Supplemental Digital Content is available in the text.
Key Words: opioids, milligrams of morphine equivalents (MME), definitions, epidemiology, Prescription Drug Monitoring Programs (PDMP)
Objective:
Morphine-standardized doses are used in clinical practice and research to account for molecular potency. Ninety milligrams of morphine equivalents (MME) per day are considered a “high dose” risk threshold in guidelines, laws, and by payers. Although ubiquitously cited, the “CDC definition” of daily MME lacks a clearly defined denominator. Our objective was to assess denominator-dependency on “high dose” classification across competing definitions.
Methods:
To identify definitional variants, we reviewed literature and electronic prescribing tools, yielding 4 unique definitions. Using Prescription Drug Monitoring Programs data (July to September 2018), we conducted a population-based cohort study of 3,916,461 patients receiving outpatient opioid analgesics in California (CA) and Florida (FL). The binary outcome was whether patients were deemed “high dose” (>90 MME/d) compared across 4 definitions. We calculated I 2 for heterogeneity attributable to the definition.
Results:
Among 9,436,640 prescriptions, 42% overlapped, which led denominator definitions to impact daily MME values. Across definitions, average daily MME varied 3-fold (range: 17 to 52 [CA] and 23 to 65 mg [FL]). Across definitions, prevalence of “high dose” individuals ranged 5.9% to 14.2% (FL) and 3.5% to 10.3% (CA). Definitional variation alone would impact a hypothetical surveillance study trying to establish how much more “high dose” prescribing was present in FL than CA: from 39% to 84% more. Meta-analyses revealed strong heterogeneity (I 2 range: 86% to 99%). In sensitivity analysis, including unit interval 90.0 to 90.9 increased “high dose” population fraction by 15%.
Discussion:
While 90 MME may have cautionary mnemonic benefits, without harmonization of calculation, its utility is limited. Comparison between studies using daily MME requires explicit attention to definitional variation.
Morphine-standardized analgesic doses are calculated in clinical practice and research routinely. And, in support of safer opioid prescribing, clinical guidelines suggest limits or cautions above 90 mg of morphine equivalents (MME) to prevent respiratory depression. Yet, subtle variations in MME per day calculations have been overlooked.1 Therefore, we sought to quantify the practical impact of definitional variants to provide clarity.
Equianalgesic conversion factors between opioids were intended to guide dosing when switching patients by accounting for potency.2,3 Conceptually, an equianalgesic dose is that at which 2 opioids provide the same pain relief. Contrary to conventional wisdom, conversion values are not based on pharmacologic properties. Instead, they arose 60 years ago from small single-dose clinical studies in postoperative or cancer populations with pain score outcomes; toxicologic effects (eg, respiratory depression) were not evaluated.4
Amid concerns about opioid overdose, the concept of equianalgesic potency resurfaced.5 In 2016, the US Centers for Disease Control and Prevention (CDC) issued a guideline for chronic noncancer pain management including strong cautions above 90 daily MME based on population-level mortality studies.6 The CDC Guideline formalized a shift in the MME concept from antinociception to toxicology. The 90 daily MME recommendation was not absolute; however, some state laws, policies, and insurance requirements now invoke the threshold explicitly. For example, the State of Maine prohibits “any combination of opioid medication in an aggregate amount in excess of 100 MME of opioid medication per day.”7 CDC recognized this misapplication with a statement softening the “hard limits” inferred.8 The American Medical Association has expressed similar concerns.9
Definitional issues in opioid management10,11 and MME criticism are longstanding.1,12–19 Studies used by CDC to establish the 90 mg threshold employed approaches to calculating daily MME that differed silently. Total MME can be divided by days supply to calculate the average daily MME per prescription. However, the CDC Guideline does not address measurement per patient. Therefore, we quantified how daily MME definitions impact clinical practice, as well as interpretation of the evidence base.
METHODS
Sources of Definitions
Because of their considerable impact on opioid prescribing and frequent citation in the literature, we examined the 27 studies cited in the CDC Guideline to identify definitions of daily MME, based on our previous review.20 Despite documentation challenges, we identified 4 distinct approaches among 18 studies1,21–37 and applied them to dispensing data from California and Florida. Supplemental Digital Content 1 (http://links.lww.com/CJP/A783) contains verbatim extracts from the original studies. Other approaches were identified,38–40 but described inadequately or infrequently.
In demonstrating how to calculate MME, the online continuing medical education module associated with the CDC Guideline41 presents the following clinical scenario, to which we added an additional prescription for illustrative purposes.
A patient receives 30 mg extended-release oxycodone twice a day for around-the-clock pain for 30 days (60 tablets), and one 5 mg oxycodone twice a day as needed for breakthrough pain for 7 days (14 tablets). Both prescriptions are dispensed on the first day of a 30-day month, with no subsequent dispensing. Assume 1.5 as the conversion factor for oxycodone-to-morphine.42
Alarmingly, for this simple scenario, 4 definitional variants return daily MME inconsistently: 75.8 or 93.5 or 31.2 or 105 mg/d.
Definitions
Total MME for the first prescription equals (60 tablets)×(30 mg per tablet)×(1.5 conversion factor from oxycodone-to-morphine),42 resulting in 2700 mg. For the second prescription (14 tablets)×(5 mg per tablet)×(1.5 conversion factor) results in 105 mg. Total MME across both prescriptions is 2805 mg, appearing as the numerator in the first 3 definitions. Formulas are provided in Supplemental Digital Content 2 (http://links.lww.com/CJP/A783).
Definition 1—Total Days Supply
This common definition appears in studies1,26,43 cited in the CDC Guideline and elsewhere.44,45 The numerator is the sum of MMEs across all prescriptions (2805 mg), and the denominator is the sum of days supply across all prescriptions (37 d), for 75.8 mg/d. The same day may contribute multiple times to the denominator (ie, prescriptions overlap), allowing the denominator to potentially exceed the number of unique calendar days.
Definition 2—On-therapy Days
Consistent with standard practice in pharmacoepidemiology, this definition identifies on-therapy days to account for overlapping prescriptions. This method is used in studies33,37 cited in the CDC Guideline and elsewhere.46 The numerator is the sum of MMEs across all prescriptions, and the denominator is the total unique person-days explicitly exposed according to days supply, counting overlap days once. No gap allowances are made for early refills. Applying this definition, 2805 is divided by 30 days, resulting in 93.5 mg/d.
