1. Introduction
Myocarditis is the progressive inflammation of the middle layer of the heart followed by a myocardial injury without ischemic events [1,2]. The infectious and non-infectious causes of myocarditis determine its prognostic outcomes. The (focal/diffuse) degrees of myocardial inflammation determine the severity of symptoms in patients with myocarditis [1]. The age/gender-appropriate burden of myocarditis was recorded as 6.1/100,000 for men and 4.4/100,000 for women (within the age range of 35-39 years) in 2019 [3]; however, myocarditis-related mortality impacted 0.2/100,000 men and 0.1/100,000 women in the same year. The clinical studies reveal the worst outcomes with poorly understood pathological pathways in 20-30% of hospitalized COVID-19 (coronavirus disease) patients with myocardial injury [4].
These adversities continue to challenge the medical management of myocarditis during the COVID-19 pandemic. The US Food and Drug Administration (FDA) approved two mRNA vaccines to prevent COVID-19 in December 2021. The BNT162b2 mRNA vaccine by Pfizer-BioNTech and the mRNA-1273 vaccine by Moderna aimed to reduce COVID-19-related fatal complications and mortality. The US FDA subsequently approved the Janssen COVID-19 vaccine in February 2021 to strengthen the vaccination drive [5]. This case review investigates myocarditis scenarios that developed after the administration of COVID-19 vaccines to individuals.
1.1. Case presentation
A 70-year-old Caucasian female with a history of multiple sclerosis presented to the hospital after two days of receiving the Janssen COVID-19 vaccine. The patient developed dyspnea at home and eventually required an ambulance for hospital transfer. The vital signs on arrival included a heart rate of 145 bpm, a 75% oxygen saturation level on room air, a blood pressure of 117/70 mmHg, a respiratory rate of 39, and a BMI of 27.5. Table 1 lists the significant laboratory results for the patient. The electrocardiogram (ECG) on admission revealed sinus tachycardia with a heart rate of 125bpm and T-wave inversions in leads V4-V6 without any ST-segment change. The patient arrived at the emergency department in severe respiratory distress that warranted immediate intubation. She was admitted to the intensive care unit (ICU) with the provisional diagnoses of acute hypoxic hypercapnic respiratory failure and septic shock. The laboratory screening and blood culture proved negative for all viruses, Mycoplasma pneumonia, and Chlamydophila pneumonia. A repeat investigation revealed marked elevations in procalcitonin [185.71(ng/mL)] and troponin [1.260-2.050 ng/mL] levels on the second day of admission.
Table 1.
Lab results with normal reference range.
| Lab | Result | Normal Reference |
|---|---|---|
| Creatinine | 1.21 | 0.50–1.20 mg/dL |
| Bicarbonate | 16 | 22–29 mmol/L |
| Troponin | <0.010 | ≤0010 ng/mL |
| Creatinine Phosphokinase (CK) | 53 | 20–190 U/L |
| Procalcitonin | 0.07 | 0.02–0.10 ng/mL |
| C-Reactive Protein | 7.2 | 0–3.00 mg/L |
| pH | 7.02 | 7.35–7.45 |
| PaCO2 | 94 | 35–48 mm Hg |
| Lactate | 8.3 | 0.6–1.4 mmol/L |
| PaO2 | 27 | 83–108 mmHg |
| Respiratory Virus Panel with polymerase chain reaction (PCR) to detect for [adenovirus; coronavirus (HKU1, NL63, 229E, OC43), SARS-CoV-2; human metapneumovirus; human enterovirus/rhinovirus, influenza A; influenza A/H1; influenza A/H3, influenza A/H1-2009; influenza B; parainfluenza viruses 1, 2, 3, 4; respiratory syncytial virus] | Negative | Negative |
The patient required multiple vasopressors to maintain the mean arterial pressure above 65 mmHg. The transthoracic echocardiogram on admission revealed 2+ aortic regurgitation and diffuse left ventricular hypokinesis with severely decreased left ventricular ejection fraction (10%). A repeat echocardiogram with contrast medium showed diffuse left ventricular hypokinesis with severely reduced contraction in the apex/distal anterior wall. The diagnostic monitoring via Swan-Ganz catheter revealed a pulmonary wedge pressure (PWP) of 14 mmHg. The patient continued receiving vasopressors and antibiotic therapy, while her renal function deterioration since admission warranted the prompt administration of renal replacement therapy. Further decline in renal function was marked by oliguria and worsening of creatinine levels. The patient declined cardiac catheterization and remained on medical therapy until her death on the eighth day of admission.
