Abstract
The use of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in coronavirus disease 2019 (COVID-19) patients has been claimed as associated with the risk of COVID-19 infection and its subsequent morbidities and mortalities. These claims were resulting from the possibility of upregulating the expression of angiotensin-converting enzyme 2 (ACE2), facilitation of SARS-CoV-2 entry, and increasing the susceptibility of infection in such treated cardiovascular patients. ACE2 and renin-angiotensin-aldosterone system (RAAS) products have a critical function in controlling the severity of lung injury, fibrosis, and failure following the initiation of the disease. This review is to clarify the mechanisms beyond the possible deleterious effects of angiotensin II (Ang II), and the potential protective role of angiotensin 1–7 (Ang 1–7) against pulmonary fibrosis, with a subsequent discussion of the latest updates on ACEIs/ARBs use and COVID-19 susceptibility in the light of these mechanisms and biochemical explanation.
Keywords: COVID-19, ACE2, Angiotensin II, Angiotensin 1–7, ACEIs, ARBs
Abbreviations: ACE1, angiotensin-converting enzyme 1; ACE2, angiotensin-converting enzyme 2; ACEIs, angiotensin-converting enzyme inhibitors; AEC-II, alveolar epithelial type II cells; Ang 1-7, angiotensin 1-7; Ang 1-9, angiotensin 1-9; AngI, angiotensin I; AngII, angiotensin II; ARBs, angiotensin receptor blockers; AT1R, angiotensin type 1 receptor; AT2R, angiotensin type 2 receptor; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; ERK, extracellular signal-regulated kinase; ICU, intensive care unit; MAPK, mitogen-activated protein kinase; miR-21, microRNA-21; NLRP3, (NOD, LRR, and pyrin domain-containing protein 3); RAAS, renin-angiotensin-aldosterone system; TGF-β, transforming growth factor-beta
1. Introduction
Coronavirus disease 2019 (COVID-19) was firstly reported on December 8th, 2019 in Hubei province, Wuhan, with subsequent spreading throughout china with pandemic alarming risks. Tentatively, the virus was named novel coronavirus-2019 (2019-nCoV) by the WHO on January 12th, 2020. Along with disease progression and spreading, the WHO has announced its epidemic concern over 2019-nCoV by the end of January (Samudrala et al., 2020, Velavan and Meyer, 2020).
After revealing its genomic sequence and discovering its close homology to its ancestor SARS-CoV, which belongs to coronavirus β-family, the virus was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses on February 11th, 2020, this was coincided with WHO naming of the disease to be COVID-19 (Jin et al., 2020, Samudrala et al., 2020). Due to the global devastating evolution of COVID-19, the WHO has declared COVID-19 as a pandemic disease on March 11th, 2020, which was followed by global full and/or partial lockdown to control the disease transmission (Guan et al., 2020).
SARS-CoV-2 resembles other coronaviruses as a respiratory virus. It is transmitted by droplets to infect the upper and lower respiratory tracts causing flu-like symptoms which may progress into the severe acute respiratory syndrome, which has higher mortality rates at this condition (Jin et al., 2020, Thachil and Srivastava, 2020, Salyer et al., 2021).
Since the 1st SARS-CoV, it has been believed that the site of entry of the virus to the alveolar cells, is the membrane-bound enzyme; angiotensin-converting enzyme 2 (ACE2), which is a binding site for coronavirus spike protein, facilitating its adhesion to the cell surface, followed by internalization of SARS-CoV/ACE2 complex to form an endosome, with subsequent release of the viral RNA to start its journey of transcription and replication to propagate the infection (Atri et al., 2020, McCreary and Pogue, 2020).
Renin-angiotensin-aldosterone system (RAAS) is one of the major systems controlling cardiovascular performance through the actions of aldosterone, angiotensin II (Ang II), and angiotensin 1–7 (Ang 1–7) (Kuster et al., 2020).
The key enzyme in this system is an angiotensin-converting enzyme (ACE), which has two isoforms; the first one is ACE1 which is highly expressed in the pulmonary blood vessels to produce Ang II which has potent vasopressing effects, enhances sympathetic tone, and has pro-inflammatory and mitogenic properties over the endothelial and epithelial cells. The second one is ACE2 that produces a heptapeptide called; Ang 1–7 which is opposite to Ang II, has vasodilator effects, anti-inflammatory activities as well as cardioprotective roles (Atri et al., 2020, Bonow et al., 2020, Meng et al., 2020).
