Warming and elevated ozone alter root traits and mycorrhizal fungal community and stimulate organic carbon decomposition.
Abstract
Climate warming and elevated ozone (eO3) are important climate change components that can affect plant growth and plant-microbe interactions. However, the resulting impact on soil carbon (C) dynamics, as well as the underlying mechanisms, remains unclear. Here, we show that warming, eO3, and their combination induce tradeoffs between roots and their symbiotic arbuscular mycorrhizal fungi (AMF) and stimulate organic C decomposition in a nontilled soybean agroecosystem. While warming and eO3 reduced root biomass, tissue density, and AMF colonization, they increased specific root length and promoted decomposition of both native and newly added organic C. Also, they shifted AMF community composition in favor of the genus Paraglomus with high nutrient-absorbing hyphal surface over the genus Glomus prone to protection of soil organic C. Our findings provide deep insights into plant-microbial interactive responses to warming and eO3 and how these responses may modulate soil organic C dynamics under future climate change scenarios.
INTRODUCTION
Global surface temperature has increased by 0.74°C since the Industrial Revolution and is predicted to further increase by another 1.8° to 3.6°C by the year 2100 (1). Concurring with climate warming, the tropospheric ozone concentration had more than doubled (2). Climate warming may increase plant growth in humid ecosystems (3) but often enhances microbial decomposition of organic carbon (C) in soil and thus soil CO2 emissions into the atmosphere (4, 5), positively feeding back to climate warming (6). On the other hand, elevated ozone (eO3) can damage stomatal function and reduce plant growth and C allocation belowground, thereby reducing detritus inputs into soil and subsequent formation of soil organic matter (7–9). Warming enhancement of microbial decomposition and O3 reduction of plant-derived C inputs to soil raise a concern that concurring warming and eO3 may aggravate losses of soil organic C, the largest active C pool on Earth’s surface, and constrain the capacity of terrestrial ecosystems as a C sink. However, experimental evidence in the field is meager.
Warming and eO3 can affect plant photosynthesis and further photosynthate allocation belowground to roots and their symbiotic microbes, rhizobia, and mycorrhizal fungi in particular (7, 10). Arbuscular mycorrhizal fungi (AMF) form symbiotic associations with most terrestrial plants and acquire their C solely from their host plants in return for mineral nutrients (11). Warming has been shown to have positive (12, 13), negative (14, 15), or even neutral effects (16, 17) on plant roots and AMF. In ecosystems with moderate water availability, warming often increases evapotranspiration and water stress to plants, reducing C allocation belowground and suppressing the growth and/or activities of roots and AMF (14, 15). When water is not limiting, however, warming often enhances root cell elongation (18, 19) and promotes root growth, increasing the plant’s ability to enhance soil exploration for nutrients (20). Warming may also enhance AMF colonization of roots directly through stimulating hyphal growth and/or indirectly by promoting plant growth and subsequent C allocation belowground to roots and AMF (10, 13). In comparison, ozone effects on roots and AMF are mostly indirect through inhibiting plant physiologies and growth, as O3 is unable to penetrate into the soil to exert direct effects (7). Elevated O3 diffuses into the intercellular air space of the mesophyll and reacts rapidly in the apoplast with a diverse set of molecules to generate reactive oxygen species (ROS) (21, 22). These apoplastic ROS can react with components of the cell wall and plasma membrane and activate several signal transduction pathways that regulate the responses of the cells to the increased oxidative load, leading to chloroplast degradation and damaged plant leaves (21, 23). These responses are modulated by plant hormones such as abscisic acid, salicylic acid, jasmonic acid, and ethylene (22, 24). Damaged leaves not only reduce plant photosynthesis but also require more photosynthates for cell repairing, thereby reducing photosynthate allocation belowground (7, 25) and altering root traits and AMF community composition and activities (26, 27).
Changes in roots and AMF may critically modulate microbial decomposition in the mycorrhizosphere. While roots are a major source of soil organic C (28, 29), labile C derived from live roots also provides the energy for saprophytic microbes that produce extracellular enzymes responsible for organic matter decomposition (i.e., priming effect) (30–32). However, live roots may also suppress microbial decomposition by outcompeting microbes for soil nutrients and water (28, 33). Effects of AMF on soil organic C can be equally variable. On one hand, AMF may enhance soil C sequestration directly by facilitating the formation and stability of soil aggregates via their secondary compounds (e.g., chitin and glomalin) (34) and hyphal enmeshment (35, 36) and indirectly by promoting plant growth and C inputs (11). On the other hand, increasing evidence has shown that AMF may enhance soil organic C decomposition likely by stimulating saprophytic microbes and contributing to the priming effect (30, 37). A decrease in photosynthate allocation belowground under warming and/or eO3 may promote plants to construct roots with high specific root length (SRL) and low root diameter or root tissue density (RTD) and reduce AMF colonization of roots and/or favor AMF species with lower C requirements (38, 39). However, roots with high SRL and/or low RTD are often associated with high root turnover or short life span (40, 41), and reduced AMF may decrease their contribution to soil aggregation and subsequent protection of soil organic C (35, 36, 42), favoring soil saprotrophs and their decomposition of soil organic C. However, very few field experiments have investigated how concurring warming and eO3 may modulate root and AMF mediation of organic C decomposition in the field, although various observational studies have examined the responses of plant roots and AMF to climate warming (10, 20, 43) or eO3 (7, 44, 45).
Taking advantage of an existing long-term field manipulation experiment, we investigated the effects of warming, eO3, and their combination on soybean roots and their associated AMF as well as the resulting impact on residue decomposition. In particular, we integrated field temperature and O3 manipulations with the Illumina MiSeq sequencing and stable isotope tracing to explore the linkages among root traits, AMF community composition, and microbial decomposition of organic C in soil. We hypothesized that (i) both warming and eO3 would increase SRL, reduce RTD, and shift AMF community composition in planta; (ii) warming- and eO3-induced changes in root traits and AMF would promote root-mediated microbial decomposition of organic C in soil; and (iii) concurring warming and eO3 would aggravate their effects on plant roots and organic C decomposition.
