Objective:
To describe the pharmacokinetics, safety, and efficacy of etravirine (ETR) in HIV-infected children 1 to less than 6 years of age.
Design:
Phase I/II, open-label, multicenter, dose-finding study.
Methods:
Antiretroviral therapy (ART)-experienced children in two age cohorts (I: 2 to <6 years; II: 1 to less than 2 years) received weight-based ETR, swallowed whole or dispersed in liquid, with optimized ART including a ritonavir-boosted protease inhibitor. Intensive pharmacokinetics occurred 7–18 days after starting ETR. Participants with ETR AUC12h less than 2350 ng h/ml had a dose increase and repeat pharmacokinetics.
Results:
Twenty-six children enrolled and 21 (15 in cohort I and 6 in cohort II) had evaluable intensive pharmacokinetics sampling at the final weight-based dose. On the final dose, the geometric mean ETR AUC12h was 3823 ng h/ml for cohort I and 3328 ng h/ml for cohort II. Seven children (33.3%) on the final dose, all taking ETR dispersed, had an AUC12 h less than 2350 ng h/ml and underwent a dose increase. ETR AUC12 h was 3.8-fold higher when ETR was swallowed whole vs. dispersed, P less than 0.0001. On the final dose, 75 and 33.3% in cohorts I and II, respectively, had HIV-1 RNA 400 copies/ml or less or at least 2 log reductions from baseline at week 48. Three children (11.5%) experienced a grade at least 3 adverse event related to ETR but only 1 discontinued.
Conclusion:
ETR was well tolerated. Predefined pharmacokinetics targets were met but overall exposures were low vs. historical data in adults, particularly in young children taking dispersed tablets. A high rate of viral efficacy was observed among those aged 2 to more than 6 years but not in those less than 2 years.
Keywords: children, etravirine, HIV, pediatrics, pharmacokinetics
Introduction
Worldwide, there are approximately 1.8 million children living with HIV infection. The majority (>90%) reside in sub-Saharan Africa. Most acquired HIV through mother-to-child transmission during pregnancy, childbirth or breastfeeding [1].
Nevirapine (NVP) and efavirenz (EFV), the two most widely used nonnucleoside reverse transcriptase inhibitors (NNRTI) globally, have a low genetic barrier for the development of drug resistance mutations [2]. This can lead to profound reductions in viral susceptibility to both drugs, while also conferring cross resistance with other agents in this class [2]. Use of either NVP or EFV can select for NNRTI resistance mutations even after a single dose. A meta-analysis estimated NVP resistance in 52.5% of infants exposed to a single NVP dose and in 16.5% of infants receiving single dose NVP with other antiretroviral drugs for prevention of maternal-to-child transmission (PMTCT) [3]. In a study of newly diagnosed infants (less than 18 months of age) from nine high incidence countries, 50% had resistance to NVP/EFV [4].
With the continued widespread use of first generation NNRTIs (NVP and EFV) in many areas as components of first-line ART, as well as NVP for PMTCT, and infant exposure to NVP during breastfeeding, the number of children harboring virus with NNRTI resistance mutations will continue to rise. Thus, there is an urgent need to develop alternative therapeutic options for children exposed to single dose NVP-containing regimens and those failing their present antiretroviral regimens.
Etravirine (ETR) is a second generation NNRTI [5], which maintains its binding affinity for HIV-1 reverse transcriptase despite binding site changes induced by the presence of common NNRTI resistance mutations [6,7].
International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1090 was a phase I/II, multicenter, open-label study designed to determine the pharmacokinetic profile, optimal dosage, safety, and tolerability of ETR in treatment-experienced HIV-infected children aged 1 to less than 6 years. A secondary objective was to assess the antiviral activity through 48 weeks of treatment.
Methods
Participants
Treatment-experienced children at least 1 year to less than 6 years of age with plasma HIV-1 RNA levels above 1000 copies/ml were eligible. Treatment experience was defined as currently on a failing combination antiretroviral regimen (containing at least three antiretrovirals, from at least two drug classes) for at least 8 weeks or on a treatment interruption of at least 4 weeks with a history of virologic failure on a combination antiretroviral regimen. Children with evidence of ETR sensitivity based on phenotypic testing (≤10-fold change) received ETR in combination with an optimized background regimen (OBR) including a ritonavir-boosted protease inhibitor (PI/r). Children had to be able to swallow ETR whole or dispersed in an appropriate liquid. Children with a new diagnosis of a CDC stage C criteria or an opportunistic or bacterial infection within 30 days prior to screening and not considered clinically stable were excluded. Those with a history of malignancy, a grade 3 or higher neutrophil count, hemoglobin, platelets, aspartate aminotransferase, alanine aminotransferase, lipase, serum creatinine, or QTc or PR interval prolongation at baseline per the DAIDS toxicity tables, use of any disallowed medications or a history of nonadherence with antiretroviral medications were ineligible.
Study design
IMPAACT P1090 was a phase I/II multicenter, open-label study (NCT01504841) of ETR in combination with an OBR containing a PI/r for children ages at least 1 year to less than 6 years of age. Children from South Africa, Brazil, and the United States were enrolled in two age cohorts, cohort I (≥2 to <6 years) or cohort II (≥1 to <2 years). Cohort I enrolled first and after meeting pharmacokinetics and safety criteria, cohort II opened for enrollment. Local institutional review boards approved the study at all participating sites, and written informed consent was obtained from participant's parent or legal guardian prior to study participation.
