Abstract
Background
Intestinal ischemia is a common complication with obscure pathophysiology in critically ill patients. Since insufficient delivery of oxygen is discussed, we investigated the influence of oxygen delivery, hemoglobin, arterial oxygen saturation, cardiac index and the systemic vascular resistance index on the development of intestinal ischemia. Furthermore, we evaluated the predictive power of elevated lactate levels for the diagnosis of intestinal ischemia.
Methods
In a retrospective case-control study data (mean oxygen delivery, minimum oxygen delivery, systemic vascular resistance index) of critical ill patients from 02/2009–07/2017 were analyzed using a proportional hazard model. General model fit and linearity were tested by likelihood ratio tests. The components of oxygen delivery (hemoglobin, arterial oxygen saturation and cardiac index) were individually tested in models.
Results
59 out of 874 patients developed intestinal ischemia. A mean oxygen delivery less than 250ml/min/m2 (LRT vs. null model: p = 0.018; LRT for non-linearity: p = 0.012) as well as a minimum oxygen delivery less than 400ml/min/m2 (LRT vs null model: p = 0.016; LRT for linearity: p = 0.019) were associated with increased risk of the development of intestinal ischemia. We found no significant influence of hemoglobin, arterial oxygen saturation, cardiac index or systemic vascular resistance index. Receiver operating characteristics analysis for elevated lactate levels, pH, CO2 and central venous saturation was poor with an area under the receiver operating characteristic of 0.5324, 0.52, 0.6017 and 0.6786.
Conclusion
There was a significant correlation for mean and minimum oxygen delivery with the incidence of intestinal ischemia for values below 250ml/min/m2 respectively 400ml/min/m2. Neither hemoglobin, arterial oxygen saturation, cardiac index, systemic vascular resistance index nor elevated lactate levels could be identified as individual risk factors.
Introduction
Intestinal ischemia (II) in critically ill patients is a life-threatening complication, leading to sepsis [1–3] caused by bacterial translocation [4–6] or direct fecal contamination of the peritoneal cavity. Congestive heart failure, diabetes mellitus, peripheral artery occlusive disease and age older than 60 years are recognized risk factors [7–9].
The mortality in these patients is increased [10–12] and it is one of the major missed diagnoses in deceased patients treated in intensive care units (ICU), implying an even higher incidence [13–15]. Overall mortality is estimated between 50% to 80% [1–3].
Surgical treatment within 24 hours (h) of diagnosis of II was identified as an independent predictor of survival, emphasizing the need for reliable risk stratification, specific markers, early detection and multidisciplinary management [16].
Acute obstruction with or without previously stenotic arterial vessels, mesenteric venous thrombosis and non-occlusive mesenteric ischemia because of impaired regional oxygen delivery are described as distinct pathophysiological entities leading to II. Although the splanchnic circulation receives approximately 20% of the cardiac output, several mechanisms like increased oxygen extraction and vascular autoregulation protect the intestines from ischemia. Nevertheless, a substantial reduction in oxygen delivery (DO2I in l/min/m²) can lead to an imbalance between oxygen supply and demand and thereby cause II [7, 17–19]. The latter is of special interest for the intensivist as it might be preventable as insufficient DO2I due to low cardiac output (cardiac index, CI in l/min/m²) combined with mesenteric and systemic vasoconstriction (systemic vascular resistance index, SVRI) caused by endo- or exogenous catecholamines leading to insufficient locoregional oxygen supply [1, 2, 9, 12, 20, 21].
Lactic acid, as well as pH, CO2 and central venous saturation (ScvO2), as routine parameters measured in the management of critically ill patients, are commonly used in a clinical setting to detect parenchymatous hypoxia, but the specificity for II is unknown. Furthermore, II per se induces lactic acid accumulation through parenchyma breakdown, as well as further pathological changes in the routine parameters.
Thus, the primary aim of this study is:
to define specific critical cut-off values for short term (the minimal DO2I during the 72 hours period before the diagnosis of II, minDO2I) or prolonged oxygen delivery (the mean DO2I during the 72 hours period before the diagnosis of II, meanDO2I) in the development of II
Secondary objectives of this study are:
to identify the role of the independent parameters (hemoglobin, Hb; arterial oxygen saturation, SaO2 and CI) of DO2I in the development of II;
to identify the independent parameter (either Hb, SaO2 or CI) whose manipulation is most beneficial in order to increase DO2I to a noncritical value to reduce the risk of II;
to evaluate the predictive power of elevated lactate levels, pH, CO2 and central venous saturation (ScvO2) for the diagnosis of II;
to identify the influence of high SVRI on the development of II
Material and methods
Study design
This study was approved by the local ethics committee (Medizinische Ethikkommission II, University Medical Centre Mannheim, Medical Faculty Mannheim of the University of Heidelberg, Mannheim) (registration number 2016-800R-MA). The study was also registered at the Deutsche Register für klinische Studien (ID: DRKS00016030).
For this retrospective observational, non-interventional, monocenter case-control study the need for informed consent was waived by the local ethics committee.
The study was conducted in the 25-bed ICU of the Department of Anaesthesiology and Critical Care Medicine, University Medical Centre Mannheim, Medical Faculty Mannheim of the University of Heidelberg.
Data were retrospectively analyzed. The inclusion period lasted from 02/2009 to 07/2017 with an average of 1869 patients per year.
All patients who stayed longer than 72 hours, were older than 18 years and had a complete electronic medical record for calculating DO2I values were included in the analysis.
Irreversible parenchymal ischemia is induced in a time frame between 6 and 12h of hypoxia in the intestinal vascular zone [12, 19, 22]. As the goal of this study was to evaluate the impact of hypoxemia on the development of II and to discriminate the diagnosis of II attributable to critical care management from the sequelae of underlying diseases associated with II and originated before ICU admission, we excluded patients with a length of stay (LOS) shorter than 72h to exclude patients with undiagnosed II at admission on the ICU.
Furthermore, patients were excluded if they were <18 years old and if the electronic medical records were incomplete for calculating DO2I values.
As the overall incidence of II is low [3, 23, 24] we opted to include commonly recognized factors and pre-existing conditions [1, 3, 9] for the stratification of the Cox proportional hazards model, that might predestine the patient for intestinal hypoxia in case of acute severe illness necessitating treatment on ICU. So, identified patients with an elevated baseline risk for II by the following criteria: 1) congestive heart failure, 2) diabetes mellitus, 3) peripheral artery occlusive disease, 4) age older than 60 years [7–9].
