Abstract
Background:
The patient suffering from urinary sepsis is often accompanied by elevated serum procalcitonin (PCT) levels and a decline in the average platelet count (PLT), which could result in a poor prognosis. This study aimed to evaluate the value of PCT and PLT in determining the severity of urinary sepsis.
Methods:
A total of 120 urosepsis patients enrolled were divided into a survival group and a death group, respectively, according to their status within 14 days after admission. Changes in PCT and PLT levels between the 2 groups were compared at different time points. A receiver operating characteristic (ROC) curve was eventually obtained to predict the prognostic value of PCT and PLT.
Results:
The PCT levels in the survival group declined gradually after admission, and the PLT decreased at first but increased rapidly in subsequence. The PCT level in the death group, however, declined in a flat-slope trend or was hardly noticeable together with the number of PLT reduced significantly. In particular, it is on the 3rd day that PCT tended to positively correlate with acute physiological and chronic health score II (APACHE II) score (r = 0.730, P < .05), but negatively with PLT (r = 0.472, P < .05). The APACHE II score and PLT (r = 0.612, P < .05) were also negatively correlated with each other. As indicated by the ROC curve, the PCT level on the 3rd day after admission was of great value for the clinical mortality prognosis, and the area under the curve was 0.858. Moreover, PLT also has a high predictive value for prognosis. Area under the curve is 0.951. When the PLT was more than 51 × 109 /L, the sensitivity was up to 90%, and the specificity was 90%.
Conclusion:
PLT and PCT levels are closely related to the APACHE II score, which could indicate the severity of urosepsis in patients. The contribution of this study was to confirm that dynamic monitoring of the changes in PCT and PLT helps determine the prognosis of urosepsis patients.
Keywords: average platelet count, procalcitonin, prognosis, urosepsis
1. Introduction
Urosepsis is the most common serious complication of urinary tract infection and is clinically characterized by occult onset, rapid progression, and poor prognosis.[1,2] If some patients suffering from it cannot be diagnosed as early as possible, their conditions would deteriorate rapidly, resulting in an increase in clinical mortality.[3] Urosepsis develops in 3 stages: systemic inflammatory response syndrome, sepsis, and septic shock each of which has a rate of clinical mortality that is apparently different from the others. Therefore, timely and correct diagnosis and judgment of illness severity can provide a basis for better clinical treatment.
Urosepsis is usually accompanied by abnormal levels of various biological markers of infection.[4] An ideal test would be able to guide treatment and improve outcomes. Serum procalcitonin (PCT) is a common clinical infectious biomarker. As shown in previous studies completed in the past, serum PCT is an essential biological marker for distinguishing bacterial infections from non-bacterial infections.[5,6] Furthermore, some recent studies have pointed out that the elevation of serum PCT level is not only related to bacterial infection but also takes place to a greater extent when patients are in surgery, trauma, tumor, and stress state.[7,8] It remains necessary to analyze the relationship between serum PCT levels and the severity of illness with regard to urosepsis. The average platelet count (PLT) is also a commonly used laboratory index for clinical diagnosis. Some studies have indicated that severely infected patients often have to face a significant decrease in the PLT.[9] However, for those who suffer from urosepsis at different stages of illness development, it is still necessary to clarify how serum PCT and PLT change and the relationship it has with the severity and prognosis of the illness. Some scholars have put forward such a proposition that the efficacy of PCT as a biomarker in the management of sepsis: slaying dragons or tilting at windmills?[10,11] Since it is convenient to test PCT and PLT in the ward, and the repetitive test data are reliable, the study will be conducted to conclude the tendency on which serum PCT and average platelet levels would change in the clinical diagnosis and treatment of urinary sepsis patients, and analyze the predictive value of the tendency to judge the severity and prognosis of these patients.
2. Materials and methods
2.1. Clinical data
2.1.1. Study subjects
A total of 120 patients with urosepsis were enrolled in this study from June 2017 to June 2019, including 62 men and 58 women aged 56.33 ± 5.85 years old. Among them, there were 33 cases of diabetes, 34 cases of obstruction-related urosepsis, 26 cases of urosepsis after percutaneous nephroscopy, and 27 cases of urosepsis after ureteroscopy.