All participants underwent 12 h intensive pharmacokinetics sampling 7–18 days after initiating ETR. The intensive pharmacokinetics visit occurred approximately 12 h after their previous ETR dose. An age-appropriate (nonstandardized) meal was consumed and the ETR dose taken within 30 min of the start of the meal. Whole blood for quantification of ETR in plasma was obtained at predose, and 1, 2, 4, 6, 9, and 12 h postdose during the intensive pharmacokinetics visit. In addition, a single plasma sample for ETR quantification for population pharmacokinetics evaluations was obtained at weeks 4, 8, 12, 24 and 48, and at any visits to confirm virologic failure.
Individual children with an ETR area under the concentration time curve for the dosing interval (AUC12 h) of less than the 10th percentile in adults (<2350 ng h/m) at the intensive pharmacokinetics visit had the ETR dose increased to target an AUC12 h of 2864 ng h/ml. Initial pharmacokinetics-guided dose increases were capped at the adult dose of 200 mg twice daily. A confirmatory intensive pharmacokinetic assessment (as above) was performed 7–14 days after any pharmacokinetics-guided dose adjustment.
For each cohort, pharmacokinetics and safety through week 4 were evaluated in the first six participants (mini-cohort) before enrolling additional children at the same dose. The target geometric mean ETR AUC12 h for the cohort was 2713--6783 ng h/ml (60–150% of adult AUC12 h).
ETR was initially evaluated in six children in cohort I at a dose of 5.2 mg/kg twice daily based on data from the PIANO trial in children aged 6--17 years [8]. ETR exposures with this dose failed to meet the protocol-defined pharmacokinetics criteria, and thus the ETR starting dose was revised. The revised ETR dose was weight-band based: 75 mg twice daily for 8 to less than 10 kg, 100 mg twice daily for 10 to less than 20 kg, and 125 mg twice daily for 20 to less than 25 kg.
Study drug
ETR was supplied by Janssen as 25 mg scored tablets and 100 mg tablets, to be swallowed whole or dispersed in liquid. For children unable to swallow the tablet whole, families were instructed to disperse ETR in a minimum of 5 ml of water and if needed, to further dilute to a maximum volume of 30 ml with water, orange juice, milk, or formula. Families were instructed to rinse the container with at least 5 ml of water or other beverage and swallow completely to ensure the entire dose was consumed.
Palatability and adherence
At the intensive pharmacokinetics visit and weeks 4, 8, and 16, the participant or participant's caregiver ranked the overall taste and texture of ETR using a 5-point Likert scale (1 -- very bad, 2 -- bad, 3 -- average, 4 -- good, 5 -- very good) and whether issues with medication refusal, vomiting, or gagging occurred.
ETR adherence was assessed via self-report (3-day recall) and pill counts (number of tablets taken/number of tablets expected).
Bioanalysis and pharmacokinetics analysis
Blood for the determination of ETR in plasma was processed by centrifugation with the plasma stored (−70 °C) within 1 h of collection. ETR plasma concentrations were determined using a validated ultrahigh-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method with a linear range of 5.00--5000 ng/ml. The assay has a minimum quantifiable limit of 5.00 ng/ml when 0.100 ml of human plasma is analyzed.
ETR AUC12h, based on intensive pharmacokinetics visits, was determined using noncompartmental methods (the linear trapezoidal summation). Maximum concentration (Cmax), time to Cmax (Tmax) and the last concentration measured in the dosing interval (Clast) were determined visually. Apparent oral clearance (CL/F) was calculated as ETR dose divided by AUC12 h.
Efficacy
Virologic success was defined as either having an HIV-1 RNA 400 copies/ml or less or at least 2 log reduction in HIV-1 RNA at week 48. Participants with missing HIV-1 RNA at week 48 were considered virologic failures.
Statistical analyses
Descriptive statistics were used to summarize demographic, pharmacokinetics, and safety data. ETR AUC12 h was compared in those who swallowed tablets whole vs. dispersed, in those who dispersed tablets in water vs. other liquid, and in those with virologic success vs. failures using unpaired t-tests with loge-transformation to reduce skew.
Results
Participants
Twenty-six HIV-infected children (46.2% girls) enrolled, 13 (50%) from South Africa, 9 (34.6%) from Brazil and 4 (15.4%) from the United States. Table 1 shows the demographic characteristics of study participants. A study flow chart for enrolled participants is provided (Fig. 1). The majority of children were black (73.1%) and 38.5% were of Hispanic ethnicity. The median age was 4 years with a range of 1.5--5.9 years at the time of their intensive pharmacokinetics visit. The median weight was 14.9 kg with a range of 8.3--24.3 kg. The most common concomitant PI/r was lopinavir/ritonavir in 65.4%. In terms of OBR, 20 children (76.9%) took an NRTI with ETR and a PI/r. The remaining six children (23.1%) took raltegravir with ETR and PI/r. All six of the children on raltegravir with ETR and PI/r were in cohort I. Zidovudine (73.1%) and lamivudine (61.5%) were the most frequent NRTIs. One child in cohort I took stavudine as part of the OBR.
