Abstract
Introduction
The effectiveness of umifenovir against COVID-19 is controversial; therefore, clinical trials are crucial to evaluate its efficacy.
Methods
The study was conducted as a single-center, randomized, open-label clinical trial. Eligible moderate-severe hospitalized patients with confirmed SARS-Cov-2 infection were randomly segregated into intervention and control groups. The intervention group were treated with lopinavir/ritonavir (400 mg/100 mg bid for 10–14 days) + hydroxychloroquine (400 mg single dose) + interferon-β1a (Subcutaneous injections of 44 µg (12,000 IU) on days 1, 3, 5) + umifenovir (200 mg trice daily for 10 days), and the control group received lopinavir/ritonavir (same dose) + hydroxychloroquine (same dose) + interferon-β1a (same dose).
Results
Of 1180 patients with positive RT-PCRs and positive chest CT scans, 101 patients were finally included in the trial; 50 were assigned to receive IFNβ1a + hydroxychloroquine + lopinavir/ritonavir group and 51 were managed to treat with IFNβ1a + hydroxychloroquine + lopinavir/ritonavir + umifenovir. Since all patients received the intended treatment as scheduled, the analysis just included as the ITT population.
Time to clinical improvement (TTCI) did not hold a statistically significant difference between intervention and control groups (median, 9 days for intervention group versus 7 days for the control group; P: 0.22).
Besides, Hazard Ratio for TTCI in the Cox regression model was 0.75 (95% CI: 0.45–1.23, P:0.25) which also confirmed that there was no statistically significant difference between the treatment group and the control group. The mortality was not statistically significant between the two groups (38% in controls vs 33.3% treatment group).
Conclusions
Our findings shed new lights on the facts that additional umifenovir has not been found to be effective in shortening the duration of SARS-CoV-2 in severe patients and improving the prognosis in non-ICU patients and mortality.
Trial registration
The trial was confirmed by the Ethics in Medical Research Committee of the Shahid Beheshti University of Medical Sciences. signed informed consents were obtained from all the participants or their legally authorized representatives. This trial has been registered as ClinicalTrials.gov, NCT04350684.
Keywords: SARS-CoV-2, Umifenovir, Arbidol, COVID-19
Abbreviations: ABG, Atrial blood gas; COVID-2019, Coronavirus Disease 2019; GCS, Glasgow Coma Scale; HR, Hazard Ratio; ICU, Intensive care unit; LFT, liver function test; SARS-CoV-2, severe acute respiratory syndrome coronavirus2; TTCI, Time to clinical improvement; VBG, Venous blood gas
1. Introduction
Coronavirus Disease 2019 (COVID-2019) caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus2) has remained as a major menace for health in the world. As of 27 September 2020, >991,220 deaths were reported worldwide [1], [2]. Several antiviral drugs as well as other medications have been evaluated as possible treatment choices for SARS-CoV-2. However, there are currently no proven treatments for COVID-19 [3].
Inasmuch as SARS-CoV-2 shares high identity with SARS-CoV, it is assumed that effective antiviral drugs against SARS-CoV may also be useful in fighting with COVID-19 infection [4]. An invitro study illustrated that umifenovir (Arbidol) as an antiviral drug could represent inhibitory activity against SARS-CoV [5].
Umifenovir has been also used against influenza A, B viruses, and other human pathogenic respiratory viruses in Russia and China [6]. Umifenover exerts its antiviral activities by impeding of virus-cell membrane fusion and virus endosome [7]. Also known as Arbidol, Umifenover showed activity against SARS-Cov-2 virus in an in vitro [8].
A recent cohort study has proved the beneficial effects of umifenovir in COVID-19 patients. The study has shown that patients who received combination of umifenovir + lopinavir/ritonavir exhibited rapid improvements in chest CT and shortened positive PCR duration as opposed to those patients treated with lopinavir/ritonavir [9]. On the other hand, a retrospective study suggested that umifenovir might have no effect on improvement of COVID-19 patients’ prognosis [10]. Information regarding the effectiveness of umifenovir as a trustworthy remedy for COVID-19 infection is sporadic and ambiguous; therefore, clinical trials are deemed necessary to evaluate the efficacy of umifenover alone or in combination with other drugs.
We performed a randomized, open-label, controlled trial to evaluate the efficacy of umifenovir when prescribing in combination with lopinavir/ritonavir, interferon-beta1a (IFN-β 1a), and hydroxychloroquine in moderate to severe cases of hospitalized COVID-19 patients.
2. Material and methods:
In this single-center, randomized, open-label clinical trial, moderate to severe confirmed Covid-19 cases by RT-PCR (Reverse Transcriptase Polymerase-Chain Reaction) and/or CT-scan (Computed Tomography Scan) at Loghman Hakim hospital and Shahid Beheshti University of Medical Sciences were recruited. Inclusion criteria for the current study were as follows (1) age > 18 years (2) Presence of at least one of the following manifestation: (radiation contactless body temperature ≥ 37.5 °C, cough, shortness of breath, nasal congestion/discharge, myalgia/arthralgia, diarrhea/vomiting, headache or fatigue) (3) Peripheral capillary oxygen saturation level (SpO2) ≤ 93% on pulse oximetry (4) A respiratory frequency ≥ 24/minute while breathing ambient air (on admission day) (5) Acute onset of symptoms (≤14 days).
