, Lu TX. Analysis of dosimetric factors associated with temporal lobe necrosis (TLN) in patients with nasopharyngeal carcinoma (NPC) after intensity modulated radiotherapy. Radiat Oncol. 2013;8:17. doi: 10.1186/1748-717X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng M, Huang Y, Fan X, Xu P, Lang J, Wang D. Prognostic variables for temporal lobe injury after intensity modulated-radiotherapy of nasopharyngeal carcinoma. Cancer Med. 2018;7:557–64. doi: 10.1002/cam4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitpanit S, Lee A, Pitter KL, Fan D, Chow JCH, Neal B, Han Z, Fox P, Sine K, Mah D, Dunn LA, Sherman EJ, Michel L, Ganly I, Wong RJ, Boyle JO, Cohen MA, Singh B, Brennan CW, Gavrilovic IT, Hatzoglou V, O'Malley B, Zakeri K, Yu Y, Chen L, Gelblum DY, Kang JJ, McBride SM, Tsai CJ, Riaz N, Lee NY. Temporal lobe necrosis in head and neck cancer patients after proton therapy to the skull base. Int J Part Ther. 2020;6:17–28. doi: 10.14338/IJPT-20-00014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proton versus photon radiotherapy for nasopharyngeal carcinoma. ClinicalTrials.gov.identifier NCT04528394. https://clinicaltrials.gov/ct2/show/NCT04528394 Updated August 27, 2020. Accessed October 8, 2020.
- 35.Proton radiotherapy with chemotherapy for nasopharyngeal carcinoma. ClinicalTrials.gov.identifier NCT00592501. https://clinicaltrials.gov/ct2/show/NCT00592501 Updated August 14, 2019. Accessed October 8, 2020.
- 36.Individualized treatment in treating patients with stage II-IVB nasopharyngeal cancer based on EBV DNA. ClinicalTrials.gov.identifier NCT02135042. https://clinicaltrials.gov/ct2/show/NCT02135042 Updated December 9, 2020. Accessed October 8, 2020.


