Abstract
The incidence of primary metastatic breast cancer (PMBC) has not decreased despite the increasing popularity of mammography screening and data on the survival among these patients are limited. Therefore, we conducted an extensive population-based study to investigate the factors influencing the survival of patients with PMBC.
We identified 14,306 patients with de novo stage-IV breast cancer using the Surveillance, Epidemiology, and End Results data from 2010 to 2015. The overall survival (OS) time and breast cancer-specific survival (BCSS) time were compared by the Kaplan-Meier method. Univariate and multivariate analyses were performed to determine the effect of different prognostic factors.
Patients with hormone receptor positive/human epidermal growth factor receptor 2 positive showed the longest median survival time in OS (39 months) and BCSS (43 months), and those with triple negative exhibited the shortest in OS (11 months) and BCSS (12 months). We concluded that patients who had undergone primary tumor surgery had better survival than those who did not. The incidence of distant visceral metastasis in the whole cohort was as follows: bone, lung, liver, and brain. This study also substantiated that patients with only brain metastasis had poorer survival than patients with metastasis at multiple sites metastasis, not including brain metastasis (P < .0001).
This study confirmed that molecular subtypes, metastatic site and primary tumor surgery were associated with the survival of PMBC patients.
Keywords: metastatic site, molecular subtype, Primary metastatic breast cancer, surgery, survival
1. Introduction
There will be an estimation of 268,600 women in the United States being diagnosed with breast cancer in 2019 and it alone accounts for 30% of all new cancer diagnoses in women.[1] Approximately 6% of patients presented with Metastatic breast cancer (MBC) at initial diagnosis (de novo stage-IV breast cancer).[2,3] MBC is considered incurable; it is the underlying reason to cause death for the majority of patients.[2,4] The prognosis for primary metastatic breast cancer (PMBC) patients is very poor and the median survival time with brain metastases is reported to 13.8 months[5]; therefore, we should pay more attention to prolong the survival time, improve the quality of life of the patients with de novo stage-IV breast cancer. Like early-stage breast cancer, MBC is a highly complex and heterogeneous disease, the therapeutic goals are to develop appropriate regimens, ameliorate symptom, improve quality of life, and extend survival time for patients.[3,4,6,7] Actually, there existed many risk factors affecting the prognosis of MBC, among which molecular subtype was one of the most important high risk factors.[8,9] Breast cancer can be categorized into four distinct molecular subtypes according to presence of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2).[10] It has been reported that the prognosis of triple negative breast cancer is the worst for both early and advanced breast cancer.[11–13] Several studies have also suggested a range of other relative factors of outcomes with MBC including factors such as race, age at diagnosis, and distant metastatic sites.[9,14,15] The role of excision of primary tumor has been controversial due to inconsistent reports. A few retrospective studies suggested the longer OS of local treatment of primary stage IV breast cancer.[6,8,16,17] In addition, patients with different molecular types tended to have their preferential sites of distant metastases. Those with hormone receptor positive (HR+)/HER2− subtype have a higher probability of bone metastases. HER2-riched cancers have a propensity to give rise to liver and triple negative subtype to lung and brain. In general, the sites of metastases with all subtypes were most prone to bone, followed by lung, liver, and brain.[8,14,15,18–20]
Although there are many studies on MBC, mostly aiming at secondary metastatic breast cancer, the data on the primary MBC are limited. It has been reported that de novo MBC has longer disease-specific survival time than recurrent MBC[21]; in addition, it is not clear the effect of operation on the prognosis.
The purpose of this study was to illuminate factors influencing survival of patients presenting with PMBC using data from the Surveillance, Epidemiology, and End Results (SEER).
2. Methods
2.1. Patient information
We abstracted data from the SEER 18 registries research database (National Cancer Institute, Bethesda, MD) and searched patients for primary metastatic female breast cancer diagnosed from 2010 to 2015 (https://seer.cancer.gov/). A total of 19,913 cases were included who had to be microscopically confirmed as malignant including histology, exfoliative, and thus we excluded 788 women. We selected patients with only one primary malignancy and excluded 4806 patients who had >1 primary cancer. We precluded 13 patients who diagnosed with only autopsy and death certification, the remaining 14,306 patients eligible for survival analyses. We collected information as follows: age at diagnosis, race, year of diagnosis, tumor grade, laterality, lymph nodes, HR, and HER2 status, breast subtypes, marital status, insurance, surgery, distant metastatic site, and survival. And the cancer was classified into 4 molecular subtypes according to HR and HER2 status: HR+/HER2+, HR–/HER2+, HR+/HER2−, and HR−/HER2−. We use overall survival (OS) and breast cancer-specific survival (BCSS) to evaluate the prognosis of the primary metastatic breast cancer.
2.2. Ethics statement
This study was mainly based on the SEER database and was conducted in compliance with the Helsinki Declaration. We obtained permission to access the SEER program research data files. The need for informed patient consent was waived because of the retrospective nature of the study. This study was approved by the ethics committee of Tianjin Medical University Cancer Institution and Hospital.
2.3. Statistical analysis
Descriptive statistics were performed to examine the baseline characteristics. We divided the age into 3 groups: <40 years, 40–70 years, and ≥70 years. Race included American Indian/Alaska Native/Pacific Islander. These variables were stratified by molecular subtypes: HR+/HER2+, HR–/HER2+, HR+/HER2−, and HR−/HER2−. The χ2 test for categorical variables (age, race, grade, lymph node, among others) was adopted to compare the differences among different groups.
We defined OS as the time from diagnosis to death from any cause or last follow-up. BCSS was calculated as the time from the date of diagnosis to the date of death from breast cancer or last follow-up. The OS and BCSS were summarized by Kaplan-Meier survival curves. The log-rank analyses were used to assess the differences among the four groups. We performed univariate and multivariate Cox proportional hazard model to examine the influence of different variables on OS and BCSS. Hazard ratios and their associated 95% confidence intervals (95% CIs) were obtained from the Cox regression analysis. The Kaplan-Meier method was conducted to study the OS and BCSS of primary surgery and different distant metastatic sites. All statistical tests were 2-sided and P values <.05 were considered statistically significant. Analyses were conducted using SPSS 22.0 (IBM SPSS, New York).
