Purpose of review
Malnutrition is frequent in patients with acute kidney injury. Nutrient clearance during renal replacement therapy (RRT) potentially contributes to this complication. Although losses of amino acid, trace elements and vitamins have been described, there is no clear guidance regarding the role of micronutrient supplementation.
Recent findings
A scoping review was conducted with the aim to review the existing literature on micronutrients status during RRT: 35 publications including data on effluent losses and blood concentrations were considered relevant and analysed. For completeness, we also included data on amino acids. Among trace elements, negative balances have been shown for copper and selenium: low blood levels seem to indicate potential deficiency. Smaller size water soluble vitamins were found in the effluent, but not larger size liposoluble vitamins. Low blood values were frequently reported for thiamine, folate and vitamin C, as well as for carnitine. All amino acids were detectable in effluent fluid. Duration of RRT was associated with decreasing blood values.
Summary
Losses of several micronutrients and amino acids associated with low blood levels represent a real risk of deficiency for vitamins B1 and C, copper and selenium: they should be monitored in prolonged RRT. Further Research is urgently required as the data are insufficient to generate strong conclusions and prescription recommendations for clinical practice.
Keywords: adsorption, carnitine, effluent losses, metabolism, micronutrient deficiency, selenium, thiamine, Vitamin C
INTRODUCTION
The increased risk of both mortality and morbidity is well recognized in hospitalized patients who develop acute kidney injury (AKI) [1]. The pathophysiological causes are complex, but one aspect that is often overlooked is the profound effect of AKI on nutritional status resulting in significant depletion of lean body mass. Indeed, low caloric and protein intake, a negative nitrogen balance and low serum albumin are all associated with increased hospital mortality [2]. To date, there is no specific treatment for AKI, instead management is aimed at correcting the resulting metabolic and volume disturbances, which may result in the use of renal replacement therapy (RRT) [3]. However, exposure to an extracorporeal circuit has potential drawbacks, not least in terms of nutritional status, wherein removal of essential dietary components may not be replenished adequately. This is particularly relevant to micronutrients, which play a central role in metabolism and maintenance of cellular function [4]. The term ‘micronutrient’ includes essential trace elements (inorganic metals and metalloids) and organic vitamins (fat-soluble and water-soluble molecule). Daily recommended intakes (DRIs) have been validated [5]. Their key functions are summarized in Table 1. However, the latter are based on the observed intakes in healthy individuals where ‘normal’ defines ranges below which a clinical deficiency state is increasingly likely, and intakes above upper tolerable limits are those where toxicity state is likely to develop [6]. Such levels are relevant at a population level but translate less well to the individual, particularly if exposed to critical illness and its management, including continuous renal replacement therapy (CRRT), prolonged intermittent therapies as well as intermittent haemodialysis (IHD). Independent of RRT modality, micronutrients may be removed through convection or diffusive techniques potentially leading to a deficiency state in at-risk individuals.
Table 1.
Key functions of the vitamins, and trace elements reported in CRRT studies
| Vitamin | Name | Molecular weight | Key functions |
| B1 | thiamine | 265.4 g/mol | Carbohydrate metabolism, ATP production |
| B2 | riboflavin | 376.36 g/mol | Essential component of 2 major coenzymes, flavin mononucleotide (FMN; also known as riboflavin-5’-phosphate) and flavin adenine dinucleotide (FAD); these coenzymes play major roles in energy production, cellular function, growth, and development, and metabolism of fats, drugs and steroids |
| B3 | Niacinamide | 123.12 g/mol | Component of the coenzyme nicotinamide adenine dinucleotide (NAD) involved in redox reaction |
| B5 | pantothenic acid | 219.23 g/mol | Required for the synthesis of CoA (coenzyme A) and the citric acid cycle |
| B6 | pyridoxine | 169.2 g/mol | Active forms: Pyridoxal 5’ phosphate (PLP) pyridoxamine 5’ phosphate (PMP). Involved in > 100 enzyme reactions, mostly related to protein metabolism; role in metabolism of one-carbon units, carbohydrates, and lipids, biosynthesis of neurotransmitters and in maintaining normal levels of homocysteine; gluconeogenesis and glycogenolysis, immune function (lymphocytes, interleukin-2), haemoglobin |
| B8 | biotin | 244.3 g/mol | coenzyme for five carboxylase enzymes which are involved in the digestion of carbohydrates, synthesis of fatty acids, and gluconeogenesis |
| B9 | folic acid / Folate | 441.4 g/mol | Contributes to iron metabolism, B9 works with B6 and B12 to control levels of the amino acid homocysteine, DNA and RNA synthesis |
| B12 | cobalamin | 1355.36 g/mol | Cofactor in DNA synthesis and in both fatty acid and amino acid metabolism; role in neurotransmitter synthesis and myelin synthesis |
| C | ascorbic acid | 176.12 g/mol | Water soluble antioxidant (can quench reactive oxygen and nitrogen species); cofactor of enzymes required for synthesis of collagen, carnitine, and neurotransmitters |
| Vitamin A | retinol | 286.45 g/mol | Role in gene expression, vision, reproduction, growth and immune function |
| Vitamin D | D2 calciferol D3 calcidiol | 397 g/mol 394.6 g/mol | Role in modulation of cell growth, neuromuscular and immune function, glucose metabolism, maintenance of calcium and phosphorus homeostasis, bone growth and remodelling, and reduction of inflammation |
| Vitamin E | a-tocopherol | 430.7 g/mol | Chain-breaking antioxidant (prevents propagation of lipid peroxidation) |
| Trace element | |||
| Cr | Chromium | Insulin sensibility, glucose metabolism, regulation of Cholesterol HDL and triglycerides | |
| Cu | Copper | Hemoglobin synthesis, collagen and elastin synthesis, conversion of dopamine to noradrenaline, structure of Cu-Zn superoxide dismutase, melanine synthesis | |
| Fe | Iron | Hemoglobin and myoglobin synthesis/structure, O2 transport, Cytochrome function, catalases, thyroid function, innate and adaptive immunity | |
| Se | Selenium | Antioxidant via selenoproteins (glutathione peroxidase family and other selenoproteins), immunity, cognitive function, thyroid function, liver detoxification | |
| Mn | Manganese | Mitochondrial superoxide dismutase, pyruvate carboxylase, | |
| Rb | Rubidium | Proper biological function unknown, but is said to stimulate the metabolism | |
| Va | Vanadium | Regulates the intracellular signalling via second messengers. Adenylate cyclase acts as catalyst in the formation of cAMP (cyclic adenosine monophosphate) from ATP (adenosine triphosphate) | |
| Zn | Zinc | All cell replications (DNA, RNA), immunity, Redox balance, cutaneous integrity, vision, gustative function, cerebral function, bone metabolism | |
(Note: g/mol =Da).