RESULTS AND DISCUSSION
Field manipulations of temperature and O3 concentration were achieved through the air exclusion system (AES) (fig. S1), leading to an average temperature across the growing season at 24.7° and 27.6°C in the ambient temperature and warming plots, respectively, and O3 concentrations at 19.5 and 65.8 parts per billion by volume (ppbv) in the low (CF and W) and elevated O3 (eO3 and W + eO3) plots, respectively (Table 1).
Table 1. Daily mean air temperature, ozone concentration (12 hours), air humidity, and soil moisture and temperature during the experimental period for experimental climate treatments.
Data are means ± SE (n = 3). CF, charcoal-filtered ambient air; W, warming; eO3, elevated O3; W + eO3, warming + eO3. Significant P values (P < 0.05) are shown in bold, ANOVA mixed model.
| Climate treatments | Mixed ANOVA | |||||
| CF | W | eO3 | W + eO3 | Warming | O3 | Warming × O3 |
| Air temperature (°C) | ||||||
| 24.7 ± 0.1 | 27.4 ± 0.3 | 24.7 ± 0.0 | 27.8 ± 0.4 | <0.001 | 0.353 | 0.482 |
| O3 (ppb) | ||||||
| 18.1 ± 0.3 | 20.8 ± 0.1 | 66.4 ± 0.8 | 65.2 ± 0.1 | 0.140 | <0.001 | 0.002 |
| Air humidity (%) | ||||||
| 72.0 ± 0.1 | 67.6 ± 0.8 | 71.5 ± 0.2 | 66.3 ± 0.9 | <0.001 | 0.149 | 0.496 |
| Soil moisture (V%) | ||||||
| 24.7 ± 0.6 | 23.9 ± 0.4 | 23.4 ± 0.5 | 23.7 ± 0.2 | 0.380 | 0.047 | 0.123 |
| Soil temperature (°C) | ||||||
| 22.2 ± 0.0 | 24.2 ± 0.1 | 22.1 ± 0.0 | 24.1 ± 0.2 | <0.001 | 0.509 | 0.959 |
Warming and eO3 significantly influenced root biomass, although they did not affect shoot biomass (Fig. 1, A and B, and table S2). Effects of warming and eO3 in the combined treatment appeared to be additive with no warming-ozone interaction observed (table S2). In particular, eO3 alone reduced root biomass by 23%, and its combination with warming additively reduced root biomass by 44% (Fig. 1B and tables S2 and S4). Warming, eO3, and their interaction significantly increased SRL by 38, 31, and 27% (Fig. 1C and table S2) and root surface area by 30, 25, and 22% (fig. S4C and table S2), respectively. In contrast, they significantly reduced RTD by 27, 22, and 23%, respectively (Fig. 1D and table S2). These tradeoffs between root length/surface area and root density indicate that warming and eO3 prompted plants to construct long, less dense roots with limited biomass investment and increase root surface that would allow to optimize belowground resource acquisition.
Fig. 1. Effects of warming and eO3 on plant traits.
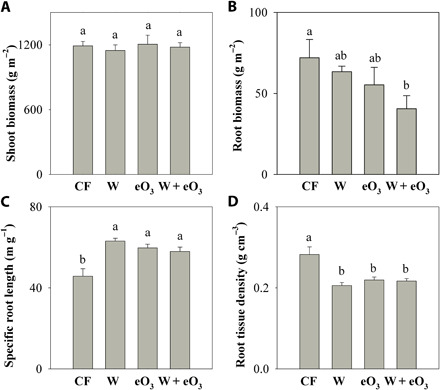
Shoot biomass (A), root biomass (B), SRL (C), and RTD (D). Data are means ± SE (n = 3). The four treatments are as follows: CF, charcoal-filtered ambient air; W, warming; eO3, elevated O3; W + eO3, warming + eO3. Different letters mean significant differences at P = 0.05 level under different treatments.
We then examined how warming and eO3, alone or in combination, affected mycorrhizal colonization of host plant roots. Warming and eO3, alone or in combination, all significantly reduced the proportion of root length colonized by AMF (Fig. 2A and table S2), which contrasted with the positive effects of warming, eO3, and their combination on SRL (Fig. 1C). Regression analysis showed that root specific length was significantly negatively related with the AMF colonization rate of roots, indicating a tradeoff between fine roots and AMF (Fig. 2B). Plant species with long, narrow-diameter roots tend to have low dependence on mycorrhizal fungi at both local and global scales (38, 39). These results, together with reduced root density (Fig. 1D), suggest that plants may optimize their C allocation for resource acquisition through developing roots with higher SRL and surface area over AMF under future climate warming and eO3 conditions.
Fig. 2. Effects of warming and eO3 on mycorrhizal colonization of soybean roots.
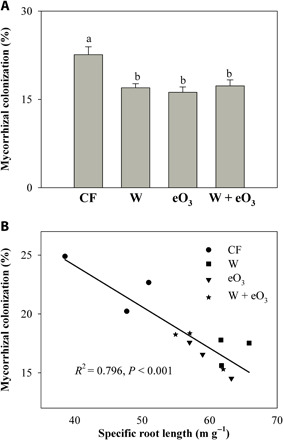
Mycorrhizal colonization rates (A) and the relationship between mycorrhizal colonization of soybean roots and SRL (B) Data are means ± SE (n = 3). The four treatments are as follows: CF, charcoal-filtered ambient air; W, warming; eO3, elevated O3; W + eO3, warming + eO3. Different letters mean significant differences at P = 0.05 level under different treatments. Relationship was considered significant if P < 0.05.
