Given the variable severity of coronavirus disease 2019 (COVID-19), clinicians need biomarkers that identify high-risk patients at the time of hospitalization. While elevated troponin is associated with greater mortality in COVID-19,1,2 studies of natriuretic peptides (NPs) have been inconclusive.2,3
We investigated the independent prognostic value of NPs and troponin in COVID-19 inpatients discharged (alive or dead) between April 1 and November 30, 2020, with laboratory data in Premier Healthcare Database (n=48 510). COVID-19 infection, comorbidities and outcomes were defined using the International Classification of Diseases, Tenth Revision codes. Only biomarkers at admission (days 1 or 2) were considered. Associations between biomarkers and in-hospital death were assessed by logistic regression, adjusted for 18 covariates: age, sex, race/ethnicity, month, region, obesity, chronic renal/pulmonary disease, diabetes, hypertension, smoking, cancer, and 6 cardiovascular conditions (heart failure, myocardial infarction, peripheral vascular and cerebrovascular disease, valve disease, and atrial fibrillation). Supporting data are available to qualified researchers from Premier. The Mass General Brigham Institutional Review Board approved the study.
NPs were available in 12 252 (25%) patients, and were analyzed in 4 groups: ≤35, 36 to 100, 101 to 400, and >400 pg/mL for BNP (B-type natriuretic peptide) and ≤100, 101 to 200, 201 to 1500, and >1500 pg/mL for NT-proBNP (N-terminal pro-B-type natriuretic peptide). Patients with higher NPs were older (mean, 74 years of age in the highest group vs 54 years of age in the lowest) and more likely to have heart failure (61% vs 5%) and atrial fibrillation (46% vs 5%). Adjusted risk of death was markedly greater in the highest versus lowest NP group (adjusted odds ratio [ORadj] 2.74 [95% CI, 2.22–3.39]; P=1.2×10-20) (Figure). This magnitude of association was comparable to an additional 27 years of age (ORadj, 1.04 [95% CI, 1.03–1.04] per year) and exceeded male sex (ORadj, 1.23; 95% CI, 1.10–1.38) or morbid obesity (ORadj, 1.91; 95% CI, 1.63–2.24 vs nonobese). Addition of NPs to the multivariable model increased the area under receiver operator characteristic curve from 0.746 (95% CI, 0.734–0.757) to 0.754 (95% CI, 0.743–0.766) (P<0.001 by DeLong method). High NPs were also associated with secondary composite end points: death or mechanical ventilation (ORadj, 3.14 [95% CI, 2.66–3.72]) and death or intensive care (ORadj, 2.87 [95% CI, 2.48–3.32]).
