Abstract
To analyze the correlation between quantitative contrast-enhanced ultrasonography (CEUS) parameters and angiogenesis in primary small hepatocellular carcinoma (sHCC) with varying degrees of differentiation.
According to varying degrees of differentiation, a total of 90 primary sHCC patients admitted to our hospital from July 2018 to January 2020 were selected and divided into poorly differentiated group (24 cases), moderately differentiated group (31 cases), and highly differentiated group (35 cases). All patients received real-time CEUS before surgery. The tumor diameter, microvascular morphology, grading of color blood flow, contrast-enhanced performance in different phases, quantitative CEUS parameters, expression of angiogenesis-related genes, and microvessel density (MVD) were compared among the 3 groups. The correlation between quantitative parameters of CEUS and angiogenesis indexes was analyzed by Spearman rank correlation analysis.
Spearman rank correlation analysis showed that vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor receptor (EGFR), and angiopoietin-2 (Ang-2) expression and MVD were negatively correlated with the time to peak (TTP), wash-out time, and peak accelerating time (PAT) (r < 0, P < .05), and were positively correlated with enhancing slope rate (ESR) and peak intensity increasing rate (PIIR) (r > 0, P < .05).
CEUS is able to identify varying degrees of differentiation in primary sHCC, and the quantitative CEUS parameters are closely related to angiogenesis.
Keywords: angiogenesis, contrast-enhanced ultrasonography, correlation, degrees of differentiation, primary small hepatocellular carcinoma, quantitative parameters
1. Introduction
Primary hepatocellular carcinoma (HCC), also known as hepatoma, refers to malignant solid tumors occurring in intrahepatic bile ductular epithelial cells and hepatocytes. Primary HCC is characterized by a poor prognosis, low surgical resection rate, and high degree of malignancy.[1,2] The occurrence and progression of HCC involve multiple genes and factors, and the increase in neovascularization, inactivation of tumor suppressor genes, and activation of proto-oncogenes are all closely related to the occurrence of HCC.[3] Malignant solid tumors have the characteristics of vessel-dependent lesions, and angiogenesis contributes to their growth, invasion, progression, and metastasis. Tumor angiogenesis can be measured based on microvessel density (MVD) and the expression of angiogenesis-related genes.[4] It has been shown that the growth activity of tumor tissue increases as tumor neovascularization increases. As a result, the release of growth factors is continuously induced, resulting in continued neovascularization. The increase in neovascularization increases vascular permeability and alters blood perfusion, resulting in a vicious cycle.[5,6] Therefore, accurate assessment of the blood supply to lesions is of great significance for diagnosing the severity of primary small hepatocellular carcinoma (sHCC) and selecting appropriate treatment regimens. Previously, ultrasound imaging was primarily adopted for the clinical diagnosis of primary sHCC. Although ultrasound imaging has the advantages of high repeatability, simple operations, and non-invasiveness, it cannot quantitatively assess the local blood supply in the tumor.[7] Contrast-enhanced ultrasonography (CEUS) is a novel technique based on traditional ultrasonography, and it can improve the contrast resolution of images based on contrast imaging techniques and contrast agents suitable for intravenous injection to clearly show the tissue vascular perfusion and micro-blood vessels and quantitatively assess the local blood supply, enabling the detection of lesions and qualitative diagnosis of tumors; in addition, neovascularization and tumor microcirculation can be evaluated based on the corresponding contrast enhancement changes in the dynamic phase.[8] Ultrasound contrast agent (UCA), a type of micron-sized pure blood pool contrast agent, can enter the circulation within the tumor and will not be absorbed by any cells or tissues. UCA combined with low mechanical index continuous imaging is conducive to reflecting the blood flow of local tissues and dynamic observation of the blood flow perfusion in tissues. In this study, the correlation between quantitative CEUS parameters and angiogenesis in primary sHCC with varying degrees of differentiation was analyzed, with the goal of providing a scientific basis for monitoring the effects of anti-tumor angiogenesis therapies that target intratumoral microvessels.
2. Materials and methods
2.1. Clinical data
A total of 90 primary sHCC patients admitted to our hospital from July 2018 to January 2020 were selected. The inclusion criteria were: compliance with the diagnostic criteria for primary HCC in Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition)[9] and diagnosis by surgery, pathology, and aspiration cytology; diameter of single lesions ≤3 cm or the sum of the diameters of multiple lesions ≤3 cm; no treatment with chemotherapy, radiotherapy, or other treatments before surgery; no previous history of HCC, fatty liver disease, cirrhosis, or hepatitis; understanding the content of this study and voluntary providing written informed consent. The exclusion criteria were: diameter of single lesions >3 cm; vital organ dysfunction (e.g., cardiac, renal, or pulmonary dysfunction); history of hepatic transplantation or liver surgery; presence of other malignant tumors or hematological diseases; lesions occupying a diffuse space in the liver; inability to tolerate CEUS. Among the 90 patients, 59 were men and 31 were women (age: 40–76 years old ( yo), mean age: 58.45 ± 4.89 yo; lesion diameter: 1.0–3.0 cm, mean lesion diameter: 1.96 ± 0.31 cm). There were 85 cases with single lesions and 5 cases with 2 lesions. This study was approved by The Affiliated Pudong New Area People's Hospital of Shanghai University of Medicine & Health Sciences.
