Abstract
Background
Autoimmune diseases affect reproductive-aged women, often require medication during pregnancy and are associated with an increased cancer risk, especially for lymphomas.
Objective
Our aim was to investigate the risk of childhood cancer among the offspring after an antenatal exposure to maternal autoimmune disease and its medication.
Study design
In this case-control study we identified all patients under the age of 20 years with their first cancer diagnosis in 1996–2014 from the Finnish Cancer Registry (n=2,037) and 1:5 population-based controls, matched for sex and birth year (n=10,185), from the Medical Birth Registry. We obtained information on maternal connective tissue disease, inflammatory bowel disease and the use of autoimmune medication from the Medical Birth Registry, Care Register for Health Care, Register of Reimbursed Drug Purchases and Medical Special Reimbursements Register. We evaluated maternal autoimmune disease and cancer risk among the offspring using conditional logistic regression, adjusting for maternal age, parity and smoking.
Results
The odds ratio for childhood cancer among the offspring following a maternal autoimmune disease exposure was 0.76 (95% confidence interval 0.47–1.23) when compared to the offspring of mothers with no autoimmune disease. The individual odds ratios for inflammatory bowel and connective tissue disease were 1.08 (95% confidence interval 0.56–2.01) and 0.50 (95% confidence interval 0.23–1.08), respectively. The odds ratio for maternal use of autoimmune disease medication was 0.95 (95% confidence interval 0.80–1.14) overall, and not increased by the drug subtype. An increased risk with medication in late pregnancy did emerge but the odds ratios were unstable owing to the small numbers.
Conclusions
Our study is not supportive of an increased cancer risk among the offspring of women with autoimmune disease in pregnancy and/or the respective medication.
Keywords: antenatal exposure, case-control, cancer risk, childhood cancer, maternal autoimmune disease, maternal medication, registry-based study
Introduction
Autoimmune diseases (AI), such as connective tissue diseases and inflammatory bowel disease (IBD), affect women in the reproductive age. In 0.7% of pregnancies ending in delivery in Finland, the mother receives drug reimbursement for treatment of a connective tissue disease and IBD is equally common among pregnant women (report of Finnish Institute for Health and Welfare, 2020).1
In Finland, up to 200 cancers are diagnosed annually among children and adolescents 20 years of age or younger. Cancer remains the most common, non-accidental cause of death among children in the developed countries. The etiology of childhood cancer is unclear with only 5–10% of the cases linked to hereditary cancer predisposition syndromes and the same proportion possibly to yet undefined environmental factors.2
AI disease, especially rheumatoid arthritis, seems to increase the risk of cancer among the adults patients.3–6 Also, therapies used to treat AI disease, such as TNF-alpha-inhibitors, have been associated with an increased risk. Both rheumatoid arthritis6,7 and its treatment8 have been associated with lymphoma.7,8 There is speculation that the risk is positively associated with an increasing severity of the AI disease.5
The relationship between a familial AI disease and the risk of childhood cancer has been previously studied, mostly in case-control settings with exposure data based either on recall3,9–11 or population-based registries.12–15There is only one study suggesting a significant association between a maternal AI disease and childhood Hodgkińs lymphoma.3 The rest found non-significant11,12,14,15 or no association9,10,13 between a maternal AI disease and childhood acute lymphoblastic leukemia (ALL) and lymphomas. The definition of autoimmune disease has varied widely between the studies warranting a population-based approach with specific subgroups of AI disease.
We investigated whether a maternal AI disease or its medication are associated with the risk of childhood cancer in the offspring up to the age of 20 years using population-based registry data.
Materials and methods
A unique personal identity code given to each Finnish citizen since 1967 allows for the linkage of information recovered from health and vital statistics registries. Permanent residents of Finland are covered under the Finnish National Health Insurance (NHI) and eligible for reimbursement for the cost of prescription medicines.
The Finnish Cancer Registry (FCR) started the systematic, nationwide registration of cancer in 1953 and includes data on treatments and causes of death. The FCR has a 95% coverage for all cancers.16 The completeness for childhood cancer is 92% for solid tumors and 97% for leukemia.17
The Finnish Medical Birth Registry (MBR), run by the Finnish Institute for Health and Welfare (THL), was founded in 1987. The MBR contains data on all mothers who have delivered a child in Finland, and the obstetric and neonatal outcomes are available until 7 days after delivery or hospital discharge.
The Register of Reimbursed Drug Purchases is maintained by the Social Insurance Institute of Finland (Kela) and retains data on all prescription drugs reimbursed since 1993. The database includes personal information on the Anatomic Therapeutic Chemical (ATC) code of the drug, date of purchase, package size, drug cost and refund category.
The Care Register for Health Care (HILMO) is maintained by THL since 1969 and contains information on patients, hospital admissions and discharges, diagnoses and treatment given in secondary and tertiary health care.
The research permits were obtained from THL (THL/252/5.05.00/2016), Kela (15/52272016) and Helsinki University. No ethical board review was required as this study was fully registry-based and no patients were contacted.