Definition 3—Fixed Observation Window
This common definition from early studies23,25,30 cited in the CDC Guideline often reference an even earlier study,47 and is still used.48,49 The US Department of Health and Human Services Office of the Inspector General recommends this method, which is one of the only definition sources with adequate documentation to allow replication.50 The numerator is again the sum of MMEs across all prescriptions, and the denominator is days elapsed during follow-up, hospital stay,51 or beneficiary enrollment.52 Although 90-day observation windows are most common,23,25 180 days43 and 365 days30 were also used in studies supporting the CDC Guideline. Applying this definition 2805 divided by 90 days results in 31.2 mg/d.
Definition 4—Maximum Daily Dose
Toxicologic framing identifies the highest single-day MME exposure, irrespective of days supply or opioid tolerance. This definition appears to underlie the calculator in the CDC Opioid Guideline mobile app.53 Prescriptions dispensed pro re nata are assumed to be consumed immediately, regardless of how long the prescription is written for. Yet, paradoxically, the “maximum” does not conceptually include consumption for intentional self-harm. This method was used by studies24,28,32,35 cited in the CDC Guideline and may be most relevant for prescriptions to patients who are opioid naive. The first prescription is 30 mg×2 (twice per day)×1.5 (the conversion factor) for 90 MME, plus the second prescription with 5 mg×2×1.5 for 15 MME, resulting in 105 mg/d.
Medication Dispensing Data
Our study used deidentified data from Prescription Drug Monitoring Programs (PDMPs) in California and Florida, which we had analyzed previously.54,55 Inclusion criteria were any complete opioid analgesic dispensing record for state residents aged 18 years and older in California (adult population: 30,571,507) and Florida (adult population: 17,071,450) from July 1, 2018, to September 30, 2018.56 To minimize left-censoring, we included fractional prescriptions dispensed before the observation period which continued past July 1. A short time period was chosen to limit seasonal variation, secular trends, and to allow stabilization of dispensing after earlier changes in Florida law to limit days supply and require checking of the PDMP.57,58 Solid oral and transdermal formulations of opioid analgesics were included (detailed in Supplemental Digital Content 2, http://links.lww.com/CJP/A783).
Primary Analysis
Descriptive statistics were calculated under the standard assumption of consumption exactly and completely as directed. We applied the 4 definitions separately to identify the prevalence of patients who would be considered “high dose” (>90 daily MME), such as would be conceptualized in a hypothetical policy evaluation. We stratified into 3 mutually exclusive subgroups: (1) patients receiving only immediate-release or short-acting opioids, generally used for acute pain, initial management, or titration of persistent pain (hereafter immediate-release); (2) patients receiving only extended-release or long-acting opioids labeled for chronic pain (hereafter extended-release); and (3) patients receiving both immediate-release and extended-release opioids contemporaneously within the 3-month observation period (eg, including, but not limited to, patients with chronic pain receiving opioids for breakthrough pain or during taper). From continuous models of daily MME, we report arithmetic means and medians by subgroup. Data management was conducted in SAS 9.4 (SAS Institute Inc., Cary, NC); code available at www.opioiddata.org.
Meta-analyses
Applying a Food and Drug Administration (FDA) method for opioid measurement dilemmas,59 we used meta-analytic techniques to quantify how much heterogeneity would have been observed across hypothetical state-comparison studies, each applying one of the 4 variants on the same sample (fixed effects). In preliminary analyses, Florida generally had higher opioid use than California, presumably due to an older population,56 scope of practice legislation,58,60 and other factors.61 Conceptualized as a comparative surveillance study, we evaluated differences between the 2 states: (1) daily MME as categorical comparing the proportion of “high dose” patients, and (2) calculating mean differences in milligrams as a continuous variable, stratified by the 3 opioid categories from subgroup analyses. To quantify heterogeneity between definitions, we computed Higgins and Thompson I 2 metric62 and χ2 statistics in Stata/MP 16.0 (Stata Corp., College Station, TX). Code and annotated output are provided in Supplemental Digital Content 3 (http://links.lww.com/CJP/A783).
Sensitivity Analysis
We explored the impact of inconsistency at the threshold borderline: Some studies use >90 daily MME (eg, 91 and higher), while others use ≥90 daily MME. Like the primary analysis, the outcome was the proportion of patients considered “high dose” with prevalence differences. The corresponding number needed to harm (NNH) represents the number of patients seen before one would be misclassified as “low dose” who should have been considered “high dose.”
Ethics Statement
The study was approved by the University of Kentucky Institutional Review Board.
Patient Involvement
The Opioid Data Lab (www.opioiddata.org) is a collaboration between the authors’ 3 institutions; professional representation by patients with chronic pain and people who use drugs is a core organizational tenet. Representatives review the portfolio of research projects, providing guidance from study conceptualization to findings dissemination. The definitional and clinical nature of this particular analysis elicited limited input from representatives, mostly on clinical plausibility and impact.
RESULTS
Descriptive Findings
The analytic sample contained 9,436,640 opioid analgesic prescriptions (California, n=5,677,277 and Florida, n=3,759,363) dispensed for use between July and September 2018, encompassing 3,916,461 unique adult residents (California, n=2,430,870 and Florida, n=1,485,591). The 3-month rate of opioid dispensing was lower in California at 7.9 per 100 adult residents than in Florida with 8.7. The prevalence of prescriptions with overlapping days supply was 39.0% in California and 44.9% in Florida, corresponding to 23.0% and 27.4% of patients, respectively. Total MME per prescription was heavily right-skewed, with divergent arithmetic means and medians. In California, average MME per prescription was 1547 mg (95% confidence interval [CI]: 1540-1554), but median was 300 (25th and 75th percentile: 100 to 1275). In Florida, total MME per prescription was higher at 2146 mg (95% CI: 2138-2154), and median 382 mg (25th and 75th percentile: 113 to 1818). Arithmetic means and medians convey dramatically different perspectives on population-level prevalence of “high dose” patients.
Primary Analysis of Definitional Variants
The 4 definitions yielded a 3-fold range of MME: 17 to 52 mg/d in California and 23 to 65 mg/d in Florida (Table 1), on the same sample. The 2 states had 2.4 and 2.9-fold differences in the number of “high dose” patients >90 daily MME (Fig. 1), respectively. In California, the 4 definitions resulted in a range of 3.6% (n=86,407) to 10.3% (n=249,471) of opioid recipients identified as “high dose.” In Florida, the range was 5.9% (n=87,295) to 14.2% (n=211,429) having >90 daily MME. In both states, Definition 4 (maximum daily dose) identified the highest number of “high dose” patients. However, in California, Definition 3 (fixed observation window) returned the fewest patients with >90 daily MME, whereas in Florida Definition 1 (total days supply) provided the least.
TABLE 1.
