Abstract
In most childhood head and neck cancers, radiotherapy is an essential component of treatment; however, it can be associated with problematic long-term complications. Proton beam therapy is accepted as a preferred radiation modality in pediatric cancers to minimize the late radiation side effects. Given that childhood cancers are a rare and heterogeneous disease, the support for proton therapy comes from risk modeling and a limited number of cohort series. Here, we discuss the role of proton radiotherapy in pediatric head and neck cancers with a focus on reducing radiation toxicities. First, we compare the efficacy and expected toxicities in proton and photon radiotherapy for childhood cancers. Second, we review the benefit of proton radiotherapy in reducing acute and late radiation toxicities, including risks for secondary cancers, craniofacial development, vision, and cognition. Finally, we review the cost effectiveness for proton radiotherapy in pediatric head and neck cancers. This review highlights the benefits of particle radiotherapy for pediatric head and neck cancers to improve the quality of life in cancer survivors, to reduce radiation morbidities, and to maximize efficient health care use.
Keywords: radiotherapy; radiotherapy, intensity-modulated; proton therapy; neoplasms, radiation-induced
Introduction
Pediatric cancers represent a unique challenge in terms of both curing the disease and minimizing long-term treatment-related complications. Radiotherapy (RT) toxicities are especially relevant to cancers involving the head and neck sites given the proximity of multiple vital organs. Because pediatric head and neck cancers are often treated with RT for improved local control because of the difficulty in achieving gross total resection, late radiation toxicities are even more relevant for this disease site because pediatric head and neck cancers represent at least 12% of all pediatric cancers and are increasing faster than pediatric cancers overall [1]. Of those patients with pediatric cancers surviving 5 years or longer, approximately 80% were treated with RT [2]. For cancers involving the head and neck area, the most common pathologic types are lymphomas, including Hodgkin lymphoma and non-Hodgkin lymphoma; neural tumors, including neuroblastoma and retinoblastoma; and soft tissue sarcomas, including rhabdomyosarcoma [1].
The types and risks of late radiation-associated toxicities are often dependent on the dose and extent of the radiation fields. Patients with leukemia, lymphoma, and neuroblastoma often receive radiation doses of less than 30 Gy and are subject to increased risk of cataracts, growth delays, dental complications, and secondary tumors. By contrast, patients with rhabdomyosarcomas, other soft tissue sarcomas, and other solid tumors, such as squamous cell carcinomas, are treated with radiation doses of 50 Gy or higher, additionally predisposing them to increased risk of hypopituitarism, bone hypoplasia, and hearing, vision, salivary, swallowing, and soft tissue toxicities.
The increased conformality and lack of exit dose in particle therapy, in particular, in proton therapy, represent an important advance to reduce the long-term radiation toxicities associated with pediatric head and neck cancers. The first part of this review discusses the advantages of proton beam therapy (PBT) over photon beam therapy in reducing late radiation toxicities, in particular, in secondary malignancies. The second part reviews the acute toxicities and long-term morbidities from PBT in pediatric head and neck cancers. Finally, we consider future applications for PBT and other modalities in pediatric head and neck cancers.
Proton versus Photon RT for Pediatric Head and Neck Cancers
Photon beam RT has been the conventional treatment for pediatric cancers using 3-dimensional conformal radiotherapy (3D-CRT) and, recently, the more-conformal intensity-modulated radiotherapy (IMRT) or volumetric-modulated arc therapy (VMAT). Compared with 3D-CRT, IMRT had comparable locoregional control approaching ≥90% at 3 years [3–5] and fewer grade ≥3 acute toxicities in pediatric head and neck cancers. The IMRT decreases high-radiation doses to critical structures at a cost of increased integral radiation doses to a larger volume of healthy tissues.
Compared with photon-based RT, including IMRT, proton-beam RT reduces or eliminates unnecessary integral low dose radiation to surrounding structures while maintaining high conformality. Table 1 compares the published mean doses to various healthy tissues for orbital and nonorbital tumors. However, a 2012 American Society for Therapeutic Radiology and Oncology (ASTRO) consensus review [11] did not find sufficient evidence to recommend PBT outside of clinical trials in head and neck cancers and pediatric non–central nervous system malignancies. This report contrasts with the consensus of most pediatric radiation oncologists who support PBT for pediatric head and neck malignancies [12]. Furthermore, most studies forming the ASTRO consensus recommendation were based on passive-scatter proton techniques and not more-recent intensity-modulated proton therapy (IMPT) techniques, which only recently became available after those guidelines were published. Figure 1 demonstrates the dose distributions between an VMAT compared with IMPT planning.
Table 1.
Comparison of mean radiation doses to indicated organs using IMRT or PBT for orbital and nonorbital head and neck cancers.
|
Organ |
Mean radiation dose, Gya |
Type of cancer |
|||
|
Orbital primary |
Nonorbital primary |
||||
|
IMRT |
Protons |
IMRT |
Protons |
||
| Brainstem | NS | NS | 18-26 | 7-8 | Rhabdomyosarcoma |
| Optic chiasm | NS | NS | 24-33 | 15-18 | Rhabdomyosarcoma |
| Pituitary | 15 | 4 | 33-43 | 24-29 | Rhabdomyosarcoma |
| Optic nerve (ipsilateral) | 37 | 29-45 | 2-37 | 0-30 | Rhabdomyosarcoma, salivary |
| Optic nerve (contralateral) | NS | 0 | 2-31 | 0-14 | Rhabdomyosarcoma, salivary |
| Eye (ipsilateral) | 40 | 25-33 | 1-16 | 1-9 | Rhabdomyosarcoma, salivary |
| Eye (contralateral) | 8 | 0 | 2-13 | 0-3 | Rhabdomyosarcoma, salivary |
| Lens (ipsilateral) | 32 | 10-44 | 7-9 | 2 | Rhabdomyosarcoma |
| Lens (contralateral) | 3 | 0-1 | 6 | 0-1 | Rhabdomyosarcoma |
| Maxilla | 12 | 7-25 | 30 | 15 | Rhabdomyosarcoma |
| Cochlea (ipsilateral) | NS | NS | 39-41 | 36-37 | Rhabdomyosarcoma |
| Cochlea (contralateral) | NS | NS | 29-32 | 4-12 | Rhabdomyosarcoma |
| Parotid (ipsilateral) | NS | NS | 38-39 | 31-37 | Rhabdomyosarcoma |
| Parotid (contralateral) | NS | NS | 11-24 | 2 | Rhabdomyosarcoma |
Abbreviations: IMPT, IMPT, intensity-modulated proton therapy; PBT, proton beam therapy; NS, not stated.
Based on references 6–10.
Figure 1.