Patients who developed II after admission with an ICU stay of at least 72h were grouped in the cases group to ensure a suitable amount of collected data for analysis.
The diagnosis of II was confirmed by clinician validation of medical records when at least one of the following criteria was fulfilled:
suggestive radiological signs for ischemia
endoscopic proof of ischemia
II specified in the pathology report
obvious ischemia detected intraoperatively without resection because of futility and an ICU stay of at least 72 hours before the surgical intervention
All other patients without the diagnosis of II during their treatment on ICU were included in the control group, were managed according to the standard operation procedures of our unit, and received radiological or endoscopic interventions respectively surgical interventions as indicated.
Collection of data
All data were collected through Philips IntelliVue Clinical Information Portfolio (ICIP) and Philips Intelli Space Critical Care and Anesthesia (ICCA) System. SaO2, Hb and lactic acid were measured routinely using a blood gas analyzer (Radiometer ABL 800 Flex, Radiometer, Willich, Germany).
According to the standard operation procedures all patients with impaired cardiopulmonary function were managed with a triple-lumen central venous catheter. Additionally, a transpulmonary thermodilution catheter (Pulsiocath™, Pulsion Medical Systems, Munich, Germany) was utilized in patients, when indicated by the attending physician.
The Pulse Contour Cardiac Output monitor (PiCCOplus™, Pulsion Medical Systems, Munich, Germany) was used for measuring CI and SVRI with routine calibrations around every 8h, averaging three daily DO2I-measurements.
DO2I was calculated using a simplified version of the standard formula:
| (Eq 1) |
And for calculating the SVRI we used the following formula:
| (Eq 2) |
In order to quantify an insufficient oxygen delivery index within 72h before the diagnosis, we calculated meanDO2I during the stay in ICU as a surrogate for a longer lasting hypoxic status and the minDO2I during the stay in ICU to capture shorter periods of hypoxia. Furthermore, we calculated the mean CI, mean SaO2, mean Hb and mean SVRI during the stay in ICU.
Lactate levels were collected in the case group 72h before the diagnosis of II and in the control group we collected all lactate values over the ICU-stay. A plasma lactate concentration of 2mmol/l or less was defined as normal finding as this represents the clearing capacity for lactic acid in normal adults [25].
Statistical analysis
Metric data is presented as mean ± standard deviation, categorical data as absolute frequency (percentage). P-values were calculated using the t-test and Fisher’s exact test.
Because some variables for the DO2I measurements were not synchronously recorded we allowed an 8h synchronization window for all variables for DO2I.
Patients in the II group were matched according to the timepoint of the diagnosis of II (in hours) with patients in the control group and an equal LOS (in hours) without II. CI, SaO2, Hb, meanDO2I, minDO2I and meanSVRI from the last 72h was recorded in the individual patient with II and in all patients in the case group with a corresponding LOS.
A stratified (by baseline risk) Cox proportional hazard model with time dependent covariates [26] was then applied to assess the relationship between these parameters and the development of II [7, 8].
We allowed a nonlinear relationship between the regressor and the hazard to develop an II by the application of smoothed regression splines [27]. For each model we assessed the general model fit, the linearity and the non-linearity of the regressor function by appropriate likelihood ratio tests (LRT).
In order to assess the effect of the individual components of DO2I (see Eq 1) we augmented the former meanDO2I model by the individual components (mean Hb, mean SaO2 or mean CI) to derive adjusted coefficients and compared them to the unadjusted coefficients derived from a model that contains only the component of DO2I (Hb, SaO2 and CI). Again, data from the last 72h of patients in the II group were matched with all control patients who had an equal LOS as the case.
Furthermore, we compared the model fit of the augment model with the mean DO2I model and the model that contains only the component of DO2I as regressor by appropriate LRT to determine the relative importance of each independent component.
We further conducted a Receiver Operating Characteristic Curve (ROC) analysis–sensitivity, specificity and Area under the ROC (AUROC) for lactate, pH, CO2, ScvO2 and their predictive value for II.
Statistical analysis was performed with R 3.3.2 (The R foundation for Statistical Computing, Vienna, Austria) [28] and the survival package and SAS 9.4 (Statistical Analysis System) [29, 30].
A p-value ≤ 0.05 was regarded as statistically significant. No adjustment for multiplicity was applied.
All dedicated statistician (MH) was responsible for the calculations.
Results
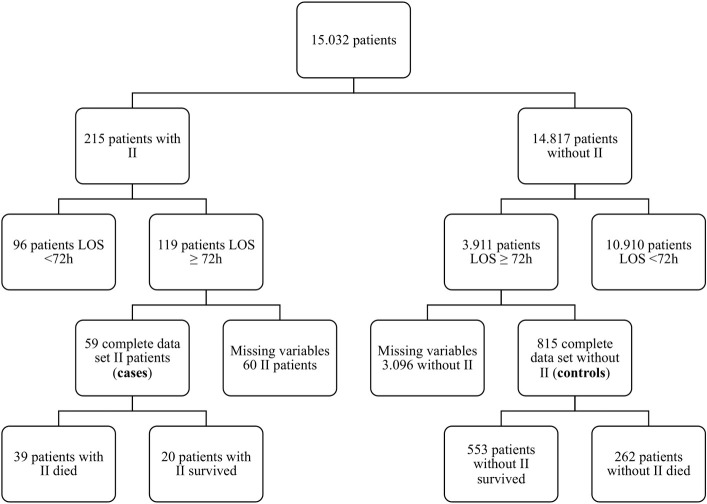
From 02/2009 to 07/2017 we analyzed 15032 patients of whom 215 patients developed II during their ICU stay. 119 patients fulfilled the minimum required ICU stay of > 72h. 60 patients had to be excluded because no advanced hemodynamical monitoring was established. Thus, a total of 59 patients fulfilled all inclusion criteria (Fig 1). Baseline characteristics are presented in Table 1. We identified 33 female and 26 male II patients with an average age of 62.4 ± 14.6 years. Patients with II had a significantly higher Simplified Acute Physiology Score (SAPS II) score on admission than controls (47.3 ± 13.7 vs. 43.1 ± 13.1, p = 0.025). II was associated with a prolongation of the ICU stay (25.0 ± 22.2 vs. 18.5 ± 16.1, p = 0.032). ICU-mortality was higher in the II group (66.1%) compared to the control group (32.1%) (p < 0.0001). None of the evaluated comorbidities were significantly more prevalent in patients with II.