2.2. Methods
All patients were subjected to CEUS at week 1 before surgery, and the CEUS images and results were satisfactory. A GE LOGIQ E9 type Color Doppler ultrasound diagnostic instrument and C2–4 type broadband convex array probe were used, and the frequency was 2 to 5 MHz. The time–intensity curve software, contrast pulse sequencing (CPS) imaging software, coded pulse inversion harmonic imaging technique, and auto tracking contrast quantification software were used. Sono Vue (Braceo, Italy) was selected as the UCA. Before use, 5 mL normal saline was added to dilute Sono Vue, followed by mixing, to prepare the suspension, and the dose was 2.4 mL. Lesions were observed under 2-dimensional ultrasonography, and the number, size, sites, and echo characteristics of the lesions were observed. The blood flow distribution characteristics were obtained by power Doppler and color Doppler imaging. The CPS imaging software was started, the imaging state was set, the double amplitude display mode was selected, and 2.4 mL of Sono Vue suspension was injected into the patient's left median elbow vein via rapid bolus injection. Meanwhile, the built-in timer in the ultrasonic instrument was started to observe the echo intensity, enhancement of the lesions, and peripheral hepatic tissues in real time. Imaging was performed for 5 to 6 minutes, and all data and images were saved. After dynamic images were recorded, auto tracking contrast quantification software was started, ROI was determined, and quantitative CEUS parameters were measured and calculated using the time–intensity curve software.
2.3. Observational indexes
-
(1)
Tumor diameter and microvascular morphology. The tumor diameters and microvascular morphologies (including dot and linear type, intermediate type, and ring type) in the 3 groups were measured.
-
(2)
Grading of color blood flow. Grade 0: No blood flow observed, no blood flow signal around or inside the tumor; Grade I: a small amount of blood flow, with 1 to 2 short rod-like or punctate blood flows around or inside the tumor; Grade II: a moderate amount of blood flow, with a long blood flow inside the tumor; Grade III: a massive amount of blood flow, with two longer or dendritic blood flows inside the tumor and blue and red blood flows inside the tumor.
-
(3)
Contrast-enhanced performance in different phases. The contrast-enhanced performance in the arterial phase, portal phase, and delayed phase, including high enhancement, heterogeneous enhancement, and low enhancement, was observed and compared among the 3 groups. Please refer to the Guidelines for the Use of Contrast Agents in Ultrasound issued by the European Federation of Societies for Ultrasound in Medicine and Biology.[10]
-
(4)
Quantitative CEUS parameters. Peak intensity, enhancement time, time to peak (TTP), wash-in time, wash-out time, peak accelerating time (PAT), enhancing slope rate (ESR), and peak intensity increasing rate (PIIR) were included.
-
(5)
Expression of angiogenesis-related genes. HCC tissues resected from patients were washed with normal saline, transferred to RIPA lysis buffer, homogenized, and centrifuged at 12,000 rpm for 20 minutes at 4 °C, with a centrifugal radius of 6 cm. After separating the supernatant, the protein expression of platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), and angiopoietin-2 (Ang-2) was measured using an enzyme-linked immunosorbent assay kit.
-
(6)
MVD. Pathological sections were immunohistochemically stained using an anti-CD34 monoclonal antibody and the SP method, and the whole section was scanned under a light microscope at 100× magnification. After 5 high-density regions of blood vessels were located, the magnification was adjusted to 400×, and the number of blood vessels positive for the staining was counted. If stained brown or brownish-yellow, several or single endothelial cells were counted as one microvessel, and the final MVD value was the average of five counts.
2.4. Statistical analysis
SPSS 23.0 (IBM Corp., Armonk, NY, US) was used for statistical analysis. Normally distributed measurement data were expressed as . Comparisons among multiple groups were conducted using single factor variance analysis, and the comparison between groups was detected using the least significant difference test. The enumeration data were expressed as percentages and detected using the chi-squared test, while ranked data were detected using the rank sum test. Correlation was analyzed using Spearman rank correlation analysis. P < .05 indicated a statistically significant difference.
3. Results
3.1. Comparison of tumor diameter and microvascular morphology among the 3 groups
There were no significant differences in tumor diameter among the 3 groups (P > .05), while there were significant differences in microvascular morphology among the 3 groups (P < .05). This indicated that there were significant differences in the microvascular morphology of primary sHCC with varying degrees of differentiation (Fig. 1).