2. Discussion
2.1. Viral myocarditis
Viral myocarditis progressively deteriorates the middle layer of the heart via myocardial injury triggered by inflammatory processes [2]. The viral etiology of myocarditis is prevalently reported in the United States and other developed nations of the world [1]. Viral myocarditis progresses with virus-mediated cardiomyocyte damage driven by inappropriate activation of innate and adaptive immune systems. The acute, subacute, and chronic phases of viral myocarditis reciprocate with the extent of cardiomyocyte deterioration and adaptive immune responses.
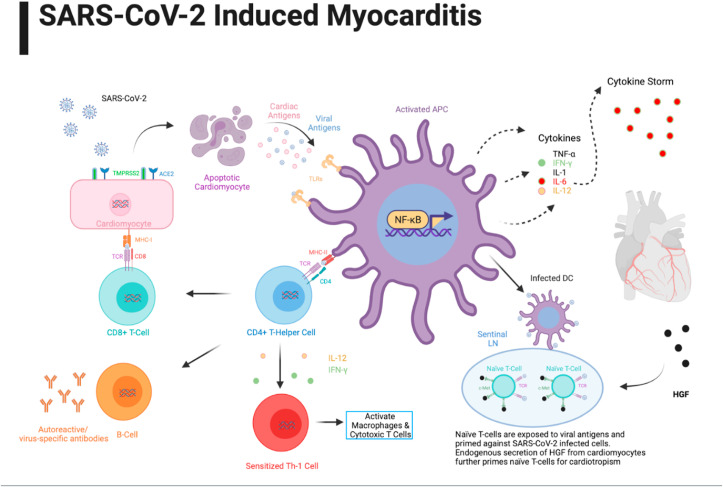
The acute phase of myocarditis progresses with the invasion of virus particles into the cardiomyocytes, followed by their cleavage, repackaging, and attachment to MHC (major histocompatibility complex)-1 receptors on the cell membrane. This event is followed by the binding of CD8+ (cytotoxic) T-cells to the class-I MHC molecules on virus-infected cardiomyocytes, thereby inducing apoptosis and subsequent release of cardiac and viral antigens (see Fig. 1 ) [6]. The binding of viral antigen to toll-like receptors (TLRs) on antigen-presenting cells (APCs) induces NF-kB transcription factor that potentiates the genes involved in the biosynthesis and secretion of proinflammatory cytokines (TNF-α, IFN-γ, IL-1, IL-6, and IL-12), thereby triggering the adaptive immune responses in the subacute phase. The virus-mediated cytotoxicity eventually induces cardiomyocyte apoptosis and early myonecrosis in the infected patient [7].
Fig. 1.
Pathophysiology of COVID-19 induced myocarditis.
The adaptive immune responses dominate the virus-mediated cardiomyocyte damage through cellular infiltration of lymphocytes during the subacute phase. The early stages of the subacute phase progress with the active repackaging of viral antigens in the antigen-presenting cells (APCs) and their interaction with MCH-II receptors. The acute phase manifests with the attachment of antigen-bound MHC II receptors (on APCs) with the CD4+ Helper T cells that triggers multiple adaptive immune responses mediated by proinflammatory cytokines [7]. The elevated cytokines (IFN-γ and IL-12) induce Th1 differentiation and promote further activation of macrophages and cytotoxic T cell-mediated damage [8]; however, IL-12 elevation potentiates the activity of natural killer (NK) cells. The activated Th cells bind to the antigen-oriented MHC II receptors on B-cells to promote the formation of virus-specific antibodies and autoreactive antibodies against the cardiac antigens and myosin [9]. The late subacute phase manifests with the sequestration of viral antigen-oriented activated dendritic cells by lymph nodes and priming of naïve T-cells against SARS-CoV-2 infected cells [10]. The chronic phase of viral myocarditis progresses with myocardial fibrosis, heart failure, and dilated cardiomyopathy.