Fig 1. Patient selection flow diagram.
II = intestinal ischemia, LOS = length of stay, h = hour.
Table 1. Patients baseline characteristics.
| Intestinal ischemia | Nonischemia controls | p-value | |
|---|---|---|---|
| n | 59 | 815 | |
| Sex (m/f) | 26/33 | 494/321 | 0.0136 |
| Age (y) | 62.4 ± 14.6 | 60.7 ± 15.9 | 0.3766 |
| SAPS II (points) | 47.3 ± 13.7 | 43.1 ± 13.1 | 0.0247 |
| Length of stay (d) | 25.0 ± 22.2 | 18.5 ± 16.1 | 0.0316 |
| ICU-mortality | 39 (66.1%) | 262 (32.1%) | <0.0001 |
| Congestive heart failure | 20 (33.9%) | 205 (25.2%) | 0.1642 |
| Diabetes mellitus | 25 (42.4%) | 378 (46.4%) | 0.5903 |
| Peripheral vascular occlusive disease | 8 (13.6%) | 56 (6.9%) | 0.0679 |
| Coronary heart disease | 8 (13.6%) | 155 (19.0%) | 0.3867 |
| COPD | 4 (6.8%) | 83 (10.2%) | 0.5041 |
| Artrial fibrillation | 29 (49.15%) | 297 (36.44%) | 0.069 |
| Chronic renal disease | 8 (13.56%) | 81 (9.94%) | 0.3714 |
| Nicotine abuse | 5 (8.47%) | 77 (9.45%) | 1.0 |
Patients baseline characteristics; n = number of patients, m = male, f = female, y = year, SAPS II = simplified acute physiology score II, d = day, ICU = intensive care unit, COPD = chronic obstructive pulmonary disease
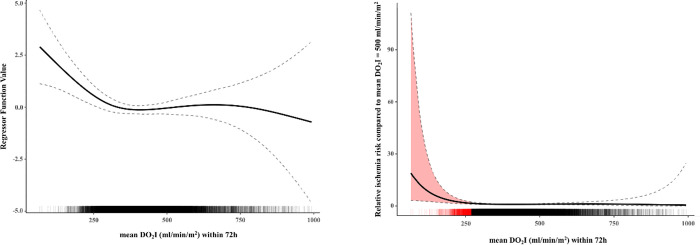
We found a significant non-linear influence of meanDO2I on ischemia hazard (LRT vs null model: χ² (df = 3.23) = 10.536, p = 0.018; LRT for non-linearity: χ² (df = 2.23) = 9.281, p = 0.012) (Fig 2A). The application of this model showed, that the relative ischemia hazard (reference: meanDO2I = 500ml/min/m2) is significantly elevated when meanDO2I falls below approximately 250ml/min/m2 and increases disproportionately with smaller values (Fig 2B).
Fig 2.
A. Regressor plot of meanDO2I within 72h. DO2I = oxygen delivery index, h = hour, the solid line shows the regressor plot for meanDO2I, dashed lines show the standard deviation, above the x-axis the 14.320 DO2I calculations are plotted as single small lines. B. Relative ischemia risk compared to mean DO2I within 72h before the onset of intestinal ischemia. Y-axis shows increasing relative ischemia risk with decreasing meanDO2I values (x-axis, solid line) by DO2I values below approximately 250ml/min/m2 (highlighted by the red marked area), dashed lines show the standard deviation, above the x-axis the 14.320 DO2I calculations are plotted as single small lines (critical DO2I values are highlighted as a red line), DO2I = oxygen delivery index, h = hours.
We observed a qualitatively similar relationship between minDO2I and relative ischemia hazard (Fig 3). First, the influence of minDO2I was shown to be linear (LRT vs null model: χ² (df = 1.39) = 6.8, p = 0.016; LRT for linearity: χ² (df = 1.00) = 5.48, p = 0.019); LRT for non-linearity: χ² (df = 0.39) = 1.103, p = 0.191), thus the increase for smaller values is less steep. Secondly, it was observed that the relative ischemia hazard is already significantly elevated at a minDO2I value of approximately 400ml/min/m2 compared to the reference value.
Fig 3. Relative ischemia risk compared to min DO2I within 72h before the onset of intestinal ischemia.
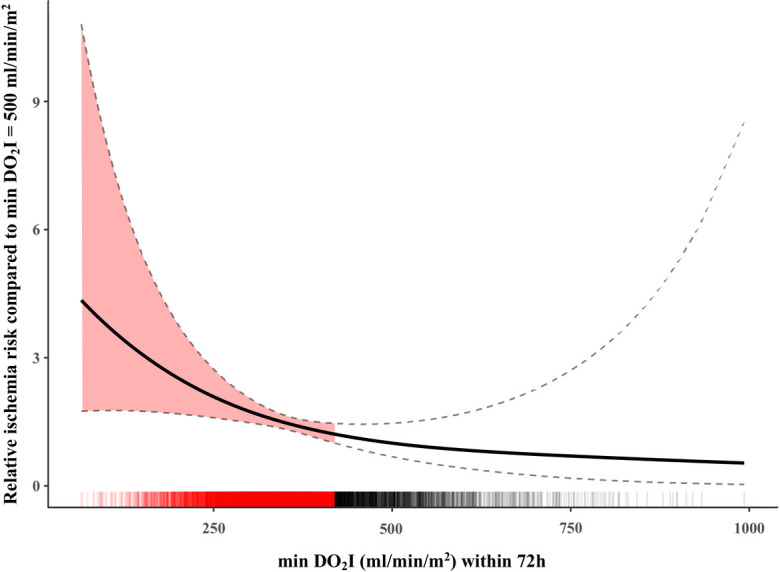
Y-axis shows increasing relative ischemia risk with lower minDO2I values (x-axis, solid line) by DO2I values below approximately 400ml/min/m2 (highlighted by the red marked area), dashed lines show the standard deviation, above the x-axis the 14.320 DO2I calculations are plotted as single small lines (critical DO2I values are highlighted as a red line), DO2I = oxygen delivery index, h = hours.
Our assessment of the individual components of DO2I showed that no single component had a significant influence on the ischemia hazard no matter if we adjust for meanDO2I or not (Table 2). Consequently, we could not improve the model fit to our data of the meanDO2I model by adding individual components (all LRT p-values were at least 0.104), but we observed a significantly poorer model fit when we dropped meanDO2I from the model for each component (all LRT p-values were below 0.024).
Table 2. Individual component of DO2I.