Study population
We identified all individuals with their first cancer diagnosis below the age of 20 years in the FCR for the years 1996–2014 (n=2,037). We also identified five population-based controls matched for sex and birth year for each patient (n=10,185) from the MBR. Due to missing data on the birthweight, eight cases and 82 controls were omitted from the analysis (Figure 1). For the descriptive characteristics of the cases and controls see Table 1.
Figure 1.
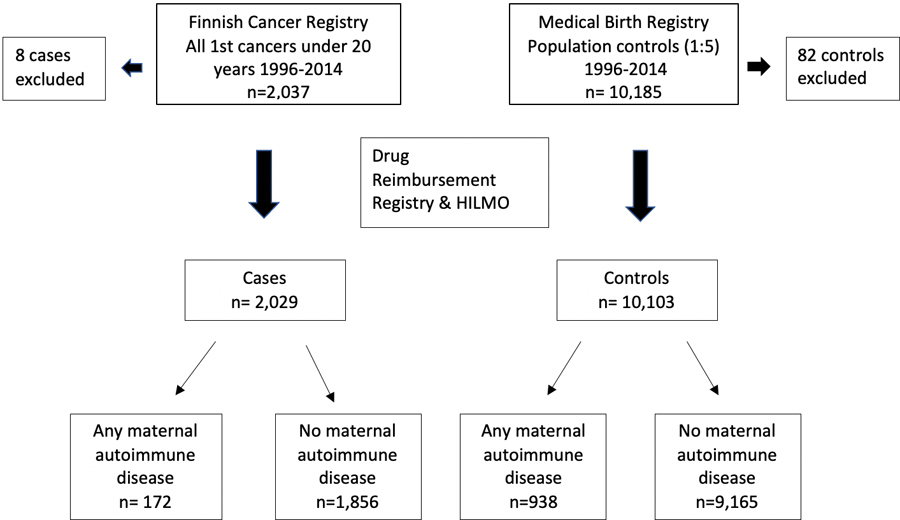
Description of Study Population.
Table 1.
Maternal and offspring characteristics of childhood cancer cases and controls, 1996–2014.
| N cases = 2,029 | Proportion (%) of cases | N controls = 10,103 | Proportion (%) of controls | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years) | ||||
| <25 | 355 | 17.5 | 1,872 | 18.5 |
| 25–29 | 639 | 31.5 | 3,246 | 32.1 |
| ≥30 | 1,035 | 51.0 | 4,985 | 49.3 |
| Parity | ||||
| Primiparous | 847 | 41.7 | 4,098 | 40.6 |
| Multiparous | 1,182 | 58.3 | 6,005 | 59.4 |
| Maternal smoking | ||||
| Yes | 291 | 14.3 | 1,447 | 14.3 |
| No | 1,680 | 82.8 | 8,404 | 83.1 |
| Unknown | 58 | 2.9 | 252 | 2.5 |
| A diagnosis of autoimmune disease (AI) prior to delivery | 19 | 0.9 | 134 | 1.3 |
| Any AI medication purchase 3 months before/during pregnancy (ATC-code) | 169 | 8.3 | 888 | 8.8 |
| IBD medication (A07E) | 13 | 0.6 | 65 | 0.6 |
| Systemic corticosteroids (H02) | 21 | 1.0 | 107 | 1.1 |
| Immunosuppressants (L04) | 3 | 0.1 | 13 | 0.1 |
| Anti-inflammatory and -rheumatic products (M01) | 141 | 6.9 | 761 | 7.5 |
| IBD | 12 | 0.6 | 61 | 0.6 |
| IBD diagnosis, no medication | 2 | 0.1 | 12 | 0.1 |
| Rheumatic disease | 7 | 0.3 | 74 | 0.7 |
| Rheumatic disease diagnosis, no medication | 1 | 0.05 | 38 | 0.4 |
| Offspring characteristics | ||||
| Offspring sex | ||||
| Male | 1,092 | 53.8 | 5,431 | 53.8 |
| Female | 937 | 46.2 | 4,672 | 46.2 |
| Multiple pregnancy | ||||
| No | 1,962 | 96.7 | 9,784 | 96.8 |
| Yes | 67 | 3.3 | 319 | 3.2 |
| Gestational age | ||||
| <37 weeks | 141 | 6.9 | 537 | 5.3 |
| ≥37 weeks | 1,888 | 93.1 | 9,566 | 94.7 |
| Weight for gestational age (size at birth) | ||||
| Small for gestational age (SGA) | 55 | 2.7 | 217 | 2.1 |
| Appropriate for gestational age (AGA) | 1,881 | 92.7 | 9,587 | 94.9 |
| Large for gestational age (LGA) | 93 | 4.6 | 299 | 3.0 |
| Delivery type | ||||
| Vaginal birth | 1,675 | 82.6 | 8,470 | 83.8 |
| Cesarean section | 354 | 17.4 | 1,627 | 16.1 |
| Unknown | 0 | 0.0 | 6 | 0.5 |
Exposure definition and classification
The medical information on an AI disease in the mother recorded any time before the delivery was obtained from the HILMO, MBR and Register of Medical Special Reimbursements. We extracted diagnoses coded using the International Statistical Classification of Disease and Related Health Problems (ICD) version ICD-9 (codes 135, 446–447, 555–556, 696, 710, 714 and 720) and ICD-10 (codes K50–K51, L40, M05–M09, M45–M49 and D86) and the special reimbursement codes (132, 202 and 208). Due to the small numbers, two subgroups were formed a priori: mothers with an IBD (ICD-9 codes 555–556, ICD-10 codes K50–K51 and special reimbursement code 208) and those with a connective tissue disease, including rheumatoid arthritis, ankylosing spondylitis, psoriasis, sarcoidosis and vasculitis (ICD-9 codes 135, 446–447, 696, 710, 714, 720, ICD-10 codes L40, M05–M09, M45–M49, D86 and special reimbursement codes 132 and 202).