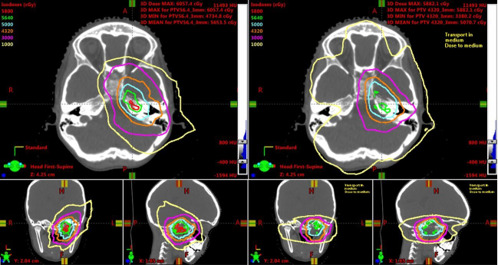
Comparison of IMPT and VMAT dose distributions. IMPT (left) and VMAT (right) plans with isodose line plans (right). Isodose lines: green: 5640 cGy; orange: 4320 cGy. Abbreviations: cGy, centigray; IMPT, intensity-modulated proton therapy; VMAT, volumetric-modulated arc therapy. Reproduced with permission from Chen et al [13].
Even with passive-scatter techniques, the volumes of low-dose RT are smaller than those of conventional photon-based RT. Leiser et al [14] demonstrated significant dosimetric sparing with passive-scatter PBT in 26 of 30 critical structures (87%) for patients with cancers in the orbital, head and neck, pelvic, and trunk and extremity sites. Similarly, a report from MD Anderson Cancer Center (Houston, Texas) compared proton and photon treatment plans for 6 children with head and neck or 18 children with brain cancers [15]. They found PBT was superior to photon RT in all cases, except for intracranial disease in which PBT demonstrated variable benefits compared with photon RT, especially for hippocampal sparing. The increasing implementation of IMPT may further increase target conformality and minimize the dose to healthy tissues. To that end, in 83 children with rhabdomyosarcoma treated with IMPT, overall nonocular grade 3 late toxicities were only 3.6%, and no grade ≥4 late toxicities were observed with PBT [14].
In head and neck rhabdomyosarcoma, PBT showed significant sparing of multiple healthy tissues, including contralateral structures such as the optic apparatus, cochlea, and both ipsilateral and contralateral parotid glands [6]. In a small case series of 7 patients with orbital rhabdomyosarcoma, PBT afforded greater local control rates, approaching 85%, with improved dosimetric sparing of the ipsilateral and contralateral optic structures and reduced optic toxicity compared with historical controls treated with photon RT [7]. That finding is consistent with other reports of favorable grade ≥3 late ocular toxicities, which have approximated 6.5% [14]. Extrapolating from adult head and neck cancers, PBT also likely reduces mucositis in pediatric head and neck cancers. Frank and colleagues [16, 17] reported delivery of 70 Gy to oropharyngeal cancers using IMPT had no grade ≥2 anterior mucositis and no grade 4–5 toxicities. A systematic review by Doyden et al [18] demonstrated that PBT better-minimized radiation doses to the salivary glands, spinal cord, brainstem, skull base structures, esophagus, and larynx compared with photon RT. Of note, the advantages of PBT persisted even when compared with the latest advances in photon treatments, including tomotherapy and VMAT [19–21]. When calculating the clinical advantage in terms of normal tissue complications probability (NTCP) in 45 cases of locally advanced head and neck cases, IMPT provided better than 10% reduction in xerostomia and mucositis in more than 50% of patients compared with IMRT or with VMAT. By contrast, a mixed photon-proton plan only reduced NTCP toxicities by approximately 10% in select cases. Tables 2 and 3 summarize the acute and late toxicities observed in children receiving photon or proton head and neck RT.
Table 2.
Rates of acute toxicities for pediatric head and neck cancers.
|
Toxicity |
Grade ≥2 acute toxicities,a % |
Cancer types |
|
|
IMRT |
Protons |
||
| Mucositis | 91 | 46 | Salivary |
| 50 | Esthesioneuroblastoma | ||
| 32 | Mixed head and neck tumors | ||
| 24 | Sarcoma | ||
| Dermatitis | 55 | 6.9 | Salivary |
| 62.5 | Esthesioneuroblastoma | ||
| 27 | Sarcoma | ||
| Dysphagia | 27 | 0 | Salivary |
| 37.5 | Esthesioneuroblastoma | ||
| 20 | Sarcoma | ||
| Otitis externa | 18 | 8 | Salivary |
Abbreviation: IMPT, intensity-modulated proton therapy.
Based on references 9 and 22–24.
Table 3.
Late toxicities associated with head and neck radiotherapy for pediatric cancers.a
|
Organ and types of toxicities |
Rates of toxicities, cumulative mean (Range), % |
Types of toxicities |
Reported dose when toxicity observed, Gy |
|
|
Photon radiotherapy |
Proton radiotherapy |
|||
| Dental abnormalities | 35 (32-100) | 7 (3-30) | Tooth, agenesis, microdontia, enamel dysplasia, xerostomia, TMJ dysfunction, osteoradionecrosis | 20 |
| Craniofacial malformations | 77 (5-97) | 25 (21-70) | Bone and soft tissue hypoplasia | Bone: 30 Soft tissue: 4 |
| Hypopituitarism: GH deficiency | 19 (5-40) | 19 (13-22) | Decreased height, decreased bone mineralization | GH: 18 |
| Other endocrinopathy | 9 (7-9) | 10 (5-10) | Delayed puberty, sexual dysfunction, subclinical hypothyroidism | GnRH: 40 ACTH: 24 TSH: 24 |
| Optic toxicities | 23 (10-83) | 10 (0-14) | Cataract Keratinization Retinopathy Optic neuropathy | 2 30 45 50 |
| Hearing toxicities | 19 (17-75) | 7 (0-11) | High frequency hearing loss | 45 |
| Secondary cancers | 3 (2-10) | 1 (0-6) | Breast cancer, meningioma | 1.8 |
Abbreviations: TMJ, temporomandibular joint; GH, growth hormone; GnRH, gonadotropin-releasing hormone agonist; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone.
Based on references 5, 7, 14, and 25–29.
Several confounding factors may affect adequate PBT, especially IMPT, to the head and neck region. The first complex issue is the small volumes of critical organs, especially the serial structures, such as the optic nerves and chiasm, receiving a high radiation dose. Moreover, IMPT plans are usually designed either with single-field optimization or multifield optimization (MFO) in which the weighting of the spot size for all fields are optimized together. In contrast to single-field optimized IMPT, MFO-IMPT often provides more-conformal target-dose distribution and better sparing of critical structures. However, MFO-IMPT is more susceptible to both range uncertainties at the sharp dose gradient at the end of range of a proton beam as well as setup uncertainties. In the head and neck region, those uncertainties are further amplified because of intrafractional changes in soft tissue geometries, weight loss during treatment, and interfractional changes in the paranasal sinus densities. In addition, the use of radiobiological equivalent (RBE) and linear energy transfer (LET) model-based planning may better reduce possible side effect from the RBE at the end of the range [30]. To overcome the sensitivity of MFO-IMPT plans to various uncertainties, robust optimization to account for those range uncertainties is incorporated into IMPT planning [31]. Furthermore, frequent verification simulations are performed, especially for tumors adjacent to the paranasal sinuses. In addition, the differences in spot size may affect treatment planning. Smaller spot sizes may increase dose homogeneity and reduce doses to healthy tissues at the potential costs of reducing plan robustness. Consequently, a minimum monitor unit constraint is necessary to improve plan robustness. For pediatric patients, the increased proximity of critical structures and frequent cancers involving, or in proximity to, the paranasal sinuses requires extra care during treatment planning. Furthermore, improved planning may be achieved with noncoplanar beams as well as multiple beam angles. However, for tumors impinging on certain critical organs, such as the brain or brainstem, caution with PBT may be needed because the LET at the end of the range may increase the risk of symptomatic brain necrosis. Therefore, care must be taken when treating patients with PBT.