Figure 1.
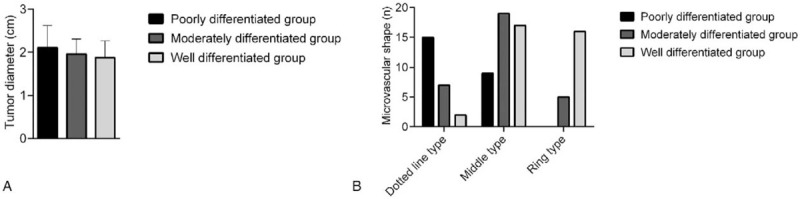
Comparison of tumor diameters and microvascular morphologies among the 3 groups. A: There were no significant differences in tumor diameter among the 3 groups; B: There were significant differences in microvascular morphology among the 3 groups.
3.2. Comparison of the grading of color blood flow among the 3 groups
There were significant differences in the grading of color blood flow among the 3 groups (P < .05). This suggested that CEUS could assess the degrees of differentiation of primary sHCC via the grading of color blood flow (Table 1).
Table 1.
Comparison of grading of color blood flow among the 3 groups (n).
| Group | Number of cases | Grade 0 | Grade I | Grade II | Grade III |
| Poorly differentiated group | 24 | 0 | 5 | 11 | 8 |
| Moderately differentiated group | 31 | 0 | 14 | 10 | 7 |
| Highly differentiated group | 35 | 0 | 15 | 18 | 2∗ |
Comparison among 3 groups.
P < .05.
3.3. Comparison of contrast-enhanced performance in different phases among the 3 groups
There were no significant differences in contrast-enhanced performance in the arterial phase, portal phase, and delayed phase among the 3 groups (P > .05). This revealed that contrast-enhanced performance in primary sHCC with varying degrees of differentiation was similar in different phases, and performance was typically characterized by a “fast-forward and fast-out” mode (Table 2).
Table 2.
Comparison of contrast-enhanced performances among the 3 groups in arterial phase, portal phase, and delayed phase (n).
| Group | Number of case | Arterial phase | Portal phase | Delayed phase | ||||||
| High | Heterogenous | Low | High | Heterogenous | Low | High | Heterogenous | Low | ||
| Poorly differentiated group | 24 | 24 | 0 | 0 | 0 | 5 | 19 | 0 | 4 | 20 |
| Moderately differentiated group | 31 | 29 | 2 | 0 | 0 | 7 | 24 | 0 | 4 | 27 |
| Highly differentiated group | 35 | 35 | 0 | 0∗ | 0 | 10 | 25∗ | 0 | 5 | 30∗ |
Comparison among the 3 groups.
P > .05.
3.4. Comparison of quantitative CEUS parameters among the 3 groups
There were no significant differences in the comparisons of peak intensity, enhancement time, and wash-in time among the 3 groups (P > .05). Regarding TTP, wash-out time, and PAT, these values were highest in the highly differentiated group and were higher in the moderately differentiated group than in the poorly differentiated group. For the ESR and PIIR, these values were lowest in the highly differentiated group and were lower in the moderately differentiated group than in the poorly differentiated group; these differences were significant (P < .05). This demonstrated that the imaging of primary sHCC with moderate and low degrees of differentiation was characterized by a “fast-forward and fast-out” mode while that of the highly differentiated group was characterized by a “fast-forward and slow-out” mode (Fig. 2).
Figure 2.
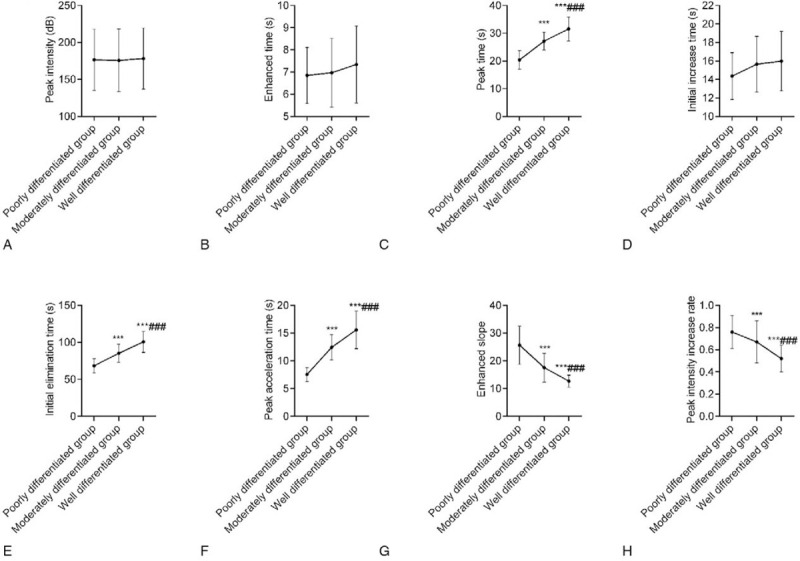
Comparison of quantitative CEUS parameters among the 3 groups. A: There were no significant differences in the peak intensity among the 3 groups. B: There were no significant differences in the enhancement time among the 3 groups. C: The TTP was highest in the highly differentiated group and was higher in the moderately differentiated group than in the poorly differentiated group; D: There were no significant differences in the wash-in time among the 3 groups; E: The wash-out time was highest in the highly differentiated group and was higher in the moderately differentiated group than in the poorly differentiated group; F: The PAT was highest in the highly differentiated group and was higher in the moderately differentiated group than in the poorly differentiated group; G: The ESR was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group; H: The PIIR was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group. Compared with the poorly differentiated group, ∗∗∗P < .001; compared with the moderately differentiated group, ∗∗∗P < .001. CEUS = contrast-enhanced ultrasonography, ESR = enhancing slope rate, PAT = peak accelerating time, PDGF = platelet-derived growth factor, PIIR = peak intensity increasing rate, TTP = the time to peak.