Box 1.
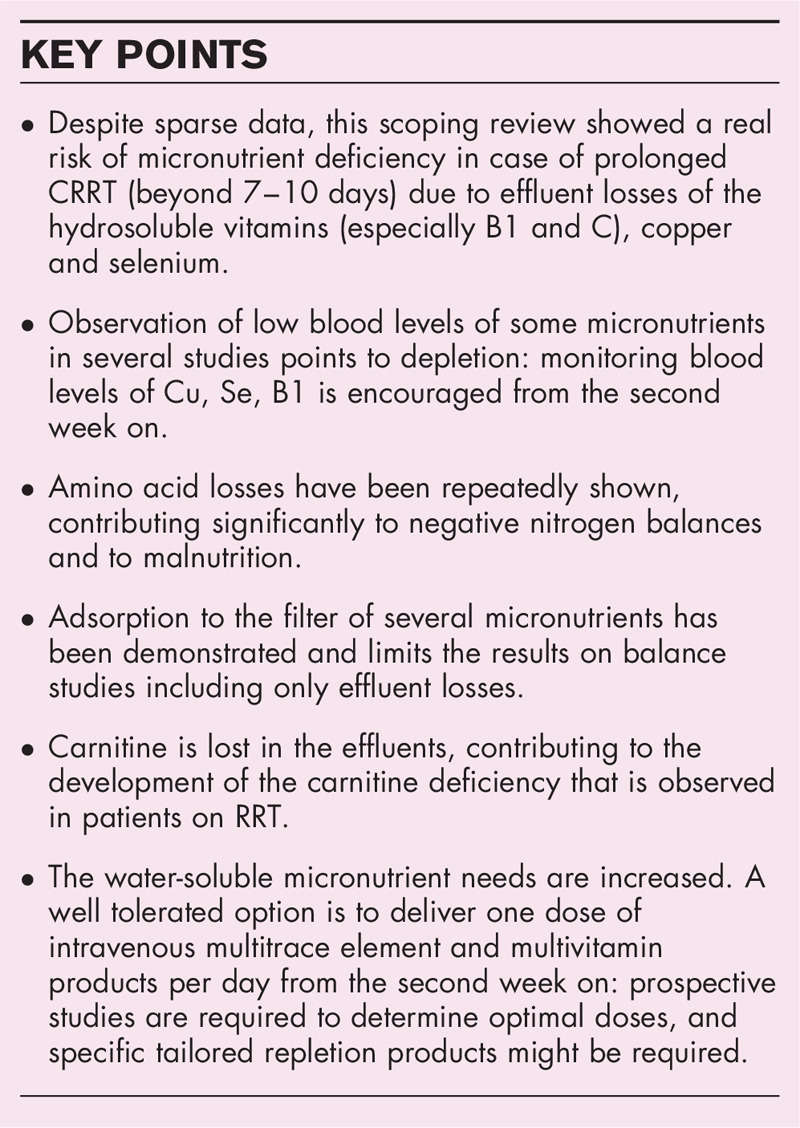
no caption available
There appears to be a relative paucity of granular data regarding micronutrient flux in continuous therapies. To clarify the current state of the art as well as identifying knowledge gaps, the following scoping review was reviewed. We examined the available evidence and explored the relationship between CRRT use and micronutrient concentrations with particular focus on the effects of therapy and supplementation [7]. Literature searches were conducted in MEDLINE, PubMed, Scopus and CINAHL (mesh words see Table 1 appendix), addressing six specific questions (Table 2).
Table 2.
Questions addressed in the scoping review
| Question | Answer | Remark | |
| 1 | Which micronutrients are lost in the effluent and at risk of depletion during CRRT? | Copper and selenium in quantities that may threaten the status; other trace elements in quantities insufficient to cause deficiency; smaller size hydrosoluble vitamins thiamine, folate, vitamin C; concomitant low blood levels have been observed | Others: either in balance or positive (e.g. zinc), due to contamination of replacement solutions |
| 2 | What is the impact of CRRT on lipid soluble vitamins? | The four lipid soluble vitamins A, D3, E and K have not been detected in effluent fluid. | Adhesion to filter is a likely hypothesis |
| 3 | Are micronutrients lost through adhesion to the filter? | No data available | Research required as this may contribute to negative balances |
| 4 | Are all CRRT modalities similar regarding the impact on micronutrients? | No strong influence | Prolonged RRT increases the losses |
| 5 | What is the impact of CRRT on carnitine and muscle loss? | Carnitine deficiency is frequent and is diagnosed in blood; contributes to sarcopenia | Carnitine not found in effluent |
| 6 | Should patients on CRRT receive routine micronutrient supplements? | Based on effluent losses, low blood levels, and adsorption, the requirements are increased particularly for the B and C vitamins, Carnitine, and for Cu and Zn. The administration of double or triple doses of parenteral nutrition multimicronutrient products does not address the specific needs | No data in favour of better outcome; adapted products likely required Monitoring in prolonged RRT required |
CRRT, continuous renal replacement therapy; RRT, renal replacement therapy.
Which micronutrients are lost in the effluent and at risk of depletion during continuous renal replacement therapy?
To address this question, observational studies, and case reports, were considered. In total, 86 publications were retrieved, of which 35 reported on effluent losses or blood concentrations during CRRT or peritoneal dialysis (n = 4), and were assessed as potentially relevant, one being only available as abstract. The quality of publications was assessed according to the Scottish Intercollegiate Guidelines Network (SIGN) grading criteria [8].
Our review showed that micronutrient losses were rarely assessed in isolation. Several studies investigated amino acids simultaneously, resulting in the following distribution of available effluent studies (Table 3):
Table 3.