2. Spots on RAAS activation pathway
Classically, RAAS was described as a regulation system responsible for blood pressure regulation and electrolyte, and fluid homeostasis, exerting most of its actions through kidneys (Skeggs et al., 1956). Afterward, its components were likewise found in extrarenal tissues, this indicates that there is a local paracrine system coexisting with the circulating RAAS (Paul et al., 2006). Thus, it was found that ACE is expressed on the surface of many organs endothelial cells mainly lungs, intestines, kidneys, placenta, choroid plexus (Hamming et al., 2004), and, to a lesser extent, in hepatic, cardiac, adrenal, and pancreatic tissues (Colucci et al., 2011).
Renin-angiotensin-aldosterone system consists of renin, an aspartyl protease, which cuts the circulating angiotensinogen to produce inactive decapeptide angiotensin I (Ang I) (1–10) (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu) which is then further cleaved by a zinc metallopeptidase called ACE1 producing the active octapeptide Ang II (1–8) by removing the C-terminal dipeptide His-Leu. Further processing (or degradation) by aminopeptidase A and N gives angiotensin 3 (2–8) and angiotensin 4 (3–8) respectively (Erdös and Skidgel, 1987).
The actions of Ang II result from activating its two specific receptors, the angiotensin type 1 receptor (AT1R) and type 2 receptor (AT2R). The activation of AT1R causes increased sodium retention, stimulation of thirst and desire for salt, vasoconstriction, enhanced activity of the sympathetic nervous system, and release of aldosterone from the adrenal gland. While stimulation of AT2R produces actions, which are counter-regulatory to those of the AT1R, causing anti-inflammatory, anti-fibrotic, and vasodilatory effects (Fig. 1). The AT2R plays a key role in development as it is the dominant receptor type in the fetus while it is less relevant in the normal adult. However, the AT2R might be upregulated in certain diseases (De Mello and Frohlich, 2014, De Mello, 2015).
Fig. 1.
RAAS activation pathway. Ang I: angiotensin I; Ang II: angiotensin II; Ang 1–7: angiotensin 1–7; Ang 1–9: angiotensin 1–9; ACE1: angiotensin-converting enzyme 1; ACE2: angiotensin-converting enzyme 2; AT1R: angiotensin type 1 receptor; AT2R: angiotensin type 2 receptor; (+): stimulatory signal; (-): inhibitory signal.
Both AT1R and AT2R have a wide tissue-specific distribution and are present in the kidneys, brain, lungs, and adrenal gland (Eguchi and Inagami, 2000, Sayeski and Bernstein, 2001). Both receptors have substrate affinity in the nanomolar range, and they have different maximal binding capacities according to source tissues. AT1R has heptahelical transmembrane domains that help in coupling to G-proteins. Stimulation of AT1R by Ang II induces hydrolysis of inositol phosphates by the action of phospholipase C, increases intracellular calcium (Ca2+) and diacylglycerol, inhibits adenylate cyclase, and releases arachidonic acid metabolites (Guo et al., 2001). While AT2R has seven transmembrane domains but not found to be coupled to G-proteins. The glycosylated protein part of AT2R is only 32% homologous to that of the AT1R (Dasgupta and Zhang, 2011). It was suggested that AT2R binding activates a phosphotyrosine phosphatase, thereby inactivating mitogen-activated protein kinase (MAPK) and decreasing cyclic guanosine monophosphate levels (Norwood et al., 2004).
3. The distribution and functions of ACE isoforms
Angiotensin-converting enzyme has two isoforms. Firstly, ACE1 is a dipeptidyl carboxypeptidase that cleaves dipeptides from the carboxyl-terminal of many peptides. It has two active sites, and its most significant substrates are both Ang I and bradykinin. It converts Ang I to Ang II and causes inactivation of bradykinin. ACE1 is widely distributed in the body organs and located on the luminal surface of vascular endothelial cells and in this manner becomes in close contact with the circulation (Acharya et al., 2003).