3.5. Comparison of the expression of angiogenesis-related genes among the 3 groups
Regarding the protein expression of PDGF, VEGF, EGFR, and Ang-2, expression levels were lowest in the highly differentiated group and were lower in the moderately differentiated group than in the poorly differentiated group (P < .05). This indicated that there were significant differences in the expression of angiogenesis-related genes in primary sHCC with varying degrees of differentiation (Fig. 3).
Figure 3.
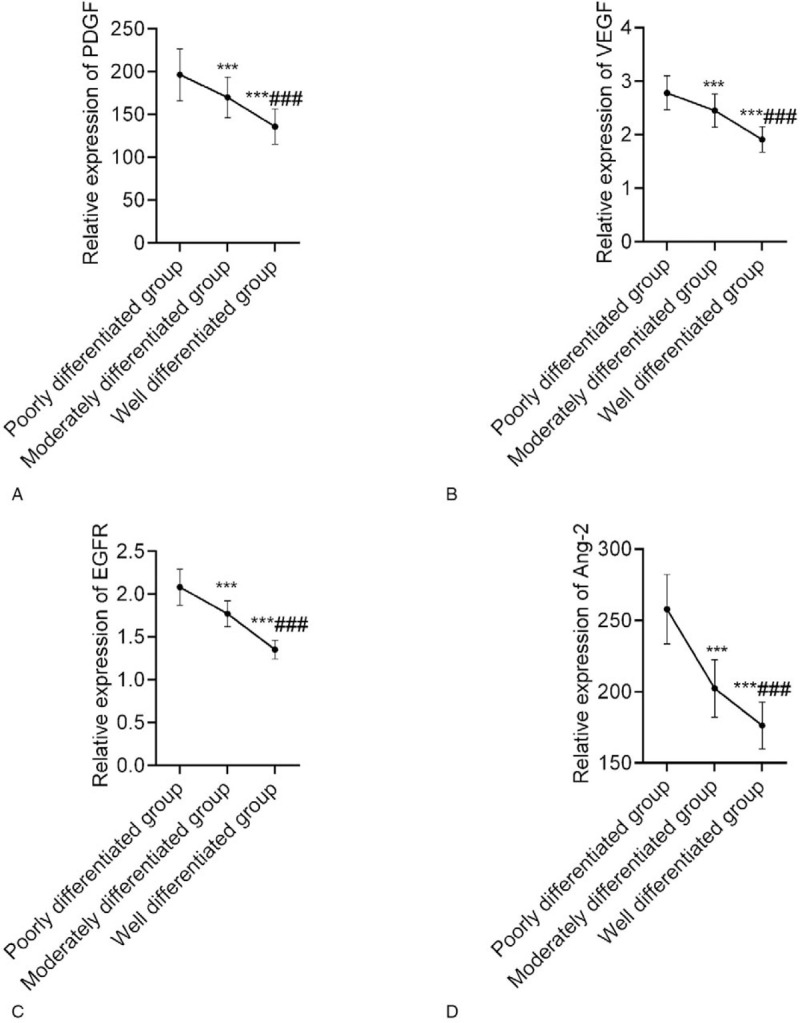
Comparison of the expression of angiogenesis-related genes among the 3 groups. A: The protein expression of PDGF was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group; B: The protein expression of VEGF was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group; C: The protein expression of EGFR was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group; D: The protein expression of Ang-2 was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group. Compared with the poorly differentiated group, ∗∗∗P < .001; compared with the moderately differentiated group, ∗∗∗P < .001. EGFR = epidermal growth factor receptor, PDGF = platelet-derived growth factor, VEGF = vascular endothelial growth factor.