Information on the medication the mothers purchased three months prior to conception and/or during pregnancy was obtained from the Register of Reimbursed Drug Purchases. Medications used to treat the AI disease were identified with the ATC classification using the second and third level codes: intestinal anti-inflammatory agents, i.e. IBD medication, (A07E), systemic corticosteroids (H02), immunosuppressants (L04) and anti-inflammatory and -rheumatic products (M01). We analyzed the associations for the AI disease and medications separately, because the women did not necessarily purchase the medication or alternatively did purchase the medication without a diagnosis in the registries. The dosage was not available to us in an analyzable format.
The date of conception was calculated as the date of delivery minus gestational age at birth in days based on ultrasound (or best clinical estimate if there was no ultrasound confirmation) as registered in the MBR. Birth weight for gestational age was categorized as small (SGA), appropriate (AGA) or large for gestational age (LGA). SGA was defined as a birthweight under −2 SD and LGA as a birth weight over +2 SD of the standard, population-based growth-curves.18
Cancer definition and classification
Cancer was defined as a malignant neoplasm, but we also included benign or borderline tumors of the central nervous system (CNS), for example, pilocytic astrocytomas, considered borderline, and the most common CNS tumors in childhood. The FCR uses the International Classification of Childhood Cancer: with morphology (ICD-0–3), and with morphology and site (ICCC3) (codes 011 for ALL, 011–015 for all leukemias, 021–025 for lymphomas, 031–036 for CNS tumors and 037–122 for other cancers).19
Statistical analysis
We evaluated the association between the AI disease of the mother and the risk of childhood cancer in her offspring. Specifically, we used conditional logistic regression to estimate the odds ratios (OR) and 95% confidence intervals (CI) for each autoimmune variable (any AI disease diagnosis or medication; any AI disease diagnosis; IBD; rheumatic disease; any AI disease medication; systemic corticosteroids; immunosuppressants ; anti-inflammatory and -rheumatic products) with cancer risk; this model was repeated adjusting for maternal age (<25, 25–29, ≥30 years), parity (primiparous, multiparous) and maternal smoking status during pregnancy (yes/no). To account for the incomplete information on smoking status, we employed a complete case approach restricting the analysis to those with non-missing data.
We then considered multiple sensitivity and secondary analyses. Both low and high birth weight have been associated with childhood cancer risk20,21, and mothers with an AI disease are known to deliver smaller babies. 22,23 Thus, birth weight is potentially a mediator of the relationship between a maternal AI and offspring cancer risk. To account for this, we conducted a sensitivity analysis adjusting the birth weight for gestational age (size at birth). We also performed subgroup analyses limited to cases diagnosed with specific childhood cancers. To evaluate the role of matching, we performed a stratified sensitivity analysis with unmatched data for birth year and sex. To estimate the impact of non-specific analgesics on the overall association, we performed a sensitivity analysis for AI disease medication excluding the anti-inflammatory and -rheumatic products. We also analyzed data on medication by stratifying the drug purchases into two groups, i.e. those during the 3 months before pregnancy and/or during the 1st trimester(yes/no) and drug purchase during the 2nd and/or 3rd trimester (yes/no). Those on medication throughout the pregnancy contributed to both categories. The statistical analyses were performed with the STATA MP14 (StataCorp LLC, Texas, USA).
Results
There was a total of 172 (8.5%) cases with cancer and prenatally exposed to any maternal AI disease and/or medication used to treat the disease. Of these, 16 (0.8%) had mothers with an AI disease diagnosis and medication, 3 (0.1%) with a diagnosis but without medication, and 150 (7.4%) without a diagnosis but with medication. In the control group there were 938 (9.3%) children exposed to maternal AI disease and/or medication. Of these, 85 (0.8%) had a diagnosis of AI disease and used medication, 50 (0.5%) with a diagnosis but without medication, and 753 (7.5%) without a diagnosis but with medication.
The maternal AI diseases included in the analyses were not associated with an increased risk for childhood cancer (crude OR 0.70, 95% CI 0.43–1.14) when compared to the offspring of mothers with no AI disease. The result was similar after adjusting for maternal age, parity and smoking status (OR 0.76, 95% CI 0.47–1.23) or when further adjusted for birth weight for gestational age (0.76, 95% CI 0.47–1.24). For an IBD, the adjusted OR was 1.08 (95% CI 0.56–2.01) and 0.50 (95% CI 0.23–1.08) for the connective tissue diseases (Table 2).
