Abstract
Increased neutrophil extracellular trap (NET) formation associates with high cardiovascular risk and mortality in patients with end-stage renal disease (ESRD). However, the effect of transplantation on NETs and its associated markers remains unclear. This study aimed to characterize circulating citrullinated Histone H3 (H3cit) and Peptidyl Arginase Deiminase 4 (PAD4) in ESRD patients undergoing transplantation and evaluate the ability of their neutrophils to release NETs.
This prospective cohort study included 80 healthy donors and 105 ESRD patients, out of which 95 received a transplant. H3cit and PAD4 circulating concentration was determined by enzyme-linked immunosorbent assay in healthy donors and ESRD patients at the time of enrollment. An additional measurement was carried out within the first 6 months after transplant surgery. In vitro NET formation assays were performed in neutrophils isolated from healthy donors, ESRD patients, and transplant recipients.
H3cit and PAD4 levels were significantly higher in ESRD patients (H3cit, 14.38 ng/mL [5.78–27.13]; PAD4, 3.22 ng/mL [1.21–6.82]) than healthy donors (H3cit, 6.45 ng/mL [3.30–11.65], P < .0001; PAD4, 2.0 ng/mL [0.90–3.18], P = .0076). H3cit, but not PAD4, increased after transplantation, with 44.2% of post-transplant patients exhibiting high levels (≥ 27.1 ng/mL). In contrast, NET release triggered by phorbol 12-myristate 13-acetate was higher in neutrophils from ESRD patients (70.0% [52.7–94.6]) than healthy donors (32.2% [24.9–54.9], P < .001) and transplant recipients (19.5% [3.5–65.7], P < .05).
The restoration of renal function due to transplantation could not reduce circulating levels of H3cit and PAD4 in ESRD patients. Furthermore, circulating H3cit levels were significantly increased after transplantation. Neutrophils from transplant recipients exhibit a reduced ability to form NETs.
Keywords: citrullinated histone H3, end-stage renal disease, NETosis, neutrophils, peptidyl arginase deiminase 4, transplantation
1. Introduction
Renal replacement therapy (RRT) is lifesaving in patients with End-Stage Renal Disease (ESRD). Nonetheless, patients on peritoneal dialysis (PD), hemodialysis (HD), and transplantation have a lower life expectancy and quality of life than the general population and are at increased risk of cardiovascular events and infections associated with immune system dysfunction.[1–3] RRT restores kidney function but cannot wholly reverse the alterations in innate and adaptive immunity present in ESRD patients. Moreover, RRT's choice can induce additional immune changes and influence the patient's inflammatory state.[4,5] Hence, advancing our knowledge in the mechanisms responsible for the immune abnormalities observed in ESRD patients, with and without RRT, could help us develop new therapeutic strategies to reduce their susceptibility to cardiovascular disease and bacterial and viral infections.
Neutrophils, innate immune cells that act as the first line of defense at sites of infection and tissue damage, execute their functions through phagocytosis, degranulation, cytokine production, and Neutrophil Extracellular Trap (NET) formation.[6,7] NETs are extracellular, mesh-like structures formed by decondensed chromatin and various proteins that can neutralize and eliminate pathogens to prevent them from spreading. The hypercitrullination of histones catalyzed by Peptidyl Arginine Deiminase Type 4 (PAD4) is crucial for chromatin release and NET formation, making citrullinated histone 3 (H3cit) and PAD4 useful markers of NETosis.[8,9] Since NETs’ first official characterization by Brinkmann V. et al in 2004, several studies revealed the unexpected role of NETs as drivers of autoimmunity and tissue damage in sterile inflammation.[6,7,10,11] NETs also provide a platform for complement activation and thrombus formation during coagulation and have been associated with glomerular injury, vascular dysfunction, and fibrosis in several renal diseases.[12–14]
As patients with chronic kidney disease progress to a terminal stage, their neutrophils become more abundant and activated.[15] Neutrophils from ESRD patients exhibit enhanced phagocytosis, degranulation, autophagy, and oxidative stress, though their transmigration ability and bactericidal efficiency appear to be diminished.[16–20] Moreover, patients on maintenance HD have increased NET formation and circulating nucleosomes than healthy individuals, events associated with chronic inflammation, endothelial dysfunction, coronary artery disease, and increased mortality.[17,21–23]
The study of neutrophils and NETosis in transplanted organs has centered on the ischemia-reperfusion injury, a consequence of transplant surgery.[24–26] Nonetheless, neutrophils are well-established regulators of inflammation, tolerance, and allograft damage in transplant recipients.[27] NETs are pathogenic and accumulate in bronchoalveolar lavage fluid from human lung transplant recipients with primary graft dysfunction and occur perioperatively during liver transplantation.[28,29] Interestingly, a cross-sectional study by Torres-Ruiz J. et al found enhanced spontaneous NETosis in neutrophils from kidney transplant recipients even in the absence of rejection and NETs in biopsies of patients diagnosed with acute antibody-mediated rejection.[30] Despite this evidence, the role of NETosis in renal transplantation has not been thoroughly investigated.
This study's primary objectives were to characterize circulating NET-associated markers, H3cit and PAD4, in ESRD patients undergoing transplantation and determine whether transplantation influences neutrophils’ ability to form NETs in vitro.
2. Materials and methods
2.1. Study design
In this prospective cohort study, we enrolled ESRD patients that fulfilled the inclusion criteria (any gender, aged ≥18 years, candidates for transplantation independent of their dialysis status or the cause of chronic kidney disease) and their living kidney donors. Patients were recruited between May 2018 and April 2019 in the Transplantation Unit at the UMAE- Hospital de Especialidades, Instituto Mexicano del Seguro Social in Guadalajara, Jalisco, Mexico. In addition, a 6 month follow-up was performed in the subset of ESRD patients who received a renal transplant after enrollment. Patients that experienced allograft loss or death within the follow-up period were excluded from the study. All transplant recipients received a triple immunosuppressive scheme based on prednisone (PDN), mycophenolate mofetil, and a calcineurin inhibitor, either tacrolimus (TAC) or Cyclosporine A (CYA). Calcineurin inhibitors were individually adjusted according to serum levels by their clinicians.
This study evaluated the biochemical parameters and NET-associated events at 2 different time points: pre-transplantation and the first 6 months after the transplant surgery. Evidence suggests that many allografts exhibit detrimental molecular changes and histological damage noticeable as soon as the first year post-surgery.[31,32] Thus, we hypothesized that NET-associated changes that arise early, within the first 6 months after transplantation, could reflect immunological processes that precede pathological evidence in the allograft.
The guidelines for conducting observational studies Strengthening the Reporting of Observational studies in Epidemiology were followed. The final sample size was determined by the number of renal transplant candidates and their living donors that accepted to participate in the present study enrolled in our hospital between May 2018 and April 2019. Bias from loss to follow-up was prevented by selecting a study population, transplant recipients, that is closely monitored in our hospital during the first year post-transplantation. The Research and Ethics Committee at Instituto Mexicano del Seguro Social approved the study (CNIC protocol number: R-2018-785-003). The procedures comply with the 2013 Fortaleza amendment of the Declaration of Helsinki, local regulations regarding Health Research, and institutional regulations. All subjects provided written informed consent before enrollment.
2.2. Data collection
Baseline information of ESRD patients was collected at enrollment. Laboratory measurements, corresponding to each sample collection time, were assayed by routine laboratory techniques and retrieved from the patient's electronic medical records with the transplant procedure's information. Data collected for ESRD patients included age, gender, weight, height, systolic and diastolic blood pressure, and RRT type. Additional data was collected for transplant recipients including donor type (living/deceased), donor age, donor sex, number of transplants, induction therapy, and immunosuppression. Laboratory measurements included hemoglobin (Hb), white blood cell (WBC) count, neutrophil count, lymphocyte count, platelet count, urea, serum creatinine (sCr), and glucose. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.[33]
2.3. Quantification of NETosis markers
Blood samples collected into dry tubes were centrifuged at 3500 x g, and serum was retrieved, aliquoted, and stored at –80°C until processing. NETosis markers, H3cit and PAD4, were quantified by enzyme-linked immunosorbent assay using commercially available kits according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI). An Epoch microplate spectrophotometer system (Biotek, Winooski, VT) was used to measure absorbance at 450 nm. Their upper quartile defined the high levels of H3Cit and PAD4 in ESRD patients before and after transplantation.