2.1.2. Enrollment criteria
The enrollment criteria for this study were as follows: First, patients had clinical symptoms and signs of urinary system infection. Second, the diagnosis of urosepsis was conducted in compliance with the 2014 guidelines for the diagnosis and treatment of severe sepsis and septic shock. Third, there was specific evidence of urinary tract infection available from laboratory examinations, such as pyuria, positive indicators of infection in urine biochemical examination, urine bacterial colony counts ≥105 colony-forming units/mL, and positive pathogenic bacterial growth of the bacterial culture.
The exclusion criteria were as follows: patients aged <18 years, acute coronary syndrome and acute heart failure, liver cirrhosis, chronic renal insufficiency, or severe illness with blood system. Immune connective tissue diseases, thyroid disease, and other serious infectious diseases, such as pulmonary infection.
2.1.3. Ethics
The study conformed to the standards of ethical medical research with the approval of the Medical Ethics Committee of Rongcheng People's Hospital (Research Approval No. 2017-01). Written informed consent was obtained from every patient and his/her family members.
2.1.4. Study grouping
We divided all patients into survival and death groups according to their survival state within 14 days after admission. All patients received empirical antibiotic therapy at first, followed by targeted antibiotic treatment based on antimicrobial sensitivity testing of the uropathogen cultured. Some of the patients in the survival group were observed to have postoperative infections after ureteroscopic surgery or percutaneous nephroscopy. Those in the death group were commonly found to have diabetes and urinary tract obstruction (P < .05). There were no significant differences between the 2 groups (P > .05) in terms of age and sex. All basic demographic data for the patients are presented in Table 1.
Table 1.
Comparison of patients’ demographics between the 2 groups.
| Characteristics | SG (n = 92) | DG (n = 28) | t/x2 | P value when applicable |
| Gender (n, %) | 49 (53.26) | 13 (46.43) | 0.634 | .526 |
| Age (years) (mean ± SD) | 61.79 ± 5.54 | 63.80 ± 6.03 | 1.647 | .102 |
| diabetes (n, %) | 12 (13.04) | 11 (39.29) | 14.510 | .000 |
| Urinary tract obstruction (n, %) | 19 (20.65) | 15 (53.57) | 11.460 | .000 |
| After PCNL (n, %) | 29 (31.52) | 3 (10.71) | 4.753 | .029 |
| After URL (n, %) | 32 (34.78) | 2 (7.14) | 8.076 | .004 |
DG = the death group, PCNL = percutaneous nephroscope, SG = the survival group, URL = ureteroscopy.
2.1.5. Experimental methods
Blood samples were collected from the radial veins of all patients at different time points – on the 1st, 2nd, 3rd, and 5th days after admission, and centrifuged at a speed of 3000 r/min and a radius of 12.4 cm before they were kept standing for 15 min and then stored in a refrigerator at −20°C for subsequent use. The serum PCT level was also measured by quantitative analysis using an enzyme-linked fluorescence assay (LUMI test PCT, Brahms Diagnostica, Berlin, Germany). The functional sensitivity of the PCT detection was 0.05 ng/mL (normal 0.035 ± 0.005 ng/mL). During the same period, 1 mL of whole blood sample was taken as well and measured to determine the PLT level using a fully automatic hematology analyzer (XS-100, Sysmex, Kobe, Japan). The levels of PCT and PLT at these 4 time points were compared between the 2 groups. Meanwhile, the acute physiological and chronic health score II (APACHE II) score was calculated based on a comprehensive analysis of patients’ basic vital signs, blood gas parameters, and laboratory biochemical data in the morning on the examination day.
2.1.6. Statistical methods
The statistical software IBM SPSS Version 26.0 was used to analyze all data. Continuous variables with a normal distribution were expressed as means and standard deviations. Data collected at different time points were compared using variance analysis of the repeated measurement data. Comparisons between different groups were performed using the Mann–Whitney U test or Dunn multiple comparison test. Binomial or categorical variables were expressed as numbers (percentages) and analyzed using chi-square tests (Pearson or Fisher). A receiver operating characteristic (ROC) curve was plotted to evaluate the ability of serum PCT and PLT to evaluate the prognosis of clinical death. The area under the curve (AUC) was calculated, and so was the accuracy of the parameter with the best sensitivity and specificity for given cutoff values. Spearman non-parametric correlation coefficient was used to analyze the correlation between the data. Differences were considered statistically significant at P < .05.
3. Results
3.1. Comparison of the changes in the PLT and PCT levels at different time points
The patients in the survival group experienced a significant drop in serum PCT levels on the 1st, 2nd, 3rd, and 5th days, while the PLT levels decreased slowly in a slightly downward trend at first, and then increased rapidly. In contrast, the declining trend of serum PCT in the patients of the death group developed slowly or was not noticeable, and the PLT kept changing in a downward trend (Tables 2 and 3).