With reduced toxicities, PBT in childhood head and neck cancers has been shown to have similar outcomes as photon beam therapy. Several groups have demonstrated equivalent rates of local control for orbital and other head and neck rhabdomyosarcomas of >95% and 90%, respectively [8, 32, 33]. Although the University of Pennsylvania group reported excellent 2-year local control rates of 92.1% for photon RT and 85.4% for proton RT [22], there were no differences between proton and photon modalities on multivariate analysis. Consistent with the findings of other groups, local failure in the University of Pennsylvania cohort was associated more with both lower chemotherapy dose and lower radiation dose [32]. Similar rates of local control have been observed in other series [14, 25]. For most other head and neck cancers, efficacy of PBT has been limited to small case series [22, 33, 34, 35], and it is difficult to compare differences between modalities. Thus, extrapolating from rhabdomyosarcomas, PBT for childhood head and neck cancers provides similar efficacy as photon beam therapy.
Overall, PBT is more conformal than 3D-CRT. Furthermore, IMPT provides even improved dose distributions compared with passive-scatter PBT, and likely IMRT, as well as VMAT, especially with lower isodose lines. However, VMAT is often technically more conformal than IMPT in high-dose regions as well as in lateral dose gradients. Consequently, IMPT and VMAT planning should be compared, when possible, for optimal planning.
The Benefit of Proton RT in Reducing Radiation-Induced Toxicities
Acute Toxicity
Irradiation of the head and neck region is often associated with severe acute toxicities, leading to mucositis, dysphagia, and weight loss, which necessitates feeding tube placement in at least 30%–50% of cases. In a study of adults, Grant et al [9] demonstrated PBT compared with photon beam therapy reduced the rates of grade 2-3 mucositis (46% versus 91%), grade 2-3 dysphagia (0% versus 21%), and weight loss (1.2% weight gain versus 5.3% weight loss). Although rates of feeding tubes after photon RT in the pediatric population are not well established, Betchel et al [36] described 33% of pediatric patients required nutritional support after PBT. Weight loss >5% of baseline was associated with a maximum esophageal dose >50 Gy or a mean oropharyngeal dose >30 Gy. Because those patients were treated with passive scatter-beam proton RT, IMPT may potentially lower feeding tube rates. Vogel et al [22] reported on 69 children, treated with PBT to the head and neck region, predominantly with rhabdomyosarcoma or Ewing sarcoma. Grade 3 mucositis, dysphagia, and weight loss were very low at 4%, 7%, and 22%, respectively [22]. However, the benefits of PBT on mucositis, dysphagia, weight loss, and feeding tube placement require further study in the pediatric population. As a caveat, at least 1 study did not find any difference between photons and protons for acute mucosal toxicity or late mucosal toxicity, which occurred in 57% and 10% of patients, respectively [36].
Acute and late salivary toxicity is another major side effect in head and neck RT affecting young children. Acute and late salivary toxicities in children irradiated to the head and neck region are approximately 25% and 10%, respectively. Bölling et al [23] demonstrated that the dose to the submandibular gland may be important in pediatric patients because maximum doses to the submandibular gland, but not the parotid gland, were associated with acute salivary toxicity [36]. Furthermore, PBT was associated with 8.3-fold less salivary toxicity compared with photon-based RT. Similarly, for children with salivary gland tumors, PBT was associated with lower doses to the salivary glands as well as optic apparatus and the pituitary, spinal cord, mandible, oral cavity, and larynx compared with photon RT.
Although protons may reduce many of the common side effects associated with head and neck RT, one underappreciated acute side effect of PBT is increased skin toxicity; PBT is well known for the increased skin toxicity because of the challenges in controlling skin dose. This lack of skin sparing by photons over protons likely results from both the effect of additive range uncertainties for proton therapy as well as the use of more field angles and/or arc therapies for photon therapy. To that end, Phillips et al [37] reported that proton irradiation was associated with >5.7-fold risk for alopecia compared with photon irradiation. Skin sparing may be better with IMPT compared with passive-scatter proton therapy. Furthermore, the skin dose in IMPT, as in IMRT- and VMAT-based photon techniques, may be optimized by reducing target volumes several millimeters from the skin surface. However, the skin dose with IMPT is still often more robust compared with photon-based techniques, which may be even more pronounced with head and neck cancer, in which target volumes often are close to the skin.
Late Toxicities
Given the potential for long-term survivors of childhood cancer, late toxicity must be carefully considered, both in quality and quantity of life. This concern is supported by the observation that the cumulative mortality attributable to nonrecurrence causes increases from 2% at 15 years to 7% at 30 years, whereas the mortality from recurrent cancer increases from 6.3% to 7.8% during that same time frame [38]. With a median follow-up of 10.5 years, 77% of long-term survivors of childhood head and neck cancer experience grade ≥3 late toxicities [39]. In a long-term follow-up of 17 children receiving head and neck photon RT for rhabdomyosarcoma, late effects of treatment were seen in all patients and included facial-growth retardation in 11 (65%) and dental abnormalities in 7 patients (41%) [26]. Similarly, Meazza et al [40] reported assessment of late toxicity in 36 patients treated for head and neck rhabdomyosarcoma with photon-based RT. The most common side effects were facial growth retardation (72%; 26 of 36) and dental abnormalities (69%; 25 of 36). Xerostomia occurred in 38% of all patients [40]. Furthermore, visual or orbital toxicities occurred in 3 of 11 (27%) 5-year survivors of nonorbital head-and-neck rhabdomyosarcoma treated with photon RT [26]. In addition, in a similar patient population, auditory toxicities were observed in 20% of patients treated with photon RT.
By contrast, PBT appears to substantially reduce late radiation complications because grade ≥2 and ≥3 late toxicities were 35% and 17% at 10 years and 45% and 17% at 20 years, respectively [41]. Fukushima et al [27] followed 60 patients 15 years or younger treated between 1983 to 2011 with PBT. A total of 32% (19 of 60) of patients had ≥1 grade ≥3 toxicity, most commonly associated with facial deformities and/or central nervous system damage. By contrast, the severity of other late toxicities, including hormone deficiencies, hair loss, hypothyroidism, and dental dysgenesis were mostly grade 1-2. Similarly, Leiser et al [14] reported low rates of grade 3 toxicities at 5 years for ocular and nonocular rhabdomyosarcoma of 18.4% and 3.6%, respectively.