2.2. COVID-19 and myocarditis
The activated spike (S) proteins of SARS-CoV-2 particles interact with angiotensin-converting enzyme-2 (ACE2) on the target cells to mediate their entry into the host system. The ACE2 receptor expression occurs in cardiomyocytes after the intrusion of SARS-CoV-2 into the epithelial cells lining the respiratory tract and type II pneumocytes [12]. The cardiomyocyte damage by SARS-CoV-2 may resemble aberrant immune responses that develop in other types of viral myocarditis. Future clinical studies still require delineating the pathophysiological processes governing myocardial injury and myocarditis in patients with COVID-19.
The direct cell injury and T-lymphocyte cytotoxicity augmented by IL-6 mediated cytokine storm (CS) govern the pathophysiology of viral myocarditis [7]. The marked elevation in the proinflammatory cytokines including, IL-6, IL-8, and TNF-α in severely ill SARS-CoV-2 patients suggests that CS development may play an important role in the clinical progression of COVID-19 [13,14]. The activity of monoclonal-antibody (like tocilizumab) against the IL-6 receptors in COVID-19 pneumonia patients adds to their medical management in the current scenario [15,16]. The clinical studies also emphasize the role of the HGF-c-MET (transmembrane tyrosine kinase) axis in the pathogenesis of SARS-CoV-2 induced myocardial damage [17]. The localized inflammation within the heart progresses with cardiomyocyte secretion of hepatocyte growth factor (HGF) and its interaction with the c-MET receptors on naïve T cells in lymph nodes [17,18]. The intrinsic myocardial processes and immune-mediated hyperinflammatory responses following viral exposure also determine the pathophysiological mechanisms of SARS-CoV-2 myocarditis [19].
3. Management of myocarditis due to COVID-19 infection or vaccine
The symptomatology of COVID-19 infection-induced or post-vaccine-related myocarditis includes shortness of breath, fatigue, and chest pain. Patients with high severity myocarditis often report the signs of right-sided heart failure, including elevated jugular venous pressure, right upper quadrant pain, and peripheral edema [20]. Few patients with COVID-19 also develop severe diffuse cardiac inflammation leading to fulminant myocarditis, ventricular arrhythmias, and cardiogenic shock. Fulminant myocarditis usually develops within 2-3 weeks of contracting the virus and presents with ventricular dysfunction and acute onset of heart failure [18]. CDC advocates myocarditis screening for patients who develop shortness of breath, chest pain, or palpitations within 7 days of receiving the mRNA COVID-19 vaccine [21]. The younger patients with COVID-19 symptoms also require myocarditis screening to rule out their coronary attribution.
4. Approach to diagnosis of myocarditis
The diagnostic interventions for myocarditis include laboratory testing concerning serum lactate, CRP, serum troponin natriuretic peptide, erythrocyte sedimentation rate, and procalcitonin. The fulminant myocarditis predominantly progresses with elevated serum cardiac troponins [20]; however, the absence of cardiac marker elevation does not rule out myocarditis. A recent systemic review revealed marked elevations in cardiac troponins, CK-MB, BNP, and CRP in COVID-19 patients with myocarditis [22].
The low sensitivity of an electrocardiogram (ECG) for myocarditis makes it a secondary modality to investigate the cardiovascular abnormalities in COVID-19 patients. The ECG results for few patients with COVID-19; however, reflect abnormalities including ST elevation, PR depression, bundle branch block (new-onset), QT prolongation, pseudo-infarct patterns, premature ventricular complexes, and bradyarrhythmia with an advanced atrioventricular nodal block [18].
The differential assessment of conditions with similar presentation (as myocarditis) is conducive to avoid diagnostic errors. These conditions include acute coronary syndrome (ACS), sepsis-induced cardiomyopathy, and stress-induced cardiomyopathy. The diagnostic assessment should also rule out obstructive coronary disease based on its resemblance with myocarditis complications including, chest pain, elevated cardiac markers, and ECG abnormalities [23]. The myocarditis assessment relies on biopsy for COVID-19 patients who do not develop coronary artery obstruction and may acquire other ischemic and non-ischemic complications.