Exclusion criteria were consumption of potentially interacting medications with lopinavir/ritonavir or IFN-β1a, pregnancy and breastfeeding, history of alcohol use disorder, or any illicit drug dependence within the past five years, blood AST/ALT levels ≥ 5-fold higher relative to maximum limit of normal range on laboratory findings and participation refusal who needed invasive ventilation from the beginning.
The cases were randomly categorized into intervention group and control groups. The intervention group (Arms1) received lopinavir/ritonavir (400 mg/100 mg bid for 10–14 days) (Kaletra) + hydroxychloroquine (400 mg single dose) + interferon-β1a (subcutaneous injections of 44 µg (12,000 IU) on days 1, 3, 5) (Recigen) + umifenovir (200 mg TDS for 7 days) (Arbidol), and the control group were treated with lopinavir/ritonavir (400 mg/100 mg bid for 10–14 days) (Kaletra) + hydroxychloroquine (400 mg single dose) + interferon-β1a (subcutaneous injections of 44 µg (12,000 IU) on days 1, 3, 5), (Recigen). All two groups received standards of care consisting of necessary oxygen support, non-invasive or invasive mechanical ventilation.
Unstratified randomization was done in a 1:1 ratio utilizing a block balance randomization method. The investigator (IAD) enrolled the patients and only then opened envelopes to assign patients to the different treatment groups. This method of randomization and allocation concealment results in minimum selection and confounding biases.
The trial was confirmed by the Ethics in Medical Research Committee of the Shahid Beheshti University of Medical Sciences. signed informed consents were obtained from all the participants or their legally authorized representatives. This trial has been registered as ClinicalTrials.gov, NCT04350684.
2.1. Clinical and laboratory monitoring
Vital signs (pulse rate, respiratory rate, body temperature, and blood pressure), SpO2, Glasgow Coma Scale (GCS) were recorded every four hours. Daily recordation of a seven-step ordinal scale using a protocol-defined checklist was also performed. Regarding safety concerns, daily monitoring for adverse effects and laboratory testing were carried out. Nasopharyngeal swab samples were obtained before enrollment and tested using Liferiver (W-RR-0479–02, China) for E, N, and Rdrp genes.
2.2. Outcome measures
2.2.1. Primary outcome
Time clinical improvement was evaluated based on improvement of two points of the seven-category ordinal scale (recommended by the World Health Organization: Coronavirus disease (COVID-2019) R&D. Geneva: World Health Organization) or discharge from the hospital, whichever comes first.
2.2.2. Secondary outcomes
Mortality from the first day of randomized trial until the last day of the study which was the day all of the patients have had at least one of the following outcomes: (1) Development of two points of the seven-category ordinal scale. (2) Discharge from the hospital (3) Death. Improvement of SPO2 during the hospitalization, duration of hospitalization from date of randomization until the date of hospital death or discharge, whichever comes first. The incidence of new mechanical ventilation uses from date of randomization until the last day of the study and its duration was extracted. Follow-ups of discharged patients were done utilizing telemedicine visits, online, or using telephone.
2.3. Statistical analysis
The total sample size was calculated according to the Latouche and colleagues approach for estimating sample size in survival analyses with 80% power, alpha = 0.05, Hazard Ratio (HR) of 2.5 (as the ratio of the hazard rates of TTCI corresponding to the intervention group compared to the control group) and assuming that 70% of patients would reach the primary outcome. The calculations were carried out using Package ‘powerSurvEpi’ in R and accounted for a dropout rate of 10%. According to above-mentioned assumptions, 100 patients should have been recruited for this trial (50 patients in each arm). The TTCI was determined when all the patients had reached day 21. Patients who failed to reach the primary endpoint or died prior to day 21 were regarded as right-censored.
Kaplan–Meier (compared with a log-rank test) was used to analyze the TTCI. Cox proportional-hazards model was also applied to calculate the HRs with 95% Confidence Intervals (CIs). All the participants who had undergone randomization were included in Intention-To-Treat (ITT) analysis (Fig. 1 ). Frequencies and percentages were employed for categorical variables. For normally and none-normally distributed continuous variables Mean (SD) and median (interquartile range) were used, respectively. Differences of continuous variables between studied groups were evaluated using T-test (for normally distributed) and Mann-Whitney U test (for non-normally distributed). Categorical variables were analyzed using the chi-squared test, or the Fisher’s exact test (when the expected frequency was<5 in one or more cells). A p-value of < 0.05 was considered to be statistically significant. All of the carried-out tests were two-tailed. R software version 3.6.1 was used to perform the statistical analyses.