Definitional Variation in MME by Type of Pain Medication
| Average Daily MME | Daily MME, Median (IQR) | |||
|---|---|---|---|---|
| Definition | California | Florida | California | Florida |
| All patients on opioid analgesics (mg) | ||||
| Total days supply | 33 | 39 | 25 (18, 40) | 30 (20, 45) |
| On-therapy days | 38 | 46 | 25 (18, 40) | 30 (20, 46) |
| Fixed observation window | 17 | 23 | 3.3 (1.1, 13.9) | 4.2 (1.2, 19.8) |
| Maximum daily dose | 52 | 65 | 30 (20, 50) | 33 (20, 60) |
| No. patients | 2,430,870 | 1,485,591 | 2,430,870 | 1,485,591 |
| Average on-therapy days (d) | 30 | 34 | 13 (5, 56) | 17 (3, 69) |
| Immediate-release only (mg) | ||||
| Total days supply | 30 | 34 | 24 (17, 38) | 30 (19, 40) |
| On-therapy days | 31 | 35 | 25 (18, 38) | 30 (19, 43) |
| Fixed observation window | 10 | 13 | 2.7 (1.1, 10.2) | 3.3 (1.1, 13.0) |
| Maximum daily dose | 40 | 45 | 30 (20, 45) | 30 (20, 50) |
| No. patients | 2,273,028 | 1,338,828 | 2,273,028 | 1,338,828 |
| Average on-therapy days (d) | 27 | 30 | 10 (5, 46) | 12 (3, 58) |
| Extended-release only (mg) | ||||
| Total days supply | 90 | 87 | 60 (30, 120) | 60 (30, 120) |
| On-therapy days | 104 | 97 | 62 (31, 121) | 63 (32, 120) |
| Fixed observation window | 73 | 67 | 42 (15, 90) | 41 (14, 90) |
| Maximum daily dose | 154 | 143 | 90 (45, 180) | 90 (55, 180) |
| No. patients | 40,038 | 26,039 | 40,038 | 26,039 |
| Average on-therapy days (d) | 61 | 60 | 75 (30, 89) | 73 (29, 89) |
| Extended-release and immediate-release (mg) | ||||
| Total days supply | 74 | 83 | 55 (38, 90) | 66 (44, 108) |
| On-therapy days | 144 | 160 | 100 (63, 172) | 123 (75, 210) |
| Fixed observation window | 123 | 133 | 82 (42, 151) | 98 (51, 181) |
| Maximum daily dose | 251 | 268 | 173 (105, 300) | 200 (120, 345) |
| No. patients | 117,804 | 120,724 | 117,804 | 120,724 |
| Average on-therapy days (d) | 74 | 74 | 88 (63, 92) | 88 (67, 92) |
IQR indicates interquartile range; MME, milligrams of morphine equivalents.
FIGURE 1.
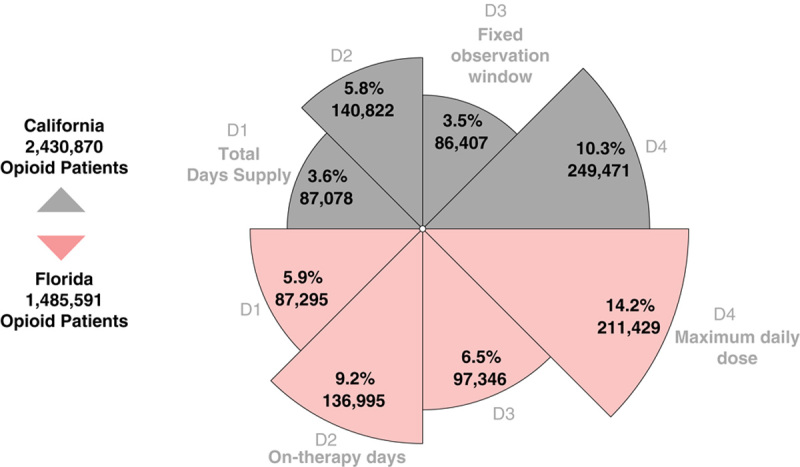
Inconsistency in identifying “high dose” patients on opioids. The proportion of patients on opioids considered “high dose” (>90 mg of morphine equivalents [MME]/day) varies by definition alone, from July to September 2018. Four definitions were identified from the literature and clinical tools. Total days supply (D1) divides the sum of MMEs by the sum of days supply, allowing the denominator to be longer than the prescribed duration. On-therapy days (D2) divides total MME by the number of calendar days. Fixed observation window (D3) uses a fixed denominator, typical 30 to 90 days in research studies. Maximum daily dose (D4) identifies the day with the highest total possible exposure.
Subgroup Analysis
We found that 92.2% of adult opioid patients were treated only with immediate-release opioids, nearly identical to national estimates.59 In addition, 78.3% of patients with extended-release opioids also received concurrent immediate-release opioids.
We next analyzed the impact of definition choice among mutually exclusive opioid patient subgroups: immediate-release only (n=3,611,856), extended-release only (n=66,077), and any combination of extended-release and immediate-release (n=238,528). Patients receiving only extended-release opioids showed the least variation, with about 2-fold relative differences between the highest and lowest definitions (Table 1).
At a clinical level, the definitional variants led to different conclusions. If assessing whether a single patient was receiving a “high dose” of opioids, on average some definitions would say yes, others no. For patients receiving only extended-release, 2-out-of-4 definitions returned an average dose >90 daily MME. For patients receiving both extended-release and immediate-release opioids, 3-out-of-4 variants returned average dose >90 mg/d.
Meta-analyses
In the first meta-analysis, we compared the proportion of patients receiving “high dose” (>90 daily MME) opioids between California and Florida. MME formula was the only source of variation. While Florida consistently had more “high dose” patients, the magnitude of the difference varied solely on how daily MME was calculated: 64% or 59% or 84% or 39%. A hypothetical surveillance study or policy evaluation would reach different conclusions based on which definition was used. Meta-analytic metrics confirmed very high heterogeneity (χ2=3257, 3 df, P<0.0001), with I 2 of 99.9% (Table 2).
TABLE 2.