4.1. Imaging modalities
The guidelines proposed by the American Heart Association (AHA) recommends further testing for patients with clinical and biochemical abnormalities of myocarditis determined by cardiac imaging, echocardiogram, and cardiovascular magnetic resonance (CMR) [20]. The first-line imaging by echocardiography helps determine potential complications of myocarditis in COVID-19 patients. The cardinal signs of myocarditis determined by an echocardiogram include an elevated wall thickness, chamber dilation, pericardial effusion, and ventricular systolic dysfunction. The patients may also develop global or regional hypokinesia [19]. The normal left ventricular ejection fraction in COVID-19 patients, however, does not exclude myocarditis and its clinical complications [23].
Myocarditis assessment in hemodynamically stable patients relies on CMR or coronary computed tomography (CCT). The tissue characterization via CMR and guided by the Lake Louise Criteria helps determine myocardial edema, myocardial injury, and pericardial effusion in COVID-19 patients (Table 2 ). It also includes T1/T2-weighted imaging criteria for myocardial inflammation [23,24]. The diagnostic results for myocarditis further reflect elevated T2 values and short T1 inversion recovery (STIR) [19].
Table 2.
CMR diagnostic criteria for myocarditis [24].
| Diagnostic Target | Lake Louise Criteria |
|---|---|
| Myocardial Edema | T2-weighted imaging, Increased Bright signal Intensity |
| Myocardial Injury -Hyperemia (intracellular/extracellular edema, capillary leak). -Myocardial Necrosis, Scar formation. |
-Increased global early gadolinium enhancement ratio between myocardium and skeletal muscle. -At least one focal lesion with non-ischemic regional distribution on late gadolinium enhancement. |
| Supportive Criteria | -Pericardial Effusion. -Systolic Left ventricular (LV) wall motion abnormality. |
| If 2 Lake Louise Criteria are positive, CMR is considered indicative of active myocardial inflammation. | |
The endomyocardial biopsy (EMB) for myocarditis is a class-I procedure (defined by AHA and ACC) that excludes giant-cell, eosinophilic, and hypersensitive myocarditis in patients with new-onset heart failure and hemodynamic instability [25]. The lymphocytic myocarditis in COVID-19 patients manifests with activated macrophages and T lymphocyte infiltrates [26].
5. Management of COVID-19 infection or vaccine related myocarditis
The current treatment strategies reportedly do not prove beneficial for patients with COVID-19 infection/vaccine-related myocarditis. The current medical management of COVID-19-related myocarditis relies on corticosteroids and intravenous immunoglobulins (IVIG) to challenge the progression of diffuse non-specific immune system activation [[18], [27]].
The efficacy and safety of corticosteroids in COVID-19 scenarios, however, warrant further investigation. The evidence-based myocarditis management guidelines by AHA and ESC restrict the use of nonsteroidal anti-inflammatory drugs (NSAIDs) based on their attribution for renal impairment and sodium retention that may exacerbate acute ventricular/LV systolic dysfunction in COVID-19-related myocarditis patients [[1], [18], [23]]. The COVID-19 patients may further require heart failure therapy based on their hemodynamic stability and cardiac output [19].
The diagnostic investigation should rule out fulminant myocarditis in COVID-19 patients with sepsis before administering intravenous fluid resuscitation to minimize the risk of fatal complications. Furthermore, cardiogenic shock in fulminant myocarditis often accompanies ventricular tachyarrhythmias and bradyarrhythmia dominated by a heart block, syncope, and sudden cardiac death [20].
The current AHA guidelines advocate the implementation of cardiogenic shock management treatment protocol for patients with fulminant myocarditis. The mechanical circulatory support by extracorporeal membrane oxygenation (ECMO), a ventricular assist device (VAD), or an intra-aortic balloon pump may assist the long-term medical management of hemodynamically unstable COVID-19 patients with myocarditis [18].
6. COVID-19 vaccine-induced myocarditis
We tracked seventeen cases of myocarditis concerning the COVID-19 vaccine in the medical literature (Table 3 ). We further retrieved the electrocardiographic findings, echocardiographic results, presence/magnitude of cardiac biomarker elevation, radiographic changes (if present), presenting symptoms (if reported), significant demographic information (for age/gender), and comorbidities of patients.
Table 3.