Table 1.
Participant demographics.
| Final weight-band based ETR dose with evaluable pharmacokinetics (n = 21) | |||
| All enrolled (n = 26) | Cohort I (2 to<6 years) (n = 15) | Cohort II (1 to <2 years) (n = 6) | |
| Sex [n (%)] | |||
| Female | 12 (46.2) | 7 (46.7) | 3 (50.0) |
| Male | 14 (53.8) | 8 (53.3) | 3 (50.0) |
| Race [n (%)] | |||
| Black or black African | 19 (73.1) | 10 (62.5) | 5 (83.3) |
| Hispanic | 10 (38.5) | 7 (43.8) | 2 (33.3) |
| Country [n (%)] | |||
| South Africa | 13 (50.0) | 6 (40.0) | 3 (50.0) |
| Brazil | 9 (34.6) | 7 (46.7) | 2 (33.3) |
| United States | 4 (15.4) | 2 (13.3) | 1 (16.7) |
| Age at intensive pharmacokinetics (years) | |||
| Median (range) | 4.1 (1.5--5.9) | 4.8 (2.8--5.9) | 1.8 (1.5--2.0) |
| Weight (kg) Median (range) | 14.9 (8.3, 24.3) | 16.1 (12.5, 24.3) | 10.4 (8.3--13.3) |
| Body surface area (BSA) (m2) Median (range) | 0.64 (0.42--0.85) | 0.68 (0.55--0.85) | 0.48 (0.42--0.55) |
| Dose (mg) [n (%)] | |||
| 75 | 7 (26.9) | 0 (0) | 3 (50.0) |
| 100 | 16 (61.5) | 12 (80.0) | 3 (50.0) |
| 125 | 3 (11.5) | 3 (20.0) | 0 |
| Administration at intensive pharmacokinetics visit [n (%)] | |||
| Dispersed | 18 (69.2) | 11 (73.3) | 5 (83.3) |
| Swallowed whole | 7 (26.9) | 3 (20.0) | 1 (16.7) |
| Combination | 1 (3.8) | 1 (6.7) | 0 (0.0) |
| Concomitant protease inhibitor [n (%)] | |||
| Ritonavir-boosted lopinavir | 17 (65.4) | 8 (53.3) | 6 (100.0) |
| Ritonavir-boosted darunavir | 8 (30.7) | 6 (40.0) | 0 (0.0) |
| Ritonavir-boosted atazanavir | 1 (3.8) | 1 (6.7) | 0 (0.0) |
| Baseline plasma HIV-1 RNA Median (range) (copies/ml) | Log10 4.4 (2.5--6.0) | Log10 4.4 (2.5--6.0) | Log10 4.4 (3.2--6.0) |
| Baseline CD4+ cell count Median (range) (cells/μl) | 863 (179--2936) | 268 (179–2936) | 1492 (388–2629) |
| Baseline CD4+ percentage Median (range) | 27.6 (7.0--42.0) | 28.2 (14.0--41.0) | 26.9 (7.0--42.0) |
ETR, etravirine.
Fig. 1.
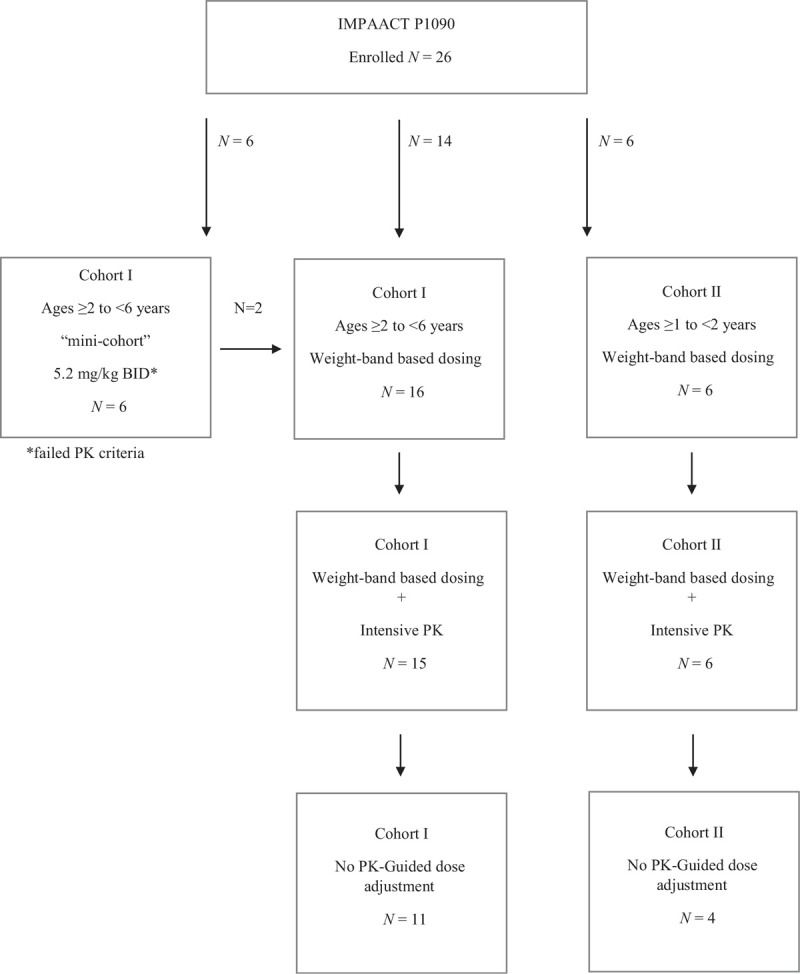
Study flow chart.