Hearing loss represents another significant morbidity for children irradiated for head and neck cancers. The combined Intergroup Rhabdomyosarcoma Studies (IRS) II and III reported a 17% rate of hearing loss [28]. Neuro-otologic morbidity is, in part, related to cochlear irradiation with maximum radiation doses kept to <32 Gy to minimize the risk of hearing loss [42]. Extrapolating from medulloblastoma, IMRT reduced cochlear doses compared with conventional RT among 26 children treated for medulloblastoma [43]. However, there were no differences in hearing loss between photons and protons likely because of the inclusion of cisplatin and/or other ototoxic chemotherapies [29, 44]. By contrast, Moeller et al [45] reported a very low rate of ototoxicity in children with medulloblastoma treated with PBT. These results must be interpreted cautiously, however, because of the role of ototoxic chemotherapy in medulloblastoma management.
Radiation-Induced Malignancies
Radiation-associated malignancies are one of the most feared complications in irradiating pediatric cancer patients. For survivors of childhood cancers, the 20- and 30-year risk for developing second malignancies approximates 3.2% and 7.9%, respectively [46, 47]. The risk of second malignancies continues to increase, even after the age of 50 years, with a cumulative incidence of 16.3% by age 55 years [48]. However, PBT has consistently decreased the estimated radiation-induced cancer risk in pediatric patients. Leiser et al [14] observed only 1 radiation-induced malignancy in 83 patients (1%) with rhabdomyosarcoma. Reporting the 10-year cumulative incidences of secondary tumors is survivors of retinoblastoma, PBT was associated with significantly lower in-field malignancies (0% versus 14%) and all malignancies (5% versus 14%) compared with photon RT [49]. Mizumoto et al [41] reported long-term follow-up for 62 patients treated with PBT followed for more than 5 years; of which, 46 (74%) were treated to the head and neck and brain regions. No secondary tumors occurred within the irradiated field.
Because secondary malignancies are difficult to assess in longitudinal studies, given the relative recent widespread implementation of proton therapy, much effort has been devoted to estimating metrics for cancer risk based on dosimetric assessment of RT plans. However, Ngyuen et al [50] has questioned the reliability of those cancer risk models because of high degrees of uncertainty in dosimetry. Even though those uncertainties limit the validity of cancer risk models for a single modality, comparisons of cancer risks between proton and photon treatment plans can still be made because the ratio of absolute risks between 2 modalities is less sensitive to those uncertainties. In a prospective, phase II study comparing the integral dose for passive-scatter PBT versus IMRT for rhabdomyosarcoma in 54 patients, the integral dose for IMRT was 1.8 times greater in the head and neck region and 3.5-fold greater for orbital site [10, 51]. In addition, IMPT in parameningeal head and neck rhabdomyosarcoma reduced the estimated risk of secondary cancers by 1.75-fold compared with passive-scattered protons, 2-fold compared with IMRT, and 2.5-fold compared with 3D-CRT [52]. Similarly, compared with photon plans, Stokkevåg et al [53] demonstrated that IMPT achieved significantly better dose conformity, resulting in a 6-fold reduction in risk of second malignancies.
Moteabbed et al [54] calculated the lifetime attributable risk (LAR) of second cancers in pediatric patients irradiated with passive-scatter PBT, IMPT, IMRT, or VMAT. The LAR for soft tissue or skull malignancies ranged from 0.01% to 2.8% for PBT and 0.04% to 4.9% for photon-based therapy [54]. Of note, that LAR was independent of the number of fields used for proton or photon RT. Although the authors did not find a difference in cancer risk between passive-scatter PBT or IMPT, their study calculated the lifetime risk for proton modalities using a 12-mm spot size. That larger spot size may not have fully realized the advantages of IMPT delivered with smaller spot sizes. To that end, Moteabbed et al [55] demonstrated that IMPT using larger spot sizes did not provide a dosimetric advantage over passive-scattered proton beams. Decreasing the spot size lowered the mean doses to healthy tissues by up to 11.6%, providing a maximum NTCP reduction of 5.4% relative to passively scattered PBT [55].
Not all reports have demonstrated reduced risks of secondary cancer with PBT. Tamura et al [56] calculated the LAR for PBT and IMRT for 4 anatomic sites, including the head and neck region. For the brain, head, and neck region, the difference in lifetime risk between PBT and IMRT was 1.02% ± 0.52%. Many factors may have caused the discrepancies between this report and other series. First, of the 8 cases used to estimate the LAR for irradiation of the head and neck region, 7 cases (88%) were primary brain tumors, which are associated with similar LARs between photon and proton modalities. One case was a patient with Ewing sarcoma involving the head and neck. Second, the LAR was estimated for passive-scatter PBT only and may have missed a greater benefit with IMPT. Thus, most of the evidence indicates that PBT reduces integral dose and thereby likely reduces the risk of secondary malignancies.
Children with Genetic Conditions That Sensitize Them to Radiation Side Effects
Pediatric head and neck malignancies are rare, with an annual rate of approximating 1 in 100 000 person-years [1]. Although genetic diseases associated with DNA repair and the maintenance of genomic stability are also rare, cancers including those involving the head and neck region frequently arise in pediatric and young adult patients. One of the most well-known genetic diseases is Fanconi anemia, which occurs from defects in a cluster of ≥17 genes involved in homologous recombination and occurs with an incidence of 1 in 130 000 births. Adolescents and young adults with Fanconi anemia frequently develop head and neck squamous cell carcinomas, with an incidence of up to 50% by 40 years old [57]. Because of defects in DNA repair, patients with Fanconi anemia and head and neck squamous cell carcinoma tolerate chemotherapy and radiation poorly, even though they frequently present with locally advanced disease [58]. Similarly, Li-Fraumeni cancer syndrome, a germline disorder in p53, has been associated with many pediatric head and neck cancers, including rhabdomyosarcomas [59], soft tissue sarcomas [60], and squamous cell carcinomas. Clinical and preclinical evidence suggest that Li-Fraumeni syndrome may be associated with increased treatment toxicities, including secondary malignancies [61].
Consequently, PBT may benefit children with cancers resulting from germline disorders by increasing conformality and reducing integral dose. At this point, only case reports support the feasibility of PBT in these settings. Hartman and Hill-Kayser [62] describe the experience using proton therapy to treat an oropharyngeal squamous cell carcinoma in a child with dyskeratosis congenita, which is frequently caused by a mutation in the telomere-associated protein DKC1. Although the patient still experienced greater dermatitis and mucositis, the patient remained disease free and was no longer dependent on a feeding tube. Similarly, PBT successfully controlled a recurrence of a choroid plexus tumor in a 3-year-old child with Li-Fraumeni syndrome [63]. Finally, Beckham et al [64] described the first Fanconi anemia patient treated with proton RT for head and neck cancer who tolerated the treatment remarkably well. Although PBT may benefit patients with cancers associated with genetic syndromes, care must be taken when determining radiation dose with PBT. Namely, disruption of DNA repair pathways, especially those involving homologous recombination, may alter the relative biologic effectiveness of particle therapy beams [65, 66]. Consequently, further study is necessary to define the advantages of proton therapy in children with germline mutations.