Fig. 1.
Trial Flow Diagram.
3. Results
3.1. Patients
Of 1180 patients with positive RT-PCRs and positive chest CT scans, 101 patients were finally included in the trial; 50 were assigned to receive IFNβ1a + hydroxychloroquine + lopinavir/ritonavir group and 51 were managed to treat with IFNβ1a + hydroxychloroquine + lopinavir/ritonavir + umifenovir. Since all patients received the intended treatment as scheduled, the analysis just included as the ITT population (Fig. 1).
The mean (SD) age of participants was 61.2 (15.8) years. The frequency of males in the trial was slightly higher relative to females (57 vs 44). Majority of patients (88.0%) were entered to the randomization less than one week from the onset of symptoms. Demographic, clinical characteristics did not reach a statistically significant difference between two groups at baseline (table 1 )
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Total (N = 101) | Infbeta1 + HQ + lopinavir/ritonavir (N = 50) | Infbeta1 + HQ + lopinavir/ritonavir + umifenovir (N = 51) | P-value |
|---|---|---|---|---|
| Age (year) | 61.2 (15.8) | 60.2 (16.5) | 62.1 (15.3) | 0.55 |
| Male sex — no. (%) | 57 (56.4%) | 26 (52.0%) | 31 (60.8%) | 0.37 |
| BMI (kg/m2) | 28.5 (6.5) | 28.8 (6.7) | 28.3 (6.3) | 0.72 |
| Duration of symptoms before presentation < 7 days | 88 (88.0%) | 44 (89.8%) | 44 (86.3%) | 0.59 |
| Underlying conditions | ||||
| Diabetes | 31 (31.6%) | 14 (29.2%) | 17 (34.0%) | 0.61 |
| Hypertension | 45 (46.4%) | 22 (45.8%) | 23 (46.9%) | 0.91 |
| Coronary Heart Disease | 11 (11.2%) | 6 (12.5%) | 5 (10.0%) | 0.69 |
| Chronic Kidney Disease | 7 (7.1%) | 4 (8.3%) | 3 (6.0%) | 0.65 |
| Malignancy | 1 (1.0%) | 0 (0.0%) | 1 (2.0%) | 1.00 |
| Ischemic Heart Disease | 23 (23.5%) | 12 (25.0%) | 11 (22.0%) | 0.81 |
| Asthma | 6 (6.1%) | 1 (2.1%) | 5 (10.0%) | 0.20 |
| Paramedical history | ||||
| Anti-viral drug | 8 (7.9%) | 3 (6.0%) | 5 (9.8%) | 0.48 |
| Steroid | 3 (3.0%) | 1 (2.0%) | 2 (3.9%) | 1.00 |
| CQ | 4 (4.0%) | 1 (2.0%) | 3 (5.9%) | 0.62 |
| NSAID | 2 (2.0%) | 0 (0.0%) | 2 (3.9%) | 0.49 |
| ACE & ARB | 38 (37.6%) | 18 (36.0%) | 20 (39.2%) | 0.74 |
| Risk factors | ||||
| RF pul dis | 10 (10.0%) | 2 (4.1%) | 8 (15.7%) | 0.053 |
| RF Chronic Kidney Disease | 12 (12.0%) | 7 (14.3%) | 5 (9.8%) | 0.49 |
| RF Diabetes | 21 (21.0%) | 11 (22.4%) | 10 (19.6%) | 0.73 |
| RF Hypertension | 49 (49.0%) | 24 (49.0%) | 25 (49.0%) | 0.99 |
| RF CVD | 20 (20.0%) | 11 (22.4%) | 9 (17.6%) | 0.55 |
| RF spO2 (<90) | 71 (71.0%) | 34 (69.4) | 37 (72.5%) | 0.73 |
| RF D.dimer | 1 (1.0%) | 1 (2.0%) | 0 (0.0%) | 0.49 |
| RF CPK | 28 (30.1%) | 16 (34.8%) | 12 (25.5%) | 0.33 |
| RF Ferritin | 65 (65.7%) | 33 (68.8%) | 32 (62.7%) | 0.53 |
| Heart Rate median (>125) | 16 (16.0%) | 5 (10.2%) | 11 (21.6%) | 0.12 |
| Respiratory factors | ||||
| Respiratory Rate > 24/min | 31 (31.0%) | 12 (24.5%) | 19 (37.3%) | 0.17 |
| Oxygen Saturation (SpO2) — median (IQR) | 86 (80–88) | 86 (80–88) | 85 (80–85) | 0.30 |
| PH— median (IQR) | 7.40 (7.38–7.45) | 7.41 (7.39–7.49) | 7.40 (7.38–7.43) | 0.32 |
| PCo2— median (IQR) | 35 (27.8–45.2) | 36 (29.7–46.4) | 35 (26.7–43.1) | 0.33 |
| White Blood Cell count (×10−9/liter) | ||||
| <4 × 10−9/liter — no. (%) | 18 (20.0%) | 10 (21.7%) | 8 (18.2%) | 0.36 |
| 4–10 × 10−9/liter — no. (%) | 62 (68.9%) | 29 (63.0%) | 33 (75.0%) | |
| >10 × 10−9/liter — no. (%) | 10 (11.1%) | 7 (15.2%) | 3 (6.8%) | |