Warming- and eO3-induced changes in plant photosynthate allocation belowground to roots may differentially affect AMF species as they have distinct life history strategies and C requirements (43, 44). To examine whether warming and eO3 may also lead to shifts in the AMF composition in roots, we characterized the AMF community composition via MiSeq sequencing. Our results showed that across all treatments, Glomus (46.6%) and Paraglomus (48.2%) were the most abundant, followed by Claroideoglomus (3.7%) and Gigaspora (1.0%) (Fig. 3A, fig. S5, and table S5). Nonmetric multidimensional scaling (NMDS) ordination and permutational multivariate analysis of variance (PERMANOVA) revealed a significant effect of warming and eO3 on the taxonomic composition of AMF communities, leading to a clear separation in the AMF communities between the control and warming, eO3, or their combination treatment (Fig. 3B). In particular, warming reduced Glomus but increased Paraglomus (Fig. 3A and table S2). Paraglomus or Gigaspora species often have more extensive extraradical hyphal network than Glomus ones (46, 47). As soybean plants mainly rely on N fixation by symbiotic rhizobia for N nutrient (48), eO3 may negatively affect the level of root nodulation and reduce the rate of N fixation (49). Also, warming and eO3 may significantly affect soybean N fixation through affecting the tripartite association of plant, rhizobia, and AMF (50), although these effects were not the focus of this study. Soybean plants can also better obtain N through water uptake under warming, and an increase in Paraglomus species may help plant acquisition of less mobile nutrients such as phosphorus (P) (51, 52). We also observed that eO3 reduced Glomus abundance but increased Paraglomus abundance (Fig. 3A and table S2), providing direct evidence of O3-led alteration of in planta AMF community composition. Previously, Cotton et al. (44) observed no significant eO3 effects on AM fungal community composition, richness, and evenness from the SoyFACE experiment at the University of Illinois, but their plants sampled may be too young (around 8 weeks) to fully reflect the eO3 impact on AMF. In comparison, Wang et al. (53) found that eO3 significantly altered the general soil microbial community under a hybrid maize but did not assess the impact on the AMF composition. The warming- and O3-induced shift in favor of Paraglomus over Glomus observed in our study (Fig. 3A) likely stems from their different C requirements. Since Paraglomus in general form smaller spores and have smaller C demand than Glomus species (46, 51, 54), they may likely more efficiently use scarce C resources under environmental stresses while obtaining nutrients at lower energy costs.
Fig. 3. Effects of warming and eO3 on AMF community composition in soybean roots.
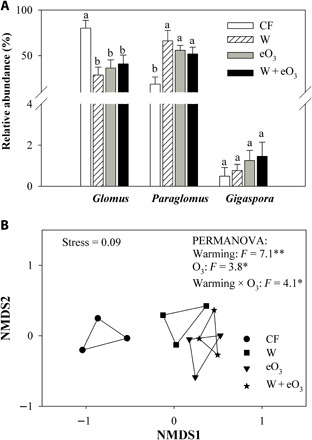
Relative abundance of Glomus, Paraglomus, and Gigaspora (A) and NMDS of community composition of AM fungal VT colonizing soybean roots among different treatments (B). Data are means ± SE (n = 3). The four treatments are as follows: CF, charcoal-filtered ambient air; W, warming; eO3, elevated O3; W + eO3, warming + eO3. In (A), different letters denote significant differences at P = 0.05. In (B), *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01.
To examine the potential impact of warming- and/or eO3-induced changes in roots and AMF on ecosystem functioning, we assess microbial decomposition of organic C in the mycorrhizosphere. Given that AMF are obligately biotrophic, we used different sizes of mesh to isolate the effect of AMF from their host roots (30). While the hyphae-ingrowth chambers (mesh size at 20 μm) only allow AMF hyphal penetration, the root-ingrowth chambers (mesh size at 1.8 mm) permit both roots and AMF to grow inside (fig. S2). Exact 1.80 g of 13C-enriched materials [aboveground residues of switchgrass, a C4 plant with δ13C = −11.51‰ (per mil)] was thoroughly mixed with 180.0 g of the field soil (with δ13C = −23.79‰), and the soil-residue mixture was then introduced into each chamber to trace the C dynamics. Thirteen weeks after the placement of hyphae- or root-ingrowth chambers, the presence of AMF alone or AMF with roots significantly stimulated soil C decomposition within hyphae- and root-ingrowth chambers (Fig. 4A and table S3; also see 13C in fig. S6). Neither warming nor eO3 had any significant impact on soil C in the no-AMF control (Fig. 4A and table S3; also see 13C in fig. S6). In the presence of AMF alone, however, warming, eO3, and their interaction significantly reduced total soil C by 8, 7, and 9%, respectively (Fig. 4A and table S3). In the presence of both plant roots and AMF, warming, eO3, and their interaction reduced total soil C more significantly by 11, 14, and 12%, respectively (Fig. 4A and table S3). Also, mycorrhizal colonization rate was positively correlated with total C remaining in the AMF- and root-ingrowth chambers (Fig. 5, B and C). Further, the relative abundance of Glomus was positively correlated with total C remaining in the AMF- and root-ingrowth chambers (Fig. 6, C and E). In contrast, the relative abundance of Paraglomus was negatively correlated with total C remaining in the AMF- and root-ingrowth chambers (Fig. 6, D and F). Through quantifying 13C remaining within hyphae- and root-ingrowth chambers, we further found that warming, eO3, and their interaction significantly enhanced the loss of the residue-derived organic C in the presence of AMF alone and roots with AMF (Fig. 4B and table S3).
Fig. 4. Warming and eO3 modulated the effects of plant roots and their associated AMF on organic C decomposition.
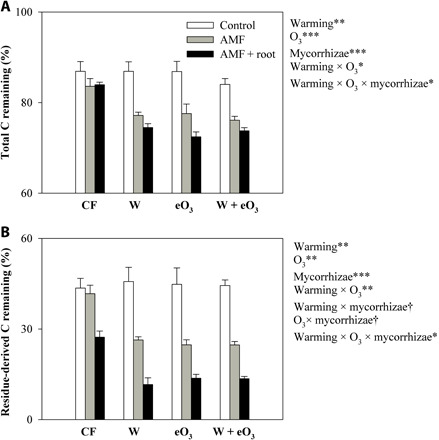
(A) Effects on total soil C remaining (%) and (B) effects on the residue organic C remaining (%) as determined by 13C remaining within no-hyphae–, hyphae-, and root-ingrowth chambers. Data are means ± SE (n = 3). Control, neither AMF nor roots growing into the chamber; AMF, AMF hyphae but not roots growing into the chamber; AMF + root, both AMF hyphae and roots growing into the chamber. The significance levels are labeled with †0.05 < P ≤ 0.10; *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01; ***P ≤ 0.001.
Fig. 5. Warming and eO3 modulated the relationships between AMF colonization rates of soybean roots and total C remaining.