COVID-19 Vaccine and myocarditis.
| Case | Age/Sex | Comorbidities | Echo | ECG | CRP | Trop | Coronary Angio |
Symptom | Symptoms onset |
cMRI |
|---|---|---|---|---|---|---|---|---|---|---|
| Albert [29] | 24 M | N/A | Normal | Sinus Rhythm | 26.4 | Trop 18.94 | Normal | CP Fever Chills Myalgia |
4 d 2nd | T2- Mild myocardial/epicardial delayed gadolinium; Patchy |
| Mouch 1 [28] | 24 M | None | Normal | Diffuse ST elevation | 58.1 | Trop: 589 | NA | CP | 3d 2nd | T2- Mild myocardial edema; LGE in subepicardial region |
| Mouch 2 [28] | 20 M | None | LVEF 50–55% Apical Hypokinesia |
Sinus tachycardia ST elevation: V2–V6 |
100 | Trop: 1062 | Normal | CP | 1d 2nd | T2- Mild myocardial edema; LGE in subepicardial region of basal/middle anterolateral and inferolateral walls |
| Mouch 3 [28] | 29 M | Obesity | Normal | Diffuse PR depression/ ST elevation |
86 | Trop: 876 | Normal | CP | 2d 2nd | T2- Mild, diffuse myocardial edema; LGE in subepicardial region of the basal inferolateral, anterolateral, and anteroseptal walls |
| Mouch 4 [28] | 45 M | HLD | LVEF 50–55% | ST elevation: I, aVL Inverted T: V3–V5 ST depression: III, aVF |
56.2 | Trop: 392 | Normal | CP | 16d 1st | T2- Subepicardial edema of middle anterolateral, inferolateral and apical anterior walls; LGE present in same segments |
| Mouch 5 [28] | 16 M | None | Normal | ST elevation: V2–V4 | 1.6 | Trop: 14350 | Normal | CP Myalgia |
1d 2nd | T2- Midmyocardial/Subepicardial edema of basal inferolateral and middle anterolateral walls; LGE present in same segments |
| Mouch 6 [28] | 17 M | Obesity | Normal | N/A | 54.7 | Trop: 1130 | NA | CP | 3d 2nd | T2- Subepicardial edema of basal/middle inferolateral, inferior-septal, apical anterior & inferior walls; LGE present in same segments; Mid-myocardial enhancement of the middle inferolateral/anterolateral, apical anterior, and lateral walls c/w myopericarditis |
| García [30] | 39 M | Asthma Autoimmune hypothyroidism Chronic atrophic gastritis Atrial fibrillation |
Normal | Sinus tachycardia Narrow QRS Diffuse ST-segment |
N/A | Trop: 854 | NA | CP | 6hrs 2nd | T2- Myocardial edema; Subpericardial enhancement of the lateral mediastinal |
| Ammirati 1 [33] | 56 M | N/A | N/A | Minimal ST elevation of Precordial Leads Peaked T waves |
2.9 | Trop: 515 | Normal | N/A | 3d 2nd | T2- Subepicardial edema of basal and apical inferolateral walls; Focal subepicardial-intramyocardial (non-ischemic pattern) LGE present in same segments c/w acute myocarditis |
| Marshall 1 [34] | 16 M | N/A | Normal | AV dissociation with junctional escape ST elevation |
12.3 | Trop: 12.43 | Normal | N/A | 2d 2nd | T2- LGE c/w acute myocarditis |
| Marshall 2 [34] | 19 M | N/A | Normal | Sinus tachycardia Diffuse ST elevation |
6.7 | Trop: 232 | Normal | N/A | 3d 2nd | T2- mid-wall LGE along basal inferolateral wall segment; Patchy |
| Marshall 3 [34] | 17 M | N/A | Borderline basal lateral/posterior strain | Abnormal T wave Diffuse ST elevation c/w acute pericarditis | 2.53 | Trop: 5550 | Borderline basal lateral/posterior strain | N/A | 2d 2nd | T2- LGE of LV subepicardial basal anterolateral and basal to mid-ventricular inferolateral segments c/w myonecrosis |
| Marshall 4 [34] | 18 M | N/A | Normal | ST elevation | 12.7 | Trop: 1090 | Normal | N/A | 3d 2nd | T2- Edema, hyperemia, and fibrosis of segments c/w acute myocarditis |
| Marshall 5 [34] | 17 M | N/A | Normal | ST elevation | 18.1 | Trop: 3200 | Normal | N/A | 3d 2nd | T2- Subepicardial edema; LGE revealed complete transmural LV free wall |
| Marshall 6 [34] | 16 M | N/A | Normal | ST-elevation | 1.8 | Trop: 660 | Normal | N/A | 3d 2nd | T2- Diffuse subepicardial edema; LGE present in same segment |
| Marshall 7 [34] | 14 M | N/A | Mildly depressed LV/RV systolic function | Diffuse ST elevation c/w acute pericarditis | 12.7 | Trop: 22100 | Mildly depressed LV/RV systolic function LVEF 47% |
N/A | 2d 2nd | T2- Subepicardial edema of middle and apical LV free wall; LGE present in same segments revealed fibrosis. |