Secondly, ACE2 which is a homolog of ACE1 and was firstly recognized in 2000 (Donoghue et al., 2000), has gotten growing interest in several disease contexts and represents the second counterbalancing RAAS pathway. ACE2 shares about a 61% homology sequence with the catalytic domains of its homolog ACE1. On the contrary to ACE1, ACE2 has only one active site and acts as a carboxypeptidase rather than a dipeptidyl carboxypeptidase by removing a single amino acid from the C-terminal of Ang I and Ang II forming two biologically active products of the RAAS cascade, namely angiotensin 1–9 (Ang 1–9) and Ang 1–7 respectively with high efficiency (Velkoska et al., 2016).
Both Ang 1–9 and Ang 1–7 act by promoting vasodilation and inhibiting inflammatory responses and endothelial cell proliferation (Fig. 1). The Vasodilator activity of Ang 1–7 has been mediated by the orphan heterotrimeric guanine nucleotide-binding protein-coupled receptor (Mas receptor) and serves to oppose the vasoconstrictor activity of Ang II. ACE2 shows a more restricted distribution pattern, mainly in the lungs, kidneys, and heart than ACE1 (Tipnis et al., 2000, South et al., 2020). Another distinctive feature of ACE2 is its inability to hydrolyze and deactivate bradykinin in contrast to ACE1 (Ferrario, 2011).
4. The deleterious effects of Ang II in lung injury and pulmonary fibrosis
Pulmonary fibrosis is described by an accumulation of large amounts of extracellular matrix proteins and proliferation of fibroblasts (Hsu et al., 2017). The lung has been recognized as a remarkable source of circulating Ang II as ACE1 is abundantly expressed in the pulmonary capillaries (Patel et al., 2017).
Due to its impact on pulmonary vascular remodeling, and fibroblast activity modifications as a result of AT1R activations, Ang II was thought to be implicated in lung injury and pulmonary fibrosis pathogenesis (Kuba et al., 2006).
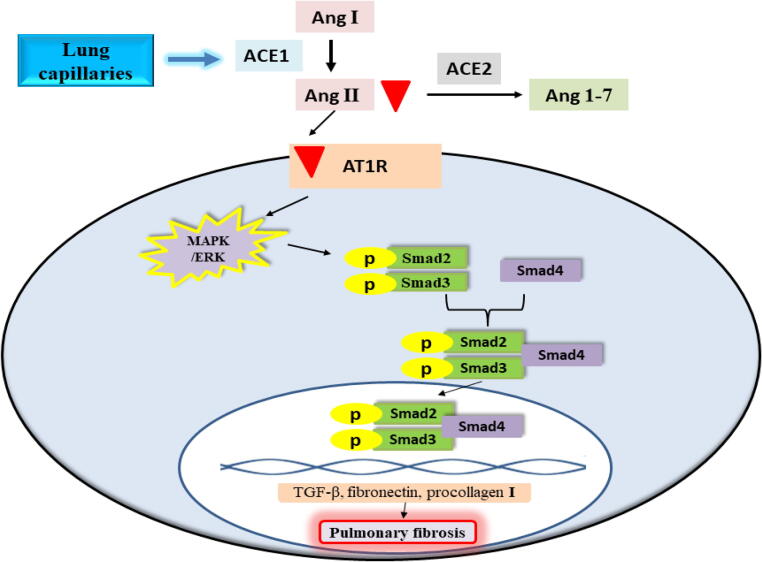
The main signaling pathway by which Ang II is implicated in pulmonary fibrosis is the activation of the signaling cascade of Smad/transforming growth factor-beta (TGF-β) (Wang et al., 2017). Upon binding of Ang II to AT1R, Smad2 and Smad3 are phosphorylated through the activation of the MAPK/extracellular signal-regulated kinase (ERK) pathway. The phosphorylated Smad2 and Smad3 combine with Smad4 forming a complex that gets transported to the nucleus resulting in the transcription of TGF-β, fibronectin, and procollagen I genes (Fig. 2) (Xu et al., 2018, Tarbit et al., 2019). TGF-β participates in cell growth and proliferation in addition to its significant role in the regulation of inflammation and extracellular matrix accumulation (Xu et al., 2019). Among TGF-β isoforms, TGF-β1 is recognized as a profibrotic cytokine that activates the myofibroblasts generation from fibroblasts and collagen deposition (Kim et al., 2018). Moreover, the combination of fibronectin with type I collagen is a basic portion of collagen fibrillogenesis (Saunders and Schwarzbauer, 2019).