Table 2.
The odds ratios (OR) and 95% confidence intervals (CI) calculated, using conditional logistic regression modeling, as the ratio of the proportion of children exposed to maternal autoimmune disease and its medication in offspring with childhood cancer (cases) and the proportion of exposure to maternal autoimmune disease and its medication in healthy offspring (controls), 1996–2014. Both crude and adjusted ORs are presented.
| N cases (%) | N controls (%) | OR* | 95% CI | OR** | 95% CI | |
|---|---|---|---|---|---|---|
| No autoimmune disease | 2,010 (99.1) | 9,969 (98.7) | 1.00 | --- | 1.00 | --- |
| Any autoimmune disease-related exposure (diagnosis or medication) | 172(8.5) | 938(9.3) | 0.91 | 0.76–1.08 | 0.92 | 0.77–1.09 |
| Any autoimmune disease diagnosis | 19(0.9) | 134 (1.3) | 0.70 | 0.43–1.14 | 0.76 | 0.47–1.23 |
| IBD | 12 (0.6) | 61 (0.6) | 0.98 | 0.53–1.82 | 1.08 | 0.56–2.01 |
| Rheumatic disease | 7(0.3) | 74 (0.7) | 0.47 | 0.21–1.02 | 0.50 | 0.23–1.08 |
| N cases (%) | N controls (%) | OR* | 95% CI | OR** | 95% CI | |
| No AI medication purchases | 1,860 (91.7) | 9,215 (91.2) | 1.00 | --- | 1.00 | --- |
| Any AI disease medication purchase | 169 (8.3) | 888 (8.8) | 0.95 | 0.80–1.12 | 0.95 | 0.80–1.14 |
| IBD medication | 13 (0.6) | 65 (0.6) | 0.97 | 0.55–1.81 | 1.08 | 0.59–1.97 |
| Systemic corticosteroids | 21 (1.0) | 107 (1.1) | 0.98 | 0.61–1.57 | 1.01 | 0.64–1.63 |
| Immunosuppressants | 3 (0.1) | 13 (0.1) | 1.15 | 0.33–4.05 | 1.28 | 0.36–4.60 |
| Anti-inflammatory and -rheumatic products | 141 (6.9) | 761 (7.5) | 0.92 | 0.76–1.11 | 0.92 | 0.76–1.11 |
From conditional logistic regression models without adjustment
From conditional logistic regression models that included maternal age (categorized), parity and smoking status
The results remained unaltered in an unmatched analysis stratified by birth year and sex (adjusted OR for any AI disease 0.70, 95% CI 0.43–1.15), except for a maternal connective tissue disease showing a lower risk for childhood cancer (OR 0.41, 95% CI 0.18–0.94) (Appendix A1). No change in the results was seen in a sensitivity analysis with a further adjustment for the birthweight (data not shown).
Any maternal medication used to treat the AI disease purchased up to 3 months before or during the pregnancy was not associated with an increased risk of childhood cancer (adjusted OR 0.95, 95% CI 0.80–1.14). The results by class of drugs were similar: maternal IBD medication (adjusted OR 1.08, 95% CI 0.59–1.97), systemic corticosteroids (adjusted OR 1.01, 95% CI 0.64–1.63), immunosuppressants (adjusted OR 1.28 with 95% CI 0.36–4.60) or anti-inflammatory and -rheumatic drugs (adjusted OR 0.92, 95% CI 0.76–1.11) compared with no medication (Table 2). In a sensitivity analysis for any maternal autoimmune medication excluding the anti-inflammatory and -rheumatic products, the adjusted OR was 1.06 (95% CI 0.72–1.56, data not shown).
Our analyses by cancer subtype (Table 3) also generally showed results compatible with no association between a maternal AI disease and childhood cancer in the offspring. We found no association with ALL (adjusted OR for maternal AI disease 0.72, 95% CI 0.30–1.71, for medication OR 0.93, 95 % CI 0.66–1.32) nor lymphomas (adjusted OR for AI disease medication 0.80, 95% CI 0.09–6.80). Yet, an increased, but non-significant, OR for lymphomas after AI disease medication was observed (adjusted OR 1.32, 95% CI 0.70–2.48).
Table 3.