3. Results
3.1. Patients characteristics
A total of 14,306 patients with de novo stage IV breast cancer were enrolled in this study from 2010 to 2015. The clinical characteristics were summarized in Table 1. Among the population, 51.6%, 15.0%, 8.2%, and 11.8% of patients had HR+/Her2−, HR+/HER2+, HR−/HER2+ and HR−/HER2− subtypes, respectively, the fewest cases had HR–/HER2+ (8.2%) tumors. Patients age < 70 years accounted for 80.6% of all the HR-/Her2+ subtype. As for race, more black patients (n=436, 25.8%) within HR-/HER2- tumors than the others were observed. In addition, more patients were to be married (n = 6039, 42.2%), insured (n = 11138, 77.9%) and usually had 1 to 3 metastases in axillary lymph nodes (n = 4831, 33.8%). Patients with HR+/HER2– subtype had a lower tumor grade (P < .001), a predilection to metastasize to bone (P < .001), and were less likely to undergo surgery (P < .001). In contrast, triple negative breast cancer patients had a higher tumor grade (P < .001), more possibilities for surgical treatment (P < .001), and were more frequently observed in lung metastases (P < .001).
Table 1.
Demographic characteristics of patients in the SEER database according to molecular subtypes.
| Patients characteristics according to molecular subtypes | |||||||||||||
| Patients characteristics | Molecular subtypes | ||||||||||||
| HR+/HER2− | HR+/HER2+ | HR−/HER2+ | Triple negative | Unknown | Total | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | P | |
| All patients | 7381 | 51.6 | 2146 | 15.0 | 1166 | 8.2 | 1690 | 11.8 | 1923 | 13.4 | 14306 | 100 | |
| Age, y | <.001 | ||||||||||||
| <40 | 422 | 5.7 | 237 | 11.0 | 132 | 11.3 | 141 | 8.3 | 64 | 3.3 | 996 | 7.0 | |
| 40–70 | 4740 | 64.2 | 1481 | 69.0 | 808 | 69.3 | 1121 | 66.3 | 1078 | 56.1 | 9228 | 64.5 | |
| ≥70 | 2219 | 30.1 | 428 | 19.9 | 226 | 19.4 | 428 | 25.3 | 781 | 40.6 | 4082 | 28.5 | |
| Race | <.001 | ||||||||||||
| White | 5671 | 76.8 | 1583 | 73.8 | 838 | 71.9 | 1138 | 67.3 | 1482 | 77.1 | 10712 | 74.9 | |
| Black | 1103 | 14.9 | 381 | 17.8 | 206 | 17.7 | 436 | 25.8 | 311 | 16.2 | 2437 | 17.0 | |
| Others | 575 | 7.8 | 178 | 8.3 | 116 | 9.9 | 113 | 6.7 | 115 | 6.0 | 1097 | 7.7 | |
| Unknown | 32 | 0.4 | 4 | 0.2 | 6 | 0.5 | 3 | 0.2 | 15 | 0.8 | 60 | 0.4 | |
| Year of diagnosis | <.001 | ||||||||||||
| 2010 | 1104 | 15.0 | 291 | 13.6 | 163 | 14.0 | 282 | 16.7 | 393 | 20.4 | 2233 | 15.6 | |
| 2011 | 1244 | 16.9 | 334 | 15.6 | 183 | 15.7 | 281 | 16.6 | 338 | 17.6 | 2380 | 16.6 | |
| 2012 | 1186 | 16.1 | 370 | 17.2 | 180 | 15.4 | 265 | 15.7 | 308 | 16.0 | 2309 | 16.1 | |
| 2013 | 1311 | 17.8 | 358 | 16.7 | 198 | 17.0 | 302 | 17.9 | 325 | 16.9 | 2494 | 17.4 | |
| 2014 | 1272 | 17.2 | 376 | 17.5 | 221 | 19.0 | 286 | 16.9 | 290 | 15.1 | 2445 | 17.1 | |
| 2015 | 1264 | 17.1 | 417 | 19.4 | 221 | 19.0 | 274 | 16.2 | 269 | 14.0 | 2445 | 17.1 | |
| Grade | <.001 | ||||||||||||
| I | 671 | 9.1 | 48 | 2.2 | 4 | 0.3 | 23 | 1.4 | 70 | 3.6 | 816 | 5.7 | |
| II | 3011 | 40.8 | 697 | 32.5 | 257 | 22.0 | 240 | 14.2 | 321 | 16.7 | 4526 | 31.6 | |
| III/IV | 2135 | 28.9 | 1061 | 49.4 | 710 | 60.9 | 1166 | 69.0 | 408 | 21.2 | 5480 | 38.3 | |
| Unknown | 1564 | 21.2 | 340 | 15.8 | 195 | 16.7 | 261 | 15.4 | 1124 | 58.5 | 3483 | 24.4 | |
| Laterality | <.001 | ||||||||||||
| Right | 3527 | 47.8 | 994 | 46.3 | 550 | 47.2 | 803 | 47.5 | 708 | 36.8 | 6582 | 46.0 | |
| Left | 3552 | 48.1 | 1103 | 51.4 | 590 | 50.6 | 836 | 49.5 | 826 | 43.0 | 6907 | 48.3 | |