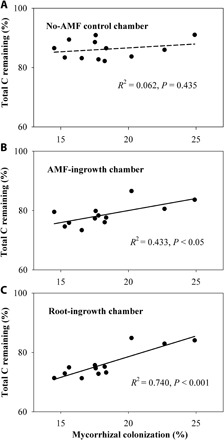
Relationships between mycorrhizal colonization of soybean roots and total C remaining (%) within (A) no-hyphae–, (B) hyphae-, and (C) root-ingrowth chambers across different climate change treatments. Relationships were considered significant if P < 0.05.
Fig. 6. Warming and eO3 modulated the relationships between AMF composition and total C remaining.
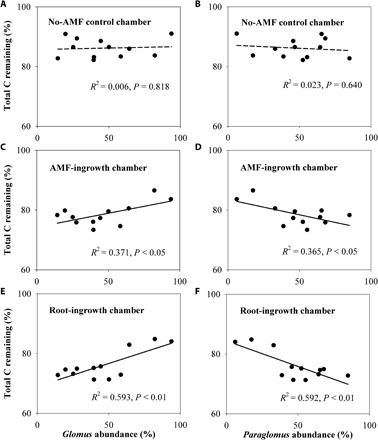
Relationships between the relative abundance of Glomus (A, C, and E) or Paraglomus (B, D, and F) in soybean roots and total C remaining (%) within no-hyphae–, hyphae-, and root-ingrowth chambers across different climate change treatments. Relationships were considered significant if P < 0.05.
The significantly increased organic C decomposition in the presence of AMF or roots (Fig. 4) indicated that changes in roots and AMF under warming and eO3 can modify C and N dynamics. The changes in root traits (increased SRL and reduced RTD), decreased colonization of roots by AMF, and shifts in the AMF community composition may cascade up to affect ecosystem C dynamics through several mechanisms. First, roots with lower density are more fragile and turn over faster, as root life span often correlates negatively with SRL and positively with RTD (40, 41). Second, reduced AMF colonization under warming and eO3 (Fig. 2A) may increase root exudation (55). Significantly increased microbial biomass C and N under warming, despite having no effects on plant root biomass and decreased AMF colonization (Figs. 1B and 2A and table S1), provide direct evidence indicating increased labile C availability for saprophytic microbes, which likely prime the decomposition of organic C in soil. Third, plant roots and AMF are major binding agents for soil aggregating through enmeshing microaggregates into macroaggregates (35, 36), and decreased RTD and AMF under warming and eO3 (Figs. 1D and 2A) would reduce the capacity of roots and AMF for aggregation (36, 42). Last, AMF produce secondary compounds such as polysaccharides that function as important binding agents and can protect organic C from microbial decomposition (35, 56). In particular, Glomus species can produce glycoprotein-type compounds (e.g., glomalin) that likely enhance soil organic C stability (34), as supported directly by the positive correlations between organic C remaining and AMF colonization rate (Fig. 5, B and C) or Glomus abundance (Fig. 6, C and E) and indirectly by the negative correlations between the organic C remaining and Paraglomus abundance (Fig. 6, D and F). Consequently, enhanced C availability for saprophytic microbes due to increased root turnover and reduced protection of soil organic materials due to decreases in root strength and AMF colonization may jointly facilitate the microbial turnover of soil organic C.
Our findings illustrate that concurring climate warming and eO3 under future climate change scenarios may elicit a cascade of events that alter root traits and AMF communities, thereby stimulating root turnover and organic C decomposition. What is interesting is that these two different factors converged in driving similar effects on plant traits and root symbionts. This convergence may likely occur because both factors function as a stress to plants that often reduces plant C fixation and/or plant carbohydrate allocation belowground (25, 57). A reduction in plant photosynthate supply likely prompts changes in root traits and root symbionts that allow to optimize resource acquisition at the minimum energy cost. Warming and O3 enhancement of soil organic C decomposition as a result of the root-AMF tradeoffs observed in our study may have some important implications for soil C dynamics. A meta-analysis found that ambient O3 concentration (ca. 40 ppb on average) in the Northern Hemisphere may have reduced the total biomass of trees in the temperate forests by 7%, compared with trees grown in charcoal-filtered controls (26). A further increase of O3 concentration to 64 ppb would reduce total biomass by 11%, compared with trees grown at the current ambient concentration (26). In agroecosystems, model analyses estimated that ozone (mean concentration of 2010 to 2012) reduced global yield annually by 12.4, 7.1, 4.4, and 6.1% for soybean, wheat, rice, and maize, respectively (58). Results from meta-analyses showed that compared to their respective controls in charcoal-filtered air, chronic exposure to ozone of 62 ppb, on average, reduced rice yield by 14% (59), and an increase to 70 ppb reduced soybean shoot biomass by 34% (60). Moreover, soybean plants exposed to warming (57, 61) and eO3 (49, 60) may reduce the level of root nodulation and/or the rate of nitrogen fixation, negatively feeding back to plant production. Reduced detritus inputs and warming and O3 stimulation of the priming effect may likely affect soil C dynamics. For example, Loya et al. (8) documented that a 50% increase in the ozone concentration for 4 years caused a 6.5% decrease in total soil C in the aspen and mixed aspen-birch forests. O3 stimulation of decomposition may have contributed to this decrease as reduced litter input alone cannot fully explain the observed total soil C loss (8). In agroecosystems, O3 suppression of biomass production and/or yield likely occurs with reduced return of crop residues, the primary organic matter source of soil organic C (62). Together, O3 suppression of residue inputs, combined with warming and O3 stimulation of root turnover and organic C decomposition, may exacerbate soil C losses, constraining the capacity of terrestrial ecosystems for C sequestration in a warmer world.