The odds ratios (OR) and 95% confidence intervals (CI) calculated, using conditional logistic regression modeling, as the ratio of the proportion of children exposed to maternal autoimmune disease and its medication in offspring with childhood cancer, leukemia and other subtypes and the proportion of exposure to maternal autoimmune disease and its medication in healthy offspring (controls), 1996–2014. Both crude and adjusted ORs are presented.
| Cancer subtypes | N cases (%) | Crude OR* | 95% CI | OR ** | 95% CI |
|---|---|---|---|---|---|
| Any maternal autoimmune disease | |||||
| No autoimmune disease | 1.00 | --- | 1.00 | --- | |
| All leukemias | 6 (0.2) | 0.69 | 0.29–1.63 | 0.72 | 0.30–1.71 |
| Acute lymphoblastic leukemia | 5 (0.2) | 0.78 | 0.30–2.01 | 0.84 | 0.32–2.19 |
| Lymphomas | 1 (0.04) | 0.71 | 0.09–5.81 | 0.80 | 0.09–6.80 |
| CNS tumors | 8 (0.4) | 1.29 | 0.59–2.81 | 1.44 | 0.65–3.19 |
| Other cancers*** | 4(0.2) | 0.37 | 0.13–1.04 | 0.41 | 0.15–1.13 |
| Any maternal autoimmune disease medication | |||||
| No AI medication purchases | 1.00 | --- | 1.00 | --- | |
| All leukemias | 55 (2.7) | 0.88 | 0.65–1.19 | 0.85 | 0.63–1.16 |
| Acute lymphoblastic leukemia | 45 (2.2) | 0.97 | 0.69–1.35 | 0.93 | 0.66–1.32 |
| Lymphomas | 14 (0.7) | 1.29 | 0.70–2.38 | 1.32 | 0.70–2.48 |
| CNS tumors | 40 (2.0) | 1.01 | 0.71–1.44 | 1.01 | 0.70–1.45 |
| Other cancers*** | 60 (3.0) | 0.91 | 0.69–1.22 | 0.94 | 0.71–1.26 |
From conditional logistic regression models without adjustment
From conditional logistic regression models that included maternal age (categorized), parity and smoking status
Other cancers including non-CNS and all cancers not categorized to other subgroups
In our analyses by trimester, the adjusted OR was 0.89 (95% CI 0.73–1.09) for cancer risk associated with AI disease medication before and/or during the 1st trimester being, and 1.32 (95% CI 0.74–2.44) for the 2nd and/or 3rd trimesters (Appendix B1). The same pattern was also seen in our subgroup analyses on the use of corticosteroids or anti-inflammatory and -rheumatic medication (Appendix B2 and B3).
Comment
In our study, with detailed and accurate exposure information on the autoimmune disease diagnoses and medication, there was little evidence of an increased risk for childhood cancer among the offspring of women with an IBD or connective tissue disease during pregnancy compared with mothers with no AI disease. These results are in line with previous publications.9,10,13
Furthermore, maternal connective tissue diseases, including rheumatoid arthritis, however, appeared to be associated with a lower risk of childhood cancer as shown in our stratified analysis. A lower risk for childhood ALL has been demonstrated only for rheumatoid arthritis in one previous study with self-reported data,10 but the underlying biology remains unclear.
Our findings were not consistent with an increased risk of childhood ALL or lymphomas following a maternal AI disease exposure as previously postulated by some, 3,23but not all studies.9,10,12,14,15,24 The impact on the risk for cancers other than ALL or lymphomas also remained inconclusive possibly due to the varying definitions of an autoimmune disease, with some studies including maternal diabetes known to be associated with childhood cancer.25,26 This emphasizes the need for more detailed, prospectively collected data in future analyses.
The genetics and biology behind the hypothesis of maternal AI or its medication associating with the risk of childhood cancer in the offspring remain elusive, even in the presence of an established risk of cancer, particularly the lymphomas, among patients with an AI disease.5,6,12,27 The cases were under the age of 20 years in our study, and an increase in cancer risk later in life cannot be ruled out.
Maternal AI disease, and the resulting glucocorticoid stress, appeared not a risk factor for childhood cancer in our study.28 AI disease medication can partially cross the placenta and is associated with an elevated risk of malformations. Despite this, it does not seem to be associated with childhood cancer in the offspring. 7,29 Our subgroup analysis by trimester, however, suggested a potentially harmful impact rendered by the AI disease medication later in pregnancy, for corticosteroids as well as anti-inflammatory and -rheumatic drugs. We, however, lacked data on steroids administered antenatally at the hospital, shown previously to be associated with an increased childhood cancer risk.30
Research implications
The impact of AI disease medication on the cancer risk of the offspring in different trimesters warrants further investigation also including therapy given in the hospital (antenatal steroids) and the full spectrum of maternal autoimmune disease entities including, e.g., thyroid diseases.
Strengths and limitations
The inclusion of detailed and comprehensive data on maternal AI disease medication is a strength of our study. Additional strengths include the population-based and nationwide structure of Finland’s registry data with detailed definitions of the exposure and outcome.
We found the use of relevant medications among pregnant mothers to be eight times more prevalent than the actual diagnosis of an AI disease, likely due to other indications for some of the medications, especially analgesics. Some of the medication in this group M02 can also be bought without prescription in Finland, most likely resulting in the capture of drug utilization in this group being incomplete. Furthermore, our data is solely based on drug purchase information and does not include information on dosage. We were also limited by the small numbers especially for the cancer subtypes also effecting the evaluation of medications by trimester. Yet, our study provides additional data on maternal AI disease medication, even by trimester, and childhood cancer risk.