Nutrients and micronutrients lost in the effluent
| Reference | N | Amino acids (AA) | Vitamins | Trace elements | Other (carnitine ..) | Mode | SIGN category |
| Ostermann et al.[9▪▪] | 31 | all AA were lost | B1, B6, B9, B12, C and D3 but only B9 and C found in effluent | Fe, Se, Cu, Zn lost | CRRT | 2 | |
| Lumlertgul et al.[19▪▪] | 33 | all AA were lost | B9 losses, but B1, B6, B12, D2, D3, and K undetected | Cu, Fe, Se, Zn | Carnitine not found | CRRT | 2 |
| Oh et al.[20▪▪] | 72 | AA loss greatest in CVVH, followed by SLEDf and IHD (e.g. 18.69 ± 3.04 g, 8.21 ± 4.07 g and 5.13 ± 3.1 g per session, respectively), | B1, B2, B3, B6, B9, B12 analysed but undetectable In vitro model shows adsorption | Zn, Fe, Mn, Cr, Va, Mo, Rb, Li analysed. Negligible losses during IHD and CVVH. Cu and Zn loss variable | – | IHD (n = 33) CVVH (n = 24) SLEDF (n = 15) | 2 |
| Stapel et al.[21▪] | 10 | AA loss in the filter = 10.4 g/day, median AA loss by UF = 13.4 g/day | – | – | – | CVVH | 2 |
| Broman et al.[10] | 31 | – | marked loss of Se, Cu and Rb; uptake of Cr, Mn, and Zn | – | CRRT | 2 | |
| Ben-Hamouda et al. 2017 [13] | 1 burns | – | – | Se and Cu loss | – | CRRT | 3 |
| Berger et al. 2004 [12] | 11 | – | B1 lost | Cu, Se, Zn amounts close to PN doses | – | CRRT | 2 |
| Chua et al. Blood Pur 2012;33 : 292 | 7 | AA loss 4.2 g/day (IQR 1.4–12.3); high GLN loss | – | – | – | extended daily diafiltration | 2 |
| Datzmann et al.[14] | 40 | Loss of B9 and B12 detected, but not vitamin A and E | Zn detected but no Cu | – | CRRT | 2 | |
| Datzmann et al.[15] | 40 | – | No: B9, B12 | No significant Zn loss | – | CRRT different doses | 2 |
| Davenport &Roberts CCM, 1989;17 : 1010 | 8 | positive correlation between serum AA value and UF loss (r = 0.84, P < 0.001) | – | – | – | High flux CRRT | 2 |
| Davies et al. CCM 1991;19 : 1510 | 6 | Detailed clearance GLU, 3MH, TRY, TYR | – | – | – | CRRT | 2 |
| Klein et al. JPEN 2002 ;26 :77 | 6 | – | – | Zn 7.7 ± 2.8 μmol/day) (no supplement needed) | – | CRRT | 3 |
| Klein et al. JPEN 2008 ;32 :129 | 6 | – | – | Losses higher in CVVHD than in CVVH: Bo 1.50, and 2.13 mg/day, Mn 62.7 and 218.9, Se 51.7 and 25 μg/day, Ni 52 and 35 μg/day, Si 12 and 16 mg/day | – | CVVH // CVVHD | 2 |
| Kuttnig et al. Child Neph Uro 1991;11 : 74 | 11 | Mean AA loss in UF = 0.159 ± 0.008 (SEM) g/kg/day | – | – | – | CAVH | 2 |
| Maxvold et al. CCM 2000;28 : 1161 | 6 (–>12) | AA clearances greater in CVVH than CWHD, except glutamic acid. Total loss: 12.50 ± 1.29 g/day/1.73 m2 | – | – | – | CVVH –CVVHD crossover | 2 |
| Nakamura et al. JRNut 2004;14 : 214 | In vitro | – | – | Cu, Cr, Mn, Se and Zn detected in UF; T | – | 2 polysulfone haemodiafilters | 2 |
| Oh et al. COCNM 2015;18 : 593 | review | Variation with RR type | – | – | – | CRRT | 4 |
| Pasko et al. Ped Nephro 2009;24 : 807 | 5 | – | – | median transmembrane clearance: Cr, Cu, Mn, Se and Zn; 0 ml, 0.59 ml, 2.48 ml, 1.22 ml, and 1.90 ml/1.73 m2, respectively | – | CVVHDF | 2 |
| Schmidt et al. Nephron 2014;126 : 62 | 10 | 19 AA: total loss 10.5 g/10 h of treatment; higher losses of glutamine than other AA | – | – | – | RRT | 2 |
| Scheinkestel et al.; Nutrition 2003; 19:733 | 11 | Increasing doses of AA administered by parenteral nutrition (1.5--2.5 g/kg): AA losses dependent on blood concentration. Overall, 17% (range, 13--24%) of infused AAs and 4% of infused glucose were lost in dialysate | – | – | – | CRRT | 2 |
| Story et al., 2004 [16] | 8 | – | Vitamin C detected, Undetectable Vit E | Cr and Cu detected | – | CVVH | 2 |
| Umber et al. Clin Neph 2014;81:93 abstract | ? | AA loss 15.7 (23.4 ± 19.2) g/treatment, like CRRT | – | – | – | SLEDf | 3 |
| Hynote et al. JPEN 1995;19 : 15 | 5 | Mean AA losses of 5.2 ± 0.6 g - conventional dialysis, 60% of the total infused, and 7.3 ± 1.8 g per high-flux dialysis, or 80% of infusion | – | – | – | HD (conventional / high flux) | 3 |
| Freudiger et al. Nephro 1990;11 : 129 | 10 | Fractional losses of AAs given by PN averaged 8 ± 1% | – | – | CVVHDF | 3 | |
| Berlyne et al. Lancet 1967;7504 : 1339 | 8 | AA loss (4.96 g/27.2 l exchange) | – | – | – | Peritoneal dialysis | 3 |
| Rector et al. Liver 1984;4 : 341 | 1 - Wilson | – | Cu: 14 mg /day | – | Peritoneal dialysis | 4 | |
| Cole BJM, 1978;6104 : 50–1 letter | 1 - Wilson | – | – | Cu: 0.633 μmol/l to 5–98 μmol/l | – | Peritoneal dialysis | 4 |
| Hamlyn et al. BMJ 1977;6088 : 660 | 2 - Wilson | – | – | Cu: 36 μmol/day (2287 μg/day) | Peritoneal dialysis | 4 | |
| Böhmer et al., 1978 [36] | 9 | Carnitine | IHD | 3 |
AA, amino acid; CAVH, continuous arterio-venous haemofiltration; CRRT, continuous renal replacement therapy; CVVH, continuous veno-venous haemofiltration; CVVHDF, continuous veno-venous haemodiafiltration; IHD, intermittent haemodialysis; RRT, renal replacement therapy; SLEDf, slow extended dialfiltration.