Socioeconomic Implications of PBT in Children
Although proton therapy can be implemented for many different types of cancer, PBT requires greater health care expenditures and may not be accessible to all individuals at this time. Dvorak et al (unpublished data) demonstrated that, for a single institution, treating patients with PBT instead of IMRT or VMAT increased costs by 22%. However, in the entire patient population, head and neck pediatric cancers represented only 16% and 2% of the entire population treated with proton or photon radiation, respectively. Verma et al [67] demonstrated the cost effectiveness of PBT for both pediatric patients as well as high-risk head and neck cancers. However, disparities exist for access to PBT for children, which depend, in part, upon insurance, race, household income, and/or parental educational level [68, 69]. Consequently, the benefit of proton therapy for children is likely tempered by issues with access-appropriate treatment facilities.
Despite the theoretic and empiric advantages of PBT over photon-based therapy, it remains unclear what the ethical limitations are for ensuring pediatric head and neck cancers are treated with PBT. Johnstone et al [70] argued that the dosimetry, as well as emerging clinical evidence, argues that PBT is the only ethical approach for craniospinal irradiation of children. By contrast, Wolden [71] argues that the existing data should be balanced with the burden of relocation for treatment. Consequently, the downstream costs of treating secondary cancers, craniofacial abnormalities, and other late complications, including dental hypoplasia, likely outweigh the upfront financial toxicity of PBT.
Alternative Particle-Based Modalities
Moreover, PBT remains the “gold standard” for particle-based therapies that minimize toxicities with equivalent efficacy as photon RT in children with cancer. The recent emergence of IMPT may further improve proton beam delivery for head and neck cancers. The advancement of MFO-IMPT and the potential for LET optimization may hold additional promise to provide greater conformality, reduce heterogeneity, and further reduce toxicities as well as secondary malignancies.
Carbon ion therapy has demonstrated impressive control rates in a small series of children and adolescents with rhabdomyosarcomas [72], as well as in chordomas and chondrosarcomas [73]. However, the few centers offering carbon ion therapy worldwide limits current research into this modality in pediatric head and neck cancers. In addition, the dosimetry of very high energy electrons (VHEEs), with energy ranging from 100 to 200 MeV, has been shown to be superior to the dosimetry of VMAT. Figure 2 demonstrates potential benefits of VHEE dosimetry compared with proton and photon RT. Furthermore, VHEEs have demonstrated better theoretic sparing of central nervous structures compared with pencil-beam proton therapy [75]. Although both carbon ion therapy and VHEE require much further study, both modalities present interesting theoretic benefits to further reduce the toxicities associated with pediatric head and neck RT.
Figure 2.
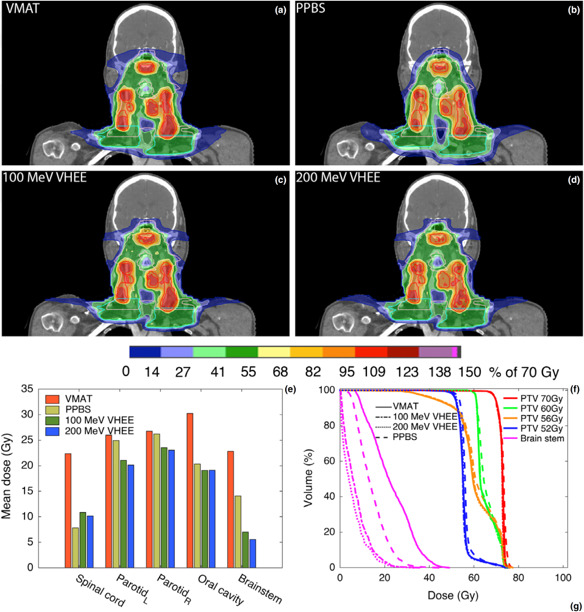
Comparison of VMAT, PPBS, and VHEE planning. (a–d) Coronal images through PTV for the different modalities: (a) VMAT, (b) PPBS, (c) 100 MeV VHEE and (d) 200 MeV VHEE. (e) mean doses to the spinal cord, parotid glands, oral cavity, and brain stem, (f) dose volume histogram for the PTVs and brain stem. Abbreviations: PPBS, proton pencil-beam scanning; PTV, planning target volume; VHEE, very high energy electron; VMAT, volumetric-modulated arc therapy. Reproduced with permission from Schuler et al [74].
Conclusions
As long-term survival rates of children with cancer increase, so do the children at risk for long-term radiation morbidities. Irradiation of the head and neck region is associated with multiple radiation complications afflicting the vision, hearing, eating, and growth. It remains unclear the extent to which particle beam therapy, especially proton therapy, represents a cost-effective approach to minimize several dosimetric measures of toxicity that have been realized in the clinic. However, PBT has demonstrated few acute and late radiation toxicities and provides similar rates of locoregional control for pediatric patients with head and neck cancer. In addition, PBT may benefit children with genetic syndromes that both sensitize them to radiation side effects and predispose them to head and neck cancers. Emerging technologies provide theoretic benefit for further reducing radiation toxicities. Thus, improving the technical precision of radiation and medical management of radiation toxicities gives hope to the survivors of all pediatric cancers, including those of head and neck, who are at risk for, or are suffering from, the complications of RT.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Steven J. Frank, M.D., is an Associate Editor of the International Journal of Particle Therapy. Dr Frank reports grants and personal fees from Hitachi. Outside the submitted work, Dr Frank is a cofounder of C4 Imaging, LLC, for which he reports grants and personal fees, and he reports personal fees from Varian; grants from Eli Lilly, Elekta, and Breakthrough Chronic Care; and personal fees from Augmenix and the National Comprehensive Cancer Center (NCCN). The authors have no additional conflicts of interest to disclose.
Funding: This work was supported by NIH/NIDCR R01DE027445-01 (M.T.S.).
Ethical Approval: This review was exempt from institutional review board approval because it did not involve human subject research.