2.3.1. Neutrophil isolation
Human neutrophils were isolated from peripheral blood collected into tubes containing ethylenediaminetetraacetic acid. The anticoagulated blood was layered over an equal volume of Polymorphoprep (Axis-Shield, Oslo, Norway) in sterile polypropylene 15 ml tubes. Tubes were centrifuged for 30 minutes at 450 × g with no brake, and neutrophils were recovered from the lower band. The purity of neutrophil preparations (> 85%) was confirmed by nuclear morphology.
2.4. In vitro NETosis assays
Sytox Green (Thermo Fisher Scientific, Inc, Waltham, MA), a nucleic acid stain, was used to observe NETosis. Briefly, purified neutrophils were resuspended in RPMI 1640 medium (Thermo Fisher Scientific, Inc, Waltham, MA). 2 x 105 cells were seeded onto glass coverslips placed into 24-well plates and treated with dimethyl sulfoxide or phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich; Merck Millipore) at a concentration of 50 nM. Sytox Green was added to a final concentration of 500 nM. Cells underwent NETosis for 2 hours at 37°C and 5% CO2. Then, the cells were fixed in 4% paraformaldehyde for 10 minutes at room temperature. Fluoroshield Mounting Medium (Abcam, Cambridge, UK) was added before placing the coverslips on top of glass slides. Images were collected with a Cytation 5 Cell Imaging Multi-Mode Reader (BioTek, Winooski, VT) using a 10X objective. Total cells and NET-forming cells were manually quantified using the ImageJ 1.51 Software (National Institutes of Health, Bethesda, MD) in 5 fields per individual. NETosis was calculated as follows: % NETs = (Number of NET-forming cells /Number of total cells) x 100.
2.5. Statistical analysis
Continuous variables are expressed as mean ± standard error of the mean or median (interquartile range). Categorical variables are presented as numbers (percentages) and compared by Pearson chi-squared test or Fisher exact test when appropriate. Normality was assessed with the Shapiro–Wilk test. Levene test was used to determine the equality of variances. Differences between groups for normally distributed variables were compared using the Student or the Welch t-test depending on the equality of variances. The Mann–Whitney U test was used to compare 2 non–normally distributed continuous variables, while more than 2 groups were compared using the Kruskal–Wallis test, followed by Dunn test for each pairwise comparison. Correlations were tested by Spearman correlation coefficient, as indicated. Data pre- and post-transplantation were assessed with the Wilcoxon rank test. NETosis markers were divided into 2 groups based on their upper quartile to define high H3cit and PAD4 in ESRD patients. All the transplant recipients included in the study were followed-up, there was no missing data. P < .05 is considered statistically significant. Data were analyzed using the IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY) and GraphPad Prism software version 6 (GraphPad Software Inc, CA).
3. Results
This study included one hundred five ESRD patients and eighty healthy donors. ESRD patients had a mean age of 30.8 ± 8.6 years, seventy-three (69.5%) were male, and thirty-two (30.5%) were female. The eGFR of only 3 (2.9%) ESRD patients was 15 ml/min/1.73 m2 or higher; the remaining one-hundred two patients (97.1%) had Chronic Kidney Disease stage 5. The mean time for sampling before transplantation was 19 ± 36 days. A subset of ninety-five (90.5%) ESRD patients that received a transplant after enrollment were further studied. Eighty living donors with a mean age of 39.7 ± 10.9 years, forty (50%) males and forty (50%) females, comprised the healthy donor group.
3.1. Detection of NETosis markers in ESRD patients
We quantified the NETosis markers H3cit and PAD4 in serum of ESRD patients and healthy donors. Both markers were significantly higher in ESRD patients (H3cit, 14.4 ng/mL [5.8–27.1]; PAD4, 3.2 ng/mL [1.2–6.8]) when compared to controls (H3cit, 6.5 ng/mL [3.3–11.7], P < .0001; PAD4, 2.0 ng/mL [0.9–3.2], P = .0076) (Fig. 1A, B). There was a positive correlation between serum levels of H3Cit and PAD4 (r = 0.5928, P < .0001) (Fig. 1C).
Figure 1.
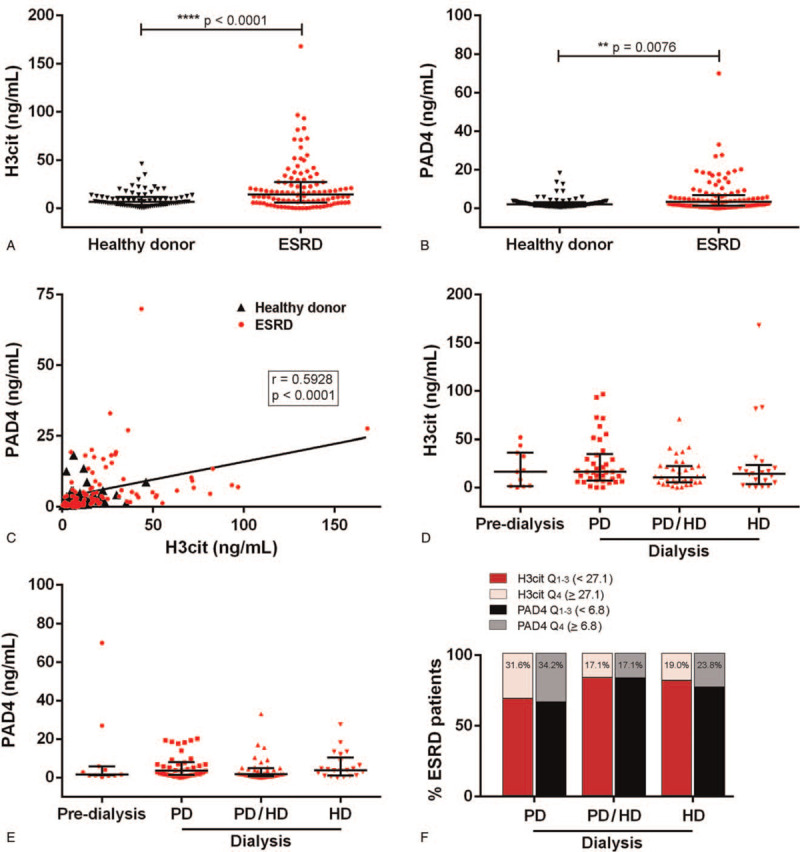
Detection of NETosis markers in ESRD patients and healthy donors. (A) H3cit and (B) PAD4 were quantified in serum from ESRD patients (n = 105) and healthy donors (n = 80) by ELISA. Results are shown as median and IQR, each data point represents a unique patient/healthy donor; ∗∗P < .01, ∗∗∗∗P < .0001 by Mann-Whitney U test. (C) For all samples, correlation of H3cit with PAD4 was assessed. Spearman's correlation coefficient was calculated and is shown in the panel. (D) H3cit and (E) PAD4 serum levels were compared according to dialysis status (pre-dialysis, PD, PD/HD, and HD). Results are shown as median and IQR, each data point represents a unique ESRD patient. (F) Percentage of ESRD patients with high H3cit and PAD4 (Q4) according to dialysis modality. ELISA = enzyme-linked immunosorbent assay, ESRD = end-stage renal disease, IQR = interquartile range.
Our ESRD cohort consisted of eleven (10.5%) pre-dialysis patients and ninety-four (89.5%) patients on different forms of RRT. Thirty-eight (36.2%) were on PD, thirty-five (33.3%) had received PD and HD, and twenty-one (20.0%) were on HD. Serum levels of H3cit and PAD4 were not significantly different between pre-dialysis patients and ESRD patients grouped by RRT (Fig. 1D, E). However, we did observe that a larger percentage of patients on PD, over 30%, had a high concentration of NETosis markers defined by the upper quartile, H3cit (≥ 27.1 ng/mL) and PAD4 (≥ 6.8 ng/mL), as shown in Figure 1F.
