Abstract
Objectives
The objective of our study was to conduct a systematic literature review of estimates of costs of illness of spinal muscular atrophy (SMA).
Methods
We searched MEDLINE (through PubMed), CINAHL, Embase, Web of Science, National Health Service Economic Evaluation Database, and the National Health Service Health Technology Assessment Database for studies published from inception up until 31 August, 2020, reporting direct medical, direct non-medical, and/or indirect costs of any phenotype of SMA. Two reviewers independently screened records for eligibility, extracted the data, and assessed studies for risk of bias using the Newcastle–Ottawa Scale. Costs were adjusted and converted to 2018 US dollars.
Results
The search identified 14 studies from eight countries (Australia, France, Germany, Italy, Spain, Sweden, the UK, and the USA). The mean per-patient annual direct medical cost of illness was estimated at between $3320 (SMA type III, Italy) and $324,410 (SMA type I, USA), mean per-patient annual direct non-medical cost between $25,880 (SMA types I–III, Spain) and $136,800 (SMA type I, Sweden), and mean per-patient annual indirect cost between $9440 (SMA type I, Germany) and $74,910 (SMA type II, Australia). Most studies exhibited a risk of bias.
Conclusions
The current body of evidence of costs of illness of SMA is relatively scarce and characterized by considerable variability across geographical settings and disease phenotypes. Our review provides data pertaining to the economic impact of SMA, which is of particular relevance in light of emerging treatments and ongoing research in this field, and underscores the substantial unmet medical need in this patient population.
Electronic supplementary material
The online version of this article (10.1007/s40258-020-00624-2) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| No study has systematically reviewed the literature for estimates of costs of illness of spinal muscular atrophy. |
| The body of literature of costs of illness of spinal muscular atrophy is limited to a few geographical settings and characterized by considerable variability across disease phenotypes. |
| Our review helps inform economic evaluations and future cost research, and highlights the substantial unmet medical need in spinal muscular atrophy. |
Introduction
Spinal muscular atrophy (SMA) is a rare inherited neuromuscular disease characterized by progressive muscle degeneration. There are several phenotypes of SMA, from SMA type I (Werdnig–Hoffmann disease) to SMA type IV (adult-onset SMA), with stark differences in onset and severity [1]. The most severe form, SMA type I, accounts for about 60% of all new cases [2, 3] and has been shown to be associated with a median age of death, or need for permanent ventilation support for survival, of less than 12 months [4, 5]. In contrast, patients with SMA types III or IV typically have a normal lifespan, despite significant impairment in functional ability that can include loss of independent ambulation [6]. The prevalence of SMA has been estimated at around 1–2 per 100,000 individuals, with an incidence of approximately 1 in 10,000 live births [3].
Recently, two medications have been approved for 5q-linked SMA, the most common form of the disease caused by mutations in the survival motor neuron gene that is found on chromosome 5: Spinraza® (nusinersen; Biogen Idec) and Zolgensma® (onasemnogene abeparvovec; Novartis AG/AveXis). Spinraza® is an antisense oligonucleotide administered by repeat intrathecal dosing and approved in many countries for the treatment of all disease phenotypes. Zolgensma® is a gene replacement therapy administered through a single intravenous dose approved in the USA, Japan, Brazil, and the European Union for children with SMA. Additionally, several treatment strategies for SMA are currently being explored, including splice-modifying therapies [7]. In conjunction with this development, to help inform value-based resource allocation and economic evaluations of forthcoming therapies, as well as describe and raise awareness of the current overall cost burden of disease, there has been an increased interest in understanding the health economic context of SMA. The objective of our study was to conduct a systematic review of estimates of costs of illness of SMA globally. Specifically, this systematic literature review sought to answer the following questions: (1) in which geographical settings have costs of illness of SMA been studied? (2) For which types of SMA have costs of illness been estimated? (3) What types of costs of illness have been estimated in patients with SMA? (4) What are the known costs of illness of SMA? The aims of this study were to review the current body of evidence of direct medical, direct non-medical, and indirect costs of SMA to help inform cost-effectiveness analyses and similar evaluations of forthcoming therapies, map out data gaps to guide future cost research, and describe the current economic burden of illness.