Pharmacokinetics
Intensive pharmacokinetics data are summarized for 25 children (96.2%). One child had an extremely low AUC12 h and suspected adherence challenges, then discontinued study at day 16 because of an elevated lipase. Thus, it was not possible to repeat the intensive pharmacokinetics after addressing adherence.
The first six children enrolled in cohort 1 received a weight-based dose (5.2 mg/kg twice daily). Four children in this mini-cohort received 75 mg twice daily (66.7%) and two (33.3%) received 100 mg twice daily. Half took the ETR dispersed. Two of the six children required an ETR dose increase because of AUC12 h less than 2350 ng h/ml, one taking ETR dispersed and one swallowed. The geometric mean ETR AUC12 h for the group was 2576 ng h/ml. Thus, exposures with this weight-based ETR dose were below protocol-defined targets and a revised dosing strategy was used for subsequent children enrolled.
Using the revised ETR dose, which was weight-band based, both age cohorts passed the protocol-defined pharmacokinetics and safety criteria. ETR pharmacokinetics is shown in Table 2 for the weight-band based dose. Eleven of 15 (73.3%) children in cohort I took ETR dispersed, one dispersed the 100 mg tablet and swallowed the 25 mg tablet, and three swallowed the tablet(s) whole. Five of six (83.3%) children in cohort II took dispersed ETR. Though pharmacokinetics targets were achieved for both age cohorts overall, seven (33.3%) children, all taking ETR dispersed, had an AUC12 h of less than 2350 ng h/ml and required an ETR dose increase. The geometric mean ETR AUC12 h was also 3.8-fold higher in participants that swallowed the tablet whole vs. dispersed, 10 721 ng h/ml (n = 4) vs. 2841 ng h/ml (n = 16), respectively (P < 0.0001). The participant who took the 100 mg tablet dispersed but swallowed the 25 mg tablet, was excluded from this analysis. Children taking the ETR dispersed tended to be younger and weigh less than those swallowing ETR intact. Median age and weight were 3.8 years and 14.3 kg among those dispersing the tablets vs. 5.7 years and 16.3 kg among those swallowing ETR whole. Two participants diluted the ETR dispersion in milk/formula, two in orange juice, and the remainder used water. Median ETR AUC12h was similar whether ETR was dispersed in water vs. another liquid (2975 vs. 3128 ng h/ml). All participants reported taking food prior to the ETR dose at the intensive pharmacokinetics visit.
Table 2.
Etravirine pharmacokinetic parameters for children who received the final weight-based etravirine dose.
| GM (% CV) | Mean (SD) | Median (range) | |
| Cohort I (2-<6 years) (n = 15) | |||
| AUC12 h (ng h/ml) | 3823.1 (75.1%) | 4813.6 (3614.0) | 3709.4 (1220.5 – 12998.6) |
| Cmax (ng/ml) | 465.8 (69.0%) | 564.6 (389.4) | 457.8 (199.1 – 1494.0) |
| Clast (ng/ml) | 232.4 (87.8%) | 328.2 (288.3) | 253 (54.3--962.0) |
| Tmax (h) | 4.5 (40.3%) | 4.8 (1.9) | 4.0 (2.0--9.0) |
| CL/F (l/h/m2) | 39.8 (62.2%) | 48.9 (30.4) | 41.7 (10.6--117.8) |
| Individual ETR dose increase required (AUC12h < 2350 ng h/ml) | 5 (33%) | N/A | N/A |
| Cohort II (1 to <2 years) (n = 6) | |||
| AUC12 h (ng h/ml) | 3328.1 (75.5%) | 4158.6 (3137.8) | 3389.7 (1148.1--9989.8) |
| Cmax (ng/ml) | 390.4 (71.3%) | 489.9 (349.4) | 379.3 (121.9--1085.0) |
| Clast (ng/ml) | 225.5 (80.0%) | 278.3 (222.6) | 186.5 (101.9--706.0) |
| Tmax (h) | 2.0 (65.7%) | 2.5 (1.6) | 2.5 (1.0--4.0) |
| CL/F (l/h/m2) | 54.3 (72.7%) | 67.1 (48.8) | 54.6 (18.4--156.8) |
| Individual ETR dose increase required (AUC12 h <2350 ng h/ml) | 2 (33%) | N/A | N/A |
AUC12 h, area under the plasma concentration time curve from time of administration to 12 h after dosing; Clast, last measurable concentration in the dosing interval; CL/F, apparent oral clearance; Cmax, maximum plasma concentration; CV, coefficient of variation; GM, geometric mean; SD, standard deviation; Tmax, time to maximum plasma concentration.
