Table 1.
Classification and characteristics of dyes depending on the structure of the chromophore.
| Dye Chemical Classes | Chromophore Structure | Examples of Dyes | Characteristics | Reference |
|---|---|---|---|---|
| Azo |

|
Methyl Orange | Azo dyes are frequently used (60%). These dyes have a functional group (-N=N-) linking two alkyl or aryl radicals, symmetrical and or asymmetrical, identical or non-azoic. | [87] |
| Congo Red | ||||
| Orange G | ||||
| Amaranth | ||||
| Anthraquinone |
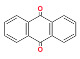
|
Remazol Brilliant Blue R | Anthraquinone dyes are the second most widely used dyes due to their low price, accessibility and performance in the dyeing process. They have anthraquinone chromophore groups comprising two carbonyl groups on either side of a benzene ring. | [88] |
| Reactive Bright Blue X-BR | ||||
| Reactive Blue 4 | ||||
| Alizarin Red S | ||||
| Triphenylmethane |
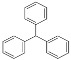
|
Malachite Green | These molecules have a central sp3 hybridised carbon atom, bonded to three aryl groups and belong to the most commonly used synthetic dyes in the textile industry. | [89] |
| Crystal Violet | ||||
| Bromophenol Blue | ||||
| Light Green SF | ||||
| Nitro and Nitroso |
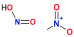
|
Naphthol Yellow S | In nitro dyes, a nitro group conjugates to an electron donor group via an aromatic system. Nitro dyes always contain a hydroxyl group as a donor. | [90] |
| Disperse Yellow 26 | ||||
| Disperse Yellow 14 | ||||
| Indigoid |
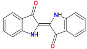
|
Indigo Carmine | Synthetic indigo is the most widely used dye in the textile industry worldwide. It is highly resistant to light and high temperatures. | [91] |
| Ciba Blue 2B | ||||
| Xanthene |
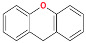
|
Rhodamine 6G | Xanthenes are dyes used in the food, cosmetics, paper and ink manufacturing industries because of their superior dyeing and colouring properties, but are poorly biodegradable, and some of them are very toxic. | [92] |
| Rhodamine 123 | ||||
| Fluorescein | ||||
| Acridine |

|
Acridine orange | Acridine dyes are heat-resistant, although they have low lightfastness. They are currently not very important commercially. | [93] |
| Basic Yellow 9 | ||||
| Phthalein |
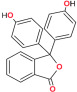
|
o-cresolphthalein | Phthalein dyes are employed to titrate weak acids. Phthalein dyes are insoluble in water but soluble in alcohol. There are frequently in the construction, coatings, electronics and electrical industries. | [94] |
| Thymolphthalein | ||||
| Dixylenolphthalein | ||||
| Phenolphthalein |
The chemical structures were elaborated in the software ACD/ChemSketch, version 2020.1.2, (ACD/ChemSketch, version 2020.1.2, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com, 2020 (accessed on 27 May 2021)).