-
(1)
15 determined losses of selected trace elements
-
(2)
seven determined selected vitamins
-
(3)
one study measured carnitine
-
(4)
15 determined specific amino acids
Eleven studies reported blood concentrations, of which four including blood levels only:
-
(1)
eight determined trace elements
-
(2)
six determined vitamins
-
(3)
five studies measured carnitine
-
(4)
four determined amino acids
Furthermore, there was wide variation in the type of RRT assessed, including modality, dose and duration. Also, some studies reported effluent concentrations without plasma concentrations.
Trace elements
Fifteen studies measured trace elements during RRT (Table 3). Copper, selenium and zinc losses were the most frequently reported. Other less frequently measured elements were boron, chromium, iron, manganese, nickel, rubidium and silicon, probably reflecting the analytical methods available. The most recent study showed significant losses of Cu, Fe, Se and Zn [9▪▪]. Broman et al.[10] analysed eight elements (Cr, Cu, Co, Mn, Mo, Rb, Se, Zn), and showed that the balances were quite different: while they were negative for Cu, Se and Rb, they were positive for Cr, Mn and Zn. This was accompanied by significantly reduced plasma levels of selenium and rubidium, whilst the concentrations of chromium, cobalt and molybdenum were significantly increased. Klein et al.[11] analysed Bo, Mn, Ni, Se and Si and showed that there was daily variation in effluent detection (7–84% depending on the element, worst for Se). Importantly, these authors showed that all prefilter dialysate samples were contaminated with boron, manganese, nickel, and silicon. Berger et al.[12] conducted a balance study (i.e. measuring difference between all inputs and outputs) for Cu, Se and Zn, and showed that contamination of replacement solutions with Zn resulted in modestly positive balances, while Cu and Se balances were negative. The same group reported the depletion effect of CRRT on Cu and Se during a case of prolonged CRRT confirming, resulting in life-threatening complications [13]. In their first study, Datzmann et al.[14] detected zinc, but no copper, but could not find significant Zn losses in their second study [15]. Pasko et al. detected Cu, Se, Mn Se and Zn in effluent of children undergoing RRT (Table 1). Copper and chromium were detected by Story et al.[16] (Table 1). Three papers reported on PD in the context of Wilsons disease (Table 1) and showed significant copper losses in the dialysate. Nakamura et al.[17] conducted an in-vitro study investigating the in-vitro clearance of trace elements: the mean sieving coefficients of two different haemodiafilters were similar for each trace element: copper, chromium, manganese, selenium and zinc were all detected in the ultrafiltrate, and the authors suggested that the losses of selenium were likely to exceed standard clinical intake [17].
Among the eight studies which measured changes in blood trace elements levels (Table 4), most reported decreased levels of copper, selenium and zinc over time, one also showed decreased rubidium (but function unknown). However, interpretation of blood levels of Se and Zn requires simultaneous determination of the intensity of inflammation: a simple marker is C-reactive protein (CRP) [18], which is generally not provided. Low levels of copper, which is linked to ceruloplasmin, a positive acute phase reactant, always reflect deficiency.
Table 4.
Studies measuring blood levels of amino acids and micronutrients
| Reference | Number of patients | Amino acids | Vitamins | Trace elements | Other (carnitine) | Mode | |
| Ostermann et al.[9▪▪] | 31 | tryptophan, taurine, histidine and hydroxyproline below reference range | vitamin D3 and C below reference range | Zn, Fe and Se below reference range throughout the 6-day period | Lower carnitine | CRRT | 2 |
| Lumlertgul et al.[19▪▪] | 33 | plasma concentrations of citrulline and glutamic acid at 24 hrs and significantly lower plasma glutamic acid (74.4 versus 98.2 μmol/l) at day 6 compared to non-CRRT | Low carnitine | CRRT | 2 | ||
| Schneider et al.[54] | 30 | Low alanine, glutamine and valine | Low vitamin A and C | – | – | CRRT | 2 |
| Oh et al.[19▪▪] | 72 | Decline of all plasma amino acids during sessions whatever the modality. amino acids are lost more from convection-based RRT than from diffusion-based treatment | B1, B2, B3, B6, B9, B12 analysed | Decrease of total trace element concentration at end of RRT sessions | – | IHD (n = 33) CVVH (n = 24) SLEDF (n = 15) | 2 |
| Broman AAS, 2017 [10] | 31 | Low Se and Rb while Cr, Co and Mo increased | CRRT | 2 | |||
| Ben-Hamouda et al.[13] | 1 | – | – | Very low Cu and Se | Carnitine deficiency | CRRT | 3 |
| Berger et al.[12] | 11 | Low B1 | Cu, Se and Zn low in plasma | CRRT | 2 | ||
| Kamel et al., 2018 [56] | 75 | 16% had below normal whole blood B1 concentrations, and 67% had below normal serum pyridoxine; ascorbic acid and folate deficiencies in 87 and 33%, respectively | 38% had Zn deficiency and 60% had Cu deficiency | CRRT | 2 | ||
| Krimetapak et al., 2016 [55] | 70 | Unchanged serum Zn, Se and Cu levels | CRRT | 2 | |||
| Sgambat and Moudgil [30▪] | 42 | Within 1 week, 64.5% (P = 0.03) and 70% (P = 0.03) were total carnitine and free carnitine deficient | CRRT | ||||
| Story et al., 2004 [16] | 8 | lower median blood Vit C and vitamin E levels | Low Se and Zn | CRRT | 3 | ||
| Fah et al. submitteda | 10 | – | B6 low in 18/26 Thiamine vit C Folate | Copper low in 35/49, Low Zn Se | 14/54 low carnitine | CRRT | 3 |
| Böhmer et al., 1978 [36] | 9 | – | – | – | Reduced muscle and plasma carnitine in all subjects postdialysis. | IHD | 3 |
| Cromphout et al. VUB Thesis 2020 [28] | A, B1, B6, B9, B12, C, D and E determined at three measuring points A and D low, B1, B6, B12 above normal, B9 and E within range | Cr, Cu, Mn, Se, Zn determined at three time points. Cr, Cu, Mn within range, Se and Zn low | CRRT | 2 |
Several references are only cited directly in this table.