Palatability and adherence
Participants or their caregivers reported no problems taking ETR at 58 of the 65 (89%) palatability assessments. Challenges taking ETR were reported for four participants (one in cohort I and three in cohort II); two reported refusing most doses and two reported infrequently refusing doses. Three of these participants were taking ETR dispersed and the other a combination of swallowed and dispersed. Participants or their caregivers rated ETR taste and texture as average, good, or very good at 86 and 93% of assessments, respectively.
Twenty-one of 26 participants (80.7%) reported no missed doses in the 3 days preceding a study visit through week 48. Of the five participants who missed one or more doses in the 3 days preceding a study visit, three were in cohort I and two were in cohort II.
Median (range) doses consumed based on pill count was 98.5% (88--115%).
Safety
Among the 26 children enrolled, 100% experienced one or more adverse events with 11 (42.3%) having grade 3 or higher adverse events through week 48. However, only three participants out of the 26 (11.5%) experienced a grade 3 or higher adverse event that was deemed probably or definitely related to ETR (Table 3). Two participants had an elevated lipase, one of which discontinued ETR because of a grade 4 lipase. The subject that discontinued because of elevated lipase was a 3-year old girl with a grade 2 lipase at baseline started on ETR with lopinavir/ritonavir and raltegravir. There were no symptoms of pancreatitis and an abdominal computed tomography (CT) was normal. The ETR AUC12 h for this child at the intensive pharmacokinetics visit was 188 ng h/ml, thus this adverse event was not associated with a high ETR exposure. Another participant had a decreased neutrophil count. All adverse events (regardless of grade and causality) are provided in the Supplemental Table. Adverse events occurring at a frequency of at least 20% (regardless of causality) included cough, nasal congestion, rhinorrhea, pharyngitis, diarrhea, vomiting, and rash. Skin and subcutaneous tissue disorders occurred in 53.8% of participants through week 48. These skin-related events were mainly rashes, occurring in 12 of 26 participants (46.2%), but the rashes were grade 1 or 2 in severity and none led to discontinuation of ETR. None of the skin-related adverse events for which a causality assessment was available were considered related to ETR.
Table 3.
Summary of grade 3 or greater events through week 48.
| Participants started on final weight-band based dose (N = 22) | Participants receiving final weight band based dose through week 48 (N = 15) | |||||||||
| All treated (N = 26) | Cohort I (N = 16) | Cohort II (N = 6) | Cohort I (N = 11) | Cohort II (N = 4) | ||||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| With grade ≥3 adverse events | 11 (42.3) | 23.4--63.1 | 5 (31.3) | 11.0--58.7 | 5 (83.3) | 35.9--99.6 | 4 (36.4) | 10.9--69.2 | 4 (100.0) | 39.8--100.0 |
| With grade ≥3 drug-relateda adverse events | 3 (11.5) | 2.4--30.2 | 2 (12.5) | 1.6--38.3 | 0 (0.0) | 0.0--45.9 | 2 (18.2) | 2.3--51.8 | 0 (0.0) | 0.0--60.2 |
Events were included if they occurred while on study drug or within 14 days after discontinuation of study drug.
N indicates number of participants in each cohort. n (%) indicates number (percentage) of participants in each subcategory.
Drug-related adverse events were determined by the Protocol Team to be possibly, probably or definitely related to ETR.
Efficacy
Viral responses are shown in Table 4. Overall, 69.2% (18/26) of children had HIV-1 RNA 400 copies/ml or less or at least 2 log reductions in HIV-1 RNA from baseline at week 48. Among the 22 children started on the final weight-band-based dose, 75% (12 of 16) in cohort I had HIV-1 RNA 400 copies/ml or less at week 48. Fewer children in cohort II (2 of 6 or 33.3%) were virologically suppressed or had at least 2 log reduction in HIV-1 RNA from baseline at week 48. Among the eight children on the final weight-band-based dose that had virologic failure at week 48, two in cohort I and one in cohort II had an ETR AUC less than 2350 ng h/ml at the intensive pharmacokinetics visit. Among those started on the final weight-band based dose, mean ETR AUC12 h was not different between the eight children who had virologic failure compared with those that did not experience virologic failure (3770 vs. 5087 ng h/ml, P = 0.40). Six had resistance testing at the time of virologic failure: two developed resistance to ETR (13-fold and 139-fold), one had partial sensitivity (3.88-fold), and the other three had retained sensitivity to ETR.
Table 4.
Number (%) with virologic failures by week 48.
| Participants started on final weight-band based dose (N = 22) | Participants without pharmacokinetics-driven dose adjustments (N = 15) | |||||||||
| All treated (N = 26) | Cohort I (N = 16) | Cohort II (N = 6) | Cohort I (N = 11) | Cohort II (N = 4) | ||||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| Week 48 | 8 (30.8) | 14.3--51.8 | 4 (25.0) | 7.3-- 52.4 | 4 (66.7) | 22.3--95.7 | 3 (27.3) | 6.0--61.0 | 3 (75.0) | 19.4--99.4 |
The criteria for virologic failure is HIV-1 RNA more than 400 copies/ml and log10 reduction in HIV-1 RNA of <2 logs. Participants with missing HIV-1 RNA values were considered as failures (N = 1).N indicates number of participants in each cohort. n (%) indicates number (percentage) of participants in each subcategory.