Fig. 2.
Ang II mediated the activation of Smad/TGF-β signaling cascade and development of pulmonary fibrosis. Ang I: angiotensin I; Ang II: angiotensin II; Ang 1–7: angiotensin 1–7; ACE1: angiotensin-converting enzyme 1; ACE2: angiotensin-converting enzyme 2; AT1R: angiotensin type 1 receptor; MAPK: mitogen-activated protein kinase; ERK: extracellular signal-regulated kinase; TGF-β: transforming growth factor-beta.
Additionally, Sun et al., (2017) reported a molecular pathway through which Ang II is involved in pulmonary fibrosis, which is the over-expression of the pro-fibrotic microRNA-21 (miR-21). miR-21 mediates the suppression of Spry1 in lung fibroblasts that results in the activation of ERK/nuclear factor kappa B (NF-κB) cascade and hence the NLRP3 (NOD, LRR, and pyrin domain-containing protein 3) inflammasome is activated. NLRP3 inflammasome induces the synthesis and deposition of collagen in the lung tissue (Meng et al., 2019) and it also activates interleukin 1β leading to severe inflammation and pulmonary fibrosis (Lasithiotaki et al., 2016).
5. The protective role of Ang 1–7 against pulmonary fibrosis
Angiotensin 1–7 is another RAAS product that results from Ang II cleavage by ACE2 (South et al., 2020). Ang 1–7 reduces pulmonary fibrosis, myofibroblasts generation, and collagen synthesis through the inhibition of various pathways involved in Ang II-mediated tissue fibrosis. Firstly, it inhibits the phosphorylation of Smad and MAPK/ERK through the induction of the different cellular phosphatases comprising Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1) and dual-specificity phosphatase 1 (DUSP-1) and hence it reduces the expression of TGF-β (Chappell and Al Zayadneh, 2017, Kim et al., 2015, Kuba et al., 2006). Secondly, it decreases the pro-fibrotic miRNA-21, and consequently, it suppresses the effect of angiotensin II-mediated activation of the ERK/NF-κB pathway and the NLRP3 inflammasome (Sun et al., 2017). Furthermore, it decreases endothelial dysfunction and cellular differentiation through inhibition of NADPH oxidase (NOX)–induced oxidative stress (Zhang et al., 2015, Peng et al., 2017).
6. The impact of ACEIs/ARBs on ACE2 expression
Many experimental animal studies reported that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) increase the expression of ACE2 in the cardiovascular system (Ishiyama et al., 2004, Agata et al., 2006, Sukumaran et al., 2012, Iwanami et al., 2014, Wang et al., 2016, Sukumaran et al., 2017) and the kidneys (Soler et al., 2009, Lakshmanan et al., 2012) with subsequent elevation in plasma level of Ang 1–7.
Theoretically, the lung ACE2 may be upregulated by the treatment with ACEIs/ARBs increasing the susceptibility to infection by SARS-CoV-2 but no experimental studies have been performed until now to establish this hypothesis. Interestingly, no human-based in vivo studies have been performed concerning the expression of ACE2 in response to treatment by ACEIs/ARBs.
7. The effect of overexpressed ACE2 on COVID-19 infection susceptibility
ACE2 was identified to be likely the cell receptor of SARS-CoV-2 (Zhou et al., 2020). Thus, ACE2 distribution and expression in the human body may demonstrate the possible routes of COVID-19 infection. Researchers have investigated the RNA expression profile of ACE2 at single-cell resolution through the developed single-cell RNA sequencing technique and single-cell transcriptomes based on the public database. High ACE2 expression was recognized in type II alveolar cells of lungs (Zou et al., 2020), absorptive enterocytes from ileum and colon (Zhang et al., 2020), Oral mucosa (Xu et al., 2020), myocardial cells, kidney proximal tubule cells, and bladder urothelial cells. It has been indicated that these organs with high ACE2 expressing cells should be expected as potential entry sites for COVID-19 infection (Zou et al., 2020).