| Bilateral | 37 | 0.5 | 10 | 0.5 | 12 | 1.0 | 6 | 0.4 | 23 | 1.2 | 88 | 0.6 | |
| Unknown | 265 | 3.6 | 39 | 1.8 | 14 | 1.2 | 45 | 2.7 | 366 | 19.0 | 729 | 5.1 | |
| Lymph node | <.001 | ||||||||||||
| N0 | 1568 | 21.2 | 401 | 18.7 | 155 | 13.3 | 308 | 18.2 | 482 | 25.1 | 2914 | 20.4 | |
| N1 | 2600 | 35.2 | 782 | 36.4 | 434 | 37.2 | 564 | 33.4 | 451 | 23.5 | 4831 | 33.8 | |
| N2 | 678 | 9.2 | 191 | 8.9 | 112 | 9.6 | 145 | 8.6 | 86 | 4.5 | 1212 | 8.5 | |
| N3 | 1859 | 25.2 | 612 | 28.5 | 388 | 33.3 | 563 | 33.3 | 365 | 19.0 | 3787 | 26.5 | |
| Nx | 676 | 9.2 | 160 | 7.5 | 77 | 6.6 | 110 | 6.5 | 539 | 28.0 | 1562 | 10.9 | |
| Marital status | <.001 | ||||||||||||
| Single | 1609 | 21.8 | 511 | 23.8 | 241 | 20.7 | 374 | 22.1 | 449 | 23.3 | 3184 | 22.3 | |
| Married | 3154 | 42.7 | 957 | 44.6 | 515 | 44.2 | 708 | 41.9 | 705 | 36.7 | 6039 | 42.2 | |
| Others | 2191 | 29.7 | 557 | 26.0 | 336 | 28.8 | 522 | 30.9 | 633 | 32.9 | 4239 | 29.6 | |
| Unknown | 427 | 5.8 | 121 | 5.6 | 74 | 6.3 | 86 | 5.1 | 136 | 7.1 | 844 | 5.9 | |
| Insurance | <.001 | ||||||||||||
| Uninsured | 288 | 3.9 | 111 | 5.2 | 42 | 3.6 | 83 | 4.9 | 106 | 5.5 | 630 | 4.4 | |
| Insured | 5829 | 79.0 | 1660 | 77.4 | 941 | 80.7 | 1336 | 79.1 | 1372 | 71.3 | 11138 | 77.9 | |
| Unknown | 1264 | 17.1 | 375 | 17.5 | 183 | 15.7 | 271 | 16.0 | 445 | 23.1 | 2538 | 17.7 | |
| Surgery | <.001 | ||||||||||||
| Surgery | 1938 | 26.3 | 631 | 29.4 | 376 | 32.2 | 611 | 36.2 | 269 | 14.0 | 3825 | 26.7 | |
| No-surgery | 5290 | 71.7 | 1476 | 68.8 | 752 | 64.5 | 1047 | 62.0 | 1627 | 84.6 | 10192 | 71.2 | |
| Unknown | 153 | 2.1 | 39 | 1.8 | 38 | 3.3 | 32 | 1.9 | 27 | 1.4 | 289 | 2.0 | |
| Bone metastases | <.001 | ||||||||||||
| No | 1526 | 20.7 | 642 | 29.9 | 536 | 46.0 | 841 | 49.8 | 558 | 29.0 | 4103 | 28.7 | |
| Yes | 5729 | 77.6 | 1459 | 68.0 | 597 | 51.2 | 816 | 48.3 | 1257 | 65.4 | 9858 | 68.9 | |
| Unknown | 126 | 1.7 | 45 | 2.1 | 33 | 2.8 | 33 | 2.0 | 108 | 5.6 | 345 | 2.4 | |
| Lung metastases | <.001 | ||||||||||||
| No | 4995 | 67.7 | 1381 | 64.4 | 674 | 57.8 | 893 | 52.8 | 1153 | 60.0 | 9096 | 63.6 | |
| Yes | 2102 | 28.5 | 684 | 31.9 | 445 | 38.2 | 756 | 44.7 | 610 | 31.7 | 4597 | 32.1 | |
| Unknown | 284 | 3.8 | 81 | 3.8 | 47 | 4.0 | 41 | 2.4 | 160 | 8.3 | 613 | 4.3 | |
| Liver metastases | <.001 | ||||||||||||
| No | 5622 | 76.2 | 1268 | 59.1 | 552 | 47.3 | 1119 | 66.2 | 1263 | 65.7 | 9824 | 68.7 | |
| Yes | 1521 | 20.6 | 817 | 38.1 | 582 | 49.9 | 529 | 31.3 | 520 | 27.0 | 3969 | 27.7 | |
| Unknown | 238 | 3.2 | 61 | 2.8 | 32 | 2.7 | 42 | 2.5 | 140 | 7.3 | 513 | 3.6 | |
| Brian metastases | <.001 | ||||||||||||
| No | 6654 | 90.2 | 1889 | 88.0 | 977 | 83.8 | 1427 | 84.4 | 1599 | 83.2 | 12546 | 87.7 | |
| Yes | 428 | 5.8 | 174 | 8.1 | 148 | 12.7 | 212 | 12.5 | 156 | 8.1 | 1118 | 7.8 | |
| Unknown | 299 | 4.1 | 83 | 3.9 | 41 | 3.5 | 51 | 3.0 | 168 | 8.7 | 642 | 4.5 | |
| Vital status | <.001 | ||||||||||||
| Alive | 3725 | 50.5 | 1245 | 58.0 | 607 | 52.1 | 402 | 23.8 | 596 | 31.0 | 6575 | 46.0 | |
| Death | 3656 | 49.5 | 901 | 42.0 | 559 | 47.9 | 1288 | 76.2 | 1327 | 69.0 | 7731 | 54.0 | |
| Cause of death | <.001 | ||||||||||||
| Alive | 3725 | 50.5 | 1245 | 58.0 | 607 | 52.1 | 402 | 23.8 | 596 | 31.0 | 6575 | 46.0 | |
| Breast cancer | 3170 | 42.9 | 801 | 37.3 | 500 | 42.9 | 1138 | 67.3 | 1076 | 56.0 | 6685 | 46.7 | |
| Others | 486 | 6.6 | 100 | 4.7 | 59 | 5.1 | 150 | 8.9 | 251 | 13.1 | 1046 | 7.3 | |
HER2 = human epidermal growth factor receptor 2, HR = hormone receptor, SEER = surveillance, epidemiology, and end results.