Conclusions
In our population- and registry-based, nationwide dataset, we did not find the risk of childhood cancer, or its key subtypes, to be increased following exposure to a maternal autoimmune disease and/or its medication. Whether the risk is impacted by the status of the fetal development (i.e. trimester), remains to be delineated.
3). AJOG at a Glance.
-
Why was this study conducted?
This study was conducted to determine whether maternal autoimmune disease and/or its medication, known to increase cancer risk in adults, are also associated with an increased childhood cancer risk in the offspring of these patients.
-
What are the key findings?
Maternal autoimmune disease, such as connective tissue disease and inflammatory bowel disease, and/or its medication, were not associated with an increased childhood cancer risk in the offspring.
-
What does this study add to what is already known?
Our findings, based on population-based registry data with definitive information on exposure, including disease type and treatment, are reassuring for the absence of an impact of maternal autoimmune disease and its medication on childhood cancer risk in the offspring.
Acknowledgments
Financial support for the research
Dr. Seppälä has received a research grant from Lastentautien tutkimussäätiö and Väreen säätiö for this study as a part of her PhD project. The funding bodies had no role in preparation or conducting the study.
Appendix
Appendix A1.
The odds ratios (OR) and 95% confidence intervals (CI) calculated, using conditional logistic regression modeling, as the ratio of the proportion of children exposed to maternal autoimmune disease and its medication in offspring with childhood cancer (cases) and the proportion of exposure to maternal autoimmune disease and its medication in healthy offspring (controls), 1996–2014. Both crude and adjusted ORs are presented. Stratified, unmatched analysis by sex and birth year.
| N cases (%) | N controls (%) | Crude OR | 95% CI | Adj. OR | 95% CI | |
|---|---|---|---|---|---|---|
| No autoimmune disease | 1.00 | --- | 1.00 | --- | ||
| Any autoimmune disease | 19 (0.9) | 134 (1.3) | 0.68 | 0.42–1.12 | 0.70 | 0.43–1.15 |
| IBD | 12 (0.6) | 61 (0.6) | 1.01 | 0.54–1.89 | 1.07 | 0.57–2.00 |
| Rheumatic disease | 7(0.3) | 74 (0.7) | 0.41 | 0.18–0.94 | 0.41 | 0.18–0.94 |
| No AI medication purchases | 1.00 | --- | 1.00 | --- | ||
| Any AI medication purchase | 169 (8.3) | 888 (8.8) | 0.95 | 0.80–1.13 | 0.96 | 0.80–1.14 |
| IBD medication | 13 (0.6) | 65 (0.6) | 1.01 | 0.56–1.84 | 1.07 | 0.59–1.95 |
| Systemic corticosteroids | 21(1.0) | 107 (1.1) | 0.91 | 0.56–1.49 | 0.93 | 0.57–1.52 |
| Immunosuppressants | 3 (0.1) | 13 (0.1) | 0.83 | 0.19–3.72 | 0.90 | 0.20–4.06 |
| Anti-inflammatory and -rheumatic products | 141 (6.9) | 761 (7.5) | 0.93 | 0.77–1.13 | 0.93 | 0.77–1.13 |
Stratified, unadjusted analysis
Stratified analysis adjusted for maternal age (categorized), parity and smoking status.
Appendix B1.
The odds ratios (OR) and 95% confidence intervals (CI) calculated, using conditional logistic regression, as the ratio of the proportion of children exposed to maternal autoimmune medication in childhood cancer (cases) and the proportion of exposure to maternal autoimmune medication in healthy controls. Multivariate analysis including purchases by trimester and the risk of childhood cancer in the offspring.
| Multivariate analysis, any AI medication and the risk of childhood cancer by trimesters | N cases (%) | N controls (%) | Crude OR | 95% CI | Adj. OR * | 95% CI |
|---|---|---|---|---|---|---|
| No AI medication purchases | 1,897 (93.5) | 9,357 (92.6) | 1.00 | ref. | 1.00 | ref. |
| AI medication purchase(s) before pregnancy and/or during the 1st trimester | 132 (6.5) | 731 (7.2) | 0.90 | 0.74–1.09 | 0.89 | 0.73–1.09 |
| AI medication purchase(s) during the 2nd and/or 3rd trimester | 22 (1.1) | 80 (7.9) | 1.36 | 0.85–2.19 | 1.39 | 0.86–2.24 |
Matched analysis, adjusted for maternal age (categorized), parity, smoking.
Appendix B2.
The odds ratios (OR) and 95% confidence intervals (CI) calculated, using conditional logistic regression, as the ratio of the proportion of children exposed to maternal systemic corticosteroid medication in childhood cancer (cases) and the proportion of exposure to maternal systemic corticosteroid medication in healthy controls. Multivariate analysis including purchases by trimester and the risk of childhood cancer in the offspring.
| Multivariate analysis, systemic corticosteroids and the risk of childhood cancer in the offspring, by trimester | N cases (%) |
N controls (%) | Crude OR | 95% CI | Adj. OR * | 95% CI |
|---|---|---|---|---|---|---|
| No systemic corticosteroid purchases | 2,012 (99.2) | 10,017 (99.1) | 1.00 | ref. | 1.00 | ref. |
| Systemic corticosteroid purchase(s) before and/or during the 1st trimester | 9 (0.4) | 58 (0.6) | 0.77 | 0.38–1.56 | 0.8 | 0.39–1.62 |
| Systemic corticosteroid purchase(s) during the 2nd and/or 3rd trimester | 8 (0.4) | 25 (0.2) | 1.61 | 0.72–3.60 | 1.63 | 0.73–3.65 |
Matched analysis, adjusted for maternal age (categorized), parity, smoking.