3.6. Comparison of MVD among the 3 groups
The MVD was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group; these differences were significant (P < .05). This demonstrated that the MVD of patients with high, medium, and low degrees of differentiation decreased successively as differentiation progressed (Fig. 4).
Figure 4.
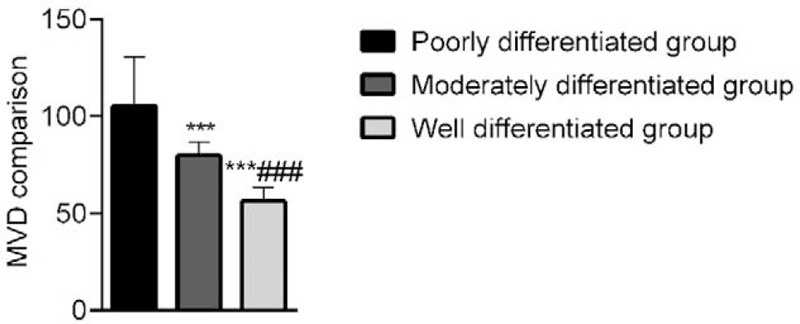
Comparison of MVD among the 3 groups. The MVD was lowest in the highly differentiated group and was lower in the moderately differentiated group than in the poorly differentiated group. Compared with the poorly differentiated group, ∗∗∗P < .001; Compared with the moderately differentiated group, ∗∗∗P < .001. MVD = microvessel density.
3.7. Correlation among quantitative CEUS parameters, angiogenesis-related gene expression, and MVD
Spearman rank correlation analysis revealed that the TTP, wash-out time, and PAT were negatively correlated with VEGF, PDGF, EGFR, and Ang-2 expression and MVD (r < 0, P < .05). The ESR and PIIR were positively correlated with VEGF, PDGF, EGFR, and Ang-2 expression and MVD (r > 0, P < .05). This demonstrated that the quantitative CEUS parameters of primary sHCC with varying degrees of differentiation were closely related to angiogenesis-related gene expression and MVD (Table 3).
Table 3.
Correlation among quantitative CEUS parameters, expression of angiogenesis, and MVD r (P).
| Parameters | VEGF | PDGF | EGFR | Ang-2 | MVD |
| TTP | –0.586 (0.001) | –0.456 (0.009) | –0.589 (0.000) | –0.641 (0.000) | –0.683 (0.000) |
| Wash-out time | –0.621 (0.000) | –0.529 (0.002) | –0.669 (0.000) | –0.731 (0.000) | –0.631 (0.000) |
| PAT | –0.520 (0.004) | –0.654 (0.000) | –0.721 (0.000) | –0.538 (0.003) | –0.592 (0.000) |
| ESR | 0.625 (0.000) | 0.599 (0.000) | 0.486 (0.007) | 0.703 (0.000) | 0.487 (0.007) |
| PIIR | 0.711 (0.000) | 0.616 (0.000) | 0.656 (0.000) | 0.664 (0.000) | 0.619 (0.000) |
Ang2 = angiopoietin-2, CEUS = contrast-enhanced ultrasonography, EGFR = epidermal growth factor receptor, ESR = enhancing slope rate, MVD = microvessel density, PAT = peak accelerating time, PDGF = platelet-derived growth factor, PIIR = peak intensity increasing rate, TTP = the time to peak, VEGF = vascular endothelial growth factor.
4. Discussion
There is a massive supply of blood in HCC lesions. Extensive neovascularization can provide nutrients for the growth, proliferation, and invasion of cancer cells, and promote vascular leakage. Therefore, the degree of vascular proliferation can be used as an indicator of the biological behavior of tumors.[11,12] MVD is considered the “gold standard” for reflecting the degree and activity of tumor neovascularization. However, measuring MVD is time-consuming and tedious and requires histological samples to be obtained. Therefore, there is a crucial need for a fast, non-invasive, and repeatedly implementable test plan for the assessment of tumor angiogenesis and efficacy of anti-angiogenic drugs.[13,14] Imaging causes no radiation damage and can be conducted repeatedly, which is conducive to comprehensively assessing tumor vascular perfusion.[15] Therefore, this study analyzed the relationship between primary sHCC and imaging from the perspective of molecular biology and dynamically monitored angiogenesis in vitro, so as to provide a basis for the non-invasive assessment of neovascularization in vivo.