| Lymphocyte count (×10−9/liter) —median (IQR) | 0.52 (0.27–0.76) | 0.51 (0.26–0.81) | 0.54 (0.28–0.72) | 0.71 |
| ≥1.0 × 10−9/liter — no. (%) | 13 (14.1%) | 7 (14.9%) | 6 (13.3%) | 0.83 |
| <1.0 × 10−9/liter — no. (%) | 79 (85.9%) | 40 (85.1%) | 39 (86.7%) | |
| Neutrophil count (×10−9/liter) — median (IQR) | 4 (2.78–6.09) | 4.02 (2.88–6.55) | 3.92 (2.76–5.23) | 0.51 |
| <1.5 × 10−9/liter — no. (%) | 2 (2.7%) | 1 (2.6%) | 1 (2.9%) | 0.16 |
| 1.5–8 × 10−9/liter — no. (%) | 67 (91.8%) | 34 (87.2%) | 33 (97.1%) | |
| >8 × 10−9/liter — no. (%) | 4 (5.5%) | 4 (10.3%) | 0 (0.0%) | |
| Platelet count (×10−9/liter) — median (IQR) | 130 (41–181) | 125 (50.2–181.5) | 141.5 (40.2–180.7) | 0.78 |
| ≥100 × 10−9/liter — no. (%) | 60 (67.4%) | 32 (71.1%) | 28 (63.6%) | 0.45 |
| <100 × 10−9/liter — no. (%) | 29 (32.6%) | 13 (28.9%) | 16 (36.4%) | |
| Serum Creatinine (μmol/liter) — median (IQR) | 1.1 (1.0–1.5) | 1.1 (1.0–1.65) | 1.1 (1.0–1.4) | 0.97 |
| ≤133 μmol/liter — no. (%) | 69 (69.7%) | 31 (64.6%) | 38 (74.5%) | 0.28 |
| >133 μmol/liter — no. (%) | 30 (30.3%) | 17 (35.4%) | 13 (25.5%) | |
| Aspartate Aminotransferase (AST) (U/liter) — median (IQR) | 53 (39–77) | 53 (41–76) | 51 (37.2–80.5) | 0.86 |
| ≤40 U/liter — no. (%) | 25 (25.8%) | 11 (23.4%) | 14 (28.0%) | 0.61 |
| >40 U/liter — no. (%) | 72 (74.2%) | 36 (76.6%) | 36 (72.0%) | |
| Alanine Aminotransferase (ALT) (U/liter) — median (IQR) | 35 (20.5–55.5) | 36 (23–52) | 32 (19.7–61.2) | 0.58 |
| ≤50 U/liter — no. (%) | 69 (71.1%) | 34 (72.3%) | 35 (70.0%) | 0.80 |
| >50 U/liter — no. (%) | 28 (28.9%) | 13 (27.7%) | 15 (30.0%) | |
| Lactate Dehydrogenase (LDH) (U/liter) — median (IQR) | 520 (422.5–719) | 565 (447.5–806.7) | 489 (381.5–684) | 0.07 |
| ≤245 U/liter — no. (%) | 29 (29.3%) | 10 (20.4%) | 19 (38.0%) | 0.054 |
| >245 U/liter — no. (%) | 70 (70.7%) | 39 (79.6%) | 31 (62.0%) | |
| Blood Urea Nitrogen (BUN) — median (IQR) | 46 (33–61) | 47.5 (34.5–61) | 43 (32–60) | 0.34 |
| CRP < 6 — no. (%) | 15 (15.8%) | 8 (17.4%) | 7 (14.3%) | 0.68 |
| CRP > 6 — no. (%) | 80 (84.2%) | 38 (82.6%) | 42 (85.7%) | |
Value for Lymphocyte count were available for 47 patients in control group and 45 patients in the treatment group. Value for Neutrophil count were available for 39 patients in control group and 34 patients in the treatment group. Value for Platelet count were available for 45 patients in control group and 44 patients in the treatment group.
Value for Aspartate Aminotransferase and Alanine Aminotransferase were available for 47 patients in control group. Blood Urea Nitrogen were available for 46 patients in control group and 49 patients in the treatment group. IQR denotes the interquartile range. Quantitative measures were compared using the Mann–Whitney U test or (if normally distributed) T-test. Categorical variables were compared using the Chi-Square test or Fisher exact test.
The values shown are based on available data. Value for PH were available for 47 patients in the control group and 43 patients in treatment group. Value for PCo2 were available for 44 patients in the control group and 42 patients in treatment group. Laboratory values for CPK were available for 46 patients in the control group and 47 patients in treatment group. Values for CRP were available for 46 patients in control group and 49 patients in the treatment group. Values for White Blood Cell count were available for 46 patients in control group and 44 patients in the treatment group.