Meta-analytic Comparison of MME Definitional Variants
| Relative Proportion More “High Dose” Patients in Florida (vs. California) | |
|---|---|
| More “High Dose” Patients, % (95% CI) | |
| Daily MME definition variant (n=3,916,461) | |
| Total days supply | 64.0 (62.5-65.5) |
| On-therapy days | 59.2 (58.0-60.3) |
| Fixed observation window | 84.3 (82.7-86.0) |
| Maximum daily dose | 38.7 (37.9-39.4) |
| I 2=99.91% | |
| Test of heterogeneity: χ2=3257, 3 df, P<0.0001 | |
| Mean difference in daily MME in Florida (vs. California) | |
| Difference in MME (95% CI) | |
| Immediate-release only (n=3,611,856) (mg) | |
| Total days supply | 3.7 (3.3-4.1) |
| On-therapy days | 3.5 (3.1-3.9) |
| Fixed observation window | 2.2 (2.2-2.3) |
| Maximum daily dose | 5.1 (4.6-5.6) |
| I 2=98.63% | |
| Test of heterogeneity: χ2=219, 3 df, P<0.0001 | |
| Extended-release only (n=66,077) (mg) | |
| Total days supply | −3.3 (−1.8 to −4.8) |
| On-therapy days | −6.8 (−4.9 to −8.7) |
| Fixed observation window | −5.9 (−4.4 to −7.4) |
| Maximum daily dose | −10.6 (−7.7 to −13.6) |
| I 2=86.38% | |
| Test of heterogeneity: χ2=22, 3 df, P=0.0001 | |
| Both extended-release and immediate-release (n=238,528) (mg) | |
| Total days supply | 8.8 (8.3-9.3) |
| On-therapy days | 16.7 (15.0-17.3) |
| Fixed observation window | 10.4 (9.2-11.5) |
| Maximum daily dose | 17.2 (15.1-19.3) |
| I 2=98.34% | |
| Test of heterogeneity: χ2=181, 3 df, P<0.0001 | |
CI indicates confidence interval; MME, milligrams of morphine equivalents.
In the second meta-analysis, we calculated mean differences in milligrams of MME per day between states. Heterogeneity was very high overall based solely on definition choice, with the extended-release only group showing the least (I 2=86%), while the other 2 subgroups had I 2 >98% (Table 2). A similar pattern was found using χ2 statistics, with extended-release only showing lower relative heterogeneity arising from definition choice (χ2=22), followed by concurrent extended-release and immediate-release (χ2=181), and immediate-release only showing greatest impact from definition choice (χ2=219). The heterogeneity in the latter subgroup appears to be driven by Definition 3 (90-d fixed observation window) which was markedly lower than other variants (Table 1). Patients receiving extended-release and immediate-release concurrently in Florida had consistently higher average doses than in California, however, the effect size was ambiguous: from 8.8 mg (95% CI: 8.3-9.3) to 17.2 mg (95% CI: 15.1-19.3). Definition 1 (total days supply) showed the least difference between states among patients receiving both immediate-release and extended-release opioids, but the second-highest difference in the immediate-release only subgroup. Definition 4 (maximum daily dose) consistently returned the most exaggerated result. The remaining 3 definitions changed in rank order. Further complicating the picture, patients in Florida receiving only extended-release opioids had lower mean MME (range: −3.3 to −10.6 mg/d) than in California. In epidemiologic terms, a claims data study using the standard incident new user design to evaluate extended-release opioids might return the opposite results to a prevalent user design.63,64
Sensitivity Analysis
Both states showed boundary effects when comparing >90 daily MME to ≥90 daily MME, with a disproportionally large increase in prevalence for 1 additional milligram of MME (Table 3). Solely including the borderline unit interval: 90.0 to 90.9 increased the “high dose” proportion by 15.4% (95% CI: 15.2% to 15.7%) on average. Definition 3 (fixed observation window) was most robust to misclassification at the 90 mg borderline. With this variant, the NNH for one misclassification was 1 in 2430 in California, and 1 in 1244 in Florida. Definition 4 (maximum daily dose) was most susceptible to boundary inclusion decisions with NNH for misclassification of 1 in 67 and 1 in 30, respectively.
TABLE 3.
Sensitivity Analysis of Boundary Inclusion at ≥90 mg
| Definition | Patients >90 Daily MME, n (%) | Patients ≥90 Daily MME, n (%) | Rate Difference Per 1000 (95% CI) | Number Needed to Harm* |
|---|---|---|---|---|
| California | ||||
| Total days supply | 87,078 (3.6) | 106,240 (4.4) | 7.9 (7.5, 8.2) | 1 in 127 |
| On-therapy days | 140,822 (5.8) | 155,254 (6.4) | 5.9 (5.5, 6.4) | 1 in 169 |
| Fixed observation window | 86,407 (3.6) | 87,407 (3.6) | 0.41 (0.07, 0.75) | 1 in 2430 |
| Maximum daily dose | 249,471 (10.3) | 285,807 (11.8) | 15.0 (14.3, 15.5) | 1 in 67 |
| Total adult opioid patients | 2,430,870 | |||
| Florida | ||||
| Total days supply | 87,295 (5.9) | 113,998 (7.7) | 18.0 (17.4, 18.6) | 1 in 56 |
| On-therapy days | 136,995 (9.2) | 157,794 (10.6) | 14.0 (13.3, 14.7) | 1 in 72 |
| Fixed observation window | 97,346 (6.6) | 98,541 (6.6) | 0.80 (0.22, 1.4) | 1 in 1244 |
| Maximum daily dose | 211,429 (14.2) | 261,335 (17.6) | 33.6 (32.7, 34.5) | 1 in 30 |
| Total adult opioid patients | 1,485,591 | |||
Number of patients seen before one would be misclassified as “low dose” who should have been considered “high dose” by using 90 mg instead of 91 mg as a threshold.
CI indicates confidence interval; MME, milligrams of morphine equivalents.
Data Sharing Statement
Data processing code used to construct each definition is available at www.opioiddata.org. Individual-level PDMP records are governed by state laws and requests must be made directly to those authorities; the authors are not permitted to transfer individual-level data to third parties. However, all aggregate data and code used for statistical analyses are publicly available at www.opioiddata.org and institutionally archived at the Carolina Digital Repository (https://doi.org/10.17615/zst5-nc25).
DISCUSSION
Over the past decade, MME have been accepted into clinical practice and adopted for opioid safety studies with limited critical assessment. The computational ease and the evocative lure of molecular fundamentals collide in an optimal level of cognitive complexity to engender MMEs with an unsubstantiated aura of immutability. Our analysis revealed definitional inconsistencies that have been overlooked. There are implications for clinical care, policy, and epidemiology, and the potential to capriciously impact many thousands of patients.
Our findings preclude a universal MME formula which suits all clinical practice. The practical utility of MME in opioid management has been questioned.12,16 Our study further suggests that when patients are handed-off between prescribers, measurement variation could lead to inconsistent experiences for patients requiring pharmacotherapy for pain relief. MME calculations are incorporated in many clinical decision support systems, yet software interfaces and clinical practice rarely allow space for probing definitional nuance.65
MMEs homogenize opioid exposure. On a policy level, the lack of definitional consensus makes it difficult to assess compliance with legislative mandates and third-party payer requirements. For example, an opioid reduction schedule was implemented by Arkansas Medicaid where beneficiaries with ≥250 MME per day were required to be tapered to ≤90 mg during an 18-month period by 50 mg intervals.66 Since these patients are clearly not opioid naive, on-therapy days or fixed observation window may be more appropriate than the exaggerated exposure from maximum daily dose (Table 4). Without a standardized definition in this setting, choice of definition will directly impact the course of a patient’s therapy arbitrarily.