Discussion
In this study of treatment-experienced children aged 1--6 years receiving ETR and an OBR including a PI/r, the geometric mean ETR exposures were within protocol-defined targets with weight-band-based dosing but numerically lower than historical data reported in adults and children older than six years. Children taking the ETR tablets dispersed had lower exposures compared with those swallowing ETR intact. Overall, 69.2% were virologically suppressed at week 48 (HIV-1 RNA ≤400 copies/ml), but a higher percentage of young children (ages 1 to <2 years) had an HIV-1 RNA more than 400 copies/ml at week 48.
Population pharmacokinetics data from registrational trials of ETR in adults (DUET-1 and DUET-2), found a mean (SD) and median (range) ETR AUC12 h of 5506 ng h/ml (4710) and 4380 ng h/ml (458–59 084), respectively in 575 adults receiving ETR with darunavir/ritonavir [9]. In the PIANO trial of 101 older children (ages ≥6 to <18 years) receiving ETR 5.2 mg/kg twice daily with a boosted protease inhibitor, mean (SD) and median (range) ETR AUC12 h based on population pharmacokinetic modeling were 5236 ng h/ml (4314) and 4499 ng h/ml (62–28 865), respectively [8]. Among the children with intensive pharmacokinetics data in the current study, the mean (SD) and median (range) ETR AUC12 h were 4383 ng h/ml (3261) and 3432 ng h/ml (1148–12 999), respectively. Children swallowing the ETR tablets intact (n = 7) had a mean and median ETR AUC12 h of 7096 and 8612 ng h/ml, respectively, whereas the mean and median in those taking ETR dispersed (n = 17) were 3340 and 3432 ng h/ml, respectively.
A prior relative bioavailability study in healthy adult volunteers found bioequivalent exposures with dispersion of the 100 mg tablet compared with swallowing the 100 mg tablet intact [10]. Possible explanations for lower AUC12 h observed with dispersed tablets in our study may relate to differences in the method for dispersion/dilution, adherence challenges, and/or inability to swallow the full volume of liquid used for dispersion. In the adult relative bioavailability study, ETR was dispersed in 100 ml of water whereas in this study, the drug was dissolved in smaller volumes (0.1--30 ml) more appropriate for young children. Though the palatability was rated as average or better and self-reported adherence using a 3-day recall and pill counts suggested excellent adherence, fluctuating viral loads and pharmacokinetics (assessed via population pharmacokinetics samples) indicate some adherence and/or administration challenges (data not shown).
Clinical adverse events in IMPAACT P1090 were similar to those observed in children ages 6--17 in PIANO including upper respiratory tract infection, rash, diarrhea, cough and vomiting [11]. More grade 3 or higher events were observed in P1090 (42%) vs. 14% in PIANO but there were fewer study discontinuations because of adverse events in P1090, 3.8 vs. 8% [11]. In the DUET trials, the most common adverse events (occurring in 11--20% of adults) were rash, diarrhea, nausea, nasopharyngitis, headache and injection site reactions (from enfuvirtide) [12]. Thirty-three percent of adults in the DUET studies had a grade 3 or higher adverse event, which led to discontinuation in 7% [12].
Rashes may occur with ETR, including severe, life-threatening rashes. Rashes of any grade were reported in 20% of adults in the DUET trials [13]. Grade 3 and 4 rashes were observed in 1.3% of adults in the DUET trials and 2.2% of participants discontinued study because of rash [13]. In the precursor to PIANO, an ETR dose finding trial in 21 children ages 6--17 years, 3 (14%) experienced a skin-related reaction after 8 days on ETR, two of which were rashes and considered related to ETR [14]. In PIANO, 13% had at least a grade 2 rash and four participants (4%) discontinued because of rash, though no Stevens--Johnson syndrome or other Grade 4 rashes occurred [11]. Though rashes were reported in 46.2% of study participants in P1090, these events were grades 1 or 2 and none for which causality data were available were attributed to ETR. The risk of rash increases with increasing ETR exposure [8], thus the overall lower exposures in P1090 and small sample size may explain the lack of severe rashes in this study.
Virologic failure at week 48 either because of a plasma HIV-1 RNA greater than 400 copies/ml, failure to achieve at least a 2 log reduction (from baseline) in HIV-1 RNA, or missing data, occurred in eight children. Seven of these children were taking ETR dispersed. Only three of eight children (37.5%) with virologic failure had an ETR AUC12 h at the intensive pharmacokinetics visit below 2350 ng h/ml suggesting virologic failures were not associated with low ETR exposures early in therapy. In adults in the DUET-1 and DUET-2 trials, 72% of participants had HIV-1 RNA less than 400 copies/ml at week 48 [12]. In children and adolescents ages 6--17 years in the PIANO trial, 67.3% had HIV-1 RNA less than 400 copies/ml at week 48 [11]. The overall rate of viral suppression in the current study (69% with HIV-1 RNA ≤400 copies/ml at 48 weeks) is within the range of viral suppression observed in other dose-finding trials of antiretroviral drugs including children less than 6 years. Virologic suppression (HIV-1 RNA ≤400 copies/ml) at week 48 among treatment-experienced children in these trials were: lopinavir/ritonavir (75%) [15], atazanavir/ritonavir (43%) [16], darunavir/ritonavir (86%) [17], and raltegravir (67--80%) [18,19]. The rate of viral suppression among those less than 2 years of age in this study was 33.3%. Historical data on the rate of viral suppression in treatment-experienced children in this age group for comparison are lacking. Nonetheless, a virologic failure rate of 66.7% coupled with lower ETR exposures vs. historical data in older children and adults indicate that ETR should not be used in those less than 2 years of age. If ETR must be used in treatment-experienced children less than 2 years of age as no other effective antiretroviral alternative exists, frequent monitoring of ETR plasma concentrations and viral loads is essential.