3.2. Metastasis pattern
Figure 1 showed that the incidence of bone metastasis was the highest, accounting for 68.9% and brain was the least common metastatic site, reflecting 7.8% of the entire cohort. The percentage with metastasis to bone, lung, liver and brain in HR+/HER2– subtype was 77.6%, 28.5%, 20.6%, and 5.8%. In the triple negative patients, the percentage was 48.3%, 44.7%, 31.3%, and 12.5%. However, patients with HR+/HER2+ and HR–/HER2+ tumors, the probability of liver metastases was higher than lung metastases.
Figure 1.
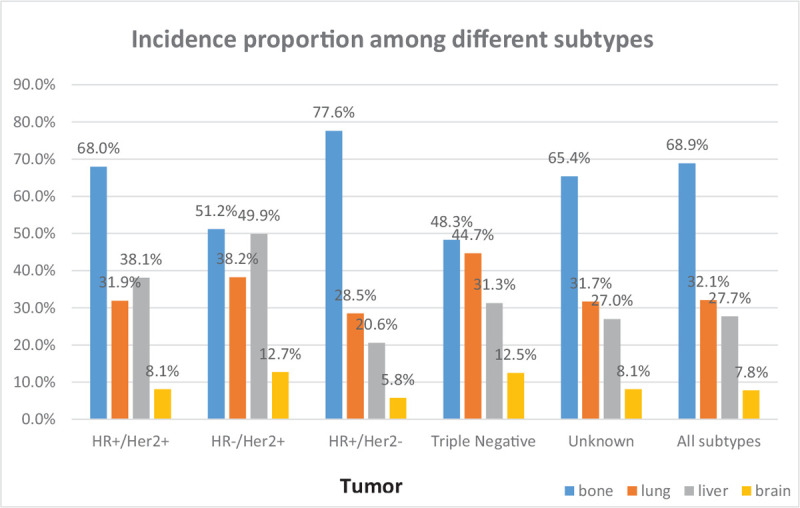
The incidence proportion of patients with initial different metastatic sites among breast cancer patients according to tumor subtype in the SEER cohort. SEER = surveillance, epidemiology, and end results.
3.3. Survival analysis
The median OS and BCSS among the whole cohort was 25 and 30 months, respectively. As shown in Figure 2, significant statistical difference in survival outcomes was observed among different tumor subtypes (P < .001). Patients with HR+/HER2+ subtype experienced the longest median OS (39 months, 95% CI: 36.1–41.9) and BCSS (43 months, 95% CI: 39.6–46.4), whereas the triple negative breast cancer had the worst survival (median OS: 11 months; median BCSS: 12 months, respectively). To further study the effect of metastasis sites and surgery on survival, we adopted the following analysis which is shown in Figures 3 and 4. We found significant difference in OS and BCSS of patients with only 1 metastasis site (Log rank P < .001), and patients with only bone metastases had the longest median survival (median OS: 36 months, median BCSS: 42 months), whereas brain metastases had the worst OS and BCSS (median OS: 12 months, median BCSS: 13 months). Furthermore, the median OS and BCSS in patients with multiple sites of metastases were 16 and 20 months. As shown in Figure 4 which represented the prognostic impact of primary tumor surgery. Kaplan–Meier curves showed higher OS and BCSS in patients undergoing primary tumor surgery compared with those who did not (P < .001). In addition, a subgroup analysis was adopted to compare the OS according to various tumor subtypes. The results showed that patients undergone primary surgery had the better OS whichever subtypes it were (P < .001). It had to be noted that unknown patients were excluded from our statistical analyses. We used univariate and multivariate Cox proportional hazard models to determine the prognostic factors, shown in Table 2. In the univariate analysis, age, race, tumor grade, marital status, insurance status, surgery treatment, molecular subtypes, and metastasis sites significantly affected OS and BCSS (P < .001). Lymph node status was significantly correlated with BCSS (P < .05) but not with OS. The multivariate Cox model confirmed that age, race, tumor grade, marital status, insurance status, surgery, molecular subtypes, and metastasis sites were independent prognostic factors for both OS and BCSS (P < .001).
Figure 2.
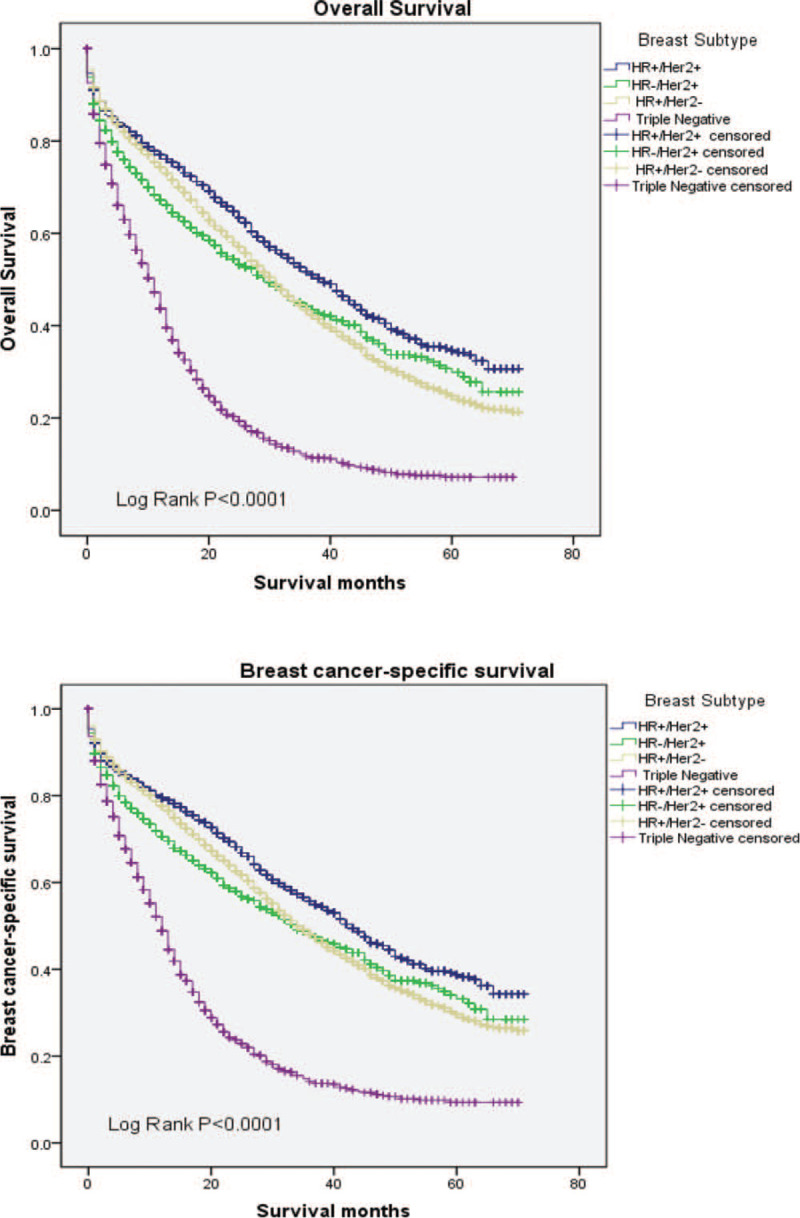
Overall survival and breast cancer-specific survival according to tumor subtypes.