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Component | HR | Coef | SD | p-value | HR | Coef | SD | p-value |
| CI | 0.838 | -0.177 | 0.154 | 0.25 | 0.997 | -0.003 | 0.277 | 0.992 |
| SaO2 | 0.90 | -0.108 | 0.065 | 0.1 | 0.885 | -0.122 | 0.066 | 0.063 |
| Hb | 1.001 | 0.001 | 0.101 | 0.994 | 1.030 | 0.03 | 0.111 | 0.787 |
Individual component of DO2I; HR = hazard ratio, coef = coefficient, SD = standard deviation, p = p-value, CI = cardiac output index, SaO2 = arterial oxygen saturation, Hb = hemoglobin
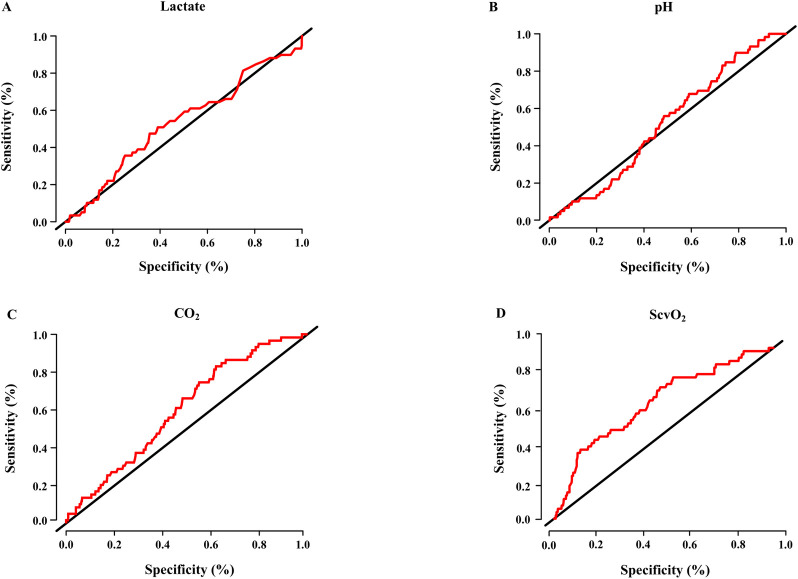
Our analysis of lactate levels showed that 51 Patients with II had lactate levels ≥ 2mmol/l (86%), eight patients showed lactate levels < 2mmol/l (14%) resulting in a sensitivity of 86.44%. In the control group there were 683 patients with lactate levels ≥ 2mmol/l (84%) and 132 (16%) patients with lactate levels < 2mmol/l, leading to a specificity of 16.2%. Receiver operating characteristics analysis was poor with an Area under the ROC of 0.5324. The analysis of pH also showed a very high sensitivity with 90%, but a very low specificity with 21%. Resulting in a poor receiver operating characteristics analysis with an area under the ROC of 0.52. Furthermore, the variables pCO2 and ScvO2 also did not perform well with a sensitivity of 83% and a specificity of 38%, with a resulting ROC analysis of 0.6017 for CO2 and 77% sensitivity, specificity of 52%, and AUROC of 0.6786 for ScvO2, respectively (Fig 4).
Fig 4. Receiver operating characteristics analysis for lactate, pH, CO2, ScvO2 and the development of intestinal ischemia.
Panel A lactate: Area under the ROC curve: 0.5324; sensitivity 86.44% and specificity 16.2% for a cut-off value of lactate ≥ 2mmol/l; Panel B pH: Area under the ROC curve: 0.52; sensitivity 90% and specificity 21%; Panel C CO2: Area under the ROC curve: 0.6017; sensitivity 83% and specificity 38%; Panel D ScvO2: Area under the ROC curve: 06786.; sensitivity 77% and specificity 52%; ROC = Receiver operating characteristic.
SVRI had no significant association with ischemia hazard (LRT vs null model: χ² (df = 1.41) = 2.522, p = 0.178) (Table 3).
Table 3. Influence of systemic vascular resistance.
| test | chisq | df | p-value |
|---|---|---|---|
| null model | 2.522 | 1.414 | 0.178 |
| linear | 1.400 | 1.000 | 0.237 |
| non-linear | 0.641 | 0.414 | 0.219 |
Influence of systemic vascular resistance; Chisq = Chi-squared test, df = degrees of freedom
Discussion
The findings in this study were:
There is a critical cut-off value for meanDO2I of approximately 250ml/min/m2 representing a longer state of insufficient oxygen delivery in a 72h timeframe before the clinical diagnosis of II which is affecting the risk for the development of II. Lower meanDO2I values strongly increase risk of II.
Even a single minDO2I value smaller than 400ml/min/m2 increases the risk for II.
Neither Hb, SaO2 nor CI as indiviual components of DO2I showed significant diagnostic superiority compared to DO2I in predicting II.
Therapeutic decisions based on lactate, pH, CO2 or ScvO2 are not useful for the prevention or early detection of II especially because of their low specificity.
In our analysis SVRI had no effect on the incidence of II.
Delivery of oxygen and survival
To our knowledge no prior study investigated whether there is a crucial DO2I cut off value for organ dysfunction like II. A meanDO2I of 250ml/min/m2 over 72h on ICU and a minDO2I value smaller than 400ml/min/m2 substantially increases the risk for developing II and could alert the attending clinician accordingly. The fact that minDO2I has an earlier effect on the development of II may be due to cellular compensatory mechanisms that have not yet been activated, in the sense of ischemic preconditioning.
Ischemic preconditioning reduces ischemia-reperfusion injury by inhibiting and reducing the inflammatory response in the reperfusion phase [31].
Single low DO2I events without ischemic preconditioning result in reduced mitochondrial ATP generation, as well as other pathological mechanisms. In the subsequent reperfusion, a pronounced inflammatory response occurs, which further exacerbates ischemia [31–33]. Guan et al. [34] showed using vivo microscopy that adverse effects due to short-term ischemia are partly completely reversible in the reperfusion phase. However, they showed that after prolonged periods of ischemia, normal cell structures and functions could not be fully restored in a large proportion of cells and during reperfusion further deterioration occurred.
In the present study, this microscopically proven pathology by Guan et al. [34] is also supported, as short-term low DO2I values have a markedly lower risk of ischemia than longer-lasting low DO2I (meanDO2I) phases.