In terms of study limitations, IMPAACT P1090 was a small safety and dose-finding study that was not powered for virologic outcomes. In addition, some participants had individual dose adjustments based on intensive pharmacokinetics results, and thus, virologic outcomes may not reflect real world use. The tests used for HIV-1 RNA were also not standardized across sites, and thus, reporting the proportion of participants with HIV-1 RNA less than 50 copies/ml was not possible for some participants. Self-report and pill-counts likely over-estimated adherence based upon fluctuating viral loads and ETR plasma concentrations in population pharmacokinetics samples being below the limits of assay detection or at least 30% less than concentrations observed at the same time postdose during the intensive pharmacokinetics visit on the same dose. Food impacts ETR exposures [20], and while participants were instructed to take ETR with food, meal content was not controlled. ETR is metabolized primarily by CYP2C19, with contributions by CYP2C9 and CYP3A4, and CYP2C19 phenotype may have a limited impact on ETR pharmacokinetics [21], but this was not assessed. Rifamycins were exclusionary and no children had active tuberculosis during the 48 weeks of P1090, thus findings are not generalizable to children coinfected with tuberculosis.
In conclusion, twice-daily weight-band-based doses of ETR achieved ETR exposures in children ages 1--6 years that overall approximate exposures previously observed in adults with good tolerability. However, lower exposures and diminished efficacy were observed in those taking ETR dispersed, particularly in children less than 2 years of age. Thus, the optimal dose of ETR was identified only in those ages 2--6 years.
Acknowledgements
We owe a considerable debt of gratitude to the children and families who participated in International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1090. We sincerely thank the clinical sites for providing excellent care to the participants and their commitment to successful completion of the study:
SOUTH AFRICA. Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand: Masebole Masenya, MBChB; Sibongile Ncube, B Cur; Faeezah Patel, MBBCh. Umlazi Clinical Research Site, Nelson R. Mandela School of Medicine: Kimesh L Naidoo, PhD; Dhayendre Moodley, PhD; Vani Govender, BSc. BRAZIL. Hospital. Geral De Nova Iguaçu: Ivete Martins Gomes, MD, MSc; Luiz Felipe Moreira, MD; José Henrique Pilotto, MD, PhD Hospital Federal dos Servidores do Estado: Maria Letícia Santos Cruz, MD, PhD; Esaú Custódio João Filho, MD, PhD; Mariza Curdo Saavedra, MD. Instituto de Puericultura e Ped. Martagao Gesteira UFRJ: Ricardo H. Oliveira, MD; Thalita Abreu, MD, PhD; Luiz Marcelo Lira, R.Ph. University of Sao Paulo: Márcia De Lima Isaac, MD, DSc; Fernanda Tome Sturzbecher, MD, DSc; Marisa Marcia Mussi-Pinhata, MD, DSc. School of Medicine Federal University Minas Gerais: Jorge Pinto, MD, DSc; Flávia Ferreira, MD; Juliana Romeiro, PhD UNITED STATES. Bronx-Lebanon Hospital: Murli Purswani, MD; Mirza Baig, MD; Martha Cavallo, NP. Jacobi Medical Center Bronx: Joanna Dobroszycki, MD; Raphaelle Auguste, RN; Marlene Burey, NP. Ann & Robert H. Lurie Children's Hospital of Chicago: Ellen Chadwick, MD; Ruth Williams, RN; Margaret Ann Sanders, MPH. University of Miami Pediatric Perinatal HIV Clinical Trials Unit: Charles Mitchell, MD; Grace Alvarez, FMD, MPH, CCRP; Monica Stone, FMD. IMPAACT Operations Center: Megan Valentine, MPA. IMPAACT Data Management Center: Mark Lojacono, MA, MSc DAIDS Pharmaceutical Affairs Branch: Katherine Shin, Pharm.D.
The efforts of the entire IMPAACT and Janssen P1090 protocol team are greatly appreciated.