3.2. Characteristics of ESRD patients in relation to circulating levels of H3cit and PAD4
ESRD patients were grouped according to their high versus low levels of H3cit and PAD4 (Table 1). Age, gender, body mass index, hypertension, and being on pre-dialysis did not associate with NETosis markers (Table 1). The laboratory measurements of the twenty-six patients with high H3Cit (≥ 27.1 ng/mL) did not differ from those of the seventy-nine patients with low H3Cit (Table 1).
Table 1.
Demographic and biochemical characteristics of ESRD patients in relation to circulating levels of H3cit and PAD4.
| Low vs High H3cit | Low vs High PAD4 | |||||
| Variable | Q1–3 n = 79 | Q4 n = 26 | P-value | Q1–3 n = 79 | Q4 n = 26 | P-value |
| NETosis markers | ||||||
| H3cit, ng/mL | < 27.1 | ≥ 27.1 | – | 9.7 [5.4–19.7] | 26.7 [16.6–50.5] | < .0001 |
| PAD4, ng/mL | 1.9 [0.9–4.9] | 6.4 [4.0–14.1] | < .0001 | < 6.8 | ≥ 6.8 | – |
| Demographic data | ||||||
| Age, years | 30.7 ± 8.8 | 31.2 ± 7.9 | .546 | 31.1 ± 8.8 | 30.0 ± 8.1 | .615 |
| Male, n (%) | 56 (70.9) | 17 (65.4) | .597 | 54 (68.4) | 19 (73.1) | .650 |
| BMI, kg/m2 | 23.6 ± 3.6 | 23.6 ± 3.6 | .093 | 23.8 ± 3.5 | 22.9 ± 3.7 | .879 |
| Hypertension, n (%) | 50 (63.3) | 15 (57.7) | .610 | 50 (63.3) | 15 (57.7) | .610 |
| Pre-dialysis, n (%) | 7 (8.9) | 4 (15.4) | .459 | 9 (11.4) | 2 (7.7) | .728 |
| Laboratory measurements | ||||||
| Hb, g/dL | 10.2 ± 1.9 | 10.4 ± 2.9 | .429 | 10.3 ± 2.2 | 10.3 ± 2.0 | .944 |
| WBC count, x 103/μL | 6.3 [5.4–8.4] | 6.7 [5.4–9.6] | .399 | 6.4 [5.4–9.0] | 6.3 [5.3–9.5] | .841 |
| Neutrophils x 103/μL | 4.0 [3.2–5.1] | 4.6 [3.5–7.4] | .429 | 4.0 [3.2–5.8] | 4.1 [3.3–6.6] | .471 |
| Lymphocytes, x 103/μL | 1.3 [0.9–1.8] | 1.5 [1.0–2.0] | .187 | 1.3 [0.9–1.7] | 1.4 [1.0–1.9] | .450 |
| Platelets, x 103/μL | 225 [194–264] | 247 [190–298] | .588 | 228 [194–274] | 221 [183–261] | .568 |
| N/L ratio | 24.0 ± 56.3 | 10.1 ± 31.2 | .806 | 20.8 ± 51.5 | 19.9 ± 52.4 | .789 |
| P/L ratio | 0.6 ± 1.1 | 0.3 ± 0.9 | .195 | 0.5 ± 1.1 | 0.5 ± 1.0 | .463 |
| Urea, mmol/L | 21 [16–25] | 19 [16–21] | .160 | 21 [16–24] | 19 [16–25] | .464 |
| sCr, μmol/L | 1085 [737–1353] | 1052 [556–1236] | .411 | 1085 [718–1353] | 1045 [690–1323] | .752 |
| eGFR, mL/min/1.73 m2 | 4.3 [3.3–6.8] | 4.7 [3.8–6.2] | .528 | 4.6 [3.3–6.3] | 4.7 [3.6–7.8] | .608 |
| Glucose, mg/dL | 95 [83–109] | 91 [80–105] | .487 | 96 [82–110] | 85 [81–100] | .113 |
Data are expressed as mean ± standard error of the mean or median [IQR]. The P-values for continuous variables were calculated with unpaired t-test (parametric comparisons) or the Mann-Whitney U test (non-parametric comparisons). The p-values for categorical variables were calculated with Pearson's chi-squared test. P < .05 is considered statistically significant. eGFR = estimated glomerular filtration rate, H3cit = citrullinated histone H3, L = lymphocyte, N = neutrophil, P = platelet, PAD4 = Peptidyl arginine deiminase 4, sCr = serum creatinine, WBC = white blood cells.
3.3. Changes in biochemical parameters and NET markers after transplantation
Transplantation data, including the combination of donor/recipient sex, the type of induction therapy, and the maintenance immunosuppression received, are shown in Table 2. Ninety (94.7%) of the ESRD patients enrolled in the study received a kidney transplant for the first time; for the remaining 5 (5.3%), this was their second transplant. Most allografts, eighty-eight (92.6%), were provided by living donors, with only 7 (7.4%) ESRD patients receiving a deceased donor graft. The most commonly used induction therapies in our cohort were basiliximab (44.2%), thymoglobulin (40.0%), and thymoglobulin plus intravenous immunoglobulin (11.6%). The maintenance immunosuppression consisted of prednisone (starting with a dose of 1 mg/kg/day with reduction doses until reaching 5 mg/day a month after transplantation) and mycophenolate mofetil (1–2 g/24 h), plus a calcineurin inhibitor. Most transplant recipients, 88.4%, received TAC (3–11 mg/d) adjusted by clinicians based on the patient's serum levels (9–15 ng/ml in the first 30 days and 8–10 ng/ml during follow-up).[34]
Table 2.
Baseline characteristics of ESRD patients undergoing transplantation.
| Variables | Transplanted ESRD patients n = 95 |
| Donor age, yr | 40.6 ± 10.9 |
| Donor sex male, n (%) | 45 (47.4) |
| Donor vital status, n (%) | |
| Living | 88 (92.6) |
| Deceased | 7 (7.4) |
| Recipient age, years | 31.0 ± 8.6 |
| Recipient sex male, n (%) | 67 (70.5) |
| Combination of donor/recipient sex, n (%) | |
| Male/Male | 30 (31.6) |
| Male/Female | 15 (15.8) |
| Female/Male | 36 (37.9) |
| Female/Female | 13 (13.7) |
| Unknown/Male | 1 (1.0) |
| Induction therapy, n (%) | |
| BSX | 42 (44.2) |
| THY | 38 (40.0) |
| THY, IVIG | 11 (11.6) |
| THY, IVIG, RIX, PPH | 1 (1.1) |
| THY, IVIG, BSX, PPH | 1 (1.1) |
| THY, IVIG, PPH | 2 (2.0) |
| Maintenance immunosuppression, n (%) | |
| TAC, MMF, PDN | 84 (88.4) |
| CYA, MMF, PDN | 11 (11.6) |
| Follow-up after transplantation, months | 4.0 ± 1.6 |
| eGFR at follow-up, mL/min/1.73 m2 | 77.5 ± 25.0 |
| H3cit at follow-up, ng/mL | 31.5 ± 29.3 |
| PAD4 at follow-up, ng/mL | 7.0 ± 11.4 |
Data are expressed as mean ± standard error of the mean. BSX = basiliximab, CYA = cyclosporine A, eGFR = estimated glomerular filtration rate, H3cit = citrullinated histone H3, IVIG = intravenous immunoglobulin, MMF = mycophenolate mofetil, PAD4 = Peptidyl arginine deiminase 4, PDN = prednisone, PPH = plasmapheresis, RIX = rituximab, TAC = tacrolimus, THY = thymoglobulin.
We then assessed changes in biochemical parameters and NETosis markers 4.0 ± 1.6 months after the transplant surgery in this subset of patients. As shown in Table 3, the Hb concentration increased after transplantation (10.2 ± 2.2 g/dL to 13.0 ± 1.7 g/dL, P < .0001) as expected due to improved renal function in our cohort. The number and function of immune cells can be affected after transplantation by multiple causes including ischemia-reperfusion injury (IRI), immunosuppressive agents, rejection, and infections.[35] Here, we found a significant reduction in WBCs (6.4 x103/μl [5.4–9.1] to 6.2 x103/μl [4.6–7.8], P = .03) and neutrophils (4.0 x103/μl [3.2–6.2] to 3.8 x103/μl [3.0–4.8], P = .026) post-transplantation; accompanied by an increase in lymphocytes (1.3 x103/μl [0.9–1.9] to 1.4 x103/μl [0.9–2.1], P = .022) and platelets (227 x103/μl [193–274] to 267 x103/μl [230–314], P < .0001). The N/L ratio improved after transplantation (20.3 ± 52.3 to 3.7 ± 3.3, P = .033), though the values remained higher than those previously reported in healthy subjects,[36] suggesting a subclinical inflammatory state in transplant recipients.