There are no established guide values for DO2I and oxygen consumption for critically ill patients. In a normal resting adult the normal DO2I is approximately 500ml/kg/m² assuming a CI of 2500 ml/min/m², a Hb of 15 g/dl and a SaO2 of 100%, from which 125ml/min/m² are consumed through the normal metabolism [35]. As a DO2I of 500ml/min/m² is commonly reported as reference value in healthy subjects and typically not associated with II and post procedural complications it was chosen as a reference value [36, 37]. On top of that a DO2I of 500ml/min/m² was tested as a safe endpoint for shock resuscitation [38].
Shoemaker et al. studied hemodynamic parameters, DO2I and oxygen consumption in critically ill patients, showing a correlation between less organ failure as well as survival and supranormal values of DO2I, oxygen consumption and cardiac index [39–43]. The authors theorized that morbidity and mortality could be reduced in critically ill patients if these parameters were used as therapeutic goals.
Subsequently, controlled randomized trials investigated the effect of hemodynamic optimization in critically ill patients, manipulating DO2I to supranormal values [43–50]. Several of these studies [43, 47–51] showed a decrease in mortality and morbidity when DO2I was manupilated to a supranormal value before surgery and during the peri- and postoperative period.
A meta-analysis by Kern et al. [52] summarized relevant prospective randomized trials analyzing hemodynamic optimization in high-risk patients. In trials with hemodynamic optimization before the onset of organ dysfunction a significant reduction in mortality [47–51] could be demonstrated. Hemodynamic optimization after the onset of organ dysfunction however caused no related mortality reduction [44–46, 53]. Our data supports the idea that hemodynamic optimization before II became clinical apparent might prevent these complications in our cases. On the other hand, it remains speculative whether there is a significant risk reduction for II due to supranormal DO2I values.
Effects of Hb, SaO2 and CO on II
Relevant studies in the field utilized standardized protocols to keep DO2I values in normal or supranormal levels by manipulating all DO2I components depending on arbitrarily elected cut-off values for CI, Hb and SaO2 [39–51, 53, 54]. None of them distinguished which component of DO2I is most effective to optimize. In this study we showed, that DO2I as a goal parameter for optimization might be relevant to prevent II, but the individual components seem equally important to prevent II.
The role of lactate, pH, CO2 and ScvO2 in the diagnosis of II
Lactate is a well-known marker of parenchymatous hypoxia regularly reported in studies revising II [55–57] and its measurement is recommended in recent guidelines [3, 9]. On the other hand, many studies and meta-analyses confirm that the classical routine parameters are of no value in distinguishing patients with II from those without [9, 58, 59]. In a retrospective multicenter study by Leone et al. [60] investigating risk factors associated with ICU-mortality in patients with II, lactate levels higher than 2.7mmol/l were found to be an independent predictor for ICU-mortality. Yet the author pointed out that lactate is not a useful tool for diagnosis or exclusion of II, because of its low sensitivity and specificity. Bourcier et al. [61] investigated patients with suspected II and also collected lactate levels, showing no statistically significant difference between lactate levels of II patients and patients without II. In a prospective trial Murray et al. [62] found a significant elevation in D-lactate levels in patients with II compared to controls. Sensitivity and specificity were 90% and 87%. In summary, lactate not differentated in its D- and L- enantiomers appears to be a good parameter for mortality estimation [60, 63] but not a reliable paramter for the diagnosis of II.
The goal of the study was to evaluate the correlation of DO2I and the development of II in patients treated on the ICU. So, we hypothesize a time dependent clinical inapparent sequence of clinical inapparent inadequate (locoregional) delivery of oxygen, inducing irreversible II and corresponding lactate accumulation leading to clinical detectable sequalae like vasomotor dysfunction and endothelial leak which then enable the clinician at the bedside to diagnose and manage II. We therefore wanted to connote our findings of a “cut-off” DO2I with the corresponding lactate levels. Our findings of relative high mean and minDO2I associated with II might help to explain the relative low sensitivity and specificity of lactate in the diagnosis of II.
Cruz et al. [64] reported an increase in the intestinal-arterial pCO2 gradient in a model of small bowel ischemia-reperfusion that corresponded with the grade of the mucosal damage. In line with that finding, Siniscalchi et al. [65] found a significant lower pH and higher PaCO2 in patients who underwent small bowel transplantation comparing their baseline measurement and 120 minutes after reperfusion of the graft. The authors hypothesized that the fall of pH after the revascularization and the concomitant rise in PaCO2 was noted due the increased metabolic activity in the new organ. We hypothesized that an increase in PaCO2 or a reduction of pH, either due to lactic acidosis or due to transient reperfusion of underperfused intestinal organs might be a valuable parameter for II. As shown by our ROC analysis unfortunately neither pH nor PaCO2 showed a clinically useful specificity for the prediction of II. This might be caused by the relative insensitivity of global changes in both parameters compared to direct measurements in the intestinal mucosa. On the other hand, in a substantial part of critical ill patients hypercapnia and the corresponding acidosis are caused by guideline-compliant management [66, 67] and not associated with intestinal ischemia at all. The measurement of the central venous oxygen saturation is discussed in the recent guidelines for the management of septic shock and represents global oxygen extraction and utilization in critically ill patients [68]. As shown by Heino et al. [69] the oxygen extraction in II is increased. Unfortunately, as shown in our ROC analysis corresponding changes in ScvO2 lack the necessary specificity to represent a useful prognostic marker of II in a clinical setting. This finding might be caused by the dichotomy of the parameter regarding oxygen delivery. A low ScvO2 is usually a sign of hypoxia or insufficient cardiac output, an increased ScvO2 usually denotes an impaired oxygen extraction [70, 71].
The role of SVRI in the development of intestinal ischemia
The hypothesis that endo- or exogenouos catecholamines may induce II because of reduced oxygen delivery to intraabdominal organs due to mesenteric vasoconstriction is proposed in many guidelines and trials [1, 3, 9, 72]. In this study we utilized systemic vascular resistance index as surrogate for vasoconstriction irrespectively of exogenouos catecholamines. We found no significant correlation between SVRI and the incidence of II. It should be noted, as SVRI is the result of the physiological effects of endo- or exogenouos catecholamines, our analysis is indepented of the catechoalmine therapy and other therapeutical decisions of the attending physicians.