Sources of support: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. Additional financial support and study drug were provided by Janssen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of interest
H.M.C., X.W.d.T., L.T., S.V., M.O. are employees of Janssen. For the remaining authors, none were declared.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1. US Department of Health and Human Services and Minority HIV/AIDS Fund: the Global HIV/AIDS Epidemic. 2019. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. [Google Scholar]
- 2.Jittamala P, Puthanakit T, Chaiinseeard S, Sirisanthana V. Predictors of virologic failure and genotypic resistance mutation patterns in thai children receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J 2009; 28:826–830. [DOI] [PubMed] [Google Scholar]
- 3.Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, et al. Ghent Group on HIV in Women and Children. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol 2007; 36:1009–1021. [DOI] [PubMed] [Google Scholar]
- 4. WHO HIV drug resistance report 2019. July 2019. https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/. [Google Scholar]
- 5. Product Information. Etravirine (Intelence). Janssen Pharmaceuticals, Inc. 2008. [Google Scholar]
- 6.Vingerhoets J, Azijn H, Fransen E, De Baere I, Smeulders L, Jochmans D, et al. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J Virol 2005; 79:12773–12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andries K, Azijn H, Thielemans T, Ludovici D, Kukla M, Heeres J, et al. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother 2004; 48:4680–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakuda TN, Brochot A, Green B, Nijs S, Vis P, Opsomer M, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic relationships of etravirine in HIV-1-infected, treatment-experienced children and adolescents in PIANO. J Clin Pharmacol 2016; 56:1395–1405. [DOI] [PubMed] [Google Scholar]
- 9.Kakuda TN, Peeters MP, Corbett C, De Smedt G, Sinha R, Leopold L, et al. Pharmacokinetics and pharmacodynamics of etravirine in treatment-experienced HIV-1-infected patients: pooled 48-week results of DUET-1 and DUET-2. ICAAC, Washington DC, 2008. [Google Scholar]
- 10.Kakuda TN, Berckmans C, De Smedt G, Leemans R, Leopold L, Peeters M, et al. Single-dose pharmacokinetics of pediatric and adult formulations of etravirine and swallowability of the 200-mg tablet: results from three Phase 1 studies. Int J Clin Pharmacol Ther 2013; 51:725–737. [DOI] [PubMed] [Google Scholar]
- 11.Tudor-Williams G, Cahn P, Chokephaibulkit K, Fourie J, Karatzios C, Dincq S, et al. PIANO study group. Etravirine in treatment-experienced, HIV-1-infected children and adolescents: 48-week safety, efficacy and resistance analysis of the phase II PIANO study. HIV Med 2014; 15:513–524. [DOI] [PubMed] [Google Scholar]
- 12.Katlama C, Haubrich R, Lalezari J, Lazzarin A, Madruga JV, Molina JM, et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS 2009; 23:2289–2300. [DOI] [PubMed] [Google Scholar]
- 13.Girard PM, Campbell TB, Grinsztejn B, Hartikainen J, Rachline A, Nijs S, Witek J. Pooled week 96 results of the phase III DUET-1 and DUET-2 trials of etravirine: further analysis of adverse events and laboratory abnormalities of special interest. HIV Med 2012; 13:427–435. [DOI] [PubMed] [Google Scholar]
- 14.Konigs C, Feiterna-Sperling C, Esposito S, Viscoli C, Rosso R, Kakuda TN, et al. Pharmacokinetics and short-term safety and tolerability of etravirine in treatment-experienced HIV-1-infected children and adolescents. AIDS 2012; 26:447–455. [DOI] [PubMed] [Google Scholar]
- 15.Saez-Llorens X, Violari A, Deetz CO, Rode RA, Gomez P, Handelsman E, et al. Forty-eight-week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr Infect Dis J 2003; 22:216–224. [DOI] [PubMed] [Google Scholar]
- 16.Rutstein RM, Samson P, Fenton T, Fletcher CV, Kiser JJ, Mofenson LM, et al. PACTG 1020A Study Team. Long-term safety and efficacy of atazanavir-based therapy in HIV-infected infants, children and adolescents: the Pediatric AIDS Clinical Trials Group Protocol 1020A. Pediatr Infect Dis J 2015; 34:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Violari A, Bologna R, Kumarasamy N, Pilotto JH, Hendrickx A, Kakuda TN, et al. Safety and efficacy of darunavir/ritonavir in treatment-experienced pediatric patients: week 48 results of the ARIEL trial. Pediatr Infect Dis J 2015; 34:e132–e137. [DOI] [PubMed] [Google Scholar]
- 18.Nachman S, Alvero C, Acosta EP, Teppler H, Homony B, Graham B, et al. Pharmacokinetics and 48-week safety and efficacy of raltegravir for oral suspension in human immunodeficiency virus type-1-infected children 4 weeks to 2 years of age. J Pediatric Infect Dis Soc 2015; 4:e76–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachman S, Zheng N, Acosta EP, Teppler H, Homony B, Graham B, et al. Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin Infect Dis 2014; 58:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholler-Gyure M, Boffito M, Pozniak AL, Leemans R, Kakuda TN, Woodfall B, et al. Effects of different meal compositions and fasted state on the oral bioavailability of etravirine. Pharmacotherapy 2008; 28:1215–1222. [DOI] [PubMed] [Google Scholar]
- 21.Green B, Crauwels H, Kakuda TN, Vanveggel S, Brochot A. Evaluation of concomitant antiretrovirals and CYP2C9/CYP2C19 polymorphisms on the pharmacokinetics of etravirine. Clin Pharmacokinet 2017; 56:525–536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