Figure 3.
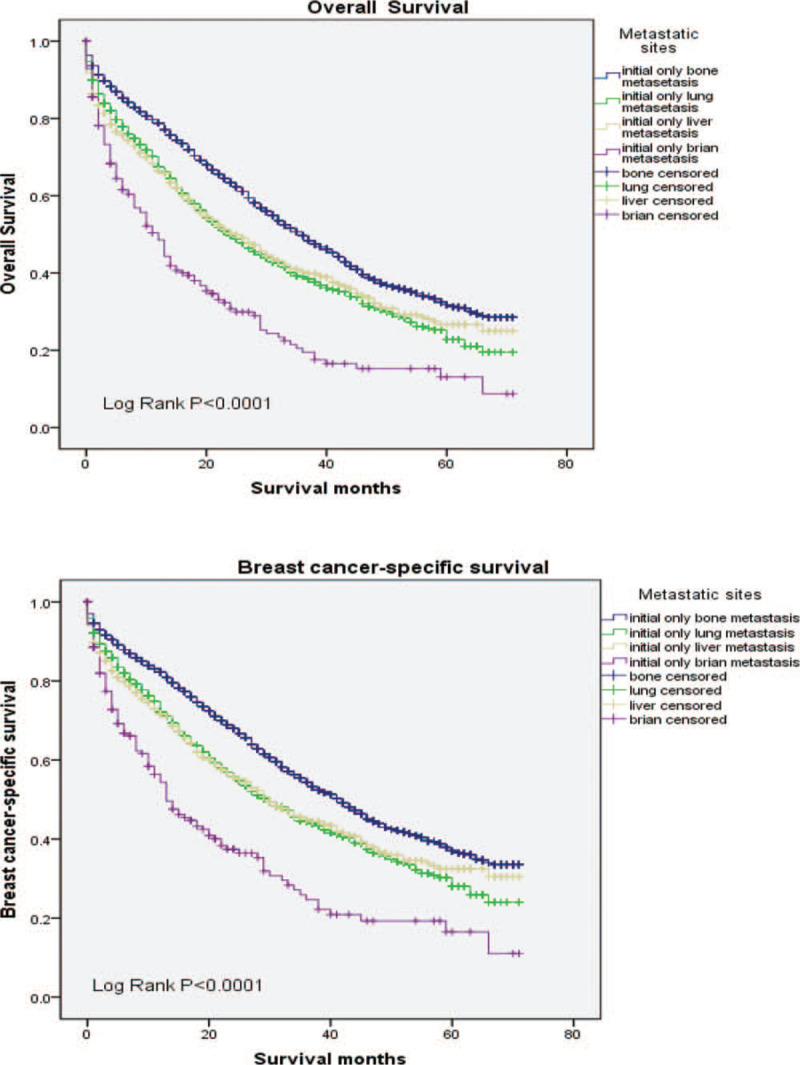
Kaplan-Meier curve for OS and BCSS in patients with primary metastatic breast cancer in different metastatic sites. BCSS = breast cancer-specific survival, OS = overall survival.
Figure 4.
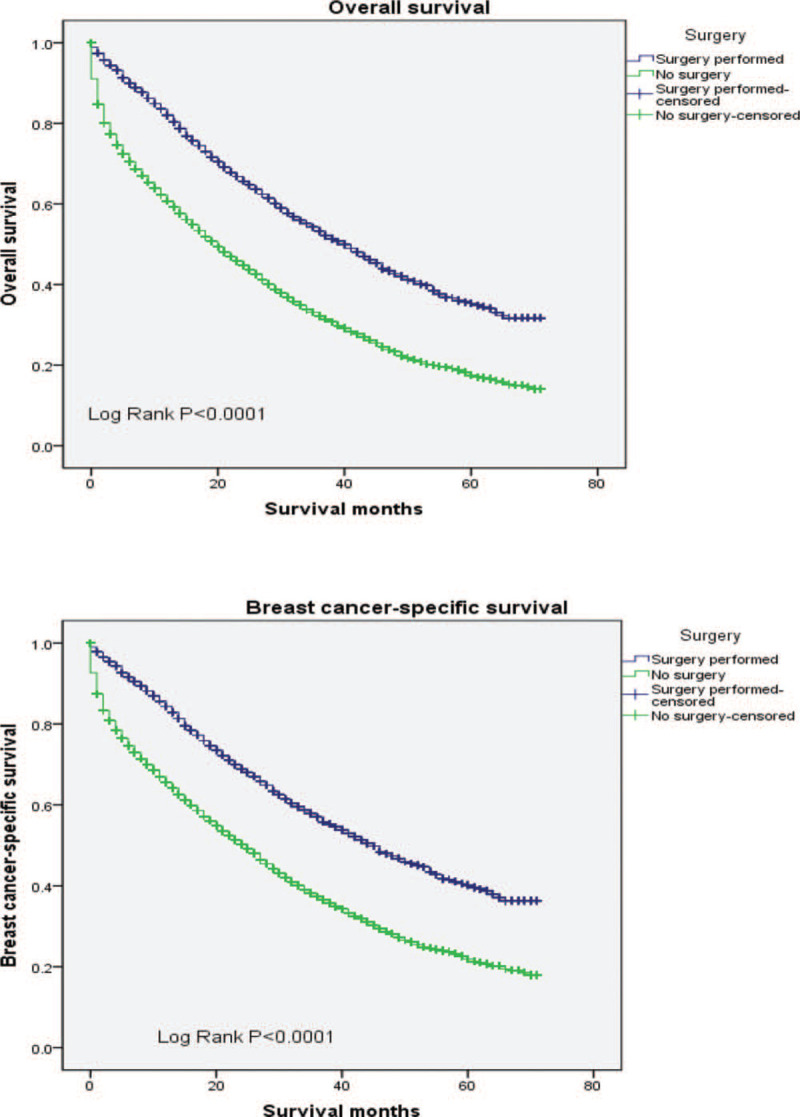
Surgery of survival analysis in OS and BCSS among patients in primary metastatic breast cancer patients. BCSS = breast cancer-specific survival, OS = overall survival.