It was demonstrated that alveolar epithelial type II cells (AEC-II) represent about 83% of ACE2-expressing cells suggesting that these cells may serve as a reservoir for viral invasion. Besides, gene ontology enrichment analysis showed that the ACE2-expressing AEC-II have high levels of multiple viral process-related genes, including regulatory genes for viral processes, viral life cycle, viral assembly, and viral genome replication, suggesting that the ACE2-expressing AEC-II facilitates coronaviral replication in the lung (Zhao et al., 2020).
8. ACEIs/ARBs in COVID-19 patients
Since the beginning of COVID-19 global dissemination, Some voices have recommended stopping the use of ACEIs/ARBs for cardiovascular patients who are highly susceptible to be infected by COVID-19, as these medications would upregulate ACE2 production, facilitating SARS-CoV-2 attachment and consequent progression of COVID-19 (Watkins, 2020). This recommendation has been followed by opposite statements from most of the national and global healthcare and cardiovascular medicine concerned organizations, societies, and authorities to continue the use of ACEIs/ARBs for their corresponding patients due to their proven cardiovascular health benefits versus the unconfirmed and inevident harms toward COVID-19 patients (Kuster et al., 2020).
Recently reported observations on COVID-19 patients with cardiovascular disease (CVD) and were on ACEIs/ARBs did not find a relationship between the use of ACEIs/ARBs before COVID-19 infection and the increased risk of infection. Moreover, a retrospective single-center study included hypertensive patients on ACEIs/ARBs reported that the use of ACEIs/ARBs for COVID-19 hospitalized patients was not associated with disease severity, progression, or increased mortality rate among those patients (Li et al., 2020).
Another retrospective study reported that the provision of ACEIs/ARBs in the intensive care unit (ICU) for COVID-19 elderly patients was associated with a marked reduction in the mortality rates (Oddy et al., 2021).
In a recent study aimed to investigate the impact of ACEIs/ARBs use on the hospital admission time of COVID-19 patients and the disease outcomes (disease severity, ICU admission, and mortality), the authors reported that ICU admission and COVID-19 mortality rates were inversely associated with ACEIs/ARBs use (ElAbd et al., 2021).
Interestingly, the use of ACEIs/ARBs for COVID-19 hospitalized hypertensive patients was found to improve the clinical outcomes with less morbidity in comparison to other antihypertensive medications. In this report, patients on ACEIs/ARBs were characterized by low plasma level of the major driver of COVID-19 associated cytokine storm; interleukin-6, increased peripheral leucocytes count, particularly cluster of differentiation (CD) 3 and 8 T-cells, in addition to the lower peak of SARS-CoV-2 viral load (Meng et al., 2020). This causal relationship between RAAS suppression and IL-6 suppression needs further elucidation.
The potential benefits of ACEIs/ARBs may not be exclusive to their control of blood pressure and cardiovascular performance, because of their higher benefits in COVID-19 patients than those received other antihypertensives such as calcium channel blockers (Choksi et al., 2021). These benefits may be due to the overexpression of ACE2 with its anti-inflammatory and antifibrotic activities.
These findings explain the effect of RAAS overactivity in COVID-19 and its inflammatory consequences in COVID-19 pathogenesis and the potential benefits of RAAS suppression by ACEIs/ARBs to restore the balance between ACE1/ACE2 actions and their corresponding Ang II/Ang 1–7 products, to favor the anti-inflammatory and antifibrotic effects of ACE2/Ang 1–7 to reduce COVID-19 complications among hypertensive patients.
9. Conclusions
Although ACE2 was reported as a site of SARS-CoV-2 entry, there is no sufficient evidence to support the harmful effect of ACE2 overexpression. As a counter-regulatory of RAAS with anti-inflammatory and antifibrotic actions, inhibitors of RAAS such as ACEIs/ARBs would be potential modulators of COVID-19 associated lung injury and acute respiratory distress syndrome, with no evidence of increased infectivity, morbidities or case fatality rates among their users. Moreover, the use of ACEIs/ARBs is recommended for CVD patients either as a continuation of previous treatment or initiation for CVD management for COVID-19 patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used during the present review article are available from the corresponding author on reasonable request.
Authors’ contributions
AE, EGK, AAE and MHG were contributed equally to the writing, revision of the manuscript as well as collection of related references. All authors have read and approved the final review article.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ahmed Elshafei, Email: Ahmed.elshafei@azhar.edu.eg.