References
- 1.Albright JT, Topham AK, Reilly JS. Pediatric head and neck malignancies: US incidence and trends over 2 decades. Arch Otolaryngol Head Neck Surg. 2002;128:655–9. doi: 10.1001/archotol.128.6.655. [DOI] [PubMed] [Google Scholar]
- 2.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit ME, Packer RJ, Potter JD, Sklar CA, Smith MA, Stovall M, Strong LC, Yasui Y, Zeltzer LK. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 3.Hein PA, Gladstone DJ, Bellerive MR, Hug EB. Importance of protocol target definition on the ability to spare normal tissue: an IMRT and 3D-CRT planning comparison for intraorbital tumors. Int J Radiat Oncol Biol Phys. 2005;62:1540–8. doi: 10.1016/j.ijrobp.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 4.McDonald MW, Esiashvili N, George BA, Katzenstein HM, Olson TA, Rapkin B, Marcus RB., Jr Intensity-modulated radiotherapy with use of cone-down boost for pediatric head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2008;72:884–91. doi: 10.1016/j.ijrobp.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 5.Wolden SL, Wexler LH, Kraus DH, Laquaglia MP, Lis E, Meyers PA. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1432–8. doi: 10.1016/j.ijrobp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Kozak KR, Adams J, Krejcarek SJ, Tarbell NJ, Yock TI. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179–86. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 7.Yock T, Schneider Friedmann A, Adams J, Fullerton B, Tarbell N. Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63:1161–8. doi: 10.1016/j.ijrobp.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Indelicato DJ, Rotondo RL, Mailhot Vega RB, Uezono H, Bradfield S, Agarwal V, Hol ML, Bradley JA. 45 GyRBE for group III orbital embryonal rhabdomyosarcoma. Acta Oncol. 2019;58:1404–9. doi: 10.1080/0284186X.2019.1627412. [DOI] [PubMed] [Google Scholar]
- 9.Grant SR, Grosshans DR, Bilton SD, Garcia JA, Amin M, Chambers MS, McGovern SL, McAleer MF, Morrison WH, Huh WW, Kupferman ME, Mahajan A. Proton versus conventional radiotherapy for pediatric salivary gland tumors: acute toxicity and dosimetric characteristics. Radiother Oncol. 2015;116:309–15. doi: 10.1016/j.radonc.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladra MM, Edgington SK, Mahajan A, Grosshans D, Szymonifka J, Khan F, Moteabbed M, Friedmann AM, MacDonald SM, Tarbell NJ, Yock TI. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol. 2014;113:77–83. doi: 10.1016/j.radonc.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, Plastaras J, Bucci MK, Yock TI, Bonilla L, Price R, Harris EE, Konski AA. An evidence based review of proton beam therapy: the report of ASTRO's Emerging Technology Committee. Radiother Oncol. 2012;103:8–11. doi: 10.1016/j.radonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Indelicato DJ, Merchant T, Laperriere N, Lassen Y, Vennarini S, Wolden S, Hartsell W, Pankuch M, Brandal P, Law CK, Taylor R, Laskar S, Okcu MF, Bouffet E, Mandeville H, Bjork-Eriksson T, Nilsson K, Nystrom H, Constine LS, Story M, Timmermann B, Roberts K, Kortmann RD. Consensus report from the Stockholm Pediatric Proton Therapy Conference. Int J Radiat Oncol Biol Phys. 2016;96:387–92. doi: 10.1016/j.ijrobp.2016.06.2446. [DOI] [PubMed] [Google Scholar]
- 13.Chen TW, Sison J, Lee B, Olch AJ, Chang A, Giebeler A, Wong K. A dosimetric comparison of intensity-modulated proton therapy, volumetric-modulated arc therapy, and 4π non-coplanar intensity-modulated radiation therapy for a patient with parameningeal rhabdomyosarcoma. Cureus. 1673. 9:e. [DOI] [PMC free article] [PubMed]
- 14.Leiser D, Calaminus G, Malyapa R, Bojaxhiu B, Albertini F, Kliebsch U, Mikroutsikos L, Morach P, Bolsi A, Walser M, Timmermann B, Lomax T, Schneider R, Weber DC. Tumour control and quality of life in children with rhabdomyosarcoma treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120:163–8. doi: 10.1016/j.radonc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Munck af Rosenschold P, Engelholm SA, Brodin PN, Jorgensen M, Grosshans DR, Zhu RX, Palmer M, Crawford CN, Mahajan A. A retrospective evaluation of the benefit of referring pediatric cancer patients to an external proton therapy center. Pediatr Blood Cancer. 2016;63:262–9. doi: 10.1002/pbc.25768. [DOI] [PubMed] [Google Scholar]
- 16.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, Munsell MF, Palmer MB, Sahoo N, Zhang X, Liu W, Zhu XR. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–53. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holliday EB, Kocak-Uzel E, Feng L, Thaker NG, Blanchard P, Rosenthal DI, Gunn GB, Garden A, Frank SJ. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis. Med Dosim. 2016;41:189–94. doi: 10.1016/j.meddos.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Doyen J, Falk AT, Floquet V, Hérault J, Hannoun-Levi JM. Proton beams in cancer treatments: clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat Rev. 2016;43:104–12. doi: 10.1016/j.ctrv.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Jakobi A, Bandurska-Luque A, Stutzer K, Haase R, Lŭck S, Wack LJ, Monnich D, Thorwarth D, Perez D, Lühr A, Zips D, Krause M, Baumann M, Perrin R, Richter C. Identification of patient benefit from proton therapy for advanced head and neck cancer patients based on individual and subgroup normal tissue complication probability analysis. Int J Radiat Oncol Biol Phys. 2015;92:1165–74. doi: 10.1016/j.ijrobp.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Kandula S, Zhu X, Garden AS, Gillin M, Rosenthal DI, Ang KK, Mohan R, Amin MV, Garcia JA, Wu R, Sahoo N, Frank SJ. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim. 2013;38:390–4. doi: 10.1016/j.meddos.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 21.van de Water TA, Lomax AJ, Bijl HP, de Jong ME, Schilstra C, Hug EB, Langendijk JA. Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79:1216–24. doi: 10.1016/j.ijrobp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Vogel J, Both S, Kirk M, Chao HH, Bagatell R, Li Y, Womer R, Balamuth N, Reilly A, Kurtz G, Lustig R, Tochner Z, Hill-Kayser C. Proton therapy for pediatric head and neck malignancies. Pediatr Blood Cancer. 2018;65:e26858. doi: 10.1002/pbc.26858. [DOI] [PubMed] [Google Scholar]
- 23.Bölling T, Weege J, Eich HT, Timmermann B, Meyer FM, Rube C, Kortmann RD, Fischedick K, Rödel C, Koch R, Willich N. Acute and late side effects to salivary glands and oral mucosa after head and neck radiotherapy in children and adolescents: results of the “Registry for the Evaluation of Side Effects after Radiotherapy in Childhood and Adolescence.”. Head Neck. 2015;37:1137–41. doi: 10.1002/hed.23715. [DOI] [PubMed] [Google Scholar]