Fah M & Wischmeyer PE. CRRT leads to severe micronutrient deficiencies in critically ill patients: A retrospective study. A4017, International Anesth. Res. Soc., 2020 https://archive.aievolution.com/2020/ars2001/index.cfm?do=abs.viewAbs&abs=4670.
In summary, the trace element data show that copper and selenium are at high risk of negative balances. Others are either in balance or even positive (e.g. zinc), due to the contamination of the replacement solutions. The reporting of low copper and selenium in blood seems to confirm that the losses result in deficiency.
Vitamins
Seven studies reported vitamin losses in the effluent. The most recent showed losses of vitamin C [9▪▪,19▪▪] but B1, B6, B12, D2, D3 could not be detected [19▪▪]. Searching for vitamins A, B9, B12 and E, Datzmann et al. could not detect any in effluent fluid [14,15]. Similarly, Story et al. found that vitamin C was detectable in the effluent, but vitamin E was not (Table 1). Berger et al. found significant B1 losses [12] amounting to two times the usual parenteral nutrition doses. Importantly, measurement of vitamins depends on the analytical methods, which may account for some for some differences between studies. Molecular size, weight and solubility are other important aspects (Table 2) and explain why some nutrients are less likely to be found in the effluents, and the four largest being liposoluble. In the studies reported in Table 1, none could detect any vitamin larger than thiamine in size (i.e. >265 Da). Importantly, the impact of the lipophilic or hydrophobic properties has not been studied.
Seven studies looked at blood levels of vitamins after a variable duration of CRRT (Table 2). Fah et al. reported (as abstract only) that 89% of the patients on CRRT presented at different times with reduced levels of several micronutrients (B1, B6, Folate), compatible with deficiency (see note Table 4). Abnormal low values being associated with higher mortality. Ostermann et al.[9▪▪] reported low values of folate and vitamin D compared to non-CRRT patients. Schneider et al. demonstrated a significant decrease of vitamin C during an 8-day period, while vitamin A remained stable and low. Story et al. had reported low levels of vitamin C and E (Table 2) and Berger et al. confirmed low thiamine levels [12].
In summary, vitamin losses in the effluent have been described for the smaller size water soluble vitamins, but not for larger size liposoluble ones. Low blood values have been frequently reported mainly for thiamine, folate and vitamin C. As for trace elements, CRP values need to be reported to interpret vitamin concentrations [18], but considering the consistency of losses and low levels, it is likely that water soluble vitamins are at risk of being deficient.
Amino acids
Fifteen studies reported significant amino acid losses. As the molecular weight of amino acids varies between 74 and 204 g/mol, their small size makes them highly ultrafiltrable, which contributes to negative nitrogen balances. The most recent studies [9▪▪,20▪▪,21▪] confirmed significant losses of all measured amino acids, ranging from 5 to 20 g/day, most results pointing to the upper range. Modality of RRT plays a role. Oh et al.[20▪▪] compared different techniques and showed that the amino acid losses were largest with continuous veno-venous hemofiltration (CVVH) compared with both slow efficiency diafiltration (SLEDf) and IHD. In case of parenteral nutrition, authors have shown that about 10% of infused amino acids are lost in the effluent (Table 3). These findings support the higher nitrogen requirements during CRRT.
What is the impact of continuous renal replacement therapy on lipid-soluble vitamins?
There are very little data available on how lipid soluble vitamins A, D3, E and K behave during CRRT. In a critical care setting levels of vitamin A and E seem to be lowered, and activation of vitamin D3 is impaired in patients with AKI [22–24]. Two studies reported that they did not find vitamin A or E in effluent fluid [14], and a third study did not find vitamin D [19▪▪]. In general, vitamin A, D and E are not prone to be removed by convective and diffusion-based processes, in contrast to water soluble molecules. However, there detailed data is missing.
Are micronutrients lost by adhesion to the filter?
In addition to elimination by diffusion and convection adsorptive processes may also take place, both on the filter membrane and the tubing material itself. Adsorption to the RRT circuit and dialyzer/filter of vitamins was evoqued as a possibility by Oh et al.[25]. After only 5 min of pseudo-filtration, a marked difference in vitamin B concentration of replacement solution and effluent was observed, which could only be explained by adsorption to the circuit and/or filter [25]. This represents a net loss to the patient but does not appear in case of balance studies (no micronutrient in effluent). Lately specific adsorptive techniques have been introduced such as sorbent columns. However, adsorption of micronutrients to the filter has not been investigated, and to date, we cannot answer the question.
Are all continuous renal replacement therapy modalities similar as to impact on micronutrients?
Only three studies compared the impact of RRT modality. Maxvold et al.[26] investigated nitrogen balances in children, but no micronutrients. Oh et al.[26] reviewed the reported impact of different modalities but provided no detailed data. The same group conducted a prospective study measuring micronutrients and amino acids [20▪▪] and compared the impact of IHD with CVVH and SLEDf. They showed that total losses were affected by modality. Generally amino acid losses were highest during CVVH, followed by SLEDf and IHD (e.g. amino acid loss was 18.69 ± 3.04, 8.21 ± 4.07 and 5.13 ± 3.1 g, respectively; P < 0.001): the pattern of overall loss was largely driven by plasma concentrations, such as glutamine. B vitamins were undetectable in effluent. Losses of Cu and Zn were marked, with considerable heterogeneity between IHD and SLEDf whereas their losses were lowest during CVVH. Datzmann et al.[15] studied three different corresponding blood flow and dialysate flow rates (BF/DF: 100 ml/min, 2000 ml/h; 80 ml/min, 1500 ml/h; 120 ml/min, 2500 ml/h). They could not find any correlation between losses and dialysis flow rate for folic acid, vitamin B12 and Zn.
A study investigating high dose (incremental postdilution fluid load to 50 ml/kg/h effluent dose) versus low dose setting in CVVH, as well as the use of citrate or NaCl 0.9% predilution demonstrated significant effluent CO2 removal [27]. The effect on micronutrients was not reported but preliminary data from the same group revealed unsurprisingly that both duration of CVVH treatment and concurrent parenteral nutrition influences losses [28].