Limitations
Results of this study were potential biased due to a different pre-existing disease profile, the heterogeneity of medical history and the clinical course, for example, new or different comorbidities in the investigated cohort. As it was the goal of this study to evaluate the effects of the delivery of oxygen and its independent parameters (Hb, arterial SaO2 and CI) on the development of II we tried to attenuate these factors by identifying and adjusting for anamenstic proxies and conditions for a higher risk of II like diabetes mellitus, peripheral vascular disease, chronic heart failure, coronary heart and pulmonary diseases and used them for the stratification of the Cox model. Therefore we opted not to include factors like prognostic scores evaluating physiological criteria like APACHE II or SOFA score in the Cox model as they reflect acute severity of illness of the patient and not necessarily predestine the patient for II per se.
As we had no opportunity to acquire advanced hemodynamic data like cardiac output from the patients included in this retrospective study prior to admission to the ICU we explicitly excluded patients with a length of stay shorter than 72h from the study. Naturally we suspect that patients suffering II prior to admission on the ICU might present a significant lower meanDO2I and minDO2I then the II group in this study. Therefore, we acknowledge that we probably evaluated a distinct subgroup of patients suffering II and our findings cannot be extrapolated to all patients with II.
Furthermore we did not account for therapeutic interventions to manage II once the clinical diagnosis was made.
Lastely, in the analysed cohort, surgical patients with abdominal pre-existing conditions might have biased the results.
Conclusion
To our knowledge, this is the first study to show a direct correlation between the incidence of II and a critical DO2I value. Our findings emphasize the need to keep DO2I at an adequate level to prevent deterioration of the patients condition as well as their outcome due to the development of II. This crucial cut-off value for DO2I may enable intensive care physicians to identify patients at risk and also allow for optimization of therapy by manipulation of the DO2I parameters to prevent II.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank the staff of the intensive care unit for their support.
Abbreviations
- AUROC
Area under the Receiver Operating Characteristic
- CI
cardiac output index
- d
day
- DO2I
oxygen delivery index
- h
hour
- Hb
hemoglobin
- IAP
intra-abdominal pressure
- ICCA
Philips Intelli Space Crital Care and Anesthesia
- ICD
International Classification of Diseases
- ICIP
Philips IntelliVue Clinical Information Portfolio
- ICU
intensive care unit
- II
intestinal ischemia
- LOS
length of stay
- LRT
likelihood ratio test
- meanDO2I
mean oxygen delivery index
- minDO2I
minimal oxygen delivery index
- OPS
Operation- and Procedures-code
- PDMS
patient data management system
- ROC
Receiver Operating Characteristic
- SaO2
arterial oxygen saturation
- SAPS II
Simplified Acute Physiology Score II
- SAS
Statistical Analysis System
- ScvO2
central venous saturation
- SVRI
systemic vascular resistance index
- y
year
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the foundation Klaus Tschira Stiftung. The funding source had no role in the design and conduct of the study.
References
- 1.Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164(10):1054–62. Epub 2004/05/26. doi: 10.1001/archinte.164.10.1054 [DOI] [PubMed] [Google Scholar]
- 2.Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374(10):959–68. Epub 2016/03/11. doi: 10.1056/NEJMra1503884 [DOI] [PubMed] [Google Scholar]
- 3.Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, et al. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. Epub 2017/08/11. doi: 10.1186/s13017-017-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31(4):334–42. Epub 2013/09/26. doi: 10.4103/0255-0857.118870 [DOI] [PubMed] [Google Scholar]
- 5.Nagpal R, Yadav H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann Nutr Metab. 2017;71 Suppl 1:11–6. Epub 2017/09/28. doi: 10.1159/000479918 [DOI] [PubMed] [Google Scholar]
- 6.Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22(4):464–71. Epub 2007/03/23. doi: 10.1111/j.1440-1746.2007.04933.x [DOI] [PubMed] [Google Scholar]
- 7.Al-Diery H, Phillips A, Evennett N, Pandanaboyana S, Gilham M, Windsor JA. The Pathogenesis of Nonocclusive Mesenteric Ischemia: Implications for Research and Clinical Practice. J Intensive Care Med. 2019;34(10):771–81. Epub 2018/07/25. doi: 10.1177/0885066618788827 [DOI] [PubMed] [Google Scholar]
- 8.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12(5):1179–87. Epub 2002/04/27. doi: 10.1007/s00330-001-1220-2 [DOI] [PubMed] [Google Scholar]
- 9.Tilsed JV, Casamassima A, Kurihara H, Mariani D, Martinez I, Pereira J, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253–70. Epub 2016/01/29. doi: 10.1007/s00068-016-0634-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. Epub 2010/10/28. doi: 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler A, Wulf DA, Lu Y, Iwashyna TJ, Escobar GJ, Shah NH, et al. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit Care Med. 2018;46(6):843–9. Epub 2018/02/13. doi: 10.1097/CCM.0000000000003023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn F, Schiergens TS, Klar E. Acute Mesenteric Ischemia. Visc Med. 2020;36(4):256–62. Epub 2020/10/03. doi: 10.1159/000508739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aalten CM, Samson MM, Jansen PA. Diagnostic errors; the need to have autopsies. Neth J Med. 2006;64(6):186–90 [PubMed] [Google Scholar]
- 14.Fares AF, Fares J, Fares GF, Cordeiro JA, Nakazone MA, Cury PM. Clinical and pathological discrepancies and cardiovascular findings in 409 consecutive autopsies. Arq Bras Cardiol. 2011;97(6):449–55. Epub 2011/10/28. doi: 10.1590/s0066-782x2011005000111 [DOI] [PubMed] [Google Scholar]
- 15.Tejerina EE, Padilla R, Abril E, Frutos-Vivar F, Ballen A, Rodriguez-Barbero JM, et al. Autopsy-detected diagnostic errors over time in the intensive care unit. Hum Pathol. 2018. Epub 2018/03/14. doi: 10.1016/j.humpath.2018.02.025 [DOI] [PubMed] [Google Scholar]