3.2. Primary outcome
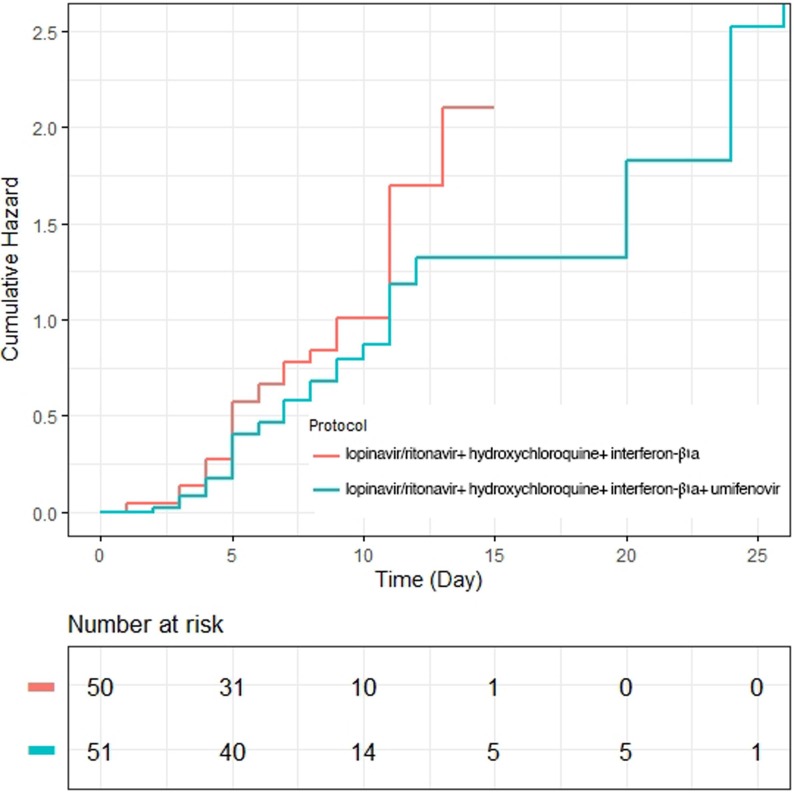
Median day for time to clinical improvement in intervention group was 9 (5.8–12.1) which did not hold a significant difference with the corresponding value for control group based on log-rank test (P = 0.22). According to the corresponding 95% CI) and the Kaplan–Meier plot, no difference for TTCI was explored between two studied groups (Table 2 (Fig. 2 ). In addition, the HR for TTCI in the Cox regression model was 0.75 (95% CI: 0.45–1.23, P: 0.25) revealing that there was no statistically significant difference between the treatment group and control group (see Table 3 ).
Table 2.
Outcomes in the Intention-to-Treat Population.*
| Total (N = 101) | Infbeta1 + HQ + lopinavir/ritonavir (N = 50) | Infbeta1 + HQ + lopinavir/ritonavir + umifenovir (N = 51) | P.value | |
|---|---|---|---|---|
| Time to clinical improvement — median (IQR) | 8 (5–11) | 7 (4–10) | 9 (5–11) | 0.22 |
| Mortality at day 21 — no. (%) | 36 (35.6%) | 19 (38.0%) | 17 (33.3%) | 0.62 |
| Mortality earlier (presentation ≤ 7 days of symptom onset) — no. (%) | 29 (33.0%) | 16 (36.4%) | 13 (29.5%) | 0.49 |
| Mortality later (presentation > 7 days of symptom onset) — no. (%) | 7 (58.3%) | 3 (60.0%) | 4 (57.1%) | 1.00 |
| ICU Admission — no. (%) | 101 (100.0%) | 50 (100.0%) | 51 (100.0%) | … |
| Invasive mechanical ventilation— no. (%) | 31 (30.7%) | 14 (28.0%) | 17 (33.3%) | 0.56 |
| Time to ventilation | 3 (2–4) | 2 (1–4.25) | 3 (2–4) | 0.25 |
| Time on ventilation | 5 (2–8) | 5.5 (1–8) | 4 (2–13) | 0.62 |
| Hospital stay — median no. of days (IQR) | 6 (4–9) | 5 (4–9) | 7 (5–10) | 0.06 |
| Time from randomization to discharge — median no. of days (IQR) | 5 (4–9) | 5 (4–8) | 6 (4.7–10.2) | 0.15 |
| Time from randomization to death — median no. of days (IQR) | 7 (4–10) | 6 (4–10) | 8 (5.5–10) | 0.27 |
| Respiratory factors | ||||
| PH (worst) — median (IQR) | 7.37 (7.29–7.49) | 7.35 (7.24–7.45) | 7.38 (7.30–7.50) | 0.45 |
| PH (best) — median (IQR) | 7.40 (7.38–7.41) | 7.40 (7.40–7.41) | 7.40 (7.37–7.41) | 0.77 |
| Pco2 (worst)— median (IQR) | 37.6 (22.2–57.7) | 39 (23–57) | 35 (21.5–61) | 0.78 |
| Pco2 (best)— median (IQR) | 41 (36–46) | 41 (34–46) | 41 (36–46.5) | 0.89 |
| White Blood Cell count (×10−9/liter) | ||||
| <4 × 10−9/liter — no. (%) | 3 (3.2%) | 2 (4.2%) | 1 (2.1%) | 060 |
| 4–10 × 10−9/liter — no. (%) | 42 (44.2%) | 19 (39.6%) | 23 (48.9%) | |
| >10 × 10−9/liter — no. (%) | 50 (52.6%) | 27 (56.3%) | 23 (48.9%) | |