An in-vitro clearance study [17] compared the sieving coefficients for Cu, Cr, Mn, Se and Zn when using of two different haemodiafilters, and found no difference.
In summary, micronutrient losses do not seem to be strongly influenced by the type of RRT.
What is the impact of continuous renal replacement therapy on carnitine and muscle loss?
Carnitine is a 161-kDa amino acid derivate that is a conditionally essential nutrient. It is not a vitamin, but was included in this analysis due to its essential functions in energy metabolism and mitochondrial function [29], particular in muscle [30▪]. Being a small water-soluble, nonprotein bound molecule [31], it might potentially be found in the effluent. Carnitine plays a vital role in free fatty acid metabolism via transport of fatty acids from the mitochondrial intermembrane space into the mitochondrial matrix in cardiac and skeletal myocytes [32,33]. Deficiency leads to impaired oxidation of fatty acids, impaired muscle function, decreased wound healing, altered ventilatory response and abnormal immune function [32]. Carnitine deficiency has been associated with prolonged hospital stay in critically ill patients [34,35].
AKI and need for RRT are risk factors for carnitine deficiency through reduced endogenous carnitine synthesis due to loss of key cofactors (i.e. ascorbic acid, amino acid precursors) lost in dialysate [36], inadequate dietary intake [37], and possibly due to carnitine effluent losses: one study showed losses [19▪▪], while another did not [9▪▪]. Original early research in haemodialysis patients showed the loss of carnitine into the dialysate (190–2100 μmol/treatment) greatly exceeded the normal loss in urine in most patients [36]. Low blood concentrations have been reported in studies on adults [9▪▪,13] (M. Fah, T. Ohnuma, K. Haines, et al., in preparation) and in children [35,38–41] (See Tables 1 and 2 for specific studies). The proportion of patients exhibiting carnitine deficiency increased with duration of CRRT with deficiencies reported as early as 5–7 days. Data from one of our research groups (Wischmeyer, personal communication and [31]) has shown an initial association of carnitine deficiency with increased mortality in patients on CRRT versus those without carnitine deficiency. Further research is needed to explore the incidence, treatments and association with clinical outcomes.
Low serum free carnitine levels are associated with decreased exercise capacity in chronic dialysis patients [30▪]. Specific to the effect of dialysis on muscle loss, sham dialysis in healthy individuals clearly demonstrates that any extracorporeal circuit can activate protein catabolism. The extent of this protein breakdown depends on the biocompatibility of the membrane used. This promotion of protein degradation appears to persist until several hours posttermination of extra-corporal circulation [42].
In summary, effluent losses remain a likely hypothesis but adsorption to the dialysis membranes cannot be excluded. Carnitine deficiency is common and may contribute to sarcopenia in AKI.
Should patients on continuous renal replacement therapy receive routine micronutrient complements?
Nutrition and provision of micronutrients in AKI are an under-researched area. Studies testing micronutrient supplementation on outcome could not be found, but two studies did test the impact of higher doses on blood levels of respective micronutrients. Marik and Hooper tested 6 g vitamin C per day in four septic patients on RRT, and observed blood concentrations within reference ranges [43]. Cromphout et al.[28] tested multimicronutrient administration over time, but results are only available as thesis report: 10 patients were studied and the intervention consisted of PN enriched with double vitamin (Cernevit; Baxter) and triple trace element (Tracutil, BBraun) doses infused over 24 h. The blood levels of different micronutrients were within or above references ranges after a few days (A, B1, B6, B9, B12, C, D and E, Cr, Cu, Mn, Se and Zn). From this preliminary report, it might be concluded that supplementation with double and triple doses is excessive: this will orient future research. In critically ill children on CVVHDF, minimal effluent losses of Cr, Mn, Se and Zn were reported and daily standard supplementation of trace elements exceeded the observed losses [44].
Expert opinion and the data retrieved in this scoping review suggest that water-soluble vitamins should be administered routinely during CRRT, but controversy exists about the optimal dosage, timing, and duration [45–47]. Recommendations from experts [10–14,17], official societies [48] and authorities vary [22,46,49▪▪,50,51]. A recent review on nutrition therapy during CRRT in critically ill children proves a dearth of information and shares weak recommendations [52]. A review on vitamin C in RRT concluded that 2 g/day intravenous (i.v.) may be necessary to achieve normal plasma concentrations during RRT considering the intense oxidative stress observed in these patients [53]. Finally, the effect of carnitine administration in patients receiving CRRT for a prolonged period has not been formally investigated. Data are sufficient though to recommend monitoring blood levels of vitamins, Cu and Zn if RRT is prolonged beyond 1–2 weeks.
According to available literature, it could be suggested to provide vitamin E daily and vitamin K weekly in patients on CRRT, but large investigations are lacking. Vitamins A and D3 seem not to need extra provision [24]. In a recent study, D3 vitamin levels were below reference range irrespective of the treatment dose of CRRT [9▪▪,54]. A well tolerated and simple solution may be to double the daily lipid soluble vitamin dose during CRRT [24]. However, there are no data demonstrating beneficial effect of an increased supply of vitamins during CRRT on outcome of critically ill patients.
In summary, there is only an unpublished study testing prospectively the administration of micronutrient complements on blood levels, but none on outcome.
CONCLUSION
This scoping review shows that the data are insufficient to generate strong conclusions and recommendations. But absence of evidence is not evidence of absence, and some larger recent studies confirm losses [9▪▪,28,54] and adsorption of micronutrients to the circuit and filter [20▪▪,25]. Balance studies, the measurement of all inputs and outputs, are considered the best tool to determine needs, however in RRT, this method leads to an underestimation, due to adsorption. To base recommendations about supplementation only on blood levels is not warranted, as blood levels are highly dependent on the degree of inflammation [18]. Given that several small water-soluble micronutrients are lost during CRRT, and blood levels are low, it is reasonable to assume that needs are increased. Daily administration of parenteral nutrition doses of micronutrients seems therefore justified, but a global approach including delivery of ‘all’ micronutrients should be refined. It is possible that a specific micronutrient combination needs to be developed for patients receiving CRRT to correct deficiency without causing over-replacement or harm.