Table 3.
Changes in NETosis markers and laboratory measurements after transplantation.
| Transplantation | |||
| Variables | Pre n = 95 | Post n = 95 | P-value |
| NETosis markers | |||
| H3cit, ng/mL | 13.4 [5.8–27.3] | 22.7 [8.5–49.4] | .0008 |
| PAD4, ng/mL | 2.8 [1.2–6.8] | 4.5 [1.6–8.6] | .1544 |
| Laboratory measurements | |||
| Hb, g/dL | 10.2 ± 2.2 | 13.0 ± 1.7 | <.0001 |
| WBC count, x 103/μL | 6.4 [5.4–9.1] | 6.2 [4.6–7.8] | .030 |
| Neutrophils x 103/μL | 4.0 [3.2–6.2] | 3.8 [3.0–4.8] | .026 |
| Lymphocytes, x 103/μL | 1.3 [0.9–1.9] | 1.4 [0.9–2.1] | .022 |
| Platelets, x 103/μL | 227 [193–274] | 267 [230–314] | <.0001 |
| N/L ratio | 20.3 ± 52.3 | 3.7 ± 3.3 | .033 |
| P/L ratio | 0.5 ± 1.1 | 0.3 ± 0.3 | .806 |
| Urea, mmol/L | 20 [16–25] | 7 [6–9] | <.0001 |
| sCr, μmol/L | 1085 [714–1353] | 106 [84–137] | <.0001 |
| eGFR, mL/min/1.73 m2 | 4.6 [3.4–6.3] | 75.8 [56.9–93.6] | <.0001 |
| Glucose, mg/dL | 95 [83–107] | 87 [81–96] | .002 |
Data are expressed as mean ± standard error of the mean or median [IQR]. The P-values for continuous variables were calculated with paired-samples t-test (parametric comparisons) or the Wilcoxon Rank Test (non-parametric comparisons). P values <.05 are considered statistically significant. eGFR = estimated glomerular filtration rate, H3cit = citrullinated histone H3, L = lymphocyte, N = neutrophil, P = platelet, PAD4 = Peptidyl arginine deiminase 4, sCr = serum creatinine, WBC = white blood cells.
As a natural result of transplantation, the values of urea (20 mmol/L [16–25] to 7 mmol/L [6–9], P < .0001) and sCr (1085 μmol/L [714–1353] to 106 μmol/L [84–137], P < .0001) decreased drastically and were accompanied by a significantly improved eGFR (4.6 mL/min/1.73 m2 [3.4–6.3] to 75.8 mL/min/1.73 m2 [56.9–93.6], P < .0001). Glucose was also lower in these patients after transplantation (95 mg/dL [83–107] to 87 mg/dL [81–96], P = .002) (Table 3).
When we looked at circulating NET markers, we found that the post-transplant levels of H3cit (22.7 ng/mL [8.5–49.4]), but nor PAD4, were significantly increased when compared to pre-transplant levels (13.4 ng/mL [5.8–27.3], P = .0008) (Fig. 2A, B). In addition, circulating H3cit had a strong positive correlation with platelet count (r = 0.2132, P = .0381) (Fig. 2D). In contrast, no significant correlation was observed between H3cit levels and neutrophils (Fig. 2C) or uremic burden (Fig. 2E, F).
Figure 2.
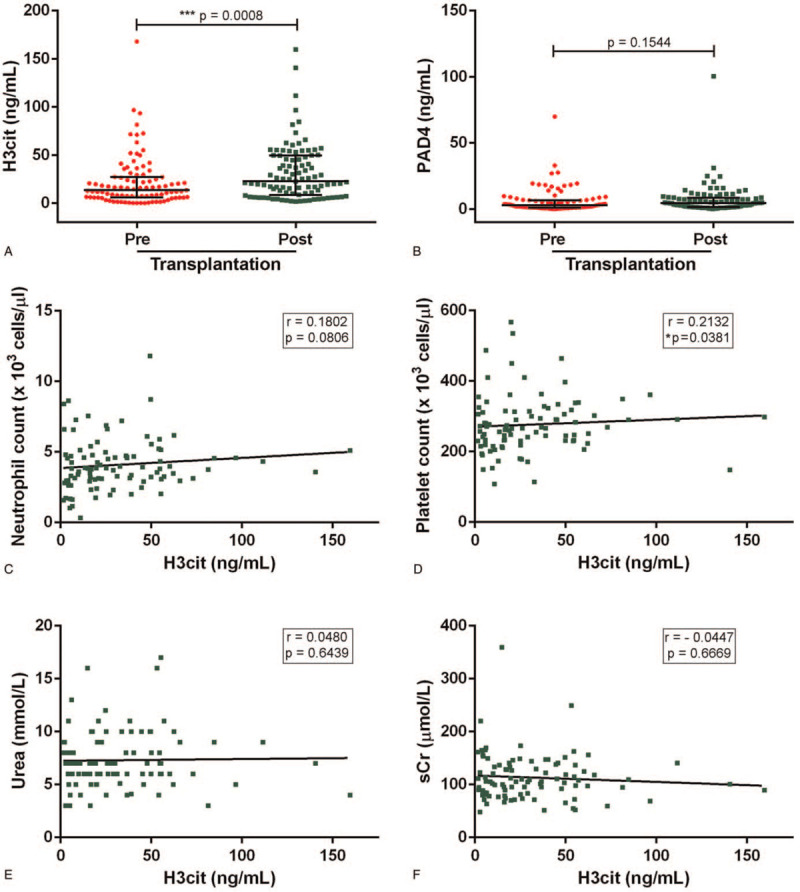
Changes in NETosis markers after renal transplantation. (A) H3cit and (B) PAD4 were quantified in serum from ESRD patients pre- and post-transplantation (n = 95) by ELISA. Results are shown as median and IQR, each data point represents a unique patient/healthy donor; ∗∗∗P < .001 by Wilcoxon rank test. For post-transplant samples, correlation of H3cit with (C) neutrophil count, (D) platelet count, (E) urea, and (F) sCr was assessed. Spearman's correlation coefficients were calculated and are shown in each panel. ELISA = enzyme-linked immunosorbent assay, ESRD = end-stage renal disease, IQR = interquartile range.
3.4. Association of NETosis markers with transplantation baseline characteristics and biochemical parameters post-transplantation
Next, we decided to compare transplant recipients’ biochemical parameters according to their circulating NET markers’ levels. As shown in Table 4, 44.2% of transplant recipients exhibit high levels of H3cit (≥ 27.1 ng/mL). Patients with high H3cit also had higher PAD4 levels post-transplantation (7.3 ng/mL [4.5–11.9]) vs 2.0 ng/mL [1.0–4.9], P < .0001). High levels of H3cit (≥ 27.1 ng/mL) or PAD4 (≥ 6.8 ng/mL) lacked association with the combination of donor/recipient sex and the induction therapy, though maintenance immunosuppression significantly associated with PAD4 (P = .021) (Table 4).
Table 4.