Table 2.
Comparison of serum procalcitonin levels at different time points.
| PCT (ng/mL) | SG (n = 92) | DG (n = 28) | t | P |
| 1 d | 38.78 ± 5.62 | 40.71 ± 3.28 | 1.727 | .087 |
| 2 d | 21.70 ± 4.32 | 33.87 ± 5.09 | 12.51 | .000 |
| 3 d | 8.74 ± 3.39 | 34.38 ± 7.52 | 25.44 | .000 |
| 5 d | 2.38 ± 0.76 | 23.93 ± 4.32 | 45.98 | .000 |
| F | 1526 | 48.11 | ||
| P | 0.000 | 0.000 |
DG = the death group, PCT = serum procalcitonin, SG = the survival group.
Table 3.
Comparison of the average platelet counts at different time points.
| PLT (×109/L) | SG (n = 92) | DG (n = 28) | t | P |
| 1 d | 73.54 ± 11.82 | 75.16 ± 10.57 | 0.650 | .517 |
| 2 d | 91.37 ± 18.75 | 56.81 ± 9.85 | 9.349 | .000 |
| 3 d | 127.89 ± 34.62 | 32.80 ± 7.63 | 14.39 | .000 |
| 5 d | 193.60 ± 32.65 | 27.95 ± 9.71 | 26.42 | .000 |
| F | 115.8 | 149.7 | – | – |
| P | 0.000 | 0.000 | – | – |
DG = the death group, PLT = the average platelet count, SG = the survival group, – = missing data.
3.2. Correlation between serum PCT, PLT, and APACHE II score
The serum PCT level was positively correlated with the APACHE II score (r = 0.730, P < .05) (see Fig. 1A), and negatively correlated with PLT (r = 0.472, P < .05) (see Fig. 1C) of the urosepsis patients. There was a negative correlation between APACHE II score and PLT (r = 0.612, P < .05) (Fig. 1B).
Figure 1.
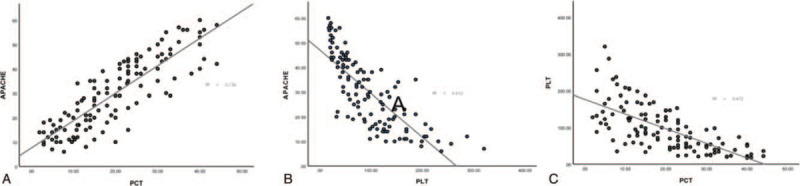
The scatter plot shows that the correlation between serum procalcitonin, the average platelet count, and APACHE II score. PCT was positively correlated with the APACHE II score (r = 0.730, P < .05) (A), and negatively correlated with PLT (r = 0.472, P < .05) (C). It has a negative correlation between APACHE II score and PLT (r = 0.612, P < .05) (B). APACHE II = acute physiological and chronic health score II, PCT = serum procalcitonin, PLT = the average platelet count.
3.3. Prognostic value
The ROC curve showed that on the 3rd day after admission, serum PCT has an excellent prognostic value for the clinical death of urosepsis patients. The AUC was 0.858 (Fig. 2A). When the serum PCT >21.00 ng/mL, the sensitivity was 73.03%, and the specificity was 83.33%. On the 3rd day after admission, PLT had a high predictive value for the survival prognosis of the disease. The AUC was 0.951 (Fig. 2B). When the PLT was more than 51 × 109/L, the sensitivity and specificity were 90% and 90%, respectively.
Figure 2.
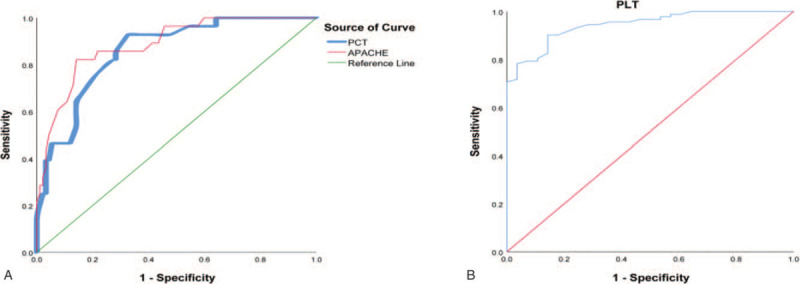
The ROC curve showed the prognostic value of PCT and PLT on the 3rd day after admission. PLT = the average platelet count, PCT = serum procalcitonin, ROC = receiver operating characteristic.