Further research is urgently required in the following areas: identification of optimal analytical techniques to determine effluent micronutrient concentrations and adhesion to filter; investigation of correlation between blood concentrations, tissue levels and physiological effects; exploration of association between nutrient losses and patient-centred outcomes, and prospective micronutrient supplementation trials.
Acknowledgements
The authors would like to express their gratitude to Ms. Marion Sammut (Baxter Healthcare) for support with literature search.
Financial support and sponsorship
There was no financial support to this publication.
Conflicts of interest
The authors have no conflict of interest to declare related to the present work.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.James MT, Bhatt M, Pannu N, Tonelli M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat Rev Nephrol 2020; 16:193–205. [DOI] [PubMed] [Google Scholar]
- 2.Bufarah MNB, Costa NA, Losilla Mprp, et al. Low caloric and protein intake is associated with mortality in patients with acute kidney injury. Clin Nutr ESPEN 2018; 24:66–70. [DOI] [PubMed] [Google Scholar]
- 3.Sarnowski A, Doyle JF, Forni LG. Interventions for improving outcomes in acute kidney injury. Curr Opin Nephrol Hypertens 2019; 28:567–572. [DOI] [PubMed] [Google Scholar]
- 4.Shenkin A. The key role of micronutrients. Clin Nutr 2006; 25:1–13. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation. Vitamin and mineral requirements in human nutrition. 2004; Geneva: Food and Agricultural Organization of the United Nations, 2. [Google Scholar]
- 6.Shenkin A. Micronutrients in health and disease. Postgrad Med J 2006; 82:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018; 18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(SIGN) Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developer's handbook. Revised version, vol. http://www.sign.ac.uk. Edinburgh: SIGN; 2014. [Google Scholar]
- 9▪▪.Ostermann M, Summers J, Lei K, et al. Micronutrients in critically ill patients with severe acute kidney injury: a prospective study. Sci Rep 2020; 10:1505. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study including 31 patients confirms significant effluent losses of Cu, Fe, Se and Zn, as well thiamine and folate, in amounts impacting the status in case of prolonged CRRT.
- 10.Broman M, Bryland A, Carlsson O, Group T. Trace Acute Study. Trace elements in patients on continuous renal replacement therapy. Acta Anaesthesiol Scand 2017; 61:650–659. [DOI] [PubMed] [Google Scholar]
- 11.Klein CJ, Nielsen FH, Moser-Veillon PB. Trace element loss in urine and effluent following traumatic injury. JPEN J Parenter Enteral Nutr 2008; 32:129–139. [DOI] [PubMed] [Google Scholar]
- 12.Berger MM, Shenkin A, Revelly JP, et al. Copper, selenium, zinc and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am J Clin Nutr 2004; 80:410–416. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Hamouda N, Charrière M, Voirol P, Berger MM. Massive copper and selenium losses cause life-threatening deficiencies during prolonged continuous renal replacement. Nutrition 2017; 34:71–75. [DOI] [PubMed] [Google Scholar]
- 14.Datzmann T, Trager K, Reinelt H, von Freyberg P. Elimination rates of electrolytes, vitamins, and trace elements during continuous renal replacement therapy with citrate continuous veno-venous hemodialysis: influence of filter lifetime. Blood Purif 2017; 44:210–216. [DOI] [PubMed] [Google Scholar]
- 15.Datzmann T, Trager K, Schroppel B, et al. Treatment dose and the elimination rates of electrolytes, vitamins, and trace elements during continuous veno-venous hemodialysis (CVVHD). Int Urol Nephrol 2018; 50:1143–1149. [DOI] [PubMed] [Google Scholar]
- 16.Story DA, Morimatsu H, Bellomo R. Strong ions, weak acids and base excess: a simplified Fencl-Stewart approach to clinical acid-base disorders. Br J Anaesth 2004; 92:54–60. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura AT, Btaiche IF, Pasko DA, et al. In vitro clearance of trace elements via continuous renal replacement therapy. J Ren Nutr 2004; 4:214–219. [PubMed] [Google Scholar]
- 18.Duncan A, Talwar D, McMillan DC, et al. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr 2012; 95:64–71. [DOI] [PubMed] [Google Scholar]
- 19▪▪.Lumlertgul N, Bear DE, Ostermann M. Clearance of micronutrients during continuous renal replacement therapy. Crit Care 2020; 24:616. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large metabolic study in 55 patients providing detailed data on sieving coefficients of the different amino acids and micronutrients. The quantities of copper, iron, selenium zinc, folate and carnitine lost in the effluent are clinically relevant and may induce deficiencies.
- 20▪▪.Oh WC, Mafrici B, Rigby M, et al. Micronutrient and amino acid losses during renal replacement therapy for acute kidney injury. Kidney Int Rep 2019; 4:1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]; A very important study with an important supplement reporting on the significant adsorption to the filter, that significantly impacts micronutrient availability with a net loss to the patients, that goes undetected in balance studies.
- 21▪.Stapel SN, de Boer RJ, Thoral PJ, et al. Amino acid loss during continuous venovenous hemofiltration in critically ill patients. Blood Purif 2019; 48:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the latest studies that confirms important amino acid losses during CVVH with a polysulfone membrane: the amino acid loss was 13.4 g/day, which corresponds to a loss of about 11.2 g of protein per day.
- 22.Druml W. Basics in clinical nutrition: nutritional support in renal disease. e-ESPEN 2010; 5:e54–e57. [Google Scholar]
- 23.Cano NJ, Aparicio M, Brunori G, et al. ESPEN Guidelines on Parenteral Nutrition: adult renal failure. Clin Nutr 2009; 28:401–414. [DOI] [PubMed] [Google Scholar]
- 24.Onichimowski D, Goraj R, Jalali R, et al. Practical issues of nutrition during continuous renal replacement therapy. Anaesthesiol Intensive Ther 2017; 49:309–316. [DOI] [PubMed] [Google Scholar]
- 25.Oh WC, Gardner DS, Devonald MA. Micronutrient and amino acid losses in acute renal replacement therapy. Curr Opin Clin Nutr Metab Care 2015; 18:593–598. [DOI] [PubMed] [Google Scholar]
- 26.Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE. Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med 2000; 28:1161–1165. [DOI] [PubMed] [Google Scholar]
- 27.Jonckheer J, Demol J, Lanckmans K, et al. MECCIAS trial: metabolic consequences of continuous veno-venous hemofiltration on indirect calorimetry. Clin Nutr 2020; 39:3797–3803. [DOI] [PubMed] [Google Scholar]
- 28.Cromphout N, Meers G, De Waele E. Vitamins and trace elements during continuous venovenous hemofiltration in critically ill patients. Brussels: Vrije University Brussels VUB; 2020. [Google Scholar]
- 29.Vanek VW, Borum P, Buchman A, et al. A. S. P. E. N. Position Paper: recommendations for changes in commercially available parenteral multivitamin and multi–trace element products. Nutr Clin Pract 2012; 27:440–491. [DOI] [PubMed] [Google Scholar]
- 30▪.Yano J, Kaida Y, Nakayama Y, et al. Carnitine deficiency is associated with decreased exercise activity in hemodialysis patients. Renal Replacement Ther 2019; 5:5. [Google Scholar]; One of the few studies addressing the issue of carnitine losses, and showing carnitine losses.