Figure.
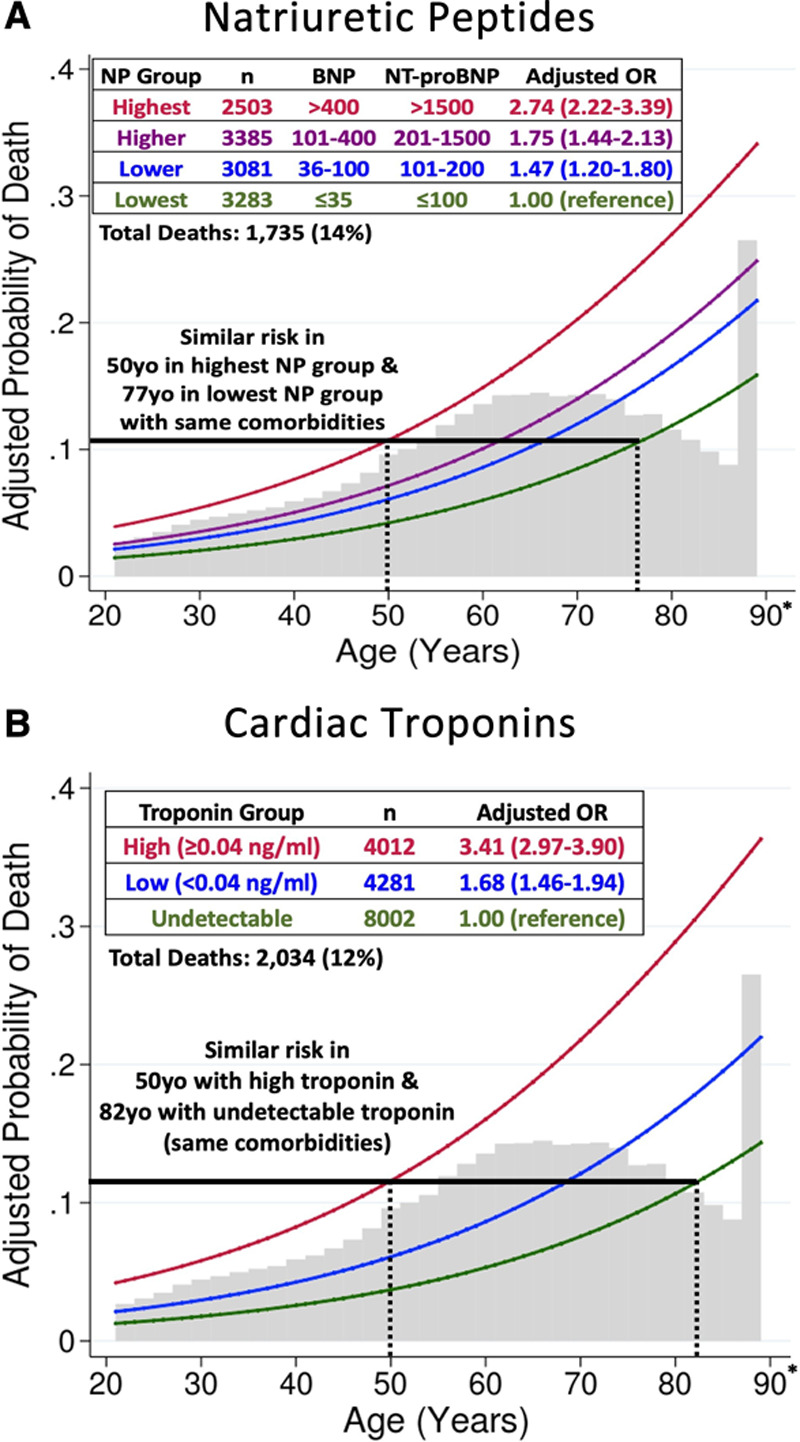
Adjusted probability of death, by age and biomarker level. A, Natriuretic peptides. B, Cardiac troponins. Curves represent estimated probability of death by multivariable logistic regression model including 18 covariates, with age as a linear predictor. Natriuretic peptide ranges are expressed in pg/mL. BNP indicates B-type natriuretic peptide; NP natriuretic peptide; NT-proBNP, N-terminal pro-B-type natriuretic peptide; and OR, odds ratio. *Patients ≥89 years of age (n=857 for NPs, n=1026 for troponin) are represented as 89 years in the dataset to preserve deidentification.
Troponin T or I was available in 16 295 (34%) patients; we classified levels as undetectable, low (<0.04 ng/mL), or high (≥0.04 ng/mL). Patients with high versus undetectable troponin were older (mean, 71 vs 60 years of age), more likely to be male (60% vs 50%), and had greater cardiovascular disease (70% vs 24%). Compared with undetectable troponin, risk of death was greater in patients with low (ORadj, 1.68 [95% CI, 1.46–1.94]; P=5.2×10-13) and high (ORadj, 3.41 [95% CI, 2.97–3.90]; P=1.7×10-70) troponin (Figure); the latter risk was comparable to 32 years of age (ORadj, 1.04 [95% CI, 1.03–1.04] per year). Adding troponin to the multivariable model increased the area under receiver operator characteristic curve from 0.768 (95% CI, 0.757–0.778) to 0.793 (95% CI, 0.784–0.803) (P<0.001). High troponin was associated with death or mechanical ventilation (ORadj, 3.08 [95% CI, 2.76–3.44]) and death or intensive care (ORadj, 3.04 [95% CI, 2.76–3.36]). In 8122 patients with both biomarkers, patients with high NPs and high troponin (n=934; 12%) had ORadj of 6.10 (95% CI, 4.25–8.76) for death compared with low NPs and troponin (n=1466; 18%). Both biomarkers together increased the area under receiver operator characteristic curve from 0.755 (95% CI, 0.741–0.769) to 0.783 (95% CI, 0.770–0.796) (P<0.001).