- 24.Lucas JT, Jr, Ladra MM, MacDonald SM, Busse PM, Friedmann AM, Ebb DH, Marcus KJ, Tarbell NJ, Yock TI. Proton therapy for pediatric and adolescent esthesioneuroblastoma. Pediatr Blood Cancer. 2015;62:1523–8. doi: 10.1002/pbc.25494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber DC, Ares C, Albertini F, Frei-Welte M, Niggli FK, Schneider R, Lomax AJ. Pencil beam scanning proton therapy for pediatric parameningeal rhabdomyosarcomas: clinical outcome of patients treated at the Paul Scherrer Institute. Pediatr Blood Cancer. 2016;63:1731–6. doi: 10.1002/pbc.25864. [DOI] [PubMed] [Google Scholar]
- 26.Paulino AC, Simon JH, Zhen W, Wen BC. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2000;48:1489–95. doi: 10.1016/s0360-3016(00)00799-9. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima H, Fukushima T, Suzuki R, Iwabuchi A, Hidaka K, Shinkai T, Masumoto K, Muroi A, Yamamoto T, Nakao T, Oshiro Y, Mizumoto M, Sakurai H, Sumazaki R. Comorbidity and quality of life in childhood cancer survivors treated with proton beam therapy. Pediatr Int. 2017;59:1039–45. doi: 10.1111/ped.13323. [DOI] [PubMed] [Google Scholar]
- 28.Raney RB, Asmar L, Vassilopoulou-Sellin R, Klein MJ, Donaldson SS, Green J, Heyn R, Wharam M, Glicksman AS, Gehan EA, Anderson J, Maurer HM. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the intergroup rhabdomyosarcoma studies (IRS)-II and -III—IRS group of the Children's Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33:362–71. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Paulino AC, Mahajan A, Ye R, Grosshans DR, Okcu MF, Su J, McAleer MF, McGovern S, Mangona VA, Chintagumpala M. Ototoxicity and cochlear sparing in children with medulloblastoma: proton vs. photon radiotherapy. Radiother Oncol. 2018;128:128–32. doi: 10.1016/j.radonc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Haas-Kogan D, Indelicato D, Paganetti H, Esiashvili N, Mahajan A, Yock T, Flampouri S, MacDonald S, Fouladi M, Stephen K, Kalapurakal J, Terezakis S, Kooy H, Grosshans D, Makrigiorgos M, Mishra K, Poussaint TY, Cohen K, Fitzgerald T, Gondi V, Liu A, Michalski J, Mirkovic D, Mohan R, Perkins S, Wong K, Vikram B, Buchsbaum J, Kun L. National Cancer Institute Workshop on Proton Therapy for Children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys. 2018;101:152–68. doi: 10.1016/j.ijrobp.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–91. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey DL, Wexler LH, Wolden SL. Worse outcomes for head and neck rhabdomyosarcoma secondary to reduced-dose cyclophosphamide. Int J Radiat Oncol Biol Phys. 2019;103:1151–7. doi: 10.1016/j.ijrobp.2018.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludmir EB, Grosshans DR, McAleer MF, McGovern SL, Harrison DJ, Okcu MF, Chintagumpala MM, Mahajan A, Paulino AC. Patterns of failure following proton beam therapy for head and neck rhabdomyosarcoma. Radiother Oncol. 2019;134:143–500. doi: 10.1016/j.radonc.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Rombi B, DeLaney TF, MacDonald SM, Huang MS, Ebb DH, Liebsch NJ, Raskin KA, Yeap BY, Marcus KJ, Tarbell NJ, Yock TI. Proton radiotherapy for pediatric Ewing's sarcoma: initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1142–8. doi: 10.1016/j.ijrobp.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Frankl J, Grotepas C, Stea B, Lemole GM, Chiu A, Khan R. Chordoma dedifferentiation after proton beam therapy: a case report and review of the literature. J Med Case Rep. 2016;10:280. doi: 10.1186/s13256-016-1076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bechtel AS, Indelicato DJ, Sandler E. Enteral nutrition in pediatric high-risk head and neck cancer patients receiving proton therapy: identifying risk factors and quality of life concerns to optimize care. J Pediatr Hematol Oncol. 2019;41:e247–53. doi: 10.1097/MPH.0000000000001364. [DOI] [PubMed] [Google Scholar]
- 37.Phillips GS, Freret ME, Friedman DN, Trelles S, Kukoyi O, Freites-Martinez A, Unger RH, Disa JJ, Wexler LH, Tinkle CL, Mechalakos JG, Dusza SW, Beal K, Wolden SL, Lacouture ME. Assessment and treatment outcomes of persistent radiation-induced alopecia in patients with cancer. JAMA Dermatol. 2020;56:963–72. doi: 10.1001/jamadermatol.2020.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, Mertens AC. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–38. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Affinita MC, Ferrari A, Milano GM, Scarzello G, De Leonardis F, Coccoli L, Pericoli R, Basso E, Zanetti I, Scagnellato A, Bisogno G. Long-term results in children with head and neck rhabdomyosarcoma: a report from the Italian Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2018;65:e26876. doi: 10.1002/pbc.26876. [DOI] [PubMed] [Google Scholar]
- 40.Meazza C, Ferrari A, Casanova M, Gandola L, Collini P, Massimino M, Luksch R, Spreafico F, Cefalo G, Polastri D, Terenziani M, Podda M, Cantù G, Scaramuzza D, Fossati-Bellani F. Evolving treatment strategies for parameningeal rhabdomyosarcoma: the experience of the Istituto Nazionale Tumori of Milan. Head Neck. 2005;27:49–57. doi: 10.1002/hed.20117. [DOI] [PubMed] [Google Scholar]
- 41.Mizumoto M, Murayama S, Akimoto T, Demizu Y, Fukushima T, Ishida Y, Oshiro Y, Numajiri H, Fuji H, Okumura T, Shirato H, Sakurai H. Long-term follow-up after proton beam therapy for pediatric tumors: a Japanese national survey. Cancer Sci. 2017;108:444–7. doi: 10.1111/cas.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merchant TE, Gould CJ, Xiong X, Robbins N, Zhu J, Pritchard DL, Khan R, Heideman RL, Krasin MJ, Kun LE. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys. 2004;58:1194–207. doi: 10.1016/j.ijrobp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Huang E, Teh BS, Strother DR, Davis QG, Chiu JK, Lu HH, Carpenter LS, Mai WY, Chintagumpala MM, South M, Grant WH, III, Butler EB, Woo SY. Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys. 2002;52:599–605. doi: 10.1016/s0360-3016(01)02641-4. [DOI] [PubMed] [Google Scholar]
- 44.Paulino AC, Lobo M, Teh BS, Okcu MF, South M, Butler EB, Su J, Chintagumpala M. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;78:1445–50. doi: 10.1016/j.ijrobp.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 45.Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, Gidley PW, Vats TS, Mahajan A. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol. 2011;6:58. doi: 10.1186/1748-717X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL, Neglia JP. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–95. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–29. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 48.Turcotte LM, Whitton JA, Friedman DL, Hammond S, Armstrong GT, Leisenring W, Robison LL, Neglia JP. Risk of subsequent neoplasms during the fifth and sixth decades of life in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2015;33:3568–75. doi: 10.1200/JCO.2015.60.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY, Munzenrider JE, Grabowski E, Rodriguez-Galindo C, Yock TI, Tarbell NJ, Marcus KJ, Mukai S, MacDonald SM. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120:126–33. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen J, Moteabbed M, Paganetti H. Assessment of uncertainties in radiation-induced cancer risk predictions at clinically relevant doses. Med Phys. 2015;42:81–9. doi: 10.1118/1.4903272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ladra MM, Szymonifka JD, Mahajan A, Friedmann AM, Yong Yeap B, Goebel CP, MacDonald SM, Grosshans DR, Rodriguez-Galindo C, Marcus KJ, Tarbell NJ, Yock TI. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32:3762–70. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–9. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 53.Stokkevåg CH, Engeseth GM, Ytre-Hauge KS, Rohrich D, Odland OH, Muren LP, Brydoy M, Hysing LB, Szostak A, Palmer MB, Petersen JB. Estimated risk of radiation-induced cancer following paediatric cranio-spinal irradiation with electron, photon and proton therapy. Acta Oncol. 2014;53:1048–57. doi: 10.3109/0284186X.2014.928420. [DOI] [PubMed] [Google Scholar]