| Lymphocyte count (×10−9/liter) —median (IQR) | 1.81 (1.18–2.48) | 1.69 (1.09–2.09) | 2.16 (1.18–2.58) | 0.19 |
| ≥1.0 × 10−9/liter — no. (%) | 78 (84.8%) | 38 (80.9%) | 40 (88.9%) | 0.28 |
| <1.0 × 10−9/liter — no. (%) | 14 (15.2%) | 9 (19.1%) | 5 (11.1%) | |
| Platelet count (×10−9/liter) — median (IQR) | 190 (76.5–272.5) | 197 (88.5–275.5) | 184 (54.5–260.2) | 0.57 |
| ≥100 × 10−9/liter — no. (%) | 71 (73.2%) | 37 (75.5%) | 34 (70.8%) | 0.60 |
| <100 × 10−9/liter — no. (%) | 26 (26.8%) | 12 (24.5%) | 14 (29.2%) | |
| Neutrophil count (×10−9/liter) — median (IQR) | 8.1 (4.87–10.78) | 8.97 (4.38–11.79) | 8.09 (5.35–9.99) | 0.88 |
| <1.5 × 10−9/liter — no. (%) | 1 (1.3%) | 1 (2.6%) | 0 (0.0%) | 0.61 |
| 1.5–8 × 10−9/liter — no. (%) | 36 (47.4%) | 18 (46.2%) | 18 (48.6%) | |
| >8 × 10−9/liter — no. (%) | 39 (51.3%) | 20 (51.3%) | 19 (51.4%) | |
| C-Reactive Protein (CRP) — median (IQR) | 65 (39.5–83.5) | 74 (45–91) | 57 (37.7–79.2) | 0.13 |
| CRP < 6 — no. (%) | 2 (2.7%) | 1 (2.9%) | 1 (2.6%) | 0.73 |
| CRP > 6 — no. (%) | 71 (97.3%) | 34 (97.1%) | 37 (97.4%) | |
| Erythrocyte Sedimentation Rate (ESR) — median (IQR) | 44.5 (27.2–59) | 43 (24.5–59.2) | 45 (31.5–59.5) | 0.38 |
The values shown are based on available data. Value for the worst PH, PCo2 and HCo3 were available for 39 patients in the control group and 38 patients in treatment group. Laboratory values for Lymphocyte count were available for 47 patients in the control group and 45 patients in treatment group. Values for White Blood Cell count were available for 47 patients in control group and 48 patients in the treatment group. Value for Lymphocyte count were available for 47 patients in control group and 45 patients in the treatment group. Value for Platelet count were available for 49 patients in control group and 48 patients in the treatment group. Value for Neutrophil count were available for 39 patients in control group and 37 patients in the treatment group. Value for C-Reactive Protein were available for 35 patients in control group and 38 patients in the treatment group. Value for Erythrocyte Sedimentation Rate were available for 30 patients in control group and 34 patients in the treatment group. Quantitative measures were compared using the Mann–Whitney U test or (if normally distributed) T-test. Categorical variables were compared using the Chi-Square test or Fisher exact test.
Fig. 2.
Kaplan–Meier plot depicted the Time to Clinical Improvement in the Intention-to-Treat Population.
Table 3.