Emad Gamil Khidr, Email: Emadgamil2003@azhar.edu.eg.
Ahmed A. El-Husseiny, Email: Ahmedabdullah1984@azhar.edu.eg.
References
- Acharya K.R., Sturrock E.D., Riordan J.F., Ehlers M.R.W. Ace revisited: a new target for structure-based drug design. Nat. Rev. Drug Discovery. 2003;2:891–902. doi: 10.1038/nrd1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata J., Ura N., Yoshida H., Shinshi Y., Sasaki H., Hyakkoku M., Taniguchi S., Shimamoto K. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertension Research. 2006;29:865–874. doi: 10.1291/hypres.29.865. [DOI] [PubMed] [Google Scholar]
- Atri D., Siddiqi H.K., Lang J.P., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. Basic to Translational Science. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow R.O., O’Gara P.T., Yancy C.W. Cardiology and COVID-19. Jama. 2020;324:1131–1132. doi: 10.1001/jama.2020.15088. [DOI] [PubMed] [Google Scholar]
- Choksi T.T., Zhang H., Chen T., Malhotra N. Outcomes of Hospitalized COVID-19 Patients Receiving Renin Angiotensin System Blockers and Calcium Channel Blockers. American Journal of Nephrology. 2021;52(3):250–260. doi: 10.1159/000515232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M.C., Al Zayadneh E.M. Angiotensin-(1-7) and the regulation of anti-fibrotic signaling pathways. Journal of cell signaling. 2017;2(1) doi: 10.4172/2576-1471.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci J.A., Yuri Arita D., Sousa Cunha T., Seno Di Marco G., Vio C.P., Pacheco-Silva A., Casarini D.E. Renin-angiotensin system may trigger kidney damage in NOD mice. J. Renin Angiotensin Aldosterone Syst. 2011;12:15–22. doi: 10.1177/1470320310375456. [DOI] [PubMed] [Google Scholar]
- Dasgupta C., Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discovery Today. 2011;16:22–34. doi: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello, W.C., 2015. Chemical Communication between Heart Cells is Disrupted by Intracellular Renin and Angiotensin II: Implications for Heart Development and Disease. Front. Endocrinol., 6, 72-72. [DOI] [PMC free article] [PubMed]
- De Mello, W.C., Frohlich, E.D., 2014. Clinical perspectives and fundamental aspects of local cardiovascular and renal Renin-Angiotensin systems. Front. Endocrinol., 5, 16-16. [DOI] [PMC free article] [PubMed]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circulation research. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Eguchi S., Inagami T. Signal transduction of angiotensin II type 1 receptor through receptor tyrosine kinase. Regul. Pept. 2000;91:13–20. doi: 10.1016/s0167-0115(00)00126-9. [DOI] [PubMed] [Google Scholar]
- ElAbd R., AlTarrah D., AlYouha S., Bastaki H., Almazeedi S., Al-Haddad M., Jamal M., AlSabah S. Angiotensin-Converting Enzyme (ACE) Inhibitors and Angiotensin Receptor Blockers (ARB) Are Protective Against ICU Admission and Mortality for Patients With COVID-19 Disease. Frontiers in Medicine. 2021;8:226. doi: 10.3389/fmed.2021.600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E.G., Skidgel R.A. The angiotensin I-converting enzyme. Lab. Invest. 1987;56:345–348. [PubMed] [Google Scholar]
- Ferrario C.M. ACE2: more of Ang-(1–7) or less Ang II? Curr. Opin. Nephrol. Hypertens. 2011;20:1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.F., Sun Y.L., Hamet P., Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–180. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.S., Liu C.C., Lin J.H., Hsu T.W., Hsu J.W., Su K., Hung Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Scientific reports. 2017;7:1–11. doi: 10.1038/s41598-017-14612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Iwanami J., Mogi M., Tsukuda K., Wang X.-L., Nakaoka H., Ohshima K., Chisaka T., Bai H.Y., Kanno H., Min L.J., Horiuchi M. Role of angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis in the hypotensive effect of azilsartan. Hypertension Research. 2014;37:616–620. doi: 10.1038/hr.2014.49. [DOI] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.S., Kim I.J., Bae E.H., Ma S.K., Lee J., Kim S.W. Angiotensin-(1-7) attenuates kidney injury due to obstructive nephropathy in rats. PloS one. 2015;10 doi: 10.1371/journal.pone.0142664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.K., Sheppard D., Chapman H.A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harbor perspectives in biology. 