- 54.Moteabbed M, Yock TI, Paganetti H. The risk of radiation-induced second cancers in the high to medium dose region: a comparison between passive and scanned proton therapy, IMRT and VMAT for pediatric patients with brain tumors. Phys Med Biol. 2014;59:2883–99. doi: 10.1088/0031-9155/59/12/2883. [DOI] [PubMed] [Google Scholar]
- 55.Moteabbed M, Yock TI, Depauw N, Madden TM, Kooy HM, Paganetti H. Impact of spot size and beam-shaping devices on the treatment plan quality for pencil beam scanning proton therapy. Int J Radiat Oncol Biol Phys. 2016;95:190–8. doi: 10.1016/j.ijrobp.2015.12.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura M, Sakurai H, Mizumoto M, Kamizawa S, Murayama S, Yamashita H, Takao S, Suzuki R, Shirato H, Ito YM. Lifetime attributable risk of radiation-induced secondary cancer from proton beam therapy compared with that of intensity-modulated x-ray therapy in randomly sampled pediatric cancer patients. J Radiat Res. 2017;58:363–71. doi: 10.1093/jrr/rrw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 58.Kutler DI, Auerbach AD, Satagopan J, Giampietro RF, Batish SD, Huvos AG, Goberdhan A, Shah JP, Singh B. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 59.Pondrom M, Bougeard G, Karanian M, Bonneau-Lagacherie J, Boulanger C, Boutroux H, Briandet C, Chevreau C, Corradini N, Coze C, Defachelles AS, Galmiche-Roland L, Orbach D, Piguet C, Scoazec JY, Vérité C, Willems M, Frebourg T, Minard V, Brugieres L. Rhabdomyosarcoma associated with germline TP53 alteration in children and adolescents: the French experience. Pediatr Blood Cancer. 2020;67:e28486. doi: 10.1002/pbc.28486. [DOI] [PubMed] [Google Scholar]
- 60.Francom CR, Leoniak SM, Lovell MA, Herrmann BW. Head and neck pleomorphic myxoid liposarcoma in a child with Li-Fraumeni syndrome. Int J Pediatr Otorhinolaryngol. 2019;123:191–94. doi: 10.1016/j.ijporl.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Kasper E, Angot E, Colasse E, Nicol L, Sabourin JC, Adriouch S, Lacoume Y, Charbonnier C, Raad S, Frebourg T, Flaman JM, Bougeard G. Contribution of genotoxic anticancer treatments to the development of multiple primary tumours in the context of germline TP53 mutations. Eur J Cancer. 2018;101:254–62. doi: 10.1016/j.ejca.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Hartman RI, Hill-Kayser CE. Proton therapy and radiation sensitivity in dyskeratosis congenita. J Pediatr Hematol Oncol. 2014;36:e51–3. doi: 10.1097/MPH.0b013e31828e5d5a. [DOI] [PubMed] [Google Scholar]
- 63.McEvoy M, Robison N, Manley P, Yock T, Konopka K, Brown RE, Wolff J, Green AL. Successful treatment of recurrent Li-Fraumeni syndrome-related choroid plexus carcinoma. J Pediatr Hematol Oncol. 2017;39:e473–5. doi: 10.1097/MPH.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beckham TH, Leeman J, Jillian Tsai C, Riaz N, Sherman E, Singh B, Lee N, McBride S, Higginson DS. Treatment modalities and outcomes of Fanconi anemia patients with head and neck squamous cell carcinoma: series of 9 cases and review of the literature. Head Neck. 2019;41:1418–26. doi: 10.1002/hed.25577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grosse N, Fontana AO, Hug EB, Lomax A, Coray A, Augsburger M, Paganetti H, Sartori AA, Pruschy M. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys. 2014;88:175–81. doi: 10.1016/j.ijrobp.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 66.Liu Q, Underwood TSA, Kung J, Wang M, Lu HM, Paganetti H, Held KD, Hong TS, Efstathiou JA, Willers H. Disruption of SLX4-MUS81 function increases the relative biological effectiveness of proton radiation. Int J Radiat Oncol Biol Phys. 2016;95:78–85. doi: 10.1016/j.ijrobp.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483–501. doi: 10.1002/cncr.29882. [DOI] [PubMed] [Google Scholar]
- 68.Shen CJ, Hu C, Ladra MM, Narang AK, Pollack CE, Terezakis SA. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer. 2017;123:4048–56. doi: 10.1002/cncr.30849. [DOI] [PubMed] [Google Scholar]
- 69.Bitterman DS, Bona K, Laurie F, Kao PC, Serezakis SA, London WB, Haas-Kogan DA. Race disparities in proton radiotherapy use for cancer treatment in patients enrolled in Children's Oncology Group Trials. JAMA Oncol. 2020;6:1465–8. doi: 10.1001/jamaoncol.2020.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnstone PAS, McMullen KP, Buchsbaum JC, Douglas JG, Helft P. Pediatric CSI: are protons the only ethical approach? Int J Radiat Oncol Biol Phys. 2013;87:228–30. doi: 10.1016/j.ijrobp.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 71.Wolden SL. Protons for craniospinal radiation: are clinical data important [comment]? Int J Radiat Oncol Biol Phys. 2013;87:231–2. doi: 10.1016/j.ijrobp.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 72.Combs SE, Kessel KA, Herfarth K, Jensen A, Oertel S, Blattmann C, Ecker S, Hoess A, Martin E, Witt O, Jäkel O, Kulozik AE, Debus J. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat Oncol. 2012;7:170. doi: 10.1186/1748-717X-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Combs SE, Nikoghosyan A, Jaekel O, Karger CP, Haberer T, Münter MW, Huber PE, Debus J, Schulz-Ertner D. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer. 2009;115:1348–55. doi: 10.1002/cncr.24153. [DOI] [PubMed] [Google Scholar]
- 74.Schüler E, Eriksson K, Hynning E, Hancock SL, Hiniker SM, Bazalova-Carter M, Wong T, Le QT, Loo BW, Jr, Maxim PG. Very high-energy electron (VHEE) beams in radiation therapy: treatment plan comparison between VHEE, VMAT, and PPBS. Med Phys. 2017;44:2544–55. doi: 10.1002/mp.12233. [DOI] [PubMed] [Google Scholar]
- 75.Simmons DA, Lartey FM, Schüler E, Rafat M, King G, Kim A, Ko R, Semaan S, Gonzalez S, Jenkins M, Pradhan P, Shih Z, Wang J, von Eyben R, Graves EE, Maxim PG, Longo FM, Loo BW., Jr Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 2019;139:4–10. doi: 10.1016/j.radonc.2019.06.006. [DOI] [PubMed] [Google Scholar]