Adverse Events in the Safety Population *
| Event | Infbeta1 + HQ + lopinavir/ritonavir (N = 50) | Infbeta1 + HQ + lopinavir/ritonavir + umifenovir (N = 51) | |
|---|---|---|---|
| Adverse Event | P Value | ||
| Nausea | 8 (16.0%) | 6 (12.2%) | 0.59 |
| Vomiting | 1 (2.0%) | 2 (4.1%) | 1.00 |
| Diarrhea | 5 (10.0%) | 4 (8.2%) | 1.00 |
| Rash | 2 (4.0%) | 0 (0.0%) | 0.49 |
| Raised liver function test | 20 (40.0%) | 18 (36.0%) | 0.68 |
| Raised total bilirubin | 11 (22.0%) | 12 (24.5%) | 0.77 |
| Increased Creatinine | 13 (26.0%) | 14 (28.0%) | 0.82 |
| Prolonged QT interval | 0 (0.0%) | 0 (0.0%) | … |
| Leukopenia | 14 (28.0%) | 12 (24.0%) | 0.65 |
| Diarrhea | 5 (10.0%) | 4 (8.2%) | 0.75 |
| Anemia | 17 (34.0%) | 17 (34.7%) | 0.94 |
| Hypoalbuminemia | 6 (12.0%) | 6 (12.2%) | 0.97 |
| Raised CPK | 6 (12.0%) | 5 (10.4%) | 0.80 |
| Abdominal pain | 8 (16.0%) | 8 (16.3%) | 0.96 |
| Lymphopenia | 25 (50.0%) | 8 (15.7%) | <0.001 |
| Serious Adverse Event | |||
| Acute Respiratory Distress Syndrome (ARDS) | 14 (28.0%) | 12 (23.5%) | 0.61 |
| Acute Kidney Failure (AKI) | 10 (20.0%) | 7 (14.3%) | 0.45 |
| Secondary Infection | 3 (6.0%) | 2 (4.1%) | 0.66 |
| Shock | 10 (20.0%) | 8 (16.3%) | 0.64 |
| Severe Anemia | 8 (16.0%) | 6 (12.2%) | 0.59 |
| Acute gastritis | 0 (0.0%) | 0 (0.0%) | … |
| Lower GI bleeding | 0 (0.0%) | 0 (0.0%) | … |
| Sepsis | 5 (10.0%) | 3 (6.1%) | 0.71 |
| Pneumothorax | 0 (0.0%) | 0 (0.0%) | … |
Adverse events that occurred in more than one patient after randomization through day 21 are shown. Some patients had more than one adverse event.
3.3. Secondary outcomes
Frequency of deceased patients during the study was 36 (35.6%) of which 19 deaths were in control group and the remainder occurred in intervention group. The in-hospital mortality was not statistically significant between two groups according to ITT population (Table 2). Mortality rates for patients who entered the study either 7 days earlier or later than the day of symptoms onset were not found to be statistically different between intervention and control groups. Incidence of need for invasive mechanical ventilation in intervention group almost resembled its incidence in control groups (33.3% vs 28%). Neither the length of hospital stays nor respiratory factors could reach a significant difference between two studied groups. All other secondary outcome measures also did not reach statistical significance between two arms (Table 2).
3.4. Safety
Lymphopenia was the most prevalent adverse effect during the study (50%), followed by Raised LFT (40.0%), anemia (34.0%), and Leukopenia (28.0%). The serious adverse event was Acute Respiratory Distress Syndrome (28.0%). With the exception to lymphopenia which was more common in control group, no differences regarding the safety aspect were observed.
There were no significant differences between the two arms regarding the safety aspect, except for lymphopenia which was statistically higher in the control group. The patients with increased LFT (liver function test) stopped receiving of lopinavir/ritonavir (20 patients in control group and 18 patients in treatment group). No patient stopped the treatment because of the adverse events.
4. Discussion
Our trial shows that umifenovir (Arbidol) did not affect time to clinical improvement and mortality when compared to the placebo group. About comorbidities and risk factors there was no significant difference between groups. The current study confirmed that treatment with umifenovir could not decrease the need for invasive mechanical ventilation.
Clinical efficacy of lopinavir/ritonavir and hydroxychloroquine is under genuine scrutiny but the combination was mandated for all severely ill COVID-19 patients by the Iranian COVID-19 national protocol, endorsed by the Iranian Ministry of Health [11], [12], [13], [14], [15], [16]. We; therefore, use the combination in both studied groups. Recently, a few articles have proved the usefulness of interferonβ-1a in management of COVID-19 infection [17], [18], [19]. Hence, we decided to added interferonβ-1a in the treatment protocol.
Umifenovir is a broad‐spectrum antiviral compound invented approximately 25 years ago by Russian scientists of Chemical‐Pharmaceutical Scientific Research Institute of Russia. It is licensed in Russia and China for the prevention and treatment of human influenza and relevant post infection complications [6].
Umifenovir, a hemagglutinin inhibitor, exerts some consequences on virus by hindering virus-host cell membrane fusion as well as inhibiting viral DNA and RNA synthesis. Moreover, it can affect interferon production as well as immune system regulation. In combination with other antiviral drugs, it may exhibit an improved efficacy, whilst as a trade-off the potential adverse effects may increase during treatment [20].
Twenty patients in control group and eighteen patients in intervention group were unable to complete the full course of adjustment. Five patients in control group and four participants in intervention group also developed diarrhea and other gastrointestinal symptoms. Moreover, self-limited skin eruptions were observed in two participants of control group. The latter may be due to inhibition of CYP3A induced by lopinavir/ritonavir [21].
A study by Deng Let al. reported that umifenovir is an effective therapeutic agent for SARS-CoV infections. They also illustrated the beneficial antiviral activity of umifenovir in fighting against Covid-19 with acceptable safety profiles [9]. In a study by Lian N, it has been proved that umifenivir was not effective in improving prognosis as well as hastening patients’ recovery [10].