Table 2.
Cox proportional hazards regression model analysis of overall survival and breast cancer-specific survival of initial metastatic breast cancer patients.
| OS | BCSS | |||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P | HR | P | HR | P | HR | P | |
| Age, y | ||||||||
| <40 | Reference | Reference | Reference | Reference | ||||
| 40–70 | 1.503 (1.354–1.668) | <.001 | 1.434 (1.291–1.594) | <.001 | 1.483 (1.328–1.656) | <.001 | 1.432 (1.281–1.601) | <.001 |
| ≥70 | 2.396 (2.151–2.668) | <.001 | 2.175 (1.943–2.435) | <.001 | 2.214 (1.975–2.482) | <.001 | 2.063 (1.830–2.326) | <.001 |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Black | 1.282 (1.211–1.358) | <.001 | 1.185 (1.117–1.257) | <.001 | 1.271 (1.194–1.352) | <.001 | 1.158 (1.086–1.234) | <.001 |
| Others | 0.880 (0.804–0.963) | .005 | 0.958 (0.875–1.049) | .352 | 0.887 (0.805–0.976) | .014 | 0.951 (0.863–1.047) | .306 |
| Year of diagnosis | ||||||||
| 2010 | Reference | Reference | ||||||
| 2011 | 0.962 (0.900–1.029) | .260 | 0.970 (0.903–1.043) | .410 | ||||
| 2012 | 0.938 (0.874–1.007) | .075 | 0.974 (0.878–1.021) | .157 | ||||
| 2013 | 0.958 (0.890–1.031) | .251 | 0.958 (0.885–1.037) | .286 | ||||
| 2014 | 0.964 (0.888–1.046) | .378 | 0.944 (0.864–1.032) | .204 | ||||
| 2015 | 0.846 (0.760–0.941) | .002 | 0.856 (0.762–0.961) | .009 | ||||
| Grade | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 1.202 (1.071–1.349) | .002 | 1.222 (1.089–1.372) | .001 | 1.297 (1.141–1.474) | <.001 | 1.310 (1.152–1.489) | <.001 |
| III/IV | 1.729 (1.545–1.935) | <.001 | 1.684 (1.499–1.892) | <.001 | 1.930 (1.703–2.186) | <.001 | 1.866 (1.641–2.122) | <.001 |
| Laterality | ||||||||
| Right | Reference | Reference | Reference | Reference | ||||
| Left | 1.013 (0.967–1.061) | .579 | 1.003 (0.958–1.050) | .902 | 1.019 (0.969–1.070) | .465 | 1.008 (0.959–1.059) | .757 |
| Bilateral | 1.520 (1.187–1.946) | .001 | 1.040 (0.811–1.334) | .758 | 1.445 (1.010–1.896) | .008 | 1.012 (0.770–1.330) | .933 |
| Lymph node metastasis | ||||||||
| N0 | Reference | Reference | Reference | Reference | ||||
| N1 | 0.863 (0.810–0.920) | <.001 | 0.899 (0.842–0.960) | .001 | 0.916 (0.855–0.981) | .013 0.930 (0.867–0.998) | 0.044 | |
| N2 | 0.842 (0.767–0.924) | <.001 | 1.026 (0.933–1.128) | .599 | 0.893 (0.809–0.986) | .026 | 1.059 (0.956–1.172) | .272 |
| N3 | 1.061 (0.994–1.133) | .074 | 1.035 (0.968–1.107) | .315 | 1.115 (1.039–1.197) | .002 | 1.054 (0.980–1.134) | .157 |
| Marital status | ||||||||
| Single | Reference | Reference | Reference | Reference | ||||
| Married | 0.759 (0.716–0.805) | <.001 | 0.804 (0.757–0.854) | <.001 | 0.768 (0.721–0.818) | <.001 | 0.816 (0.765–0.871) | <.001 |
| Others | 1.157 (1.090–1.229) | <.001 | 1.010 (0.948–1.077) | .748 | 1.137 (1.065–1.213) | <.001 | 1.016 (0.949–1.088) | .644 |
| Insurance | ||||||||
| Uninsured | Reference | Reference | Reference | Reference | ||||
| Insured | 0.751 (0.677–0.833) | <.001 | 0.788 (0.709–0.875) | <.001 | 0.739 (0.662–0.826) | <.001 | 0.787 (0.704–0.881) | <.001 |
| Surgery | ||||||||
| Surgery | Reference | Reference | Reference | Reference | ||||
| No-surgery | 1.921 (1.820–2.029) | <.001 | 1.784 (1.682–1.891) | <.001 | 1.887 (1.780–2.000) | <.001 | 1.798 (1.690–1.914) | <.001 |
| Molecular subtypes | ||||||||
| HR+/HER2+ | Reference | Reference | Reference | Reference | ||||
| HR−/HER2+ | 1.293 (1.163–1.436) | <.001 | 1.279 (1.150–1.422) | <.001 | 1.304 (1.166–1.458) | <.001 | 1.284 (1.148–1.437) | <.001 |
| HR+/HER2− | 1.223 (1.137–1.315) | <.001 | 1.309 (1.215–1.410) | <.001 | 1.194 (1.105–1.290) | <.001 | 1.309 (1.209–1.417) | <.001 |
| Triple Negative | 2.924 (2.683–3.186) | <.001 | 2.931 (2.686–3.199) | .009 | 2.942 (2.685–3.222) | <.001 | 2.977 (2.713–3.267) | <.001 |
| Metastasis sites | ||||||||
| Bone | Reference | Reference | Reference | Reference | ||||
| Lung | 1.383 (1.272–1.504) | <.001 | 1.114 (1.022–1.215) | .014 | 1.371 (1.252–1.502) | <.001 | 1.087 (0.990–1.194) | .080 |
| Liver | 1.371 (1.247–1.506) | <.001 | 1.423 (1.292–1.566) | <.001 | 1.370 (1.238–1.517) | <.001 | 1.386 (1.249–1.539) | <.001 |
| Brain | 2.318 (1.953–2.751) | <.001 | 1.904 (1.602–2.263) | <.001 | 2.340 (1.945–2.816) | <.001 | 1.904 (1.579–2.295) | <.001 |
| ≥2 Sites | 1.945 (1.841–2.054) | <.001 | 1.742 (1.646–1.843) | <.001 | 2.029 (1.914–2.152) | <.001 | 1.792 (1.686–1.904) | <.001 |
BCSS = breast cancer-specific survival, CI = confidence interval, HER2 = human epidermal growth factor receptor 2, HR = hormone receptor, OS = overall survival.