TABLE 4.
Recommendations on Equianalgesic Definitional Variants
| 1. Total days supply: The least complicated calculation appears best suited when immediate-release opioids are prescribed for short discrete times. It consistently underestimated MME per day when overlapping prescriptions were present or when immediate-release and extended-release opioids were prescribed concurrently, as with 78% of patients with chronic opioid use |
| 2. On-therapy days: Provides a smoothed measure useful in studies of dose-dependent adverse effects, including opioid-induced constipation or overdose in patients with opioid tolerance or who have been stable on opioids. The metric is time-varying and affords the greatest flexibility to define medication gap periods and leftover/unused medications to improve pharmacoepidemiologic studies |
| 3. Fixed observation window: Most suitable for studies with a known or suspected duration of risk during which adverse events are expected to occur, such as incidence of opioid use disorder. This definition may be useful when prescriptions are filled at irregular time intervals on a as-needed basis (pro re nata). The definition consistently had the lowest milligrams per day for immediate-release opioids. This is the definition recommended by the Department of Health and Human Services Office of the Inspector General.43 It is the most robust to misspecification, amenable to transformations, and has the least noise when constructing continuous functions.40 However, since it assumes uniform exposure/risk within a window, there is less scope for time-varying adjustment |
| 4. Maximum daily dose: A toxicological perspective may be appropriate for opioid naive patients with no tolerance and in the presence of comorbidities for respiratory depression. It appears to be best suited for immediate dose-dependent toxic effects, such as respiratory depression in opioid naive patients. This definition may have limited use if it includes opioids where fatal toxicity does not involve respiratory depression (eg, tramadol) or have atypical mu-opioid receptor agonism (eg, tapentadol, buprenorphine). The definition assumes uniform risk of adverse outcomes regardless of time on-therapy. More so than the others, this definition is prone to influence from early refills, unused medication, and how the 90 MME threshold is operationalized. This definition underlies the algorithm embedded in the CDC Opioid Guideline mobile app.51 There may be difficulty reconciling findings with studies using the other definitions because it returns a MME per day that is significantly higher |
CDC indicates US Centers for Disease Control and Prevention; MME, milligrams of morphine equivalents.
At the medicolegal interface, our work has implications for law enforcement and prescriber communication.67 MME alert thresholds are incorporated in “doctor shopping algorithms” and automated proactive reporting, routinely devoid of diagnosis.68 Some law enforcement use daily MME to target prescribers,69 yet we have little reason to believe that definitions are applied with fidelity. In light of our findings, penalizing clinicians solely on the basis of 90 MME limits is problematic.70
Of concern to epidemiologists, long-term intervention evaluation may be subject to an overlooked form of bias. This is because definition choice impacts immediate-release and extended-release opioids differentially. If the proportion of these 2 formulations changes over time, daily MME will produce biased time trends. For example, between 2012 and 2019 the number of extended-release prescriptions decreased quicker than immediate-release; the reduction was even more pronounced for extended-release opioids with properties intended to deter tampering.59 Definition 1 is of particular concern as it exaggerates the difference in daily MME between these 2 types of formulations: Definition 1 returned one of the highest daily MMEs for immediate-release opioids, but for the lowest for extended-release. For evaluation studies with trends over time, Definition 3 may have utility since it was the most robust to misspecification, including due to overlapping prescriptions, by formulation, and at the 90 mg inclusion boundary. The mean-median inequality also challenges assumptions in average-generating statistical models; median or geometric (eg, log-transformed) averages may be a more accurate representation because they are less prone to influence by outliers.71 The mean is not always the message; policymakers reading PDMP reports based solely on MME averages are in danger of making decisions based on metrics that are artifactually inflated. Medians and ranges may convey a more accurate picture in these scenarios.
There are standard assumptions and limitations inherent to database studies of medication use72,73: perfect specification and completeness, generic equivalence,74,75 absence of counterfeits,76 no external sources (eg, out-of-state, leftover, diverted, or illicitly manufactured).77 However, these are of less concern in our study because we were not associating with biological outcomes and are independent of definition. To relax assumptions of perfect adherence, we are exploring novel parametric methods.78 Dispensing data do not necessarily reflect actual consumption. About 60% of patients prescribed opioids retain unused medication.79 Therefore all definitions assuming medication completion systematically overestimate biological exposure. We did not have enough information to determine how unused medications would impact each definition differently. Each definition is dependent on days supply, which is subject to variations when calculated at pharmacies; we are investigating this separately. Converting transdermal formulations to oral MME can be tricky due to dosing units measured in hours, leading to prescriber, pharmacist, and researcher variation.80 No definition considered pain etiology or tolerance. We were not able to observe social determinants of health81 or unfilled prescriptions,82 and could not differentiate cancer pain. Finally, we note the debate about specific conversion factors between opioid molecules.16,19 We did not evaluate the impact of equianalgesic multipliers in a bid to reduce analytic complexity. Finally, the toxicologic framing of MME may have limited application for opioids where fatal toxicity does not involve respiratory depression (eg, serotonin depletion with tramadol), in the presence of atypical mu-opioid receptor agonism (eg, tapentadol, buprenorphine), or when consumed in the presence of synergistic nonopioid central nervous system depressants.
Our recommendations (Table 4) will benefit from collective iteration. Definition 2 appears to have face validity with routine clinical practice. Definition 4’s toxicologic focus might be useful for new opioid patients with simple regimens at risk for respiratory depression but carries the highest risk of overestimating daily MME. It remains to be seen if shifting clinical definition choice between patients may provide more practice autonomy and better patient outcomes. At a minimum, clinical guidelines, legislation, PDMP vendors, and clinical decision support systems should make formulae, conversion tables, and code explicit. Research studies should consider sensitivity analyses by definition choice, and treating MME exposure as a transformed continuous variable.83 Our findings may have implications for other drug classes (eg, benzodiazepines84 and stimulants) and the World Health Organization defined daily dose for opioids.80,85,86
The sensitivity analysis showed that 15% of patients were right on the 90 to 91 mg borderline. While our study was not designed to assess prescribing motivations, the strong clustering effect suggests that this threshold might be used as a cap to appear in compliance with external mandates. There is no particular clinical reason we could identify for patients to otherwise cluster at 90 MME per day outside of policy, health system, and payer requirements. We speculate that patients who might have otherwise received higher doses are subsumed under this threshold. Definitional choices have consequences.