| D'Angelo 1 [32] | 30 M | N/A | Preserved EF, mild pericardial effusion, segmental wall motion abnormality of apical portion of interventricular septum | ST elevation: V2–V4, Non-specific T-wave changes: V5 and V6 | 39.6 | Trop: 12546.8 | NA | N/A | 3d 2nd | T2-weighted STIR- Increased myocardial and pericardial signal intensity |
| Our Case | 70 F | Multiple Sclerosis | LVEF 10% - diffuse LV hypokinesis | sinus tachycardia – inverted T: V4–V6 | 7.2 | Trop 2050 | – | Dyspnea | 2d J&J | – |
CP: chest pain; ECG = Electrocardiogram; CXR: chest x-ray; CTA: computed tomography angiography; Echo: transesophageal echocardiography or transthoracic echocardiography; Peak CRP= Peak C-Reactive Protein, reported in; Peak Trop = Peak Troponin T or I, reported in; TTE = Trans-Thoracic Echocardiogram; cMRI = Cardiac MRI; LGE = Late Gadolinium Enhancement; NA= Not assessed; c/w = Consistent With; = After; J&J: Johnson & Johnson's single-dose COVID-19 vaccine.
All cases were associated with mRNA COVID-19 vaccine except our case which was associated with adenovirus vector-related vaccine. The reported cases exclusively included males with a mean age of 25.5 years and a median age of 22 years. The medical history of the myocarditis patients revealed multiple comorbidities, including obesity and hyperlipidemia [28]. The patient concerning our case study, however, had a history of multiple sclerosis.
The clinical data for most patients with myocarditis did not reveal their presenting symptoms (excluding eight patients with chest pain as their presenting complaint) [[28], [29], [30]]. The clinical findings further confirmed myalgia in two patients and fever in one case [28,29].
The data further clarified the onset of myocarditis in patients after several weeks of receiving the COVID-19 vaccine [31]. The patients reported myocarditis symptoms within three days of receiving the first/second dose; however, most presentations correlated with the second dose of the COVID-19 vaccine. The patient we studied developed myocarditis symptoms within two days of receiving the COVID-19 vaccine. The medical literature revealed COVID-19 vaccine-related myocarditis patients within the age group of 20-30 years, unlike our patient, who had completed her 6th decade of life.
The patient we studied presented with T-wave inversions that matched the ECG findings recorded for three cases in the medical literature. We further noticed T-wave inversions in two patients [28,32] and ST-segment elevation in twelve of the reported seventeen cases [28,30,33,34]. The ECG findings further correlated with the cardiac biomarker elevations and serum troponin peaks at varying levels in the registered patients. The findings from our patient initially revealed a normal troponin level that subsequently trended upwards during her medical management.
The seventeen cases we retrieved from the medical literature presented with a preserved ejection fraction, excluding one patient who developed apical hypokinesia [28].
The patient we managed exhibited a significantly reduced ejection fraction (10%) and left ventricular dyskinesia. She had a limited pretest probability for ACS in the absence of cardiac risk factors. The patient declined cardiac catheterization despite the medical recommendation. We further noticed cardiac catheterization undertaken for thirteen out of seventeen patients registered in the medical literature [28,29,33,34]. The patients who received cardiac catheterization had no history of coronary artery disease. The elevated cardiac markers and chest pain proved to be the greatest confounders in the diagnostic assessment of myocarditis. We administered invasive mechanical ventilation and vasopressor support to our patient unguided by a cardiac MRI. The seventeen cases reported in medical literature, however, received cardiac MRI during their therapeutic management. Our findings further revealed a marked elevation in the procalcitonin level (185ng/mL) of the myocarditis patient. The abnormal echocardiogram, EKG, and cardiac biomarker levels did not correlate with any finding concerning a source of infection other than the COVID-19 vaccine.