Appendix B3.
The odds ratios (OR) and 95% confidence intervals (CI) calculated, using conditional logistic regression, as the ratio of the proportion of children exposed to maternal anti-inflammatory and -rheumatic medication in childhood cancer (cases) and the proportion of exposure to maternal anti-inflammatory and -rheumatic medication in healthy controls. Multivariate analysis including purchases by trimester and the risk of childhood cancer in the offspring.
| Multivariate analysis, anti-inflammatory and -rheumatic products and the risk of childhood cancer in the offspring, by trimester | N cases (%) | N controls (%) | Crude OR* | 95 % CI | Adj. OR * | 95% CI |
|---|---|---|---|---|---|---|
| No purchases of anti-inflammatory and -rheumatic drugs | 1,891 (93.2) | 9,364 (92.7) | 1.00 | ref. | 1.00 | ref. |
| Purchase(s) of anti-inflammatory and -rheumatic drugs before and/or during the 1st trimester | 124 (6.1) | 685 (6.8) | 0.90 | 0.74–1.10 | 0.89 | 0.73–1.09 |
| Purchase(s) of anti-inflammatory and -rheumatic drugs during the 2nd and/or 3rd trimester | 14 (0.7) | 53 (0.5) | 1.31 | 0.73–2.36 | 1.34 | 0.74–2.44 |
Matched analysis, adjusted for maternal age (categorized), parity, smoking.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Permanent work address Stenbäckinkatu 9, PL 347, 00029 HUS, Finland
Paper presentation information
The preliminary results of this paper have been presented in e-poster format at the International Society of Pediatric Oncology (SIOP 2020) arranged virtually on October 16–19, 2020.
1) Condensation
Maternal autoimmune disease, or medication used to treat it, do not appear to be associated with childhood cancer in the offspring.
Contributor Information
Laura K SEPPÄLÄ, Helsinki, Finland, University of Helsinki, Children’s hospital.
Laura-Maria MADANAT-HARJUOJA, Helsinki, Finland; Finnish Cancer Registry; University of Helsinki, Children’s hospital.
Rebecca TROISI, Transdivisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute.
Joshua N SAMPSON, Biostatistics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute.
Maarit K LEINONEN, Helsinki, Finland; Finnish Institute of Health and Welfare, Information Services Department, Unit of Data and Analytics, Helsinki, Finland.
Kim VETTENRANTA, Helsinki, Finland, University of Helsinki, Children’s Hospital, University of Helsinki, Helsinki, Finland.
References
- 1.Leinonen M, Martikainen V, Ellfolk M, et al. Raskausajan Lääkkeiden Käyttö Ja Syntyneiden Lasten Terveys 1996–2016 [Maternal Medication Use during Pregnancy and Children’s Health 1996–2016]. Helsinki, Finland; 2020. [Google Scholar]
- 2.Lichtenstein P, Holm NV., Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer: Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000. 10.1056/NEJM200007133430201 [DOI] [PubMed]
- 3.Linabery AM, Erhardt EB, Fonstad RK, et al. Infectious, autoimmune and allergic diseases and risk of Hodgkin lymphoma in children and adolescents: A Children’s Oncology Group study. Int J Cancer. 2014. 10.1002/ijc.28785 [DOI] [PMC free article] [PubMed]
- 4.Baecklund E, Smedby KE, Sutton LA, Askling J, Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders - What are the driving forcesα. Semin Cancer Biol. 2014. 10.1016/j.semcancer.2013.12.001 [DOI] [PubMed]
- 5.Yadlapati S, Efthimiou P. Autoimmune/Inflammatory Arthritis Associated Lymphomas: Who Is at Risk? Biomed Res Int. 2016. 10.1155/2016/8631061 [DOI] [PMC free article] [PubMed]
- 6.Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: A review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev. 2006. 10.1158/1055-9965.EPI-06-0300 [DOI] [PubMed]
- 7.Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNFα drugs for the treatment of inflammatory bowel disease: Results from the multicenter European TEDDY study. Am J Gastroenterol. 2018. 10.1038/ajg.2017.501 [DOI] [PubMed]
- 8.Hellgren K, Smedby KE, Backlin C, et al. Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: A cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheumatol. 2014. 10.1002/art.38339 [DOI] [PubMed]
- 9.Wen WQ, Shu XO, Sellers T, Bhatia S, Lampkin B, Robison LL. Family history of cancer and autoimmune disease and risk of leukemia in infancy: A report from the Children’s Cancer Group (United States and Canada). Cancer Causes Control. 1998. 10.1023/A:1008830210605 [DOI] [PubMed]