Despite variation in underlying definitions, the studies cited in the CDC Guideline consistently found an increased risk of fatal overdose ≥90 daily MME. The simplest explanation is an artifact of turning a continuous metric into one that is categorical: All but 2 studies30,33 we reviewed categorized MME exposure using 90 to 120 mg as the lower bound for the highest stratum. However, for fatal overdose, not all opioid molecules exhibit a dose-dependent correlation.87 Still, our study supports FDA’s contention that overdose risk with opioid analgesics is a continuous function.88 Historically, the transition of the MME concept from pain relief to toxicology ignored the clinical concept of differential tolerance.89 With opioid dose escalation, analgesic and unintended effects emerge asynchronously. While 90 MME may have cautionary mnemonic benefits in the midst of broad societal concern, a renewed emphasis on opioid tolerance and definitional harmonization (for daily MME and long-term therapy11,90) seems overdue.
The overlooked inconsistency among daily MME definitions revealed by our study calls into question the clinical validity of a single numerical risk threshold. When measuring with inches, centimeters, and yards, the absolute number of units is arbitrary. The mix of clinical and research metrics used to calculate the 90 MME threshold is similarly convoluted. As providers, we struggle to do what we feel is right for our patients in the midst of increasing outside pressure with serious ramifications. Our findings call into question state laws and third-party payer MME threshold mandates. Without harmonization, the scientific basis for these mandates may need to be revisited. As the CDC Guideline is revised, and clinical decision tools are developed, it is critically important to reassess the evidence base in light of this previously unknown MME definitional variability.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.clinicalpain.com.
ACKNOWLEDGMENTS
The authors thank Maryalice Nocera, MS (Project Manager, Injury Prevention Research Center, University of North Carolina) and LaMonda Sykes (Budget Analyst, Office of the Vice-Chancellor for Research, University of North Carolina) for project management and actuarial support. They are also grateful to the California and Florida PDMPs for providing de-identified data access.
Footnotes
N.D., B.A.C., and C.D.: conceptualized the analysis. N.D., C.D., Y.W., J.B., and T.C.: contributed to study methodology. Y.W. and J.B.: analyzed individual-level data and generated base tables, and conducted quality assurance. N.D.: conducted meta-analyses and sensitivity analysis and created the figure, and was responsible for study integrity. A.C.K.: contributed code and wrote the statistical equations. T.C.: was responsible for all aspects of project management and dissemination.
Supported by FDA and BJA. US Food and Drug Administration, Silver Spring, MD (HHSF223201810183C) funded the efforts of N.D., B.A.C., and T.C. The US Department of Justice, Bureau of Justice Assistance, Washington, DC (2017-PM-BX-K038) funded the efforts of Y.W., J.B., and C.D. The BJA is a component of the Department of Justice’s Office of Justice Programs, which also includes the Bureau of Justice Statistics, the National Institute of Justice, the Office of Juvenile Justice and Delinquency Prevention, the Office for Victims of Crime, and the SMART Office. Funding agencies had no involvement in study design, analysis, interpretation, or decision to publish. N.D. is a part-time methods consultant to the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System of Denver Health and Hospitals Authority, a political subdivision of the State of Colorado. RADARS System had no knowledge of or involvement in this analysis. N.D. has no relationship with any pharmaceutical manufacturer or distributor. The remaining authors declare no conflict of interest.
Contributor Information
Nabarun Dasgupta, Email: nab@unc.edu.
Yanning Wang, Email: ynwang@ufl.edu.
Jungjun Bae, Email: june.bae@uky.edu.
Alan C. Kinlaw, Email: akinlaw@email.unc.edu.
Brooke A. Chidgey, Email: brooke_chidgey@med.unc.edu.
Toska Cooper, Email: toskac@email.unc.edu.
Chris Delcher, Email: chris.delcher@uky.edu.
REFERENCES
- 1. Liang Y, Turner BJ. Assessing risk for drug overdose in a national cohort: role for both daily and total opioid dose? J Pain. 2015;16:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foley KM. The treatment of cancer pain. N Engl J Med. 1985;313:84–95. [DOI] [PubMed] [Google Scholar]
- 3. Eddy NB, Lee LE. The analgesic equivalence to morphine and relative side action liability of oxymorphone (14-hydroxydihydro morphinone). J Pharmacol Exp Ther. 1959;125:116–121. [PubMed] [Google Scholar]
- 4. Fudin J, Raouf M, Wegrzyn EL. Opioid dosing policy: pharmacological considerations regarding equianalgesic dosing. A White Paper from the Academy of Integrative Pain Management. American Academy of Integrative Pain Management; 2017.
- 5. Dasgupta N, Kramer ED, Zalman M-A, et al. Association between non-medical and prescriptive usage of opioids. Drug Alcohol Depend. 2006;82:135–142. [DOI] [PubMed] [Google Scholar]
- 6. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 7. Requirements regarding prescription of opioid medication. 32 Maine Rev. Stat. Ann. § 3300-F; 2016.
- 8. Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380:2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Resneck J. Inappropriate use of CDC guidelines for prescribing opioids (resolution 235-I-18), report of the board of trustees. 2019. Available at: www.ama-assn.org/system/files/2019-04/a19-bot22.pdf. Accessed September 23, 2020.