4. Discussion
Sepsis is defined as a systemic inflammatory response syndrome caused by a dysregulated inflammatory host response induced by infection and is associated with high rates of morbidity and mortality. Typical clinical infections include lung infection, bloodstream infection, abdominal infection, and urinary tract infection. We may make a rapid diagnosis of lung and abdominal infections because of the appearance of typical symptoms and signs. However, urosepsis is mainly characterized by occult onset and poor prognosis, especially in cases of complicated urinary tract infections.[12,13] Should he/she begin to show some typical clinical characteristics, his/her status would deteriorate rather quickly, and mortality might arise in consequence. There is 1 major system available for judging the severity of sepsis, the APACHE II score, which is regarded as the most widely used and authoritative critical illness evaluation system for severe patients. Prior studies have suggested that the APACHE II score could be used to effectively assess the severity of patients’ critical conditions and predict the mortality of severe patients admitted to intensive care units.[14,15] Moreover, the maximum theoretical score of APACHE II would be up to 71. The higher the score, the more serious the illness. However, scoring system indicators are mainly dependent on a comprehensive quantitative analysis of the magnitude of abnormal changes in various acute physiological indices. Clinical judgment is lagging, which cannot reflect the severity of sepsis in a timely manner. In recent years, various biomarkers of infection have been extensively used to diagnose sepsis. Serum PCT is a commonly used infectious biomarker in clinical practice and is mainly used for the diagnosis of different bacterial infectious and non-bacterial contagious diseases. Previous studies have demonstrated that PCT concentrations in patients suffering from bacterial infectious disease are evidently higher than those in patients with viral, mycoplasma, or tuberculosis infections.[16] Studies have found that PCT has better performance characteristics in the identification of infectious inflammation and higher specificity for bacterial infection. In cases where the patients suffered from a systemic infection and sepsis, PCT concentrations would increase to a large extent. For urosepsis patients, however, it is of great clinical concern to determine a reliable bedside serum biomarker to judge the severity of the illness due to the concealment of clinical symptoms and dangerous changes in their critical conditions. A few studies have reported that PCT has received substantial interest in predicting mortality and may be helpful in the determination of illness severity.[3] Whether PCT concentrations reflect the severity of illness in patients with urosepsis remains to be confirmed. In clinical practice, it is necessary to be clear and specific in what exactly we want a biomarker or test.
Peripheral complete blood cell count analysis is the most common biological indicator used in infected patients. In cases of common bacterial infections, patients may generally show an increase in the total number of peripheral leukocyte counts and a significant increase in neutrophil percentage. On most occasions, the PLT count usually remains normal for mild infectious patients, which, however, will decrease because bacterial endotoxin might inhibit bone marrow megakaryocytes when severe infections occur. Meanwhile, the cascade burst reactions of various cytokines and inflammatory mediators can speed up the immune clearance and destruction of platelets. All of these might have resulted in a sharp reduction in PLT. An exaggerated sepsis response can lead to multi-organ failure. The severity of organ dysfunction has a prognostic value. Previous studies have also highlighted the role of platelets as immune mediators in sepsis. During sepsis, it is still unclear whether a drop in platelet count is the cause or consequence of illness severity. Some scholars have put forward such an ongoing quest: PLT, which is a promising tool or just another overhyped test? Several studies have suggested that PLT count could be a marker of severity in patients with sepsis.[17,18] Regardless of the platelet count or the mean platelet volume, they could indicate the severity of the infectious disease. It has not yet been validated whether the change in PLT count in the short term could reflect the severity of the illness in patients with urosepsis. The level of serum PCT in urosepsis patients was positively correlated with the APACHE II score but negatively correlated with PLT. As demonstrated by the results of these studies, there are some correlations among PCT, PLT, and the severity of the illness as far as urosepsis patients are concerned. The more severe the illness conditions develop, the higher the level of PCT, and the more the number of the PLT will decline. PCT and PLT levels were associated with increased mortality. It is suggested that the severity of illness could be determined by observing the levels of PCT and PLT in patients with sepsis in urology.