2018;10:a022293. doi: 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Current opinion in pharmacology. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P., Widmer A.F., Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A.P., Thandavarayan R.A., Watanabe K., Sari F.R., Meilei H., Giridharan V.V., Sukumaran V., Soetikno V., Arumugam S., Suzuki K., Kodama M. Modulation of AT-1R/MAPK cascade by an olmesartan treatment attenuates diabetic nephropathy in streptozotocin-induced diabetic mice. Mol Cell Endocrinol. 2012;348:104–111. doi: 10.1016/j.mce.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Lasithiotaki I., Giannarakis I., Tsitoura E., Samara K.D., Margaritopoulos G.A., Choulaki C., Vasarmidi E., Tzanakis N., Voloudaki A., Sidiropoulos P., Siafakas N.M. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. European Respiratory Journal. 2016;47:910–918. doi: 10.1183/13993003.00564-2015. [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary E.K., Pogue J.M. Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options. Open Forum. Infect Dis. 2020;7:ofaa105. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes. Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Pan M., Zheng B., Chen Y., Li W., Yang Q., Zheng Z., Sun N., Zhang Y., Li X. Autophagy attenuates angiotensin ii-induced pulmonary fibrosis by inhibiting redox imbalance-mediated NOD-like receptor family pyrin domain containing 3 inflammasome activation. Antioxidants & redox signaling. 2019;30:520–541. doi: 10.1089/ars.2017.7261. [DOI] [PubMed] [Google Scholar]
- Norwood V.F., Fernandez L.G., Tufro A., Gomez R.A. In: Fetal and Neonatal Physiology. Third Edition. Polin R.A., Fox W.W., Abman S.H., editors. Saunders; W.B: 2004. Chapter 128 - Development of the Renin-Angiotensin System; pp. 1249–1256. [Google Scholar]
- Oddy C., Allington J., McCaul J., Keeling P., Senn D., Soni N., Morrison H., Mawella R., Samuel T., Dixon J. Inpatient Omission of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Is Associated With Morbidity and Mortality in Coronavirus Disease 2019. Clinical therapeutics. 2021;43(4) doi: 10.1016/j.clinthera.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomedicine & Pharmacotherapy. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- Paul M., Poyan Mehr A., Kreutz R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Peng J.J., Liu B., Xu J.Y., Peng J., Luo X.J. NADPH oxidase: its potential role in promotion of pulmonary arterial hypertension. Naunyn-Schmiedeberg’s archives of pharmacology. 2017;390:331–338. doi: 10.1007/s00210-017-1359-2. [DOI] [PubMed] [Google Scholar]
- Salyer S.J., Maeda J., Sembuche S., Kebede Y., Tshangela A., Moussif M., Ihekweazu C., Mayet N., Abate E., Ouma A.O., Nkengasong J. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. The Lancet. 2021;397:1265–1275. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudrala P.K., Kumar P., Choudhary K., Thakur N., Wadekar G.S., Dayaramani R., Agrawal M., Alexander A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. European Journal of Pharmacology. 2020;883:173375. doi: 10.1016/j.ejphar.2020.173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J.T., Schwarzbauer J.E. Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing. Molecular biology of the cell. 2019;30:2218–2226. doi: 10.1091/mbc.E19-03-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeski P.P., Bernstein K.E. Signal transduction mechanisms of the angiotensin II type AT(1)-receptor: looking beyond the heterotrimeric G protein paradigm. J. Renin. Angiotensin. Aldosterone Syst. 2001;2:4–10. doi: 10.3317/jraas.2001.007. [DOI] [PubMed] [Google Scholar]