MATERIALS AND METHODS
The study site and experimental manipulations
This study was a component of a long-term field manipulation experiment at the Lake Wheeler farm of North Carolina State University, Raleigh, NC, USA (35°43′N, 78°40′W; elevation, 120 m) (63). The field experiment was established in June 2013. The field manipulation experiment had a 2 × 2 factorial design with two levels of temperature (ambient versus elevated +3°C) and two targeted concentrations of O3 (low at 20 ppbv versus high at 60 ppbv) in a soybean agroecosystem (63, 64). This led to a total of four treatment combinations: (a) charcoal-filtered air and ambient temperature (CF); (b) charcoal-filtered air plus 3.0°C increase in temperature (W); (c) charcoal-filtered air plus ambient temperature and 1.3 times ambient ozone (eO3); (d) charcoal-filtered air + warming + 1.3 times ambient ozone (W + eO3). AESs (fig. S1) (3 m by 10 m each) were used to control the temperature and O3 concentration, and all AESs were laid out in a randomized block design with three replicates of each treatment. Each AES consisted of two double-walled, open-top chamber panels attached to aluminum frames and placed in parallel. The warming treatments (W and W + eO3) were achieved by addition of warming, humidified air to increase the ambient air temperature by 3.0°C, taking the control (CF) as the baseline (63). In this system, electrical resistance heaters and solar-heated water were used to warm the air. O3 was generated from oxygen (O2) by corona discharge (model TG-20, Ozone Solutions, Hull, IA, USA) and dispensed 12 hours daily to each plot in proportion to ambient O3 concentrations. Air temperature and relative humility and O3 concentration were constantly monitored (by HOBO Pro V2 Temp/RH Datalogger and an ultraviolet photometric O3 analyzer, respectively) by an automatic system in an adjacent trailer. On the basis of these results, the feedback system automatically regulated the heat release or O3 release (please see Supplementary Materials and Methods for details). All plots were planted with a soybean cultivar Asgrow AG5232 in 2013, Asgrow AG5633 in 2014, and with soybean cultivar “Jake” in 2015 and 2016 (63, 64).
Plant and soil sampling
In September 2016, soybean plants (cultivar Jake) of approximately the same age at the pod filling stage were harvested at the soil surface from each plot to determine shoot biomass (63). Three plant roots were sampled from each plot to determine root biomass and characterize root morphological parameters and their associated AMF (see Supplementary Materials and Methods for details). Three soil cores (5 cm diameter, 0 to 10 cm depth) from each plot were randomly taken and mixed to form one composite sample.
Determinations of soil chemical and microbial properties, root morphologies, mycorrhizal colonization of roots, and AMF community composition in roots
Soil ammonium (NH4+) and nitrate (NO3−), extractable organic C, and microbial biomass C and N were measured (see Supplementary Materials and Methods for details). Each root sample was scanned on a desktop scanner, and images were processed with WinRHIZO (Regent Instruments Inc.) to determine root morphological parameters (root diameter, length, surface area, and volume). SRL was determined by dividing root length by the dry biomass weight. RTD was calculated by dividing dry mass by volume. Mycorrhizal colonization of plant roots was microscopically determined after roots were stained with acidic glycerol–trypan blue solution at 90°C for 30 min (65) and scored using gridline intersection (66).
Mycorrhizal fungal DNA was extracted from a 0.05-g (freeze-dried) subsample of fine roots with a PowerPlant DNA Isolation Kit (MoBio Laboratories Inc., Carlsbad, CA, USA). The quantity and purity of DNA samples were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The primer set AMV4.5NF (5′-AAGCTCGTAGTTGAATTTCG-3′)/AMDGR (5′-CCCAACTATCCCTATTAATCAT-3′) was used to amplify the 18S ribosomal RNA (rRNA) gene (67). The polymerase chain reaction products were purified with an agarose gel DNA purification kit (AP-GX-250G; Axygen, Union City, CA, USA) and quantified using a NanoDrop ND-1000 spectrophotometer. The purified products were pooled in equimolar amounts and then sequenced on the Illumina MiSeq platform (Shanghai Personal Biotechnology Co. Ltd., Shanghai, China) (see Supplementary Materials and Methods for details).
Raw high-throughput sequencing data were processed by using the Quantitative Insights Into Microbial Ecology (QIIME) toolkit (68). Potentially similar sequences were clustered into operational taxonomic units (OTUs) using the seed-based UCLUST algorithm at a 97% identity threshold. The most abundant sequence from each OTU was selected as the representative sequence. To compare our results with other studies, the representative sequences were checked against the MaarjAM database and defined in virtual taxa (VT), roughly corresponding to species-level taxa for further analysis (69). The MaarjAM database classifies the central part of Glomeromycotina small subunit rRNA gene sequences into phylogenetically delimited sequence clusters, VT (69).
Determination of root and mycorrhizal priming of organic C decomposition
One week after soybeans were planted in June 2016, three ingrowth chambers (a no-hyphae chamber, pore size: 0.45 μm; a hyphae-ingrowth chamber, pore size: 20 μm, and a root-ingrowth chamber, pore size: 1.8 mm) (fig. S2) were randomly buried into each AES plot to assess the impacts of AMF hyphae and roots on organic C decomposition, respectively. Each chamber was filled with 1.80 g of residues of switchgrass (a C4 plant with δ13C = −11.51‰) and 180.0 g of the field soil (with δ13C = −23.79‰) to trace the C dynamics. After incubation for 13 weeks, all the chambers were retrieved on the same day. Total C and 13C of sample were measured using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) at the University of California, Davis (30). To estimate the net losses of residue-derived C by the mycorrhizal effect, we calculated the mean proportion (fR) of the residue C in the incubated samples using the equation fR = (δ13M − δ13S)/(δ13R − δ13S), where δM, δR, and δS denote the mean isotopic signatures (δ13C) for the mixture M (incubated samples of soil/residue mixture), source R (residue, which is −11.51‰), and source S (soil, which is −23.79‰), respectively. Although this two-pool model did not allow us to quantify the exact amounts of the organic C derived from root exudates and AMF, it allowed us to estimate the impacts of AMF and roots on organic C decomposition inside the growth chambers (see Supplementary Materials and Methods for details).
Statistical analysis
All statistical analyses were performed using R 3.6.2 (70). We used linear mixed-effects models (LMMs) to examine the effects of warming, ozone, and their interactions on plant biomass, soil chemical and microbial parameters, AMF colonization of roots, and root morphological parameters. We also used LMMs to examine the effects of warming, ozone, mycorrhizae, and their interactions on C remaining within hyphae- and root-ingrowth chambers. Treatment was used as a fixed effect and block as a random effect. Linear regression was performed to examine the relationship between SRL and AMF colonization of roots.