Sensitivity analyses assessed for selection bias and confounding by indication. First, the association between higher NPs or troponin and mortality was not attenuated in patients without known cardiovascular disease (n=7049; ORadj, 4.53 [95% CI, 3.24–6.32] for high vs low NPs; n=9959; ORadj, 4.58 [95% CI, 3.71–5.65]) for high vs undetectable troponin). Second, at sites where biomarker testing was performed in more than the median proportion of COVID-19 patients (26% for NPs, 36% for troponin), risk of death associated with higher biomarker levels was slightly attenuated for NPs (n=9451; ORadj, 2.39 [95% CI, 1.86–3.06] for highest vs lowest group) and somewhat attenuated for troponin (n=12 294; ORadj, 2.81 [95% CI, 2.41–3.28] for high vs undetectable), which may suggest troponin was more subject to selection bias. However, biomarker prognostic value remained strong where testing was performed more systematically. Third, adjusted risk of death was similar in patients with and without troponin testing (ORadj, 1.02 [95% CI, 0.96–1.09]), and only slightly higher in patients with available NPs (ORadj, 1.11 [95% CI, 1.04–1.19]).
Despite modest levels in most patients with COVID-19, NP and troponin elevation strongly and independently predicted in-hospital death, including in patients without cardiovascular disease. The extent to which biomarker levels reflected preexisting cardiovascular disease or COVID-19 severity is unclear. Potential causes of biomarker elevation in COVID-19 include myocardial injury, stress cardiomyopathy, pulmonary embolism, kidney disease, or cardiopulmonary strain attributable to hypoxemia and sepsis, as observed in acute respiratory distress syndrome.1,4 Though biomarkers were obtained for clinical indications, similar adjusted mortality in patients with and without biomarkers suggests minimal residual confounding by indication. Measurement of admission NPs, troponin, or both in COVID-19 patients may help clinicians determine appropriate triage and identify high-risk patients for clinical trials.
Sources of Funding
None.
Disclosures
Dr Cunningham is supported by the National Heart, Lung, and Blood Institute (T32 postdoctoral training grant T32HL094301). Dr Claggett reports personal fees from Amgen, Boehringer Ingelheim, Corvia, MyoKardia, and Novartis. Dr Jering is supported by the National Heart, Lung, and Blood Institute (T32 postdoctoral training grant T32HL007604). Dr Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (National Institutes of Health/National Center for Advancing Translational Sciences Award UL 1TR002541); receives research grant support from Amgen and Boehringer Ingelheim; serves on advisory boards for Amgen, American Regent, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa; and participates in clinical end point committees for studies sponsored by Galmed, Novartis, and the National Institutes of Health. Dr Bhatt reports speaking fees from Sanofi Pasteur and is supported by the National Heart, Lung, and Blood Institute (T32 postdoctoral training grant T32HL007604). Dr Rosenthal is an employee of Premier Inc, which curates the Premier Healthcare Database. Dr Solomon reports grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, Neurotronik, National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Respicardia, Sanofi Pasteur, and Theracos; and personal fees from Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GlaxoSmithKline, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Sanofi-Pasteur, Tenaya, Dinaqor, Tremeau, CellProThera, and Moderna.
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- NP
- natriuretic peptide
- ORadj
- adjusted odds ratio
For Sources of Funding and Disclosures, see page 178.
Contributor Information
Jonathan W. Cunningham, Email: jcunningham3@bwh.harvard.edu.
Brian L. Claggett, Email: BCLAGGETT@BWH.HARVARD.EDU.
Karola S. Jering, Email: kjering@bwh.harvard.edu.
Muthiah Vaduganathan, Email: mvaduganathan@bwh.harvard.edu.
Ankeet S. Bhatt, Email: abhatt5@bwh.harvard.edu.
Ning Rosenthal, Email: ning_rosenthal@premierinc.com.
References
- 1.Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, et al. Myocardial injury in severe COVID-19 compared with non–COVID-19 acute respiratory distress syndrome. Circulation. 2021;143:553–565. doi: 10.1161/CIRCULATIONAHA.120.050543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omland T, Prebensen C, Røysland R, Søvik S, Sørensen V, Røsjø H, Svensson M, Berdal JE, Myhre PL. Established cardiovascular biomarkers provide limited prognostic information in unselected patients hospitalized with COVID-19. Circulation. 2020;142:1878–1880. doi: 10.1161/CIRCULATIONAHA.120.050089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajwa EK, Januzzi JL, Gong MN, Thompson BT, Christiani DC. Prognostic value of plasma N-terminal probrain natriuretic peptide levels in the acute respiratory distress syndrome. Crit Care Med. 2008;36:2322–2327. doi: 10.1097/CCM.0b013e318181040d [DOI] [PMC free article] [PubMed] [Google Scholar]