Characteristics of transplant recipients in relation to circulating levels of H3cit and PAD4.
| Low vs High H3cit | Low vs High PAD4 | |||||
| Variable | Q1–3 n = 53 | Q4 n = 42 | P-value | Q1–3 n = 65 | Q4 n = 30 | P-value |
| NETosis markers | ||||||
| H3cit, ng/mL | < 27.1 | ≥ 27.1 | – | 16.5 [5.8–31.4] | 47.6 [27.9–60.7] | < .0001 |
| PAD4, ng/mL | 2.0 [1.0–4.9] | 7.3 [4.5–11.9] | < .0001 | < 6.8 | ≥ 6.8 | – |
| Transplant characteristics | ||||||
| Donor age, years | 39.8 ± 11.7 | 41.6 ± 9.9 | .438 | 41.0 ± 11.1 | 39.6 ± 10.6 | .569 |
| Recipient age, years | 31.6 ± 9.2 | 30.2 ± 7.8 | .421 | 31.6 ± 9.6 | 29.6 ± 5.8 | .838 |
| Combination of donor/recipient sex, n (%) | .814 | .650 | ||||
| Male/Male | 17 (32.1) | 13 (31.0) | 19 (29.2) | 11 (36.7) | ||
| Male/Female | 9 (17.0) | 6 (14.3) | 10 (15.4) | 5 (16.7) | ||
| Female/Male | 18 (34.0) | 18 (42.8) | 24 (36.9) | 12 (40.0) | ||
| Female/Female | 8 (15.1) | 5 (11.9) | 11 (16.9) | 2 (6.6) | ||
| Unknown/Male | 1 (1.8) | 0 (0) | 1 (1.5) | 0 (0) | ||
| Induction therapy, n (%) | .837 | .909 | ||||
| BSX | 22 (41.5) | 20 (47.6) | 28 (43.1) | 14 (46.7) | ||
| THY | 21 (39.6) | 17 (40.5) | 27 (41.5) | 11 (36.7) | ||
| THY, IVIG | 7 (13.2) | 4 (9.5) | 7 (10.8) | 4 (13.3) | ||
| THY, IVIG, RIX, PPH | 1 (1.9) | 0 (0) | 1 (1.5) | 0 (0) | ||
| THY, IVIG, BSX, PPH | 1 (1.9) | 0 (0) | 1 (1.5) | 0 (0) | ||
| THY, IVIG, PPH | 1 (1.9) | 1 (2.4) | 1 (1.5) | 1 (3.3) | ||
| Immunosuppression, n (%) | .206 | .021 | ||||
| TAC/MMF/PDN | 49 (92.5) | 35 (83.3) | 61 (93.8) | 23 (76.7) | ||
| CYA/MMF/PDN | 4 (7.5) | 7 (16.7) | 4 (6.2) | 7 (23.3) | ||
| Laboratory measurements | ||||||
| Hb, g/dL | 12.7 ± 1.7 | 13.4 ± 1.8 | .060 | 12.6 ± 1.7 | 13.8 ± 1.6 | .002 |
| WBC count, x 103/μL | 5.8 [4.1–7.8] | 6.3 [5.4–7.6] | .215 | 6.1 [4.1–7.8] | 6.2 [5.4–7.6] | .459 |
| Neutrophils x 103/μL | 3.8 [2.8–4.8] | 4.0 [3.2–4.8] | .259 | 3.8 [2.7–5.0] | 3.8 [3.2–4.7] | .628 |
| Lymphocytes, x 103/μL | 1.1 [0.7–2.1] | 1.6 [1.1–2.1] | .152 | 1.4 [0.8–2.2] | 1.6 [1.0–2.1] | .398 |
| Platelets, x 103/μL | 256 [215–298] | 291 [245–330] | .026 | 267 [230–314] | 268 [229–319] | .933 |
| N/L ratio | 3.8 ± 3.2 | 3.6 ± 3.4 | .664 | 4.0 ± 3.7 | 3.2 ± 2.1 | .651 |
| P/L ratio | 0.3 ± 0.3 | 0.2 ± 0.2 | .374 | 0.3 ± 0.3 | 0.2 ± 0.2 | .208 |
| Urea, mmol/L | 7 [6–8] | 7 [6–9] | .557 | 7 [6–9] | 6 [6–9] | .808 |
| sCr, μmol/L | 105 [81–139] | 107 [91–137] | .851 | 105 [81–139] | 111 [95–130] | .611 |
| eGFR, mL/min/1.73m2 | 72.7 [54.1–92.9] | 78.7 [59.4–97.1] | .378 | 75.2 [56–100.7] | 76.8 [57.7–91.0] | .858 |
| Glucose, mg/dL | 88 [82–94] | 86 [81–96] | .713 | 88 [81–97] | 85 [81–91] | .210 |
Data are expressed as mean ± standard error of the mean or median [IQR]. The P-values for continuous variables were calculated with unpaired t-test (parametric comparisons) or the Mann-Whitney U test (non-parametric comparisons). The P-values for categorical variables were calculated with Pearson Chi-square test or Fisher exact test. P < .05 is considered statistically significant. BSX = basiliximab, CYA = cyclosporine A, eGFR = estimated glomerular filtration rate, H3cit = citrullinated histone H3, IVIG = intravenous immunoglobulin, L = lymphocyte, MMF = mycophenolate mofetil, N = neutrophil, P = platelet, PAD4 = Peptidyl arginine deiminase 4, PDN = prednisone, PPH = plasmapheresis, RIX = rituximab, sCr = serum creatinine, TAC = tacrolimus, THY = thymoglobulin, WBC = white blood cells.
As shown in Table 4, transplant recipients with high H3cit had significantly higher platelet counts (291 x103/μl [245–330]) than those with low H3cit (256 x103/μl [215–298], p = 0.026). Meanwhile, transplant recipients with high levels of PAD4 had increased H3cit (47.6 ng/mL [27.9–60.7] vs. 16.5 ng/mL [5.8–31.4], P < .0001), and Hb (13.8 ± 1.6 g/dL vs 12.6 ± 1.7 g/dL, P = .002). Despite evidence from previous studies suggesting spontaneous neutrophil activation and increased NET formation in uremia,[17,21] the dramatic changes in urea, sCr, and eGFR after transplantation did not associate with lower levels of NETosis markers in our cohort (Table 4).
3.5. In vitro NETosis in healthy donors, ESRD patients, and transplant recipients
In several pathological states, the inflammatory environment modulates NET release.[10] Hence, we investigated whether neutrophils isolated from healthy donors, ESRD patients, and transplant recipients exhibit a distinct ability to form NETs in vitro after being stimulated with PMA. Representative fluorescent images of neutrophils treated with dimethyl sulfoxide, as control, or PMA are shown in Figure 3A. We observed that neutrophils from several ESRD patients appeared to be highly activated and formed NETs even in the absence of a stimulus, as evidenced by changes in DNA morphology observed with Sytox green (Fig. 3A). Moreover, neutrophils from ESRD patients released more NETs in response to PMA (70.0% [52.7–94.6]) when compared to neutrophils isolated from healthy donors (32.2% [24.9–54.9], P < .001) (Fig. 3A, B). Importantly, neutrophils from transplant recipients exhibited a diminished ability to form NETs in response to PMA (19.5% [3.5–65.7], P < .05) than ESRD patients (Fig. 3A, B). These results suggest that transplantation impacts neutrophils’ ability to form NETs, though the mechanisms behind this effect remain to be explored.
Figure 3.
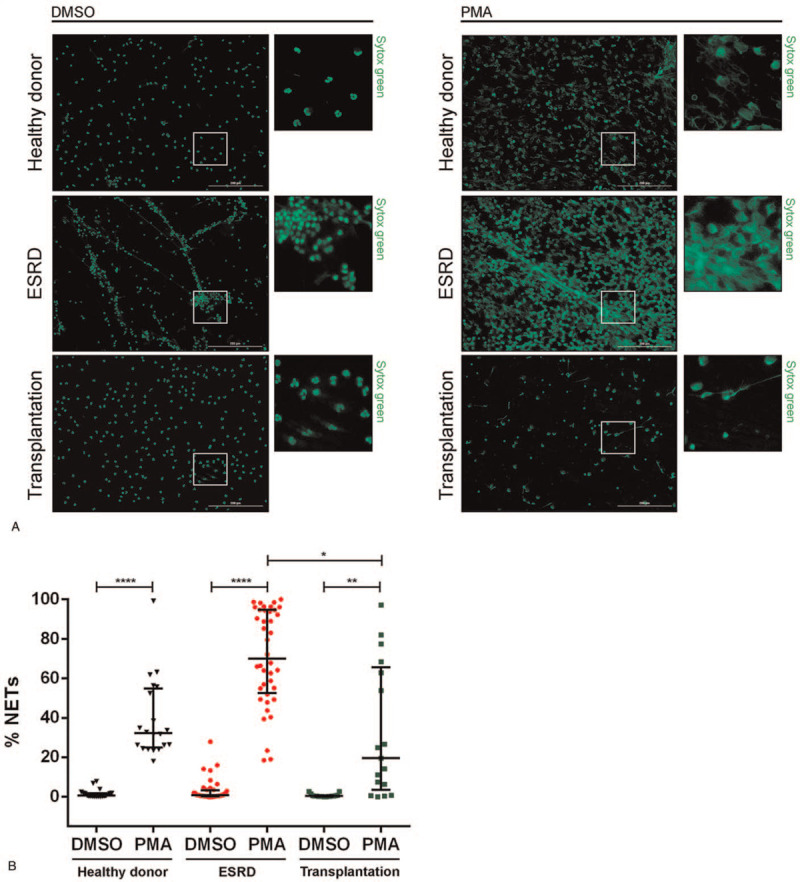
In vitro NETosis is reduced in transplant recipients. (A) Representative fluorescent images of NETosis induced in neutrophils of healthy donors (n = 20), ESRD patients (n = 40), and transplant recipients (n = 17) by DMSO (control) or PMA. DNA appears as a green stain and was detected with SYTOX Green. Scale bar: 200 μm. (B) Quantification of NETosis in 5 fields per individual. Results are shown as median and IQR, each data point represents a unique patient/healthy donor; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < .001 by Kruskal–Wallis test, followed by Dunn test for each pairwise comparison. DMSO = dimethyl sulfoxide, IQR = interquartile range.