The structure of AMF communities was compared using the proportion of different VT reads as a proxy for the relative abundance of AMF taxa per sample (69). To visualize variation in AM fungal communities across experimental treatments, NMDS ordination analysis based on the Bray-Curtis distance matrix was performed, using function “metaMDS” from R package vegan (71). To test the effects of the experimental treatments on AM fungal composition, we performed two-way PERMANOVA using the default settings, with the function “adonis” from R package vegan (71). For all tests, P < 0.05 was considered a statistically significant difference.
Acknowledgments
We thank W. Pursley and S. Ray of the USDA-ARS Plant Science Research Unit for field technical help. We would like to thank C. Tu for assistance with field sampling. We are also grateful to three anonymous reviewers whose comments and suggestions greatly helped improve the manuscript. Funding: This research was partially supported by the College of Agriculture and Life Sciences, NC State University, grants to S.H. from the USDA-NIFA (2012_02978_230561; 2018-51106-28773), USDA-ARS funding to K.O.B. and R.W.Z. from the U.S. Congressional Appropriation, a China Scholarship Council scholarship to Y.Q. (CSC no. 201306320137), and a grant to Yi Zhang from the National Natural Science Foundation of China (NSFC) (no. 31600383). Author contributions: All authors contributed to this work. S.H., Y.Q., K.O.B., R.W.Z., and H.D.S. conceived the experiment. K.O.B. and R.W.Z. designed and maintained the field long-term warming and O3 study. S.H., Y.Q., Yi Zhang, K.O.B., R.W.Z., and H.D.S. contributed to the design of the experiment. Y.Q., L.G., L.Z., M.C., K.Z., and Yexin Zhao performed the experiment. Y.Q., L.G., X.X., and Yi Zhang performed the data analyses. Y.Q., S.H., and Yi Zhang wrote the manuscript with inputs from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials (datasets S1 to S3). Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/28/eabe9256/DC1
REFERENCES AND NOTES
- 1.IPCC, Climate Change 2013: The Physical Science Basis, T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, P.M. Midgley, Eds. (Cambridge Univ. Press, 2013). [Google Scholar]
- 2.Sitch S., Cox P. M., Collins W. J., Huntingford C., Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 448, 791–794 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Reich P. B., Sendall K. M., Stefanski A., Rich R. L., Hobbie S. E., Montgomery R. A., Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 562, 263–267 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Crowther T. W., Todd-Brown K. E. O., Rowe C. W., Wieder W. R., Carey J. C., Machmuller M. B., Snoek B. L., Fang S., Zhou G., Allison S. D., Blair J. M., Bridgham S. D., Burton A. J., Carrillo Y., Reich P. B., Clark J. S., Classen A. T., Dijkstra F. A., Elberling B., Emmett B. A., Estiarte M., Frey S. D., Guo J., Harte J., Jiang L., Johnson B. R., Kröel-Dulay G., Larsen K. S., Laudon H., Lavallee J. M., Luo Y., Lupascu M., Ma L. N., Marhan S., Michelsen A., Mohan J., Niu S., Pendall E., Peñuelas J., Pfeifer-Meister L., Poll C., Reinsch S., Reynolds L. L., Schmidt I. K., Sistla S., Soko N. W., Templer P. H., Treseder K. K., Welker J. M., Bradford M. A., Quantifying global soil carbon losses in response to warming. Nature 540, 104–108 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Pries C. E. H., Castanha C., Porras R., Torn M. S., The whole-soil carbon flux in response to warming. Science 1319, 1420–1423 (2017). [DOI] [PubMed] [Google Scholar]
- 6.van Gestel N., Shi Z., van Groenigen K. J., Osenberg C. W., Andresen L. C., Dukes J. S., Hovenden M. J., Luo Y., Michelsen A., Pendall E., Reich P. B., Schuur E. A. G., Hungate B. A., Predicting soil carbon loss with warming. Nature 554, E4–E5 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Andersen C. P., Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 157, 213–228 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Loya W. M., Pregitzer K. S., Karberg N. J., King J. S., Glardina C. P., Reduction of soil carbon formation by tropospheric ozone under increased carbon dioxide levels. Nature 425, 705–707 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Agathokleous E., Feng Z., Oksanen E., Sicard P., Wang Q., Saitanis C. J., Araminiene V., Blande J. D., Hayes F., Calatayud V., Domingos M., Veresoglou S. D., Peñuelas J., Wardle D. A., De Marco A., Li Z., Harmens H., Yuan X., Vitale M., Paoletti E., Ozone affects plant, insect, and soil microbial communities: A threat to terrestrial ecosystems and biodiversity. Sci. Adv. 6, eabc1176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birgander J., Rousk J., Olsson P. A., Warmer winters increase the rhizosphere carbon flow to mycorrhizal fungi more than to other microorganisms in a temperate grassland. Glob. Change Biol. 23, 5372–5382 (2017). [DOI] [PubMed] [Google Scholar]
- 11.S. E. Smith, D. J. Read, Mycorrhizal Symbiosis (Academic Press, 2010). [Google Scholar]
- 12.Norby R. J., Jackson R. B., Root dynamics and global change: Seeking an ecosystem perspective. New Phytol. 147, 3–12 (2000). [Google Scholar]
- 13.Rillig M. C., Wright S. F., Shaw M. R., Field C. B., Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 97, 52–58 (2002). [Google Scholar]
- 14.Bai W., Wan S., Niu S., Liu W., Chen Q., Wang Q., Zhang W., Han X., Li L., Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: Implications for ecosystem C cycling. Glob. Change Biol. 16, 1306–1316 (2010). [Google Scholar]
- 15.Olsrud M., Carlsson B. Å., Svensson B. M., Michelsen A., Melillo J. M., Responses of fungal root colonization, plant cover, and leaf nutrients to long-term exposure to elevated atmospheric CO2 and warming in a subarctic birch forest understory. Glob. Change Biol. 16, 1820–1829 (2010). [Google Scholar]
- 16.Dukes J. S., Chiariello N. R., Cleland E. E., Moore L. A., Shaw M. R., Thayer S., Tobeck T., Mooney H. A., Field C. B., Responses of grassland production to single and multiple global environmental changes. PLOS Biol. 3, e319 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arndal M. F., Merrild M. P., Michelsen A., Schmidt I. K., Mikkelsen T. N., Beier C., Net root growth and nutrient acquisition in response to predicted climate change in two contrasting heathland species. Plant Soil 369, 615–629 (2013). [Google Scholar]