The lesions of most primary sHCC patients were characterized by a “fast-forward and fast-out” mode, but the CEUS of a small number of primary sHCC patients exhibited a contrast agent filling defect, resulting in a “slow-in” or “fast-forward and slow-out” mode. This may be related to their biological characteristics.[16,17] In this study, SonoVue was selected as the UCA, and the blood perfusion and distribution of tissues were clearly observed. Using CPS imaging software, the hepatic tumors were dynamically observed in real time. Based on the characteristics of hepatic perfusion and blood supply, the contrast-enhanced performance in the delayed phase, portal phase, and arterial phase was obtained, and the enhancement mode of primary sHCC was then analyzed.[18] Among 90 patients in this study, 88 patients exhibited high enhancement in the arterial phase, 2 patients with differentiated carcinoma exhibited heterogenous enhancement, and 68 patients with lesions exhibited low enhancement in the portal phase and no high enhancement. This shows a “fast-forward and fast-out” mode.
With the aggravation of the malignancy degree of tumor lesions in patients, the blood supply of local tumor cells increased significantly. Therefore, the quantitative parameters of CEUS have certain significance for the changes of tissue microvessels in patients. The enhancement time can reflect the local blood vessel pressure and vascular patency at the focal site of patients; TTP can reflect the collateral circulation ability, vascular patency and contrast agent diffusion degree of patients; ESR and PIIR can reflect the malignant proliferation of the tumor cells in patients.[19] In this study, regarding TTP, wash-out time, and PAT, these values were highest in the highly differentiated group and were higher in the moderately differentiated group than in the poorly differentiated group. This demonstrates that quantitative CEUS parameters can reflect the degrees of differentiation of primary sHCC to a certain extent, and there is massive supply of blood in moderately and poorly differentiated HCC tissues. The contrast shows a “fast-forward and fast-out” mode; with the inflow rate being high, the peak can be reached quickly and the blood also flows out quickly. In contrast, the highly differentiated HCC tissues show the enhancement mode of “fast-forward and slow-out.” Dai[20] found that the beginning enhancement time, TTP, and wash-out time of contrast agent were shortened with the decrease of differentiation degree of primary sHCC, and the decrease of wash-out time of contrast agent was particularly obvious, which was basically consistent with the results of this study, further indicating that the imaging manifestations of different differentiation degree of primary sHCC were significantly different. This may be related to the following points: most of the highly differentiated primary sHCC cells are arranged in a rope or beam shape and have numerous blood sinuses, which could easily lead to the retention of UCA microbubbles and slow clearance, whereas there are few blood sinuses in poorly and moderately differentiated HCC; the blood of highly differentiated primary sHCC is primarily supplied by the portal vein and hepatic artery or by vein, and UCA microbubbles are continuously injected from the portal vein. This easily causes the “slow-out” phenomenon. However, the blood of poorly differentiated primary sHCC is primarily supplied by the hepatic artery, most poorly differentiated primary sHCC cases are accompanied by arteriovenous fistula, and the UCA is rapidly cleared through the arteriovenous fistula, thus forming a “fast-out” mode.[21]
The growth of malignant tumors depends on angiogenesis, and tumor angiogenesis results from the interaction between angiogenesis-inhibiting and -promoting factors. Normally, the 2 factors are in a balanced state. However, when the tumor diameter is >2 mm, it has reached the stage of angiogenesis. The tumor volume gradually increases and the cell population rapidly increases. At this time, the balanced state is lost, resulting in decreased inhibitory factors or increased promoting factors.[22,23] In this study, regarding the protein expression of PDGF, VEGF, EGFR, and Ang-2, expression levels were lowest in the highly differentiated group and were lower in the moderately differentiated group than in the poorly differentiated group, among which PDGF and VEGF were angiogenesis-promoting factors, which can affect endothelial progenitor and endothelial cells and can promote the formation of vascular structure and the proliferation of endothelial cells; Ang-2 is a specific and highly expressed factor that can play a stimulatory role in angiogenesis; as a type of receptor tyrosine kinase, EGFR can promote the release and secretion of VEGF and mediate the transduction of cell proliferation signals. This indicates that angiogenesis is related to the degree of differentiation of patients with primary sHCC, and the increase in blood supply can promote the proliferation of cancer cells, resulting in the decrease in the expression of tumor suppressor factors.[24] In this study, Spearman rank correlation analysis was used to further analyze the correlation between quantitative CEUS parameters and angiogenesis. The results suggested that TTP, wash-out time, and PAT were negatively correlated with VEGF, PDGF, EGFR, and Ang-2 expression and MVD, and the ESR and PIIR were positively correlated with VEGF, PDGF, EGFR, and Ang-2 expression and MVD. This demonstrates that the quantitative CEUS parameters are closely related to angiogenesis and can indirectly indicate the state of angiogenesis. Shao et al[25] compared the CEUS parameters between a sHCC group and an intrahepatic bile duct stone group and the expression of angiogenesis-related genes in the lesion tissues. Pearson test showed that the CEUS parameters maximum intensity (IMAX) and TTP and the level of mTT in sHCC patients were directly related to the expression of angiogenesis-related genes in lesions, further indicating that specific CEUS parameters are closely related to angiogenesis-related gene expression. These findings are similar to those observed in the current study. This may be due to the larger lumen, numerous blood vessels, increased number of microvessels, and marked tumor enhancement in primary sHCC patients. Therefore, the peak can be reached quickly. In addition, most patients with poorly differentiated tumors also exhibit arteriovenous fistula, leading to an increased arterial flow and blood flow rate and shortened TTP and PAT.[26]
The innovation of this study is to effectively correlate the quantitative parameters of CEUS with the degree of differentiation and angiogenesis of patients. It is found that the quantitative parameters are closely related to the changes of tumor blood flow and the degree of differentiation, which can be used as an important basis to evaluate the ability of angiogenesis and judge the degree of angiogenesis, and it is convenient for the in vivo noninvasive evaluation and analysis on the occurrence, development and metastasis mechanism of primary sHCC, and has guiding significance for clinical selection of appropriate interventional therapy and surgical methods. However, there are some limitations in this study, such as the subjectivity regarding the selection of contrast region, the small sample size, the failure to follow up patients and analyze the relationship between various parameters and survival. Therefore, future in-depth studies with a larger sample size should be conducted.