4. Discussion
Extracellular trap formation, occurring under sterile inflammation conditions, has been implicated in the establishment and progression of various renal and autoimmune diseases.[13,14] In this study, we investigated NET formation in ESRD patients undergoing transplantation. The nuclear translocation of PAD4 in activated neutrophils induces chromatin decondensation and promotes NET formation through histone citrullination.[8] Thus, to determine the presence of NET-associated markers, we quantified H3cit and PAD4 in patients’ serum and found higher circulating levels in ESRD patients than in healthy donors. Interestingly, the levels of H3cit further increased in these patients within the first 6 months after transplant surgery. We also investigated whether ESRD and transplantation influence neutrophils’ ability to form NETs in vitro and observed that neutrophils isolated from transplant recipients have a reduced ability to release NETs than neutrophils from ESRD patients. Although previous reports have correlated NETs with chronic inflammation, endothelial dysfunction, and cardiovascular events in patients with chronic kidney disease,[17,21,23] this is, to our knowledge, the first cohort study that characterizes circulating NET-associated markers in ESRD patients before and after transplantation.
Neutrophils are activated in ESRD patients, but it is difficult to distinguish if their alterations result from enhanced inflammatory processes, RRT-related procedures such as chronic dialysis, or a combination of both.[20,37] Recurrent exposure of blood to dialysis membranes can result in transient neutropenia, increased adhesion of neutrophils to peripheral vascular walls, complement activation, and release of neutrophilic lysosomal enzymes, cytokines, and reactive oxygen species.[37–39] NETosis has been implicated in the elevated levels of cell-free DNA found in plasma after the hemodialytic procedure,[40] and high interstitial hemodynamic forces stimulate neutrophils to release NETs rapidly.[41] Recent studies have found neutrophil dysfunction and increased NETosis in patients on maintenance HD.[17,21,23] Our results extend these findings to pre-dialysis patients, ESRD patients on PD, and patients that had received a combination of HD and PD. Though we initially hypothesized that the dialysis status would affect NETosis markers, we found that pre-dialysis was not significantly associated with lower levels of H3cit or PAD4. Furthermore, whether patients were on HD, PD, or had received both did not significantly influence NETosis markers’ levels, despite evidence that HD associates with more neutrophil activation than PD.[42] However, our study had several limitations when assessing the influence of RRT on NETosis. First, the number of pre-dialysis patients included in our cohort was small. Additionally, we were unable to control for confounding factors such as the time ESRD patients had been on dialysis before transplantation and the specific details regarding each dialysis treatment. Thus, the impact of dialysis vintage on NETosis remains to be determined. More studies are needed to dissect the influence of dialysis on neutrophil activation, NETosis, and ESRD patients’ outcomes.
Here, we did not find hyperglycemia to be associated with high levels of H3cit in ESRD patients despite previous reports in which elevated blood glucose levels induce NETosis and NET-related biomarkers (neutrophil elastase, nucleosomes, and double-stranded DNA) increase in patients with type 2 diabetes compared to non-diabetic subjects.[43] In our cohort of ESRD patients undergoing transplantation, blood glucose levels decreased within the first 6 months after the surgery, suggesting that the high levels of circulating H3cit post-transplantation found in these patients are not linked to elevated blood sugar. Transplantation often leads to new-onset diabetes and worsens preexisting hyperglycemia partly due to the metabolic and cytotoxic effects of immunosuppressant drugs.[44,45] Despite our findings, interventions to monitor and manage transplant recipients’ hyperglycemia may have clinically significant implications for the long-term patient and graft survival by diminishing the risk of endothelial dysfunction, infections, and cardiovascular disease exacerbated by dysregulated NETosis.
Neutrophils are considered a marker of transplant injury since they infiltrate into transplanted organs early and have a deleterious role in IRI, sterile tissue damage that results from transplant surgery and is exacerbated by the release of neutrophil's oxidative and proteolytic effectors.[46] There is compelling evidence that in renal IRI, infiltrating neutrophils undergo NETosis and release cytotoxic damage-associated molecular patterns, like histones, that worsen inflammation, tubular epithelial cell injury, and remote organ injury.[24] Although IRI is an important trigger of NETosis, we believe that the contribution of these acute inflammatory events occurring shortly after transplant surgery is negligible as the earliest measurement of NET formation and NET-associated markers in our cohort occurred > 30 days after the surgery.
There is evidence that the uremic platelet dysfunction present in ESRD patients is partially reversed by renal transplantation.[47] In our cohort, transplantation increased platelet count, and serum levels of H3cit post-transplantation positively correlated with platelets, but not neutrophils. Activated complement proteins can stimulate NET formation, and NETs decorated with platelets, in turn, act as a platform for complement activation and thrombus formation during coagulation.[12] Platelets play an essential but often overlooked role in cardiovascular disease,[48] making NET-associated events relevant for transplant recipient outcomes.
The transition of neutrophils from a basal state into a primed one is disrupted in ESRD patients.[49] Multiple studies have suggested that uremic conditions prime neutrophils and enhance their functional responses linked to inflammation, oxidative stress, and NET formation.[50–52] In our cohort, higher levels of circulating H3cit were detected in ESRD patients within 6 months after transplant surgery despite the drastic decrease in uremic burden. Uremic toxins associate with immune dysfunction and are known to activate neutrophils spontaneously.[53] Moreover, uremia is a factor that has been directly associated with the increased NET formation and high atherosclerosis burden.[21,54] Our results challenge this notion and suggest that stimuli other than uremia are increasing circulating H3cit in transplant recipients. It is important to note that circulating H3cit levels had a broad distribution pattern in ESRD patients pre- and post-transplantation. The high H3cit levels could then be an indicator of subclinical conditions specific to each patient at sample collection time. In the 6 months of follow-up of our cohort, 1 transplant recipient suspected of acute rejection (not confirmed by biopsy) received methylprednisolone 250 mg-1 g/24 h/3 days and 2 other patients experienced cytomegalovirus infections. However, neither of these events occurred at the time of NETosis marker assessment.
In line with our findings, Torres-Ruiz J. et al also found increased circulating levels of H3cit-DNA complexes in kidney transplant recipients.[30] Furthermore, their analysis of NETosis in plasma and tissue samples revealed that kidney transplant recipients with acute antibody-mediated rejection had a higher amount of peripheral circulating NETs, and NET-associated neutrophils were observed in the kidney biopsies from these patients. More investigation regarding the prognostic significance of circulating H3cit for post-transplantation events such as calcineurin inhibitors toxicity, allograft rejection, and bacterial or viral infections is needed.