Our patient also required invasive mechanical ventilation and pressor support, for which cardiac MRI was not performed. However, in comparison to the seventeen reported cases, all underwent cardiac MRI.
In addition to cardiac biomarkers, EKG, and echocardiogram findings, our case presentation showed an elevated procalcitonin level, which reached a maximum value of 185; however, no source of infection was found, leaving only the previously administered vaccination as the source of the sudden instability.
7. Conclusions
The outcomes of this case scenario confirm myocarditis as a probable complication of COVID-19 vaccines. The differential assessment of patients with COVID-19 vaccination status and symptoms of acute cardiac decompensation must rule out myocarditis to avoid fatal complications. An early diagnosis is key to minimize COVID-19 vaccine-related adversities and improve the medical management of patients suspected of myocarditis.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., Klingel K., Linhart A., Maisch B., McKenna W., Mogensen J., Pinto Y.M., Ristic A., Schultheiss H.P., Seggewiss H., Tavazzi L., Thiene G., Yilmaz A., Charron P., Elliott P.M. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d PMID: 23824828. [DOI] [PubMed] [Google Scholar]
- 2.Krejci J., Mlejnek D., Sochorova D., Nemec P. Inflammatory cardiomyopathy: a current view on the pathophysiology, diagnosis, and treatment. Biomed Res Int, 2016. 2016:4087632. doi: 10.1155/2016/4087632. PMID: 27382566; PMC4921131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., Bonny A., Brauer M., Brodmann M., Cahill T.J., Carapetis J., Catapano A.L., Chugh S.S., Cooper L.T., Coresh J., Criqui M., DeCleene N., Eagle K.A., Emmons-Bell S., Feigin V.L., Fernández-Solà J., Fowkes G., Gakidou E., Grundy S.M., He F.J., Howard G., Hu F., Inker L., Karthikeyan G., Kassebaum N., Koroshetz W., Lavie C., Lloyd-Jones D., Lu H.S., Mirijello A., Temesgen A.M., Mokdad A., Moran A.E., Muntner P., Narula J., Neal B., Ntsekhe M., Moraes de Oliveira G., Otto C., Owolabi M., Pratt M., Rajagopalan S., Reitsma M., Ribeiro A.L.P., Rigotti N., Rodgers A., Sable C., Shakil S., Sliwa-Hahnle K., Stark B., Sundström J., Timpel P., Tleyjeh I.M., Valgimigli M., Vos T., Whelton P.K., Yacoub M., Zuhlke L., Murray C., Fuster V. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. PMID: 33309175; PMC7755038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. PMID: 32211816; PMC7097841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spring S. Janssen COVID-19 vaccine emergency use authorization. Food and Drug Administration. 2021 https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine cited 2021; Available from: [PubMed] [Google Scholar]
- 6.Dennert R., Crijns H.J., Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29(17):2073–2082. doi: 10.1093/eurheartj/ehn296. PMID: 18617482; PMC2519249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esfandiarei M., McManus B.M. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. PMID: 18039131. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z., John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. PMID: 32728222; PMC7389156 Roche, Pieris, Elstar and Surface Oncology. EJW has a patent licensing agreement on the PD1 pathway with Roche/Genentech. E.J.W. is a founder of Arsenal Biosciences. ZC declares no competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caforio A.L., Tona F., Bottaro S., Vinci A., Dequal G., Daliento L., Thiene G., Iliceto S. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity. 2008;41(1):35–45. doi: 10.1080/08916930701619235. PMID: 18176863. [DOI] [PubMed] [Google Scholar]
- 10.Obst R. The timing of T cell priming and cycling. Front Immunol. 2015;6:563. doi: 10.3389/fimmu.2015.00563. PMID: 26594213; PMC4633513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher P.E., Ferrario C.M., Tallant E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295(6):H2373–H2379. doi: 10.1152/ajpheart.00426.2008. PMID: 18849338; PMC2614534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. PMID: 32839624; PMC7869028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. PMID: 32754163; PMC7365923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. PMID: 33631066; PMC7953459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. PMID: 32234467; PMC7118634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komarowska I., Coe D., Wang G., Haas R., Mauro C., Kishore M., Cooper D., Nadkarni S., Fu H., Steinbruchel D.A., Pitzalis C., Anderson G., Bucy P., Lombardi G., Breckenridge R., Marelli-Berg F.M. Hepatocyte growth factor receptor c-met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity. 2015;42(6):1087–1099. doi: 10.1016/j.immuni.2015.05.014. PMID: 26070483; PMC4510150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., Cooper L.T., Jr., Chahal C.A.A. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. PMID: 32387246; PMC7199677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castiello T., Georgiopoulos G., Finocchiaro G., Claudia M., Gianatti A., Delialis D., Aimo A., Prasad S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2021:1–11. doi: 10.1007/s10741-021-10087-9. PMID: 33761041; PMC7988375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kociol R.D., Cooper L.T., Fang J.C., Moslehi J.J., Pang P.S., Sabe M.A., Shah R.V., Sims D.B., Thiene G., Vardeny O. Recognition and initial management of fulminant myocarditis: a scientific statement from the American heart association. Circulation. 2020;141(6):e69–e92. doi: 10.1161/cir.0000000000000745. PMID: 31902242. [DOI] [PubMed] [Google Scholar]
- 21.Clinical considerations: myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html [cited 2021 6/14/2021]; Available from:
- 22.Kariyanna P.T., Sutarjono B., Grewal E., Singh K.P., Aurora L., Smith L., Chandrakumar H.P., Jayarangaiah A., Goldman S.A., Salifu M.O., McFarlane I.M. A systematic review of COVID-19 and myocarditis. Am J Med Case Rep. 2020;8(9):299–305. PMID: 32747875; PMC7397751. [Google Scholar]
- 23.Mele D., Flamigni F., Rapezzi C., Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med. 2021:1–7. doi: 10.1007/s11739-021-02635-w. PMID: 33484452; PMC7823176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., Friedrich M.G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. PMID: 30545455. [DOI] [PubMed] [Google Scholar]
- 25.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U., Levine G.N., Narula J., Starling R.C., Towbin J., Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116(19):2216–2233. doi: 10.1161/circulationaha.107.186093. PMID: 17959655. [DOI] [PubMed] [Google Scholar]
- 26.Bracamonte-Baran W., Čiháková D. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. PMID: 28667560; PMC5706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/s0140-6736(20)30628-0. PMID: 32192578; PMC7270045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., Zoabi M., Aisman M., Goldschmid N., Berar Yanay N. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39(29):3790–3793. doi: 10.1016/j.vaccine.2021.05.087. PMID: 34092429; PMC8162819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021 doi: 10.1016/j.radcr.2021.05.033. PMID: 34025885; PMC8130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista Garcia J., Pena Ortega P., Bonilla Fernandez J.A., Cardenes Leon A., Ramirez Burgos L., Caballero Dorta E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol. 2021 doi: 10.1016/j.rec.2021.04.005. PMID: 33994339; PMC8075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawalha K., Abozenah M., Kadado A.J., Battisha A., Al-Akchar M., Salerno C., Hernandez-Montfort J., Islam A.M. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revascularization Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. PMID: 32847728; PMC7434380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Angelo T., Cattafi A., Carerj M.L., Booz C., Ascenti G., Cicero G., Blandino A., Mazziotti S. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. 2021 doi: 10.1016/j.cjca.2021.05.010. PMID: 34118375; PMC8187737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammirati E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F., Schroeder J.W., Morici N., Giannattasio C., Frigerio M., Metra M., Camici P.G., Oliva F. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Heart Vasc. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774. PMID: 33821210; PMC8011690 personal relationships that could have appeared to influence the work reported in this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., Shaughnessya R., Carona R., Fuss C., Corbin K.J.E., Emuren L., Faherty E., Hall E.K., Di Pentima C., Oster M.E., Paintsil E., Siddiqui S., Timchak D.M., Guzman-Cottrill J.A. Symptomatic acute myocarditis in seven adolescents following pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021 doi: 10.1542/peds.2021-052478. PMID: 34088762. [DOI] [PubMed] [Google Scholar]