- Skeggs L.T., Jr., Kahn J.R., Shumway N.P. The preparation and function of the hypertensin-converting enzyme. J. Exp. Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. American Journal of Physiology-Renal Physiology. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- South A.M., Shaltout H.A., Nixon P.A., Diz D.I., Jensen E.T., O’Shea T.M., Chappell M.C., Washburn L.K. Association of circulating uric acid and angiotensin-(1–7) in relation to higher blood pressure in adolescents and the influence of preterm birth. Journal of human hypertension. 2020;34(12):818–825. doi: 10.1038/s41371-020-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran V., Tsuchimochi H., Tatsumi E., Shirai M., Pearson J.T. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE-2/ANG 1–7/Mas receptor cascade. Biochemical pharmacology. 2017;144:90–99. doi: 10.1016/j.bcp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Sukumaran V., Veeraveedu P.T., Lakshmanan A.P., Gurusamy N., Yamaguchi K.i., Ma M., Suzuki K., Kodama M., Watanabe K. Olmesartan medoxomil treatment potently improves cardiac myosin-induced dilated cardiomyopathy via the modulation of ACE-2 and ANG 1–7 mas receptor. Free radical research. 2012;46:850–860. doi: 10.3109/10715762.2012.684878. [DOI] [PubMed] [Google Scholar]
- Sun N.-N., Yu C.-H., Pan M.-X., Zhang Y., Zheng B.-J., Yang Q.-J., Zheng Z.-M., Meng Y. Mir-21 mediates the inhibitory effect of Ang (1–7) on AngII-induced NLRP3 inflammasome activation by targeting Spry1 in lung fibroblasts. Scientific reports. 2017;7:1–11. doi: 10.1038/s41598-017-13305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbit E., Singh I., Peart J.N., Rose’Meyer R.B. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart failure reviews. 2019;24:1–15. doi: 10.1007/s10741-018-9720-1. [DOI] [PubMed] [Google Scholar]
- Thachil J., Srivastava A. SARS-2 Coronavirus-Associated Hemostatic Lung Abnormality in COVID-19: Is It Pulmonary Thrombosis or Pulmonary Embolism? Semin. Thromb. Hemost. 2020;46(7):777–780. doi: 10.1055/s-0040-1712155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkoska E., Patel S.K., Burrell L.M. Angiotensin converting enzyme 2 and diminazene: role in cardiovascular and blood pressure regulation. Curr. Opin. Nephrol. Hypertens. 2016;25:384–395. doi: 10.1097/MNH.0000000000000254. [DOI] [PubMed] [Google Scholar]
- Wang M., Chen D.Q., Wang M.C., Chen H., Chen L., Liu D., Zhao H., Zhao Y.Y. Poricoic acid ZA, a novel RAS inhibitor, attenuates tubulo-interstitial fibrosis and podocyte injury by inhibiting TGF-β/Smad signaling pathway. Phytomedicine. 2017;36:243–253. doi: 10.1016/j.phymed.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Wang X., Ye Y., Gong H., Wu J., Yuan J., Wang S., Yin P., Ding Z., Kang L., Jiang Q. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang (1–7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. Journal of molecular and cellular cardiology. 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368 doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.l., Gan X.x., Ni J., Shao D.c., Shen Y., Miao N.j., Xu D., Zhou L., Zhang W., Lu L.m. SND p102 promotes extracellular matrix accumulation and cell proliferation in rat glomerular mesangial cells via the AT1R/ERK/Smad3 pathway. Acta Pharmacologica Sinica. 2018;39:1513–1521. doi: 10.1038/aps.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhang Y., Chu L., Chen W., Du Y., Gu J. Long non‑coding RNA HIF1A‑AS1 is upregulated in intracranial aneurysms and participates in the regulation of proliferation of vascular smooth muscle cells by upregulating TGF‑β1. Experimental and therapeutic medicine. 2019;17:1797–1801. doi: 10.3892/etm.2018.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Kang, Z., Gong, H., Xu, D., Wang, J., Li, Z., Cui, X., Xiao, J., Meng, T., Zhou, W., Liu, J., 2020. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv 2020.01.30.927806.
- Zhang K., Meng X., Li D., Yang J., Kong J., Hao P., Guo T., Zhang M., Zhang Y., Zhang C. Angiotensin (1–7) attenuates the progression of streptozotocin-induced diabetic renal injury better than angiotensin receptor blockade. Kidney international. 2015;87:359–369. doi: 10.1038/ki.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. 2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present review article are available from the corresponding author on reasonable request.