- 10. Abdel Shaheed C, McLachlan AJ, Maher CG. Rethinking “long term” opioid therapy. BMJ. 2019;367:l6691. [DOI] [PubMed] [Google Scholar]
- 11. Shen Y, Bhagwandass H, Branchcomb T, et al. Chronic opioid therapy: a scoping literature review on evolving clinical and scientific definitions. J Pain. 2021;22:246–262. [DOI] [PubMed] [Google Scholar]
- 12. Schatman ME, Fudin J, Pratt Cleary J. The MEDD myth: the impact of pseudoscience on pain research and prescribing-guideline development. J Pain Res. 2016;9:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaheen PE, Walsh D, Lasheen W, et al. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage. 2009;38:409–417. [DOI] [PubMed] [Google Scholar]
- 14. Anderson R, Saiers JH, Abram S, et al. Accuracy in equianalgesic dosing. J Pain Symptom Manage. 2001;21:397–406. [DOI] [PubMed] [Google Scholar]
- 15. Pereira J, Lawlor P, Vigano A, et al. Equianalgesic dose ratios for opioids. J Pain Symptom Manage. 2001;22:672–687. [DOI] [PubMed] [Google Scholar]
- 16. Fine PG, Portenoy RK. Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gammaitoni AR, Fine P, Alvarez N, et al. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19:286–297. [DOI] [PubMed] [Google Scholar]
- 18. Brant JM. Opioid equianalgesic conversion: the right dose. Clin J Oncol Nurs. 2001;5:163–165. [PubMed] [Google Scholar]
- 19. Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med. 2011;25:504–515. [DOI] [PubMed] [Google Scholar]
- 20. Ranapurwala SI, Naumann RB, Austin AE, et al. Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base. Pharmacoepidemiol Drug Saf. 2019;28:4–12. [DOI] [PubMed] [Google Scholar]
- 21. Tennant FS, Rawson RA. Outpatient treatment of prescription opioid dependence: comparison of two methods. Arch Intern Med. 1982;142:1845–1847. [PubMed] [Google Scholar]
- 22. Ralphs JA, Williams AC, Richardson PH, et al. Opiate reduction in chronic pain patients: a comparison of patient-controlled reduction and staff controlled cocktail methods. Pain. 1994;56:279–288. [DOI] [PubMed] [Google Scholar]
- 23. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. [DOI] [PubMed] [Google Scholar]
- 25. Gomes T, Juurlink DN, Dhalla IA, et al. Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Med. 2011;5:e13–e22. [PMC free article] [PubMed] [Google Scholar]
- 26. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. [DOI] [PubMed] [Google Scholar]
- 27. Naliboff BD, Wu SM, Schieffer B, et al. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J Pain. 2011;12:288–296. [DOI] [PubMed] [Google Scholar]
- 28. Paulozzi LJ, Kilbourne EM, Shah NG, et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95. [DOI] [PubMed] [Google Scholar]
- 29. Mitra F, Chowdhury S, Shelley M, et al. A feasibility study of transdermal buprenorphine versus transdermal fentanyl in the long-term management of persistent non-cancer pain. Pain Med. 2013;14:75–83. [DOI] [PubMed] [Google Scholar]
- 30. Gwira Baumblatt JA, Wiedeman C, Dunn JR, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174:796–801. [DOI] [PubMed] [Google Scholar]
- 31. Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic non-cancer pain: the role of opioid prescription. Clin J Pain. 2014;30:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain. 2014;15:1911–1929. [DOI] [PubMed] [Google Scholar]
- 33. Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality: prescribed opioid dose and overdose mortality. Pain Med. 2015;17:85–98. [DOI] [PubMed] [Google Scholar]
- 34. Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175:608–615. [DOI] [PubMed] [Google Scholar]
- 35. Park TW, Saitz R, Ganoczy D, et al. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bohnert ASB, Logan JE, Ganoczy D, et al. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med Care. 2016;54:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turner BJ, Liang Y. Drug overdose in a retrospective cohort with non-cancer pain treated with opioids, antidepressants, and/or sedative-hypnotics: interactions with mental health disorders. J Gen Intern Med. 2015;30:1081–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meisenberg BR, Grover J, Campbell C, et al. Assessment of opioid prescribing practices before and after implementation of a health system intervention to reduce opioid overprescribing. JAMA Netw Open. 2018;1:e182908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hinami K, Ray MJ, Doshi K, et al. Prescribing associated with high-risk opioid exposures among non-cancer chronic users of opioid analgesics: a social network analysis. J Gen Intern Med. 2019;34:2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare Part D: Drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Centers for Disease Control and Prevention. Module 6: Dosing and titration of opioids: how much, how long, and how and when to stop? WB2861. 2018. Available at: www.cdc.gov/drugoverdose/training/dosing/accessible/index.html. Accessed August 28, 2020.
- 42. US Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2016. Available at: www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed September 23, 2020.
- 43. Sullivan MD, Edlund MJ, Fan M-Y, et al. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kimmel PL, Fwu C-W, Abbott KC, et al. Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol. 2017;28:3658–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu W, Chernew ME, Sherry TB, et al. Initial opioid prescriptions among U.S. commercially insured patients, 2012-2017. N Engl J Med. 2019;380:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raman SR, Bush C, Karmali RN, et al. Characteristics of new opioid use among medicare beneficiaries: identifying high-risk patterns. J Manag Care Spec Pharm. 2019;25:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perez HR, Buonora M, Cunningham CO, et al. Opioid taper is associated with subsequent termination of care: a retrospective cohort study. J Gen Intern Med. 2020;35:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Campbell C, Weisner C, Kline-Simon A, et al. Testing a Program to Increase Patient Activation Among Patients Prescribed Opioid Medicine for Chronic Pain. Washington, DC: Patient-Centered Outcomes Research Institute (PCORI); 2019. [Google Scholar]
- 50. Office of the Inspector General. HHS OIG toolkits for calculating opioid levels and identifying patients at risk of misuse or overdose. 2018. Available at: https://oig.hhs.gov/oei/reports/oei-02-17-00560.asp. Accessed September 23, 2020.
- 51. Fan KL, Luvisa K, Black CK, et al. Gabapentin decreases narcotic usage: enhanced recovery after surgery pathway in free autologous breast reconstruction. Plast Reconstr Surg. 2019;7:e2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007-16: retrospective cohort study. BMJ. 2018;363:k4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention. CDC Opioid Guideline Mobile App. 2016. Available at: https://www.cdc.gov/drugoverdose/prescribing/app.html. Accessed October 1, 2020.
- 54. Brown JR, Oh Gy, Wang Y, et al. Variation in abuse‐deterrent formulation opioid prescribing in California, Florida, and Kentucky in 2018. J Rural Health. 2021;37:23–28. [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Delcher C, Li Y, et al. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. [DOI] [PubMed] [Google Scholar]
- 56. US Census. American Community Survey Demographic and Housing Estimates, Table DP05. 2018. Available at: https://data.census.gov/cedsci/table?q=ACSDP1Y2018.DP05%20California&g=0400000US12&tid=ACSDP1Y2018.DP05&hidePreview=true. Accessed September 23, 2020.
- 57. Florida Health. 2018-2019 Prescription Drug Monitoring Program Annual Report. 2019. Available at: www.floridahealth.gov/statistics-and-data/e-forcse/laws-rules/2019-pdmp-annual-report.pdf. Accessed September 23, 2020.
- 58. Hincapie-Castillo JM, Goodin A, Possinger M-C, et al. Changes in opioid use after Florida’s Restriction Law for acute pain prescriptions. JAMA Netw Open. 2020;3:e200234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. US Food and Drug Administration. FDA Briefing Information for the September 10-11, 2020 Joint Meeting of the Drug Safety and Risk Management Advisory Committee (DSaRM) and the Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC). 2020. Available at: www.fda.gov/media/141914/download. Accessed September 9, 2020.