- 18.Martins S., Montiel-Jorda A., Cayrel A., Huguet S., Roux C. P.-L., Ljung K., Vert G., Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat. Commun. 8, 309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Y., Shi Y., Yang S., Molecular regulation of plant responses to environmental temperatures. Mol. Plant 13, 544–564 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Mueller K. E., LeCain D. R., McCormack M. L., Pendall E., Carlson M., Blumenthal D. M., Root responses to elevated CO2, warming, and irrigation in a semiarid grassland: Integrating biomass, length, and lifespan in a 5-year field experiment. J. Ecol. 106, 2176–2189 (2018). [Google Scholar]
- 21.Kangasjärvi J., Talvinen J., Utriainen M., Karjalainen R., Plant defence systems induced by ozone. Plant Cell Environ. 17, 783–794 (1994). [Google Scholar]
- 22.Fiscus E. L., Booker F. L., Burkey K. O., Crop responses to ozone: Uptake, modes of action, carbon assimilation, and partitioning. Plant Cell Environ. 28, 997–1011 (2005). [Google Scholar]
- 23.Kangasjärvi J., Jaspers P., Kollist H., Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 28, 1021–1036 (2005). [Google Scholar]
- 24.Rao M. V., Lee H. I., Creelman R. A., Mullet J. E., Davis K. R., Jasmonic acid signaling modulates ozone-induced hyper-sensitive cell death. Plant Cell 12, 1633–1646 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ainsworth E. A., Yendrek C. R., Sitch S., Collins W. J., Emberson L. D., The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 63, 637–661 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Wittig V. E., Ainsworth E. A., Naidu S. L., Karnosky D. F., Long S. P., Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology, and biochemistry: A quantitative meta-analysis. Glob. Change Biol. 15, 396–424 (2009). [Google Scholar]
- 27.Cotton T. E. A., Arbuscular mycorrhizal fungal communities and global change: An uncertain future. FEMS Microbial. Ecol. 94, fiy179 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Rasse D. P., Rumpel C., Dignac M.-F., Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269, 341–356 (2005). [Google Scholar]
- 29.Sokol N. W., Bradford M. A., Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 12, 46–53 (2019). [Google Scholar]
- 30.Cheng L., Booker F. L., Tu C., Burkey K. O., Zhou L., Shew H. D., Rufty T. W., Hu S., Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337, 1084–1087 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Keiluweit M., Bougoure J. J., Nico P. S., Pett-Ridge J., Weber P. K., Kleber M., Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 5, 588–595 (2015). [Google Scholar]
- 32.Adamczyk B., Sietiö O.-M., Straková P., Prommer J., Wild B., Hagner M., Pihlatie M., Fritze H., Richter A., Heinonsalo J., Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat. Commun. 10, 3982 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontaine S., Mariotti A., Abbadie L., The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 35, 837–843 (2003). [Google Scholar]
- 34.Wright S. F., Upadhyaya A. A., A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant and Soil 198, 97–107 (1998). [Google Scholar]
- 35.Tisdall J. M., Oades J. M., Organic matter and water-stable aggregates in soils. J. Soil Sci. 62, 141–163 (1982). [Google Scholar]
- 36.Miller R. M., Jastrow J. D., Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol. Biochem. 22, 579–584 (1990). [Google Scholar]
- 37.Hodge A., Fitter A. H., Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. U.S.A. 107, 13754–13759 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Z., Guo D., Xu X., Lu M., Bardgett R. D., Eissenstat D. M., McCormack M. L., Hedin L. O., Evolutionary history resolves global organization of root functional traits. Nature 555, 94–97 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Bergmann J., Weigelt A., van der Plas F., Laughlin D. C., Kuyper T. W., Guerrero-Ramirez N., Valverde-Barrantes O. J., Bruelheide H., Freschet G. T., Iversen C. M., Kattge J., McCormack M. L., Meier I. C., Rillig M. C., Roumet C., Semchenko M., Sweeney C. J., van Ruijven J., York L. M., Mommer L., The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 6, eaba3756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eissenstat D. M., Wells C. E., Yanai R. D., Whitbeck J. L., Building roots in a changing environment: Implications for root longevity. New Phytol. 147, 33–42 (2000). [Google Scholar]
- 41.Weemstra M., Mommer L., Visser E. J. W., van Ruijven J., Kuyper T. W., Mohren G. M., Sterck F. J., Towards a multidimensional root trait framework: A tree root review. New Phytol. 211, 1159–1169 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Verbruggen E., Jansa J., Hammer E. C., Rillig M. C., Vries F., Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil? J. Ecol. 104, 261–269 (2016). [Google Scholar]
- 43.Cao J., Lin T. C., Yang Z., Zheng Y., Xie L., Xiong D., Yang Y., Warming exerts a stronger effect than nitrogen addition on the soil arbuscular mycorrhizal fungal community in a young subtropical Cunninghamia lanceolata plantation. Geoderma 367, 114273 (2020). [Google Scholar]
- 44.Cotton T. E. A., Fitter A. H., Miller R. M., Dumbrell A. J., Helgason T., Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol. 205, 1598–1607 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mrak T., Eler K., Badea O., Hoshika Y., Carrari E., Paoletti E., Kraigher H., Elevated ozone prevents acquisition of available nitrogen due to smaller root surface area in poplar. Plant and Soil 450, 585–599 (2020). [Google Scholar]
- 46.Morton J. B., Redecker D., Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93, 181–195 (2001). [Google Scholar]
- 47.Treseder K. K., Allen E. B., Egerton-Warburton L. M., Hart M. M., Klironomos J. N., Maherali H., Tedersoo L., Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. J. Ecol. 106, 480–489 (2018). [Google Scholar]
- 48.Santachiara G., Salvagiotti F., Rotundo J. L., Nutritional and environmental effects on biological nitrogen fixation in soybean: A meta-analysis. Field Crop Res 240, 106–115 (2019). [Google Scholar]