- 16.Corcos O, Castier Y, Sibert A, Gaujoux S, Ronot M, Joly F, et al. Effects of a multimodal management strategy for acute mesenteric ischemia on survival and intestinal failure. Clin Gastroenterol Hepatol. 2013;11(2):158–65 e2. Epub 2012/10/30. doi: 10.1016/j.cgh.2012.10.027 [DOI] [PubMed] [Google Scholar]
- 17.Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am. 1997;77(2):289–306. Epub 1997/04/01. doi: 10.1016/s0039-6109(05)70549-1 [DOI] [PubMed] [Google Scholar]
- 18.Haglund U, Bergqvist D. Intestinal ischemia—the basics. Langenbecks Arch Surg. 1999;384(3):233–8. Epub 1999/08/07. doi: 10.1007/s004230050197 [DOI] [PubMed] [Google Scholar]
- 19.van Petersen AS, Kolkman JJ, Meerwaldt R, Huisman AB, van der Palen J, Zeebregts CJ, et al. Mesenteric stenosis, collaterals, and compensatory blood flow. J Vasc Surg. 2014;60(1):111–9, 9 e1-2. Epub 2014/03/22. doi: 10.1016/j.jvs.2014.01.063 [DOI] [PubMed] [Google Scholar]
- 20.Doulberis M, Panagopoulos P, Scherz S, Dellaporta E, Kouklakis G. Update on ischemic colitis: from etiopathology to treatment including patients of intensive care unit. Scand J Gastroenterol. 2016;51(8):893–902. Epub 2016/05/07. doi: 10.3109/00365521.2016.1162325 [DOI] [PubMed] [Google Scholar]
- 21.Gnanapandithan K, Feuerstadt P. Review Article: Mesenteric Ischemia. Curr Gastroenterol Rep. 2020;22(4):17. Epub 2020/03/19. doi: 10.1007/s11894-020-0754-x [DOI] [PubMed] [Google Scholar]
- 22.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–83. Epub 1970/10/01. doi: 10.1001/archsurg.1970.01340280030009 [DOI] [PubMed] [Google Scholar]
- 23.Acosta S, Bjorck M. Acute thrombo-embolic occlusion of the superior mesenteric artery: a prospective study in a well defined population. Eur J Vasc Endovasc Surg. 2003;26(2):179–83. Epub 2003/08/15. doi: 10.1053/ejvs.2002.1893 [DOI] [PubMed] [Google Scholar]
- 24.Duran M, Pohl E, Grabitz K, Schelzig H, Sagban TA, Simon F. The importance of open emergency surgery in the treatment of acute mesenteric ischemia. World J Emerg Surg. 2015;10:45. Epub 2015/09/29. doi: 10.1186/s13017-015-0041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18(5):503. Epub 2014/11/15. doi: 10.1186/s13054-014-0503-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eilers PH MB. Flexible smoothing with B-splines and penalties. Statistical Science. 1996;11:89–121. [Google Scholar]
- 27.Hurvich CM, Simonoff JS, Tsai C-L. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. JRSSB. 1998;60:271–93. [Google Scholar]
- 28.Team RC. R: A Language and Enviroment for Statistical Computing: R Foundation for Statistical Computing; 2016. Available from: https://www.R-project.org/.
- 29.Therneau TM. A Package for Survival Analysis in S 2015. Available from: https://CRAN.R-project.org/package=survival.
- 30.Grambsch TMTaPM. Modeling Survival Data: Extending the {C}ox Model: Springer; 2000.
- 31.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39(2):481–4. Epub 2007/03/17. doi: 10.1016/j.transproceed.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 32.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250(6 Pt 1):G749–53. Epub 1986/06/01. doi: 10.1152/ajpgi.1986.250.6.G749 [DOI] [PubMed] [Google Scholar]
- 33.Kurose I, Granger DN. Evidence implicating xanthine oxidase and neutrophils in reperfusion-induced microvascular dysfunction. Ann N Y Acad Sci. 1994;723:158–79. Epub 1994/06/17 [PubMed] [Google Scholar]
- 34.Guan Y, Worrell RT, Pritts TA, Montrose MH. Intestinal ischemia-reperfusion injury: reversible and irreversible damage imaged in vivo. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G187–96. Epub 2009/05/02. doi: 10.1152/ajpgi.90595.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang YC. Monitoring oxygen delivery in the critically ill. Chest. 2005;128(5 Suppl 2):554S–60S. Epub 2005/11/25. doi: 10.1378/chest.128.5_suppl_2.554S [DOI] [PubMed] [Google Scholar]
- 36.Futier E, Robin E, Jabaudon M, Guerin R, Petit A, Bazin JE, et al. Central venous O(2) saturation and venous-to-arterial CO(2) difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care. 2010;14(5):R193. Epub 2010/11/03. doi: 10.1186/cc9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartha E, Arfwedson C, Imnell A, Kalman S. Towards individualized perioperative, goal-directed haemodynamic algorithms for patients of advanced age: observations during a randomized controlled trial (NCT01141894). Br J Anaesth. 2016;116(4):486–92. Epub 2016/03/20. doi: 10.1093/bja/aew025 [DOI] [PubMed] [Google Scholar]
- 38.McKinley BA, Kozar RA, Cocanour CS, Valdivia A, Sailors RM, Ware DN, et al. Normal versus supranormal oxygen delivery goals in shock resuscitation: the response is the same. J Trauma. 2002;53(5):825–32. Epub 2002/11/19. doi: 10.1097/00005373-200211000-00004 [DOI] [PubMed] [Google Scholar]
- 39.Shoemaker WC, Appel PL, Kram HB. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 1988;16(11):1117–20. Epub 1988/11/01. doi: 10.1097/00003246-198811000-00007 [DOI] [PubMed] [Google Scholar]
- 40.Shoemaker WC, Appel PL, Kram HB. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 1992;102(1):208–15. Epub 1992/07/01. doi: 10.1378/chest.102.1.208 [DOI] [PubMed] [Google Scholar]
- 41.Shoemaker WC, Appel PL, Kram HB. Hemodynamic and oxygen transport responses in survivors and nonsurvivors of high-risk surgery. Crit Care Med. 1993;21(7):977–90. Epub 1993/07/01. doi: 10.1097/00003246-199307000-00010 [DOI] [PubMed] [Google Scholar]
- 42.Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398–403. Epub 1982/06/01. doi: 10.1097/00003246-198206000-00015 [DOI] [PubMed] [Google Scholar]
- 43.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176–86. Epub 1988/12/01. doi: 10.1378/chest.94.6.1176 [DOI] [PubMed] [Google Scholar]
- 44.Alia I, Esteban A, Gordo F, Lorente JA, Diaz C, Rodriguez JA, et al. A randomized and controlled trial of the effect of treatment aimed at maximizing oxygen delivery in patients with severe sepsis or septic shock. Chest. 1999;115(2):453–61. Epub 1999/02/23. doi: 10.1378/chest.115.2.453 [DOI] [PubMed] [Google Scholar]
- 45.Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333(16):1025–32. Epub 1995/10/19. doi: 10.1056/NEJM199510193331601 [DOI] [PubMed] [Google Scholar]