- 31.Sgambat K, Frank L, Ellini A, et al. Carnitine supplementation improves cardiac strain rate in children on chronic hemodialysis. Pediatr Nephrol 2012; 27:1381–1387. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan JL, Simmons PA, Vehige J, et al. Role of carnitine in disease. Nutr Metab (Lond) 2010; 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad S. L-carnitine in dialysis patients. Semin Dial 2001; 14:209–217. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z, Qiu C, Chen C, et al. [Related factor of serum carnitine deficiency and influence of its deficiency on the length of hospital stay in critical ill patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:890–894. [DOI] [PubMed] [Google Scholar]
- 35.Sgambat K, Moudgil A. Carnitine deficiency in children receiving continuous renal replacement therapy. Hemodial Int 2016; 20:63–67. [DOI] [PubMed] [Google Scholar]
- 36.Böhmer T, Bergrem H, Eiklid K. Carnitine deficiency induced during intermittent haemodialysis for renal failure. Lancet 1978; 1:126–128. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract 2005; 20:218–243. [DOI] [PubMed] [Google Scholar]
- 38.Bérard E, Iordache A. Effect of low doses of L-carnitine on the response to recombinant human erythropoietin in hemodialyzed children: about two cases. Nephron 1992; 62:368–369. [DOI] [PubMed] [Google Scholar]
- 39.Glöggler A, Bulla M, Puchstein C, et al. Plasma and muscle carnitine in healthy and hemodialyzed children. Child Nephrol Urol 1988; 9:277–282. [PubMed] [Google Scholar]
- 40.Koşan C, Sever L, Arisoy N, et al. Carnitine supplementation improves apolipoprotein B levels in pediatric peritoneal dialysis patients. Pediatr Nephrol 2003; 18:1184–1188. [DOI] [PubMed] [Google Scholar]
- 41.Khoss AE, Steger H, Legenstein E, et al. [L-carnitine therapy and myocardial function in children treated with chronic hemodialysis]. Wien Klin Wochenschr 1989; 101:17–20. [PubMed] [Google Scholar]
- 42.Druml W. Metabolic aspects of continuous renal replacement therapies. Kidney Int Suppl 1999. S56–S61. [PubMed] [Google Scholar]
- 43.Marik PE, Hooper MH. Adjuvant Vitamin C in critically ill patients undergoing renal replacement therapy: what's the right dose? Crit Care 2018; 22:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasko DA, Churchwell MD, Btaiche IF, et al. Continuous venovenous hemodiafiltration trace element clearance in pediatric patients: a case series. Pediatr Nephrol 2009; 24:807–813. [DOI] [PubMed] [Google Scholar]
- 45.Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019; 38:48–79. [DOI] [PubMed] [Google Scholar]
- 46.Druml W, Joannidis M, John S, et al. [Metabolic management and nutrition in critically ill patients with renal dysfunction: recommendations from the renal section of the DGIIN, OGIAIN, and DIVI]. Med Klin Intensivmed Notfmed 2018; 113:393–400. [DOI] [PubMed] [Google Scholar]
- 47.Brown RO, Compher C. A.S.P.E.N. clinical guidelines: nutrition support in adult acute and chronic renal failure. JPEN J Parenter Enteral Nutr 2010; 34:366–377. [DOI] [PubMed] [Google Scholar]
- 48.Brown RO, Compher C. American Society for Parenteral, Enteral Nutrition Board of Directors. A.S.P.E. N. clinical guidelines: nutrition support in adult acute and chronic renal failure. JPEN J Parenter Enteral Nutr 2010; 34:366–377. [DOI] [PubMed] [Google Scholar]
- 49▪▪.Singer P, Reintam-Blaser A, Berger MM, et al. ESPEN guidelines: nutrition in the ICU. Clin Nutr 2019; 38:48–79. [DOI] [PubMed] [Google Scholar]; The most recent guidelines for critical illness that attract attention to the micronutrients (and other metabolic) specificities of acute kidney injury patients.
- 50.Honore PM, De Waele E, Jacobs R, et al. Nutritional and metabolic alterations during continuous renal replacement therapy. Blood Purif 2013; 35:279–284. [DOI] [PubMed] [Google Scholar]
- 51.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016; 40:159–211. [DOI] [PubMed] [Google Scholar]
- 52.Jonckheer J, Vergaelen K, Spapen H, et al. Modification of nutrition therapy during continuous renal replacement therapy in critically ill pediatric patients: a narrative review and recommendations. Nutr Clin Pract 2019; 34:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honoré PM, Spapen HD, Marik P, et al. Dosing vitamin C in critically ill patients with special attention to renal replacement therapy: a narrative review. Ann Intensive Care 2020; 10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider AG, Picard W, Honore P, et al. Amino-acids and vitamins status during continuous renal replacement therapy, a prospective observational study. Anaesth Crit Care Pain Med ACCPM 2021; 40:100813. [DOI] [PubMed] [Google Scholar]
- 55.Kritmetapak K, Peerapornratana S, Srisawat N, et al. The impact of macro-and micronutrients on predicting outcomes of critically Ill patients requiring continuous renal replacement therapy. PLoS One 2016; 11:e0156634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamel AY, Dave NJ, Zhao VM, et al. Micronutrient alterations during Continuous Renal Replacement therapy in critically ill adults: A retrospective study. Nutr Clin Pract 2018; 33:439–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