In the present study, we did not determine the origin of H3Cit found in serum. Initially, we considered NETs the most probable source since multiple studies have demonstrated that ESRD patients have increased neutrophil activation and NET formation.[17,21] Our in vitro results show that neutrophils of ESRD patients release NETs more readily when stimulated with PMA than neutrophils isolated from healthy donors. We even observed that a proportion of ESRD neutrophils undergo spontaneous NETosis in the absence of PMA. However, when we evaluated in vitro NETosis in neutrophils from transplant recipients, we found that their ability to release NETs in response to PMA was significantly reduced compared to neutrophils from ESRD patients. This finding was unexpected, based on the high H3cit levels found in the serum of transplant recipients. Thus, we do not rule out that the origin of H3cit could be independent of NETosis. Interestingly, Torres-Ruiz J. et al found enhanced spontaneous NETosis in kidney transplant recipients compared to healthy donors, but their ability to release NETs was not potentiated by the presence of lipopolysaccharide.[30] In contrast with their study, we did not observe spontaneous NETosis in unstimulated neutrophils post-transplantation. The discrepancies between their findings and ours could be due to differences in patients’ characteristics, such as the fact that they included transplant recipients up to 52.4 months after transplant surgery. Additionally, NETs were induced by PMA instead of lipopolysaccharide in our study. The use of a different NET inducer could also be partly responsible for the differences among studies since evidence suggests that NETs induced by different stimuli are heterogeneous in protein content and post-translational modifications, potentially exhibiting different biological effects.[55] Additionally, our NETosis in vitro assays post-transplantation had the limitation that neutrophils could only be isolated from a small percentage of the patients included in the study.
A critical element that could be affecting neutrophil function post-transplantation is immunosuppression.[56,57] In our cohort, patients received calcineurin inhibitors (TAC or CYA). Thus, we do not exclude the idea that immunosuppressant-associated effects could be responsible for the decreased NETosis post-transplantation observed in this study. Moreover, fluctuations in the blood levels of immunosuppressants are robustly related to poor kidney graft function, whereas high drug level variability promotes toxicity, donor-specific antibody development, and allograft rejection.[58–61] Although all transplant recipients included in this study received a standard triple immunosuppressive scheme in which clinicians individually adjusted TAC or CYA levels during follow-up, we did not directly measure immunosuppressant drugs blood levels at the time of NETosis marker assessment or NET formation assays. Additionally, corticosteroids have been shown to impair the production of reactive oxygen species in granulocytes and inhibit NET formation in vitro.[62] Furthermore, a double-blind, randomized, placebo-controlled multicentre trial of community-acquired pneumonia demonstrated that prednisone treatment modulates NETosis in the circulation, highlighting a bidirectional interaction of corticosteroids and NETs.[63] Thus, we cannot rule out that fluctuations in immunosuppressant drug level and prednisone-associated effects could be partly responsible for the diminished ability of neutrophils to release NETs in vitro post-transplantation.
5. Conclusion
In conclusion, our study demonstrates that the NET-associated markers H3cit and PAD4 are increased in ESRD patients independently of their dialysis status. Transplantation further increased circulating H3cit levels, suggesting that other factors different from uremia promote its release into the circulation. Neutrophils isolated from transplant recipients have a reduced ability to form NETs in vitro in response to PMA. Further studies are needed to understand the implications of NETosis and NET-associated markers for transplantation outcomes.
Acknowledgments
The authors acknowledge the support given by Ernesto Ortiz, Minerva Pedroza Blanco, Consuelo Pontón Vázquez, Luis Nabor Barajas Gutiérrez, Pedro A. Vázquez Galván, and Monserrat García Navarro.
Author contributions
Conceptualization: Caridad Leal-Cortes, Eliseo Portilla-de Buen, Benjamín Gomez-Navarro, Raquel Echavarria.
Formal analysis: Citlalin Vega-Roman, Zesergio Melo, Adriana Franco-Acevedo, Raquel Echavarria.
Funding acquisition: Raquel Echavarria.
Investigation: Citlalin Vega-Roman, Zesergio Melo, Adriana Franco-Acevedo, Isis Gone-Vazquez, Araceli Escobedo-Ruiz.
Methodology: Luis Felipe Jave-Suarez, Sonia Luquin, Raquel Echavarria.
Project administration: Caridad Leal-Cortes, Benjamín Gomez-Navarro.
Resources: Benjamín Gomez-Navarro, Miguel Medina-Perez, Basilio Jalomo-Martinez, Petra Martinez-Martinez, Luis Alberto Evangelista-Carrillo, Jose Ignacio Cerrillos-Gutierrez, Jorge Andrade-Sierra, Juan J. Nieves.
Writing – original draft: Citlalin Vega-Roman, Raquel Echavarria.
Writing – review & editing: Eliseo Portilla-de Buen, Zesergio Melo, Jorge Andrade-Sierra, Raquel Echavarria.
Footnotes
Abbreviations: CYA = cyclosporine A, eGFR = estimated glomerular filtration rate, ELISA = enzyme-linked immunosorbent assay, ESRD = end-stage renal disease, H3cit = citrullinated histone H3, HD = hemodialysis, IRI = Ischemia-Reperfusion Injury, NET = neutrophil extracelular trap, PAD4 = peptidyl arginase deiminase 4, PD = peritoneal dialysis, PDN = prednisone, PMA= phorbol 12-myristate 13-acetate, RRT = renal replacement therapy, sCr = serum creatinine, TAC = tacrolimus, THY = thymoglobulin.
How to cite this article: Vega-Roman C, Leal-Cortes C, Portilla-de Buen E, Gomez-Navarro B, Melo Z, Franco-Acevedo A, Medina-Perez M, Jalomo-Martinez B, Martinez-Martinez P, Evangelista-Carrillo LA, Cerrillos-Gutierrez JI, Andrade-Sierra J, Nieves JJ, Gone-Vazquez I, Escobedo-Ruiz A, Jave-Suarez LF, Luquin S, Echavarria R. Impact of transplantation on neutrophil extracellular trap formation in patients with end-stage renal disease: a single-center, prospective cohort study. Medicine. 2021;100:27(e26595).
C V-R and C L-C Contributed to this work equally.