5. Conclusions
In summary, CEUS is able to differentiate the degrees of differentiation of primary sHCC. The quantitative CEUS parameters, which are closely related to angiogenesis, can be used as an indicator of angiogenesis to provide guidance for clinical diagnosis and treatment.
Author contributions
Conceptualization: Shuhao Deng, Yuan Zhang.
Data curation: Quan Jiang, Yongbing Wang, Xin Lu.
Formal analysis: Quan Jiang, Yongbing Wang, Xin Lu.
Investigation: Quan Jiang, Yongbing Wang, Xin Lu.
Validation: Quan Jiang, Yongbing Wang, Xin Lu.
Writing – original draft: Shuhao Deng.
Writing – review & editing: Yuan Zhang.
Footnotes
Abbreviations: Ang2 = angiopoietin-2, CEUS = contrast-enhanced ultrasonography, CPS = contrast pulse sequencing, EGFR = epidermal growth factor receptor, ESR = enhancing slope rate, HCC = primary hepatocellular carcinoma, MVD = microvessel density, PAT = peak accelerating time, PDGF = platelet-derived growth factor, PIIR = peak intensity increasing rate, sHCC = small hepatocellular carcinoma, TTP = the time to peak, UCA = ultrasound contrast agent, VEGF = vascular endothelial growth factor, Yo = years old.
How to cite this article: Deng S, Jiang Q, Wang Y, Lu X, Zhang Y. Relationship between quantitative contrast-enhanced ultrasonography parameters and angiogenesis in primary small hepatocellular carcinoma: a retrospective study. Medicine. 2021;100:27(e26489).
This work was supported by Pudong New Area Science and Technology Development (No: PKJ2018-Y11); Pudong New Area Health and Family Planning Commission Subject Leader Course Project (No. PWRd 2017-06); and Youth project of Shanghai Pudong New Area Municipal Health Burean (PW2018B-04).
Data availability statement: My manuscript has no associated data.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Best J, Schotten C, Theysohn JM, et al. Novel implications in the treatment of hepatocellular carcinoma. Ann Gastroenterol 2017;30:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Suh CH, Kim KW, Park SH, et al. Performing Gadoxetic acid-enhanced MRI after CT for guiding curative treatment of early-stage hepatocellular carcinoma: a cost-effectiveness analysis. AJR Am J Roentgenol 2018;210:W63–9. [DOI] [PubMed] [Google Scholar]
- [3].Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67:526–34. [DOI] [PubMed] [Google Scholar]
- [4].Yue WW, Wang S, Xu HX, et al. Parametric imaging with contrast-enhanced ultrasound for differentiating hepatocellular carcinoma from metastatic liver cancer. Clin Hemorheol Microcirc 2016;64:177–88. [DOI] [PubMed] [Google Scholar]
- [5].Tu H, Chen L, Lin J, Wang J. Liver cancer confirmation by contrast-enhanced ultrasound coupled with magnetic resonance imaging: case report of liver inflammation misdiagnosed as atypical liver cancer. J Ultrasound Med 2020;39:1453–7. [DOI] [PubMed] [Google Scholar]
- [6].Xu EJ, Lv SM, Li K, et al. Immediate evaluation and guidance of liver cancer thermal ablation by three-dimensional ultrasound/contrast-enhanced ultrasound fusion imaging. Int J Hyperthermia 2018;34:870–6. [DOI] [PubMed] [Google Scholar]
- [7].Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med 2018;39:154–80. Die EFSUMB-Leitlinien und Empfehlungen für den klinischen Einsatz des kontrastverstärkten Ultraschalls (CEUS) bei nicht-hepatischen Anwendungen: Update 2017 (Kurzversion). [DOI] [PubMed] [Google Scholar]
- [8].Westwood M, Joore M, Grutters J, et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013;17:01–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 2018;7:235–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Del Frate C, Bazzocchi M, Mortele K, et al. Detection of liver metastases: comparison of gadobenate dimeglumine–enhanced and ferumoxides-enhanced MR imaging examinations. Radiology 2003;225:766–72. [DOI] [PubMed] [Google Scholar]