A cogent reason behind the inefficacy of umifenovir in ceasing the infection may be due to the drug dosage. To obtain satisfactory results in suppressing COVID-19, high doses of the drug are recommended by in vitro studies [5]. However, owing to various side effects, it is clinically impossible to prescribe high doses. The utilizing of lopinavir/ritonavir gives rises to more gastrointestinal symptoms which may exert harmful consequences on patient’s recovery. It is noteworthy that based on drug instructions and previous experiences in treating HIV-infected patients, short-term adverse effects of lopinavir/ritonavir are chiefly diarrhea, abnormal stools, abdominal pain, nausea, vomiting, and asthenia [22]. The side effects induced by lopinavir/ritonavir may hinder the treatment effectiveness. Presence of interferon drug in both studied groups might lead to optimal clinical responses. Our study posed some limitations. Which were: (1) It was a single-center study (2) Atrial blood gas (ABG) was not taken, venous blood gas (VBG). Were used instead. (3) Thirty-eight patients were unable to complete the treatment course of administration because of liver enzyme elevation. (4) The trial was conducted on hospitalized patients with moderate-severe COVID-19 and the effectiveness of umifenovir in patients with mild Covid-19 has not been evaluated.
5. Conclusion
In summary, the umifenovir trial unveiled that intervention group had not numerically more favorable TTCIs when compared to the control group. Furthermore, the mortality rate in the two groups was not different. Additive umifenovir has not been found to be effective in shortening the duration of SARS-CoV-2 in severe patients and improving the prognosis in non-ICU patients.
6. Declarations
6.1. Ethics approval and consent to participate
The trial was confirmed by the Ethics in Medical Research Committee of the Shahid Beheshti University of Medical Sciences. signed informed consents were obtained from all the participants or their legally authorized representatives. This trial has been registered as ClinicalTrials.gov, NCT04350684.
6.2. Consent for publication
Not applicable.
6.3. Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
6.4. Fund ing
Not applicable.
6.5. Authors' contributions
All authors contributed to conception and design of study; MMR, FH, IAD, MTSH, NKH, AHK, AS, PT and HSHD contributed to the acquisition of data; MMR, FH, IAD and MAP contributed to the analysis of data; all authors contributed to the drafting of the article and/or critical revision; and all authors contributed to the final approval of manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to thank the Clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their help and support in conducting this clinical trial.
Code availability
Not applicable.
Consent to participate
Not applicable.
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Matheson N.J., Lehner P.J. How does SARS-CoV-2 cause COVID-19? Science. 2020;369(6503):510–511. doi: 10.1126/science.abc6156. [DOI] [PubMed] [Google Scholar]
- 3.Potential Antiviral Drugs Under Evaluation for the Treatment of COVID-19 (updated July 24, 2020). Available from: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/.
- 4.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease (COVID-19): current status and future perspective. Int. J. Antimicrob. Agents. 2019;2020:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khamitov R., Loginova S., Shchukina V., Borisevich S., Maksimov V., Shuster A. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr. Virusol. 2008;53(4):9–13. [PubMed] [Google Scholar]
- 6.Boriskin Y., Leneva I., Pecheur E.-I., Polyak S. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15(10):997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 7.Villalaín J. Membranotropic effects of arbidol, a broad anti-viral molecule, on phospholipid model membranes. J. Phys. Chem. B. 2010;114(25):8544–8554. doi: 10.1021/jp102619w. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Cao R., Zhang H., Liu J., Xu M., Hu H., et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discovery. 2020;6(1):1–5. doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 12.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 IMoHTo. Flowchart for Management of COVID-19 patients – first ed. Available from: http://dme.behdasht.gov.ir/uploads/Felo_Tashkish.pdf.
- 14.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin E.J., Harrington D.P., Hogan J.W., Gatsonis C., Baden L.R., Hamel M.B. The urgency of care during the Covid-19 pandemic — learning as we go. N. Engl. J. Med. 2020 doi: 10.1056/NEJMe2015903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. bmj. 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dastan F., Nadji S.A., Saffaei A., Marjani M., Moniri A., Jamaati H., et al. Subcutaneous administration of interferon beta-1a for COVID-19: a non-controlled prospective trial. Int. Immunopharmacol. 2020;85:106688. doi: 10.1016/j.intimp.2020.106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalkanen J., Hollmén M., Jalkanen S. Interferon beta-1a for COVID-19: critical importance of the administration route. Crit. Care. 2020;24(1):335. doi: 10.1186/s13054-020-03048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novel coronavirus pneumonia prevention and control program, 7th ed., 2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 21.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. New Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangum E.M., Graham K.K. Lopinavir-ritonavir: a new protease inhibitor. Pharmacother.: J. Hum. Pharmacol. Drug Ther. 2001;21(11):1352–1363. doi: 10.1592/phco.21.17.1352.34419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.