In the past, the judgment of the severity of sepsis patients mainly relied on the single time-point level detection of various infectious biomarkers, which could serve as a static value index. However, the condition of patients with sepsis continuously undergoes dynamic changes and an evolving clinical course. It is possible that we should not always consider only the initial PCT values. Whether the dynamic observations of the changes in PCT and PLT levels at different time points as opposed to a single static value is helpful in judging the prognosis remains to be analyzed in patients with urosepsis.[19,20] Observations were conducted on 2 groups of patients with different clinical adverse outcomes, which showed that during the elapse of time, the level of PCT declined significantly in the patients in the survival group. On the other hand, the level of PLT first decreased and then increased rapidly. In contrast, the downward trend of serum PCT levels in the death group was very slow, which was not visible. However, PLT followed a continuous downward trend. This study assessed the prognostic abilities of PCT and PLT. The authors found that the dynamic observations on serum PCT and PLT are of some help in determining the prognosis of patients with urosepsis. The possible reasons why the serum PCT level of uroseptic patients increased continuously are as follows: First, under normal conditions, thyroid C cells in the human body secrete and produce PCT with a minimum content of serum PCT. If the body is in severe infections, stress, and trauma, all of the tissues and organs would secrete PCT.[4] This would result in a significant increase in serum PCT. Second, the half-life of serum PCT is relatively short, approximately 25 to 30 hours. Once the body's injurious factors are relieved or the inflammatory reaction process is alleviated, the level of serum PCT concentrations will decrease. In addition, the clinical results of serum PCT concentrations are seldom disturbed by external factors. The change in serum PCT level is influenced slightly by the patient's age, sex, and the primary status of renal function.[1,21] On the other hand, platelet is a crucial regulator of inflammatory immune responses. The inflammatory response and activation of coagulation involved in PLT are 2 crucial aspects of sepsis. Platelet consumption via thrombin-mediated platelet activation is the classical mechanism. Thus far, it has not been clear whether monitoring the level of serum PCT and PLT will be helpful in judging the prognosis of uroseptic patients.[22,23] The ROC curve showed that serum PCT was of great value in the mortality of patients with urosepsis on the 3rd day after admission. The area under the curve is 0.858. When serum PCT concentrations >21.00 ng/mL, the sensitivity was 73.03%, and the specificity was 83.33%. On the 3rd day after admission, PLT had a high predictive value for patient survival. The area under the curve is 0.951. When the PLT was more than 51 × 109/L, the sensitivity and specificity were 90% and 90%, respectively. The results of the study demonstrated that dynamically monitoring the changes in serum PCT and PLT is helpful in determining the prognosis of patients with urosepsis. Numerous biomarkers have been evaluated and rejected as unsuitable for predicting disease severity. Albumin is a potent marker of infection-related diseases. There has always been much controversy regarding changes in microvascular permeability to proteins. Serum albumin concentration tends to decrease during acute phase infections due to severe infection and systemic capillary leak syndrome. More recently, Luo et al published a paper in which they described that the PCT/albumin ratio could be used as an early diagnostic predictor in discriminating urosepsis from patients with febrile urinary tract infection and may be superior to other traditional biomarkers.[24] We need further to have a thorough study on whether the disease prognosis of urosepsis by PCT and PLT have an advantage over previously established markers such as PCT/albumin ratio? However, this finding is not a double-blind, multicenter study. The bias was likely due to the small sample size, and caution must be exercised. Among the limitations of the study, only 120 patients were included, and the patients were recruited from a single department in our hospital. This limits the generalizability of our results. The conclusions of the study need to be deeply analyzed. These limitations will be addressed in future studies.
5. Conclusion
In conclusion, the most obvious finding to emerge from this study is that PLT and PCT levels in patients with urinary sepsis are closely related to the severity of their illness or APACHE II score. The contribution of this study is to confirm that continuous monitoring of the tendency of serum PCT and PLT changes will contribute to judging the prognosis of urosepsis patients.
Acknowledgments
The authors wish to thank the patients who participated in this study and the staff involved in this work. This work was also supported by the Rongcheng Hospital, affiliated with the Shandong First Medical University.
Author contributions
Conceptualization: Shao-Hua Lin.
Investigation: Ling Jiang, Jun Wang.
Methodology: Ling Jiang.
Software: Shao-Hua Lin, Jun Wang.
Writing – original draft: Shao-Hua Lin.
Writing – review & editing: Ling Jiang, Cun-Kun Chu.
Footnotes
Abbreviations: APACHE II = acute physiological and chronic health score II, PCT = procalcitonin, PLT = average platelet count, ROC curve = receiver operating characteristic curve.