- 46.Yu M, Levy MM, Smith P, Takiguchi SA, Miyasaki A, Myers SA. Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: a prospective, randomized, controlled study. Crit Care Med. 1993;21(6):830–8. Epub 1993/06/01. doi: 10.1097/00003246-199306000-00009 [DOI] [PubMed] [Google Scholar]
- 47.Lobo SM, Salgado PF, Castillo VG, Borim AA, Polachini CA, Palchetti JC, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med. 2000;28(10):3396–404. Epub 2000/11/01. doi: 10.1097/00003246-200010000-00003 [DOI] [PubMed] [Google Scholar]
- 48.Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ. 1999;318(7191):1099–103. Epub 1999/04/24. doi: 10.1136/bmj.318.7191.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma. 1995;38(5):780–7. Epub 1995/05/01. doi: 10.1097/00005373-199505000-00018 [DOI] [PubMed] [Google Scholar]
- 50.Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270(22):2699–707. Epub 1993/12/08 [PubMed] [Google Scholar]
- 51.Tuchschmidt J, Fried J, Astiz M, Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest. 1992;102(1):216–20. Epub 1992/07/01. doi: 10.1378/chest.102.1.216 [DOI] [PubMed] [Google Scholar]
- 52.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30(8):1686–92. Epub 2002/08/07. doi: 10.1097/00003246-200208000-00002 [DOI] [PubMed] [Google Scholar]
- 53.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330(24):1717–22. Epub 1994/06/16. doi: 10.1056/NEJM199406163302404 [DOI] [PubMed] [Google Scholar]
- 54.Lugo G, Arizpe D, Dominguez G, Ramirez M, Tamariz O. Relationship between oxygen consumption and oxygen delivery during anesthesia in high-risk surgical patients. Crit Care Med. 1993;21(1):64–9. Epub 1993/01/01. doi: 10.1097/00003246-199301000-00014 [DOI] [PubMed] [Google Scholar]
- 55.Lange H, Jackel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg. 1994;160(6–7):381–4. Epub 1994/06/01 [PubMed] [Google Scholar]
- 56.Kurimoto Y, Kawaharada N, Ito T, Morikawa M, Higami T, Asai Y. An experimental evaluation of the lactate concentration following mesenteric ischemia. Surg Today. 2008;38(10):926–30. Epub 2008/09/30. doi: 10.1007/s00595-007-3737-8 [DOI] [PubMed] [Google Scholar]
- 57.Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, et al. Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia: Prospective Study from an Intestinal Stroke Center. Am J Gastroenterol. 2017;112(4):597–605. Epub 2017/03/08. doi: 10.1038/ajg.2017.38 [DOI] [PubMed] [Google Scholar]
- 58.Demir IE, Ceyhan GO, Friess H. Beyond lactate: is there a role for serum lactate measurement in diagnosing acute mesenteric ischemia? Dig Surg. 2012;29(3):226–35. Epub 2012/06/16. doi: 10.1159/000338086 [DOI] [PubMed] [Google Scholar]
- 59.Derikx JP, Schellekens DH, Acosta S. Serological markers for human intestinal ischemia: A systematic review. Best Pract Res Clin Gastroenterol. 2017;31(1):69–74. Epub 2017/04/12. doi: 10.1016/j.bpg.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 60.Leone M, Bechis C, Baumstarck K, Ouattara A, Collange O, Augustin P, et al. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med. 2015;41(4):667–76. Epub 2015/03/04. doi: 10.1007/s00134-015-3690-8 [DOI] [PubMed] [Google Scholar]
- 61.Bourcier S, Oudjit A, Goudard G, Charpentier J, Leblanc S, Coriat R, et al. Diagnosis of non-occlusive acute mesenteric ischemia in the intensive care unit. Ann Intensive Care. 2016;6(1):112. Epub 2016/11/20. doi: 10.1186/s13613-016-0213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum D(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167(6):575–8. Epub 1994/06/01. doi: 10.1016/0002-9610(94)90101-5 [DOI] [PubMed] [Google Scholar]
- 63.Reissfelder C, Sweiti H, Antolovic D, Rahbari NN, Hofer S, Buchler MW, et al. Ischemic colitis: who will survive? Surgery. 2011;149(4):585–92. Epub 2011/01/21. doi: 10.1016/j.surg.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 64.Cruz RJ Jr., Correia CJ, Ribeiro CM, Poli de Figueiredo LF, Rocha e Silva M. Oxygen consumption, pCO2 gradients and regional blood flow distribution in an alternative model of intestinal autotransplantation. J Surg Res. 2006;130(1):13–9. Epub 2005/11/08. doi: 10.1016/j.jss.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 65.Siniscalchi A, Piraccini E, Cucchetti A, Lauro A, Maritozzi G, Miklosova Z, et al. Analysis of cardiovascular, acid-base status, electrolyte, and coagulation changes during small bowel transplantation. Transplant Proc. 2006;38(4):1148–50. Epub 2006/06/08. doi: 10.1016/j.transproceed.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 66.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. Epub 2012/07/17. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 67.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. Epub 2016/02/24. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 68.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. Epub 2017/01/20. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 69.Heino A, Hartikainen J, Merasto ME, Alhava E, Takala J. Systemic and regional pCO2 gradients as markers of intestinal ischaemia. Intensive Care Med. 1998;24(6):599–604. Epub 1998/07/29. doi: 10.1007/s001340050621 [DOI] [PubMed] [Google Scholar]
- 70.Perz S, Uhlig T, Kohl M, Bredle DL, Reinhart K, Bauer M, et al. Low and "supranormal" central venous oxygen saturation and markers of tissue hypoxia in cardiac surgery patients: a prospective observational study. Intensive Care Med. 2011;37(1):52–9. Epub 2010/08/07. doi: 10.1007/s00134-010-1980-8 [DOI] [PubMed] [Google Scholar]
- 71.Wittayachamnankul B, Apaijai N, Sutham K, Chenthanakij B, Liwsrisakun C, Jaiwongkam T, et al. High central venous oxygen saturation is associated with mitochondrial dysfunction in septic shock: A prospective observational study. J Cell Mol Med. 2020;24(11):6485–94. Epub 2020/05/01. doi: 10.1111/jcmm.15299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arif R, Farag M, Zaradzki M, Reissfelder C, Pianka F, Bruckner T, et al. Ischemic Colitis after Cardiac Surgery: Can We Foresee the Threat? PLoS One. 2016;11(12):e0167601. Epub 2016/12/16. doi: 10.1371/journal.pone.0167601 [DOI] [PMC free article] [PubMed] [Google Scholar]