- 10.Zierhut H, Linet MS, Robison LL, Severson RK, Spector L. Family history of cancer and non-malignant diseases and risk of childhood acute lymphoblastic leukemia: A Children’s Oncology Group Study. Cancer Epidemiol. 2012. 10.1016/j.canep.2011.06.004 [DOI] [PMC free article] [PubMed]
- 11.Perillat-Menegaux F, Clavel J, Auclerc MF, et al. Family history of autoimmune thyroid disease and childhood acute leukemia. Cancer Epidemiol Biomarkers Prev. 2003. [PubMed]
- 12.Ekström K, Hjalgrim H, Brandt L, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48(4):963–970. 10.1002/art.10939 [DOI] [PubMed] [Google Scholar]
- 13.Westbom L, Åberg A, Källén B. Childhood malignancy and maternal diabetes or other autoimmune disease during pregnancy. Br J Cancer. 2002. 10.1038/sj.bjc.6600192 [DOI] [PMC free article] [PubMed]
- 14.Mellemkjaer L, Pfeiffer RM, Engels EA, et al. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin’s lymphoma. Arthritis Rheum. 2008. 10.1002/art.23267 [DOI] [PubMed]
- 15.Mellemkjær L, Alexander F, Olsen JH. Cancer among children of parents with autoimmune diseases. Br J Cancer. 2000. 10.1054/bjoc.1999.1104 [DOI] [PMC free article] [PubMed]
- 16.Leinonen MK, Miettinen J, Heikkinen S, Pitkäniemi J, Malila N. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017. 10.1016/j.ejca.2017.02.017 [DOI] [PubMed]
- 17.Jokela M, Leinonen MK, Malila N, Taskinen M, Madanat-Harjuoja LM. Completeness of pediatric cancer registration in the Finnish Cancer Registry. Acta Oncol (Madr). July 2019:1–4. 10.1080/0284186X.2019.1638522 [DOI] [PubMed]
- 18.Pihkala J, Hakala T, Voutilainen P, Raivio K. [Characteristic of recent fetal growth curves in Finland]. Duodecim. 1989;105(18):1540–1546. [PubMed] [Google Scholar]
- 19.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005. 10.1002/cncr.20910 [DOI] [PubMed]
- 20.Spector LG, Puumala SE, Carozza SE, et al. Cancer risk among children with very low birth weights. Pediatrics. 2009. 10.1542/peds.2008-3069 [DOI] [PMC free article] [PubMed]
- 21.Paltiel O, Tikellis G, Linet M, et al. Birthweight and childhood cancer: Preliminary findings from the international childhood cancer cohort consortium (I4C). Paediatr Perinat Epidemiol. 2015. 10.1111/ppe.12193 [DOI] [PMC free article] [PubMed]
- 22.Mazzucchelli I, Decembrino L, Garofoli F, et al. Maternal and neonatal outcomes in pregnant women with autoimmune diseases in Pavia, Italy. BMC Pediatr. 2015;15:217. 10.1186/s12887-015-0532-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro-Jiménez MÁ, Cortés-Sánchez CE, Rueda-Arenas E, Tibaduiza-Buitrago LA. Acute lymphoblastic leukemia in a 2-year-old girl whose mother was previously diagnosed with antiphospholipid syndrome: A case report Case Reports. BMC Res Notes. 2015;8(1):26–28. 10.1186/s13104-015-1104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perillat-Menegaux F, Clavel J, Auclerc MF, et al. Family history of autoimmune thyroid disease and childhood acute leukemia. Cancer Epidemiol Biomarkers Prev. 2003. [PubMed]
- 25.Seppälä LK, Vettenranta K, Pitkäniemi J, Hirvonen E, Leinonen MK, Madanat-Harjuoja L-M. Maternal diabetes and risk of childhood cancer in the offspring. Int J Cancer. 2020;147(3). 10.1002/ijc.32757 [DOI] [PubMed] [Google Scholar]
- 26.Søegaard SH, Rostgaard K, Kamper-Jørgensen M, Schmiegelow K, Hjalgrim H. Maternal diabetes and risk of childhood acute lymphoblastic leukaemia in the offspring. Br J Cancer. 2018. 10.1038/bjc.2017.351 [DOI] [PMC free article] [PubMed]
- 27.Hellgren K, Baecklund E, Backlin C, Sundstrom C, Smedby KE, Askling J. Rheumatoid Arthritis and Risk of Malignant Lymphoma: Is the Risk Still Increased? Arthritis Rheumatol. 2017. 10.1002/art.40017 [DOI] [PubMed]
- 28.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011. 10.1016/j.yhbeh.2010.06.007 [DOI] [PubMed]
- 29.Skorpen CG, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. 10.1136/annrheumdis-2015-208840 [DOI] [PubMed] [Google Scholar]
- 30.Seppälä LK, Vettenranta K, Leinonen MK, Tommiska V. Preterm birth, neonatal therapies and the risk of childhood cancer. Int J Cancer. October 2020. 10.1002/ijc.33376 [DOI] [PubMed]