How to cite this article: Jiang L, Lin SH, Wang J, Chu CK. Prognostic values of procalcitonin and platelet in the patient with urosepsis. Medicine. 2021;100:27(e26555).
The authors have no conflicts of interest to disclose.
All data used to support the finding of this study are available from the corresponding author upon request.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Pescatore R, Niforatos JD, Rezaie S, et al. Evidence-informed practice: diagnostic questions in urinary tract infections in the elderly. West J Emerg Med 2019;20:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ko YH, Ji YS, Park SY, Kim SJ, Song PH. Procalcitonin determined at the emergency department as an early indicator of progression to septic shock in patient with sepsis associated with ureteral calculi. Int Braz J Urol 2016;42:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yunus I, Fasih A, Wang Y. The use of procalcitonin in the determination of the severity of sepsis, patient outcomes and infection characteristics. PLoS One 2018;13:e0206527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Akagi T, Nagata N, Miyazaki H, et al. Procalcitonin is not an independent predictor of 30-day mortality, albeit predicts pneumonia severity in patients with pneumonia acquired outside the hospital. BMC Geriatr 2019;19:03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wirz Y, Meier MA, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care 2018;22:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gluck E, Nguyen HB, Yalamanchili K, et al. Real-world use of procalcitonin and other biomarkers among sepsis hospitalizations in the United States: a retrospective, observational study. PLoS One 2018;13:e0205924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Niu R, Jiang L. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine (Baltimore) 2019;98:e16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;3:304–77. [DOI] [PubMed] [Google Scholar]
- [10].Aydemir C, Aydemir H, Kokturk F, Kulah C, Mungan AG. The cut-off levels of procalcitonin and C-reactive protein and the kinetics of mean platelet volume in preterm neonates with sepsis. BMC Pediatr 2018;18:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care 2018;22:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higashikawa T, Okuro M, Ishigami K, et al. Procalcitonin, and albumin as prognostic biomarkers in elderly patients at risk of bacterial infection. J Int Med Res 2018;46:2606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Karakioulaki M, Stolz D. Biomarkers in pneumonia-beyond procalcitonin. Int J Mol Sci 2019;20:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gao N, Yan C, Zhang G. Changes of serum procalcitonin (PCT), C-reactive protein (CRP), interleukin-17 (IL-17), interleukin-6 (IL-6), high mobility group protein-B1 (HMGB1) and d-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (CRRT) and its clinical significance. Med Sci Monit 2018;24:5881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pescatore D, Niforatos JD, Rezaie S, Swaminathan A. Evidence-informed practice: diagnostic questions in urinary tract infections in the elderly. West J Emerg Med 2019;20:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Papagiannopoulos D, Whelan P, Ahmed W, et al. Procalcitonin is a strong predictor of urine culture results in patients with obstructing ureteral stones: a prospective, pilot study. Urol Ann 2016;8:277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park SY. Procalcitonin to guide antibiotic therapy for critically ill patients in Korea. J Korean Med Sci 2019;34:e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brendan J, Kelly, Ebbing Lautenbach, et al. Combined biomarkers predict acute mortality among critically ill patients with suspected sepsis. Crit Care Med 2018;46:1106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Honore PM, De Bels D, Attou R, Redant S, Gallerani A, Kashani K. Influence of pathogen and focus of infection on procalcitonin values in sepsis: are there additional confounding factors? Crit Care 2019;23:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stalenhoef JE, van Nieuwkoop C, Wilson DC, et al. Procalcitonin, mid-regional proadrenomedullin and C-reactive protein in predicting treatment outcome in community-acquired febrile urinary tract infection. BMC Infect Dis 2019;19:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kuil SD, Hidad S, Fischer JC, et al. Sensitivity of point-of-care testing C reactive protein and procalcitonin to diagnose urinary tract infections in Dutch nursing homes: PROGRESS study protocol. BMJ Open 2019;9:e031269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gbinigie A, Onakpoya IJ, Richards GC, et al. Biomarkers for diagnosing serious bacterial infections in older outpatients: a systematic review. BMC Geriatr 2019;19:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vijayan AL, Vanimaya, Ravindran S, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care 2017;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luo X, Yang X, Li J, et al. The procalcitonin/albumin ratio as an early diagnostic predictor in discriminating urosepsis from patients with febrile urinary tract infection. Medicine 2018;97:28. [DOI] [PMC free article] [PubMed] [Google Scholar]