This research was funded by CONSEJO NACIONAL DE CIENICA Y TECNOLOGÍA (CONACyT), México, grant number PN-2016/2889. C V-R received a PhD scholarship from CONACyT-Mexico
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3(5):1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Weiner DE, Carpenter MA, Levey AS, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant 2012;12(9):2437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005;16(2):489–95. [DOI] [PubMed] [Google Scholar]
- [4].Jofre R, Rodriguez-Benitez P, Lopez-Gomez JM, Perez-Garcia R. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 2006;17: 12 Suppl 3: S274–80. [DOI] [PubMed] [Google Scholar]
- [5].Cantarin MP, Keith SW, Lin Z, et al. Association of Inflammation prior to Kidney Transplantation with Post-Transplant Diabetes Mellitus. Cardiorenal Med 2016;6(4):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011;11(8):519–31. [DOI] [PubMed] [Google Scholar]
- [7].Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- [8].Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol 2012;3:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Bont CM, Koopman WJH, Boelens WC, Pruijn GJM. Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim Biophys Acta Mol Cell Res 2018;1865(11 Pt A):1621–9. [DOI] [PubMed] [Google Scholar]
- [10].Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 2017;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 2016;12(7):402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol 2019;16(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nakazawa D, Marschner JA, Platen L, Anders HJ. Extracellular traps in kidney disease. Kidney Int 2018;94(6):1087–98. [DOI] [PubMed] [Google Scholar]
- [14].Salazar-Gonzalez H, Zepeda-Hernandez A, Melo Z, Saavedra-Mayorga DE, Echavarria R. Neutrophil extracellular traps in the establishment and progression of renal diseases. Medicina (Kaunas) 2019;55(8): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int 2007;71(2):167–72. [DOI] [PubMed] [Google Scholar]
- [16].Lin CS, Wann JG, Hsiao CW, et al. Intracellular acidification enhances neutrophil phagocytosis in chronic haemodialysis patients: possible role of CD11b/CD18. Nephrol Dial Transplant 2008;23(5):1642–9. [DOI] [PubMed] [Google Scholar]
- [17].Kim JK, Park MJ, Lee HW, et al. The relationship between autophagy, increased neutrophil extracellular traps formation and endothelial dysfunction in chronic kidney disease. Clin Immunol 2018;197:189–97. [DOI] [PubMed] [Google Scholar]
- [18].Sela S, Shurtz-Swirski R, Cohen-Mazor M, et al. Primed peripheral polymorphonuclear leukocyte: a culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol 2005;16(8):2431–8. [DOI] [PubMed] [Google Scholar]
- [19].Pindjakova J, Griffin MD. Defective neutrophil rolling and transmigration in acute uremia. Kidney Int 2011;80(5):447–50. [DOI] [PubMed] [Google Scholar]
- [20].Anding K, Gross P, Rost JM, Allgaier D, Jacobs E. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant 2003;18(10):2067–73. [DOI] [PubMed] [Google Scholar]
- [21].Kim JK, Hong CW, Park MJ, Song YR, Kim HJ, Kim SG. Increased neutrophil extracellular trap formation in uremia is associated with chronic inflammation and prevalent coronary artery disease. J Immunol Res 2017;2017:8415179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jeong JC, Kim JE, Gu JY, et al. Significance of the DNA-histone complex level as a predictor of major adverse cardiovascular events in hemodialysis patients: the effect of uremic toxin on DNA-histone complex formation. Blood Purif 2016;41(1–3):64–71. [DOI] [PubMed] [Google Scholar]
- [23].Kim JK, Lee HW, Joo N, et al. Prognostic role of circulating neutrophil extracellular traps levels for long-term mortality in new end-stage renal disease patients. Clin Immunol 2020;210:108263. [DOI] [PubMed] [Google Scholar]
- [24].Nakazawa D, Kumar SV, Marschner J, et al. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 2017;28(6):1753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ham A, Rabadi M, Kim M, et al. Peptidyl arginine deiminase-4 activation exacerbates kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 2014;307(9):F1052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Raup-Konsavage WM, Wang Y, Wang WW, Feliers D, Ruan H, Reeves WB. Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury. Kidney Int 2018;93(2):365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scozzi D, Ibrahim M, Menna C, Krupnick AS, Kreisel D, Gelman AE. The role of neutrophils in transplanted organs. Am J Transplant 2017;17(2):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sayah DM, Mallavia B, Liu F, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2015;191(4):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].von Meijenfeldt FA, Burlage LC, Bos S, Adelmeijer J, Porte RJ, Lisman T. Elevated plasma levels of cell-free DNA during liver transplantation are associated with activation of coagulation. Liver Transpl 2018;24(12):1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Torres-Ruiz J, Villca-Gonzales R, Gomez-Martin D, et al. A potential role of neutrophil extracellular traps (NETs) in kidney acute antibody mediated rejection. Transpl Immunol 2020;60:101286. [DOI] [PubMed] [Google Scholar]
- [31].O’Connell PJ, Zhang W, Menon MC, et al. Biopsy transcriptome expression profiling to identify kidney transplants at risk of chronic injury: a multicentre, prospective study. Lancet 2016;388(10048):983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med 2003;349:2326–33. [DOI] [PubMed] [Google Scholar]
- [33].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Galván-Ramírez ML, Sánchez-Orozco LV, Andrade-Sierra J, et al. Toxoplasma infection in kidney donors and transplant recipients from Western Mexico: A one-year follow-up. Transpl Infect Dis 2019;21(5):e13139. [DOI] [PubMed] [Google Scholar]
- [35].Zaza G, Leventhal J, Signorini L, et al. Effects of antirejection drugs on innate immune cells after kidney transplantation. Front Immunol 2019;10:2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore) 2018;97(26):e11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vanholder R. Relationship between biocompatibility and neutrophil function in hemodialysis patients. Adv Ren Replace Ther 1996;3(4):312–4. [DOI] [PubMed] [Google Scholar]
- [38].Ward RA, Ouseph R, McLeish KR. Effects of high-flux hemodialysis on oxidant stress. Kidney Int 2003;63(1):353–9. [DOI] [PubMed] [Google Scholar]
- [39].Ono K, Ueki K, Inose K, Tsuchida A, Yano S, Nojima Y. Plasma levels of myeloperoxidase and elastase are differentially regulated by hemodialysis membranes and anticoagulants. Res Commun Mol Pathol Pharmacol 2000;108(5–6):341–9. [PubMed] [Google Scholar]
- [40].Korabecna M, Tesar V. NETosis provides the link between activation of neutrophils on hemodialysis membrane and comorbidities in dialyzed patients. Inflamm Res 2017;66(5):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yu X, Tan J, Diamond SL. Hemodynamic force triggers rapid NETosis within sterile thrombotic occlusions. J Thromb Haemost 2018;16(2):316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bieber S, Muczynski KA, Lood C. Neutrophil activation and neutrophil extracellular trap formation in dialysis patients. Kidney Med 2020;2(6):692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Menegazzo L, Ciciliot S, Poncina N, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 2015;52(3):497–503. [DOI] [PubMed] [Google Scholar]
- [44].Chakkera HA, Weil EJ, Castro J, et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol 2009;4(4):853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Veroux M, Corona D, Giuffrida G, et al. New-onset diabetes mellitus after kidney transplantation: the role of immunosuppression. Transplant Proc 2008;40(6):1885–7. [DOI] [PubMed] [Google Scholar]
- [46].Scozzi D, Ibrahim M, Menna C, et al. The role of neutrophils in transplanted organs. Am J Transplant 2017;17(2):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kennedy C, Wong L, Sexton DJ, et al. Successful kidney transplantation normalizes platelet function. Clin Kidney J 2018;11(4):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Renga B, Scavizzi F. Platelets and cardiovascular risk. Acta Cardiol 2017;72(1):02–8. [DOI] [PubMed] [Google Scholar]
- [49].Cohen G, Horl WH. Immune dysfunction in uremia-An update. Toxins (Basel) 2012;4(11):962–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Glorieux G, Vanholder R, Lameire N. Uraemic retention and apoptosis: what is the balance for the inflammatory status in uraemia? Eur J Clin Invest 2003;33(8):631–4. [DOI] [PubMed] [Google Scholar]
- [51].Cohen G, Rudnicki M, Horl WH. Uremic toxins modulate the spontaneous apoptotic cell death and essential functions of neutrophils. Kidney Int Suppl 2001;78:S48–52. [DOI] [PubMed] [Google Scholar]
- [52].Cohen G, Raupachova J, Wimmer T, Deicher R, Horl WH. The uraemic retention solute para-hydroxy-hippuric acid attenuates apoptosis of polymorphonuclear leukocytes from healthy subjects but not from haemodialysis patients. Nephrol Dial Transplant 2008;23(8):2512–9. [DOI] [PubMed] [Google Scholar]
- [53].Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol 2012;2(11):120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Warnatsch A, Ioannou M, Wang Q, et al. Inflammation. neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349(6245):316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Petretto A, Bruschi M, Pratesi F, et al. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One 2019;14(7):e0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Imbert S, Bresler P, Boissonnas A, et al. Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogeneic hematopoietic stem cell transplant recipients. J Allergy Clin Immunol 2016;138(3):860–8. [DOI] [PubMed] [Google Scholar]
- [57].Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One 2014;9(5):e97088.doi:10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Borra LC, Roodnat JI, Kal JA, et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010;25(8):2757–63. [DOI] [PubMed] [Google Scholar]
- [59].Kahan BD, Welsh M, Urbauer DL, et al. Low intraindividual variability of cyclosporin A exposure reduces chronic rejection incidence and health care costs. J Am Soc Nephrol 2000;11(6):1122–31. [DOI] [PubMed] [Google Scholar]
- [60].Ro H, Min SI, Yang J, et al. Impact of tacrolimus intraindividual variability and CYP3A5 genetic polymorphism on acute rejection in kidney transplantation. Ther Drug Monit 2012;34(6):680–5. [DOI] [PubMed] [Google Scholar]
- [61].Rodrigo E, Segundo DS, Fernández-Fresnedo G, et al. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation 2016;100(11):2479–85. [DOI] [PubMed] [Google Scholar]
- [62].Kalleda N, Amich Elias J, Poreddy S, et al. Corticosteroids impair granulocyte transfusion therapy by targeting NET formation and neutrophil antifungal functions via ROS/Dectin1 pathways. Blood 2016;128:2506. [Google Scholar]
- [63].Ebrahimi F, Giaglis S, Hahn S, et al. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial. Eur Respir J 2018;51(4):1701389. [DOI] [PubMed] [Google Scholar]