4. Discussion
This study suggested that molecular subtypes demonstrate a strong correlation to the prognosis of patients with primary stage IV breast cancer. Patients with triple negative subtype and brain metastases had the worst outcome. Furthermore, patients undergoing primary surgery had better prognosis than those who did not.
We observed significantly differences of prognosis according to tumor subtypes. HR+/HER2+ subtype experienced the longest OS and BCSS whereas the triple negative had the worst survival. However, this result was slightly discrepant with a study conducted by Kennecke et al who included 3726 patients with early-stage breast cancer from 1986 to 1992 then 1357 developed distant metastases during subsequent follow up. The median survival (MS) of luminal A (MS = 2.2 years) and luminal B (MS = 1.6 years) subtypes were longer than that of luminal/HER2 (MS = 1.3 years) subtype.[22] The survival difference may due to the fact that the latter did not undergo HER2 targeted therapy which rapidly implemented in 1998. In addition, an observational study conducted in Netherlands had drawn similar conclusion to this study that HR+/HER2+ subtype survived the longest time than the others.[23] There was light difference in the effect of molecular subtypes on the prognosis of early and advanced breast cancer. Unlike primary stage IV breast cancer patients, 10-year OS was higher for luminal A (HR+/HER2−) subtype compared with other subtypes (P < .001) in early-stage breast cancer patients. Triple negative subtype had the worst prognosis whether in early or metastatic ones,[24] which signified triple negative breast cancer was a heterogeneous disease and still the hotpot of future researches. Our study suggested that molecular subtypes were independent prognostic factors for PMBC and therefore it is necessity to re-biopsy the metastatic tissue to identify whether the molecular subtype has changed. In conclusion, this article confirmed that molecular subtypes are great relevance to the prognosis of the de novo stage-IV breast cancer. This study demonstrated that breast cancer subtypes were associated with the unique site of distant metastases. We found that bone was the most common metastatic organ and the incidence of bone metastases was highest in patients with HR+/HER2− status. Several studies had indicated the similar results.[8,15,18–20] The strong association of HR–/HER2+ and triple negative subtypes with lung metastases were reported in our study. Previous research suggested the lung metastasis gene-expression was expressed in breast cancer cells and it developed a high risk of lung metastases. It was reported that this gene could both mediate and predict lung metastases.[25] Tumors with HR− status, basal-like molecular subtype were tended to lung metastases.[22,26] This study showed liver and brain metastases were more likely to occur in HR−/HER2+ tumors and furthermore, liver metastases were more frequently observed in HER2+ subtype. This could be deeply understanding by knowing the relationship between chemokine receptor CXCR4 and HER2. A research demonstrated that the expression of CXCR4 predicted for the development of liver metastases and another study showed that HER2 over-expression up-regulated the expression of CXCR4.[27,28] In addition, the HER2+ had been reported having a strong relationship with brain metastases.[22,29] A phase II study revealed the HER2+ was associated with central nervous system involvement[30] and Bos et al found that 6 of the 17 genes which were associated with brain relapse were shared with lung metastasis gene-expression signature.[26,31] The univariate and multivariate analyses based on OS and BCSS demonstrated statistically significant difference in primary stage IV breast cancer diagnosed with various site-specific metastasis patterns. We only selected patients with one distant metastasis site to eliminate the hybrid bias in the study, and results showing patients with bone metastases had the best OS in all other metastatic patterns and the shortest survival time belonged to brain metastases, which were consistent with the previous researches.[8,20] This study also found that the duration of OS in brain metastases were lower than that of multiple metastasis sites. Present international guidelines for breast cancer do not recommend the routine brain screening for patients without symptoms of brain metastasis,[29] which leads to a high degree of malignancy in patients with brain metastases. The significantly shorter OS with brain metastasis than that of multiple distant metastasis sites is, to our knowledge, a novel observation. There is a dispute over whether the patients with primary stage IV breast cancer should be treated surgically. Our study suggested tumor surgery improved the OS time of these patients. Several previous studies had reported the similar results.[6,8,16,17,32] Meanwhile, we found primary tumor excision with various molecular subtypes had the better OS. There were few studies reporting the relationship between surgery and tumor subtypes in de novo MBC patients. A study showed patients with either HR+ or HER2+ who experiencing surgery was associated with improved survival, which the authors thought this survival benefit in patients with HER2+ because of targeted therapy.[33] Recently a large, retrospective, population-based cohort study suggested patients with younger age, lower disease burden, smaller primary breast tumors, and improvement of systemic treatment could have greater benefit from surgery.[32] In contrast, some studies reported no benefit of the primary tumor removal.[34,35] Surgery-induced immunosuppression, circulating tumor cells to the target organs, surgery-induced angiogenic switch, and the post-operative inflammatory reaction could be the reason leading to the shorter duration of survival.[35] So more rigorous, large-scale clinical studies should be carried out to address this point in the future. However, this study also has some limitations. First, information relating to systemic treatment such as chemotherapy, endocrine therapy, targeted therapy and radiotherapy is not available in the seer database, which may exert an important impact on prognosis. Secondly, the SEER database only provides information on distant metastasis sites such as bone, lung, liver, and brain not on other sites. Thirdly, molecular subtypes were categorized according to HR and HER2 status without other marks such as Ki-67; thus, we could not further subdivide the molecular subtypes.