- [11].Dietrich CF, Tana C, Caraiani C, Dong Y. Contrast enhanced ultrasound (CEUS) imaging of solid benign focal liver lesions. Expert Rev Gastroenterol Hepatol 2018;12:479–89. [DOI] [PubMed] [Google Scholar]
- [12].Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (ceus) in non-hepatic applications: Update 2017 (Long Version). Ultraschall Med 2018;39:e2–44. Die EFSUMB-Leitlinien und Empfehlungen für den klinischen Einsatz des kontrastverstärkten Ultraschalls (CEUS) bei nicht-hepatischen Anwendungen: Update 2017 (Langversion). [DOI] [PubMed] [Google Scholar]
- [13].Dietrich CF, Potthoff A, Helmberger T, Ignee A, Willmann JK. [Contrast-enhanced ultrasound: Liver Imaging Reporting and Data System (CEUS LI-RADS)]. Z Gastroenterol 2018;56:499–506. Standardisierte Befundung und Dokumentation der Kontrastmittelsonografie der Leber (CEUS LI-RADS). [DOI] [PubMed] [Google Scholar]
- [14].Kim TK, Noh SY, Wilson SR, et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 2017;23:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dietrich CF. Contrast-enhanced ultrasound of benign focal liver lesions. Ultraschall Med 2019;40:12–29. Kontrastmittelsonografie benigner Lebertumoren. [DOI] [PubMed] [Google Scholar]
- [16].Ayuso C, Rimola J, Vilana R, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 2018;101:72–81. [DOI] [PubMed] [Google Scholar]
- [17].Zarzour JG, Porter KK, Tchelepi H, Robbin ML. Contrast-enhanced ultrasound of benign liver lesions. Abdom Radiol (NY) 2018;43:848–60. [DOI] [PubMed] [Google Scholar]
- [18].Zhang L, Zhang L, Wang H, Chen L, Sui G. Diagnostic performance of contrast-enhanced ultrasound and magnetic resonance imaging for detecting colorectal liver metastases: a systematic review and meta-analysis. Dig Liver Dis 2019;51:1241–8. [DOI] [PubMed] [Google Scholar]
- [19].Jung EM, Clevert DA. [Contrast-enhanced ultrasound (CEUS) and image fusion for procedures of liver interventions]. Radiologe 2018;58:538–44. Kontrastmittelsonographie (CEUS) und Bildfusion zur Durchführung von Leberinterventionen. [DOI] [PubMed] [Google Scholar]
- [20].Dai QH. Application of contrast-enhanced ultrasound in the diagnosis of primary small hepatocellular carcinoma with different degrees of differentiation. Chin J Rural Med Pharm 2016;23:61–2. [Google Scholar]
- [21].Nakamoto RH, Uetake H, Iida S, et al. Correlations between cyclooxygenase-2 expression and angiogenic factors in primary tumors and liver metastases in colorectal cancer. Jpn J Clin Oncol 2007;37:679–85. [DOI] [PubMed] [Google Scholar]
- [22].Liu M, Yang S, Zhang D, et al. Fructopyrano-(1→4)-glucopyranose inhibits the proliferation of liver cancer cells and angiogenesis in a VEGF/VEGFR dependent manner. Int J Clin Exp Med 2014;7:3859–69. [PMC free article] [PubMed] [Google Scholar]
- [23].Maneikyte J, Bausys A, Leber B, et al. Dietary glycine decreases both tumor volume and vascularization in a combined colorectal liver metastasis and chemotherapy model. Int J Biol Sci 2019;15:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones NM, Yang H, Zhang Q, Morales-Tirado VM, Grossniklaus HE. Natural killer cells and pigment epithelial-derived factor control the infiltrative and nodular growth of hepatic metastases in an Orthotopic murine model of ocular melanoma. BMC Cancer 2019;19:484.doi: 10.1186/s12885-019-5712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shao Z, Li Z, Quan Y, Zhang S. The correlation of small hepatocellular carcinoma ultrasonography parameters with the expression of oncogenes and angiogenesis genes. J Hainan Med Uni 2017;23:3441–4. [Google Scholar]
- [26].Yano Y, Yoshimatsu K, Yokomizo H, Sagawa M, Itagaki H, Naritaka Y. Enhancement of the marginal area in colorectal cancer liver metastasis on computed tomography correlates with microvessel density and clinicopathological factors. Anticancer Res 2019;39:1301–8. [DOI] [PubMed] [Google Scholar]