- 60. Delcher C, Wang Y, Wagenaar AC, et al. Prescription and illicit opioid deaths and the Prescription Drug Monitoring Program in Florida. Am J Public Health. 2016;106:e10–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davis CS, Carr DH. Self-regulating profession? Administrative discipline of “pill mill” physicians in Florida. Subst Abuse. 2017;38:265–268. [DOI] [PubMed] [Google Scholar]
- 62. Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J Promot Commun Stat Stata. 2008;8:493–519. [Google Scholar]
- 63. Webster-Clark M, Ross RK, Lund JL. Initiator types and the causal question of the prevalent new user design: a simulation study. Am J Epidemiol. 2020:kwaa283. Available at: https://academic.oup.com/aje/advance-article-abstract/doi/10.1093/aje/kwaa283/6043913?redirectedFrom=fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suissa S, Moodie EEM, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores: prevalent new-user designs. Pharmacoepidemiol Drug Saf. 2017;26:459–468. [DOI] [PubMed] [Google Scholar]
- 65. Lazard AJ, King AJ. Objective design to subjective evaluations: connecting visual complexity to aesthetic and usability assessments of eHealth. Int J Hum-Comp Int. 2020;36:95–104. [Google Scholar]
- 66. Arkansas Medicaid Pharmacy Program. Innovative practice to reduce chronic use of opioid MME/day [ATT6-2017-AR-IPN]. 2017. Available at: www.medicaid.gov/sites/default/files/Medicaid/Prescription-Drugs/Downloads/Drug-Utilization-Review/DUR-FFS-Reports/AR-DUR-FFY17.pdf. Accessed September 29, 2020.
- 67. Islam MM, McRae IS. An inevitable wave of Prescription Drug Monitoring Programs in the context of prescription opioids: pros, cons and tensions. BMC Pharmacol Toxicol. 2014;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Worley J. Prescription Drug Monitoring Programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33:319–328. [DOI] [PubMed] [Google Scholar]
- 69. Barthe E, DeVore D, Ward S. Scott MS, Clarke RV. Opioid abuse in Reno, Nevada. Problem-Oriented Policing: Successful Case Studies. New York, NY: Routledge Taylor & Francis; 2020:168–178. [Google Scholar]
- 70. Lawhern R. Stop persecuting docs for legitimately prescribing opioids for chronic pain. STAT News; 2019. Available at: www.statnews.com/2019/06/28/stop-persecuting-doctors-legitimately-prescribing-opioids-chronic-pain/. Accessed September 29, 2020.
- 71. Zheng S, Mogusu E, Veeranki SP, et al. The relationship between the mean, median, and mode with grouped data. Comm Stat Theory Methods. 2017;46:4285–4295. [Google Scholar]
- 72. Hall GC, Sauer B, Bourke A, et al. Guidelines for good database selection and use in pharmacoepidemiology research: good database conduct in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2012;21:1–10. [DOI] [PubMed] [Google Scholar]
- 73. Takahashi Y, Nishida Y, Asai S. Utilization of health care databases for pharmacoepidemiology. Eur J Clin Pharmacol. 2012;68:123–129. [DOI] [PubMed] [Google Scholar]
- 74. Walter C, Felden L, Lötsch J. Bioequivalence criteria for transdermal fentanyl generics: do these need a relook? Clin Pharmacokinet. 2009;48:625–633. [DOI] [PubMed] [Google Scholar]
- 75. Seoane-Vazquez E, Rodriguez-Monguio R, Hansen R. Interchangeability, safety and efficacy of modified-release drug formulations in the USA: the case of opioid and other nervous system drugs. Clin Drug Investig. 2016;36:281–292. [DOI] [PubMed] [Google Scholar]
- 76. Vo KT, van Wijk XMR, Lynch KL, et al. Counterfeit Norco Poisoning Outbreak—San Francisco Bay Area, California, March 25–April 5, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:420–423. [DOI] [PubMed] [Google Scholar]
- 77. Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues. 2011;41:283–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bødkergaard K, Selmer RM, Hallas J, et al. Using the waiting time distribution with random index dates to estimate prescription durations in the presence of seasonal stockpiling. Pharmacoepidemiol Drug Saf. 2020;29:1072–1078. [DOI] [PubMed] [Google Scholar]
- 79. Schirle L, Stone AL, Morris MC, et al. Leftover opioids following adult surgical procedures: a systematic review and meta-analysis. Syst Rev. 2020;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies: oral morphine equivalents. Pharmacoepidemiol Drug Saf. 2016;25:733–737. [DOI] [PubMed] [Google Scholar]
- 81. Park JN, Rouhani S, Beletsky L, et al. Situating the continuum of overdose risk in the social determinants of health: a new conceptual framework. Milbank Q. 2020;98:700–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pruitt LCC, Swords DS, Russell KW, et al. Prescription vs. consumption: opioid overprescription to children after common surgical procedures. J Pediatr Surg. 2019;54:2195–2199. [DOI] [PubMed] [Google Scholar]
- 83. Naumann RB, Marshall SW, Gottfredson NC, et al. Trajectories of dispensed prescription opioids among beneficiaries enrolled in a Medicaid controlled substance “lock-in” program. Pharmacoepidemiol Drug Saf. 2019;28:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kiang MV, Humphreys K, Cullen MR, et al. Opioid prescribing patterns among medical providers in the United States, 2003-17: retrospective, observational study. BMJ. 2020;368:l6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Svendsen K, Borchgrevink P, Fredheim O, et al. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25:725–732. [DOI] [PubMed] [Google Scholar]
- 86. Nielsen S, Gisev N, Bruno R, et al. Defined daily doses (DDD) do not accurately reflect opioid doses used in contemporary chronic pain treatment: defined daily doses for opioids in chronic pain. Pharmacoepidemiol Drug Saf. 2017;26:587–591. [DOI] [PubMed] [Google Scholar]
- 87. Hirsch A, Proescholdbell SK, Bronson W, et al. Prescription histories and dose strengths associated with overdose deaths: prescription histories and overdose deaths. Pain Med. 2014;15:1187–1195. [DOI] [PubMed] [Google Scholar]
- 88. Coyle DT, Pratt C-Y, Ocran-Appiah J, et al. Opioid analgesic dose and the risk of misuse, overdose, and death: a narrative review. Pharmacoepidemiol Drug Saf. 2018;27:464–472. [DOI] [PubMed] [Google Scholar]
- 89. Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia. Anesthesiology. 2016;124:483–488. [DOI] [PubMed] [Google Scholar]
- 90. Karmali RN, Bush C, Raman SR, et al. Long-term opioid therapy definitions and predictors: a systematic review. Pharmacoepidemiol Drug Saf. 2020;29:252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.clinicalpain.com.