5. Conclusions
In general, in this study, we developed a deeper understanding of the relationship between primary stage IV cancer and the various molecular subtypes. The survival time of patients with brain metastases is the shortest. Surgical excision of primary tumor can improve the prognosis. Finally, we hope to develop appropriate programs for each primary metastatic breast cancer patients to prolong survival and improve quality of life.
Author contributions
Yang Li: (1) acquisition of data and analysis and interpretation of data; (2) drafting the article;
Shuaibing Wang: substantial contributions to conception and design and revising the article critically for important intellectual content;
Wenbo Yang: Contributions to statistical assistance and final revision;
Hong Liu: study design and final approval of the version to be published.
Conceptualization: Yang Li.
Data curation: Yang Li.
Formal analysis: Yang Li, Shuaibing Wang.
Funding acquisition: Hong Liu.
Investigation: Yang Li, Shuaibing Wang.
Software: Wenbo Yang.
Supervision: Hong Liu.
Writing – original draft: Yang Li.
Writing – review & editing: Shuaibing Wang, Wenbo Yang.
Footnotes
Abbreviations: BCSS = breast cancer-specific survival, HER2 = human epidermal growth factor receptor 2, HR = hormone receptor, OS = overall survival, PMBC = primary metastatic breast cancer, SEER = surveillance, epidemiology, and end results.
How to cite this article: Li Y, Wang S, Yang W, Liu H. Prognostic significance of molecular subtype, metastatic site and primary tumor surgery for survival in primary metastatic breast cancer: A SEER-based study. Medicine. 2021;100:27(e26619).
YL, SW, and WY contributed equally to this article.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:07–34. [DOI] [PubMed] [Google Scholar]
- [2].Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol 2004;22:3302–8. [DOI] [PubMed] [Google Scholar]
- [3].Barinoff J, Heitz F, Kuemmel S, et al. Improvement of survival in patient with primary metastatic breast cancer over a 10-year periode: prospective analyses based on individual patient date from a multicenter data bank. J Cancer Ther 2013;04:1306–12. [Google Scholar]
- [4].Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134–50. [DOI] [PubMed] [Google Scholar]
- [5].Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 2010;102:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 2010;21:2169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gong Y, Liu YR, Ji P, et al. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep 2017;7:45411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502. [DOI] [PubMed] [Google Scholar]
- [10].Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. [DOI] [PubMed] [Google Scholar]
- [11].Onitilo AA, Engel JM, Greenlee RT, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009;7:04–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bonotto M, Gerratana L, Poletto E, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist 2014;19:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol 2008;19:2012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gong Y, Zhang J, Ji P, et al. Incidence proportions and prognosis of breast cancer patients with bone metastases at initial diagnosis. Cancer Med 2018;7:4156–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Warschkow R, Guller U, Tarantino I, et al. Improved survival after primary tumor surgery in metastatic breast cancer: a propensity-adjusted, population-based SEER trend analysis. Ann Surg 2016;263:1188–98. [DOI] [PubMed] [Google Scholar]
- [17].Ruiterkamp J, Ernst MF, van de Poll-Franse LV, et al. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol 2009;35:1146–51. [DOI] [PubMed] [Google Scholar]
- [18].Wu Q, Li J, Zhu S, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget 2017;8:27990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sihto H, Lundin J, Lundin M, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res 2011;13:R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang H, Zhang C, Zhang J, et al. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget 2017;8:26368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Malmgren JA, Mayer M, Atwood MK, et al. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990-2010. Breast Cancer Res Treat 2018;167:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–7. [DOI] [PubMed] [Google Scholar]
- [23].Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 2013;141:507–14. [DOI] [PubMed] [Google Scholar]
- [24].Metzger-Filho O, Sun Z, Viale G, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol 2013;31:3083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu S, Wu J, Fang Y, et al. The impact of surgical excision of the primary tumor in stage IV breast cancer on survival: a meta-analysis. Oncotarget 2018;9:11816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Minn AJ, Gupta GP, Padua D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A 2007;104:6740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Andre F, Cabioglu N, Assi H, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol 2006;17:945–51. [DOI] [PubMed] [Google Scholar]
- [28].Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 2004;6:459–69. [DOI] [PubMed] [Google Scholar]
- [29].Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 2017;3:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Crivellari D, Pagani O, Veronesi A, et al. High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann Oncol 2001;12:353–6. [DOI] [PubMed] [Google Scholar]
- [31].Bos PD, Zhang XHF, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009;459:1005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thomas A, Khan SA, Chrischilles EA, et al. Initial surgery and survival in stage IV breast cancer in the United States, 1988-2011∗∗. JAMA Surg 2016;151:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neuman HB, Morrogh M, Gonen M, et al. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer 2010;116:1226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barinoff J, Schmidt M, Schneeweiss A, et al. Primary metastatic breast cancer in the era of targeted therapy – Prognostic impact and the role of breast tumour surgery. Eur J Cancer 2017;83:116–24. [DOI] [PubMed] [Google Scholar]
- [35].Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380–8. [DOI] [PubMed] [Google Scholar]