- 49.Biancari L., Cerrotta C., Menéndez A. I., Gundel P. E., Martínez-Ghersa M. A., Episodes of high tropospheric ozone reduce nodulation, seed production and quality in soybean (Glycine max (L.) merr.) on low fertility soils. Environ. Pollut. 269, 116117 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Bonfante P., Anca I.-A., Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 63, 363–383 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Gosling P., Proctor M., Jones J., Bending G. D., Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 24, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Dai M., Hamela C., Bainarda L. D., Arnaud M. S., Grant C. A., Lupwayi N. Z., Malhi S. S., Lemkef R., Negative and positive contributions of arbuscular mycorrhizal fungal taxa to wheat production and nutrient uptake efficiency in organic and conventional systems in the Canadian prairie. Soil Biol. Biochem. 74, 156–166 (2014). [Google Scholar]
- 53.Wang P., Marsh E. L., Ainsworth E. A., Leakey A. D. B., Sheflin A. M., Schachtman D. P., Shifts in microbial communities in soil, rhizosphere and roots of two major crop systems under elevated CO2 and O3. Sci. Rep. 7, 15019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aguilar-Trigueros C. A., Hempel S., Powell J. R., Cornwell W. K., Rillig M. C., Bridging reproductive and microbial ecology: A case study in arbuscular mycorrhizal fungi. ISME J. 13, 873–884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones D. L., Hodge A., Kuzyakov Y., Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 163, 459–480 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Oades J. M., Soil organic matter and structural stability: Mechanisms and implications for management. Plant and Soil 76, 319–337 (1984). [Google Scholar]
- 57.Teixeira E. I., Fischer G., van Velthuizen H., Walter C., Ewert F., Global hot-spots of heat stress on agricultural crops due to climate change. Agric. For. Meteorol. 170, 206–215 (2013). [Google Scholar]
- 58.Mills G., Sharps K., Simpson D., Pleijel H., Frei M., Burkey K., Emberson L., Uddling J., Broberg M., Feng Z., Kobayashi K., Agraval M., Closing the global ozone yield gap: Quantification and cobenefits for multistress tolerance. Glob. Change Biol. 24, 4869–4893 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Ainsworth E. A., Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Change Biol. 14, 1642–1650 (2008). [Google Scholar]
- 60.Morgan P. B., Ainsworth E. A., Long S. P., How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ. 26, 1317–1328 (2003). [Google Scholar]
- 61.Mourtzinis S., Specht J. E., Lindsey L. E., Wiebold W. J., Ross J., Nafziger E. D., Kandel H. J., Mueller N., Devillez P. L., Arriaga F. J., Conley S. P., Climate-induced reduction in US-wide soybean yields underpinned by region- and in-season-specific responses. Nat. Plants 1, 14026 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y., Wang M., Hu S., Zhang X., Ouyang Z., Zhang G., Huang B., Zhao S., Wu J., Xie D., Zhu B., Yu D., Pan X., Xu S., Shi X., Economics- and policy-driven organic carbon input enhancement dominates soil organic carbon accumulation in Chinese croplands. Proc. Natl. Acad. Sci. U.S.A. 115, 4045–4050 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burkey K., Tisdale R., Zobel R., Ray S., Pursley W., Interactive effects of elevated ozone and temperature on growth and yield of soybean (Glycine max (L.) Merr.) under field conditions. Agronomy 10, 1803 (2020). [Google Scholar]
- 64.Qiu Y., Jiang Y., Guo L., Burkey K. O., Zobel R. W., Shew H. D., Hu S., Contrasting warming and ozone effects on denitrifiers dominate soil N2O emissions. Environ. Sci. Technol. 52, 10956–10966 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Phillips J. M., Hayman D. S., Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–161 (1970). [Google Scholar]
- 66.McGonigle T. P., Miller M. H., Evans D. G., Fairchild G. L., Swan J. A., A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 115, 495–501 (1990). [DOI] [PubMed] [Google Scholar]
- 67.Lumini E., Orgiazzi A., Borriello R., Bonfante P., Bianciotto V., Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ. Microbiol. 12, 2165–2179 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., Huttley G. A., Kelley S. T., Knights D., Koenig J. E., Ley R. E., Lozupone C. A., McDonald D., Muegge B. D., Pirrung M., Reeder J., Sevinsky J. R., Turnbaugh P. J., Walters W. A., Widmann J., Yatsunenko T., Zaneveld J., Knight R., QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Öpik M., Vanatoa A., Vanatoa E., Moora M., Davison J., Kalwij J. M., Reier Ü., Zobel M., The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 188, 223–241 (2010). [DOI] [PubMed] [Google Scholar]
- 70.R Core Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, 2017; www.R-project.org/.
- 71.J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, H. Wagner, vegan: Community ecology package. R package version 2.5-6 (2019); https://cran.r-project.org/web/packages/vegan/vegan.pdf.
- 72.Heagle A. S., Body D. E., Heck W. W., An open-top field chamber to assess the impact of air pollution on plants. J. Environ. Qual. 2, 365–368 (1973). [Google Scholar]
- 73.Vance E. D., Brookes P. C., Jenkinson D. S., An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- 74.Cabrera M. L., Beare M. H., Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci. Soc. Am. J. 57, 1007–1012 (1993). [Google Scholar]
- 75.Qiu Y., Jiang Y., Guo L., Zhang L., Burkey K. O., Zobel R. W., Reberg-Horton S. C., Shew H. D., Hu S., Shifts in the composition and activities of denitrifiers dominate CO2 stimulation of N2O emissions. Environ. Sci. Technol. 53, 11204–11213 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Zobel R. W., Lolium perenne L. root systems are a collection of Gaussian curve shaped meso diameter class length distributions. Plant Soil 363, 113–121 (2013). [Google Scholar]
- 77.Brown J. R., Blankinship J. C., Niboyet A., van Groenigen K. J., Dijkstra P., Le Roux X., Leadley P. W., Hungate B. A., Effects of multiple global change treatments on soil N2O fluxes. Biogeochemistry 109, 85–100 (2012). [Google Scholar]
- 78.Crain C. M., Kroeker K., Hapen B. S., Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/28/eabe9256/DC1