Methods
This systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) [identifier: CRD42020160020], and conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8].
Search Strategy
We searched MEDLINE (through PubMed), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Web of Science, National Health Service Economic Evaluation Database, and the National Health Service Health Technology Assessment Database for records of studies published from inception up until 31 August, 2020, reporting estimates of costs of illness of SMA. The search string contained a combination of the following medical subject heading terms, title/abstract, and topic field tags: “Spinal muscular atrophy”, “cost”, “financial”, “burden”, “economic”, “monetary”, “Cost of Illness”, “Costs and Cost Analysis”, “Cost-Benefit Analysis”, “cost-effectiveness”, “cost-utility”, “spending”, and “expenditure” (full search strings are provided in the Electronic Supplementary Material [ESM]).
Selection Criteria
We included all identified publications reporting estimates of the following cost of illness of SMA in any currency: direct medical costs (i.e., costs of medical resources directly included in the formal management of the disease), direct non-medical costs (i.e., costs of non-medical resources directly included in the formal management of the disease), informal care costs (i.e., costs associated with the informal management of the disease by non-professionals [9]), indirect costs (i.e., production losses from the perspective of society due to absenteeism and presenteeism from work [10]), and the total cost of illness. The provided descriptions represent the working definitions of these costs as employed in this study. We considered publications reporting results from any study type in any language. We excluded studies based on samples comprising fewer than ten patients in total, and also required that results were reported separately for patients with SMA (in case costs of several indications were investigated as part of the same study). We did not consider conference abstracts, as these contain too few details for meaningful synthesis. No further criteria were imposed for study eligibility.
Screening, Data Extraction, and Assessment of Risk of Bias
Two investigators (EL and AP) independently screened article titles and abstracts for eligibility, and subsequently reviewed full-text versions of selected records. The reasons for article exclusion were recorded and disagreements were resolved by the involvement of a third investigator (TS). For all articles that met the inclusion criteria upon full-text review, the following data were extracted: author, year, geographical setting, design, data sources, type of data, study periods, patient population, estimated cost categories (i.e., direct medical, direct non-medical, informal care, and/or indirect costs, as described above), perspective of analysis (as reported by the included publications, or inferred based on information of the included resources and methods of valuation), and estimated costs. Additionally, we also extracted information of resource categories (e.g., inpatient care, outpatient care, and prescription drugs) included in identified estimates of the per-patient annual direct medical cost of SMA. We did not consider costs of individual resources part of higher level cost categories (e.g., the cost of a general practitioner visit as part of the per-patient annual direct medical cost of illness).
Extracted data from each article were synthesized and reported with respect to the four review questions as stated in the Introduction. Based on the information in the reviewed publications, we structured identified estimates of costs of illness of SMA into the following cost categories: the per-patient annual direct medical cost of illness, the per-patient annual direct non-medical cost of illness (including informal care costs of illness), the per-patient annual indirect cost of illness, and the per-patient annual total cost of illness. Identified costs not estimated per annum (e.g., the per-patient cost per hospitalization) were reported separately. To facilitate comparison, identified costs were adjusted for inflation to 2018 values using country-specific consumer price index data from the World Bank and subsequently converted to US dollars. All reported cost estimates were rounded to the nearest ten.
Risk of bias was established with the Newcastle–Ottawa Scale (NOS) [11] by two investigators (EL and AP). The NOS was developed to assess the quality and risk of bias of non-randomized studies in three dimensions: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively. Each category is assigned a score rating (maximum score: ◊◊◊◊ for selection, ◊◊ for comparability, and ◊◊◊ for outcome). To ascertain selection, we required patients to be diagnosed with SMA (score: ◊), that the diagnosis was confirmed via standard International Statistical Classification of Diseases and Related Health Problems (ICD) codes for the disease (i.e., ICD-9: 335.0, 335.1X, and/or 335.21; and ICD-10: G12.0, G12.1, G12.2, G12.8, and/or G12.9 [12]) in an out- or inpatient setting (score: ◊), and that the sample was not restricted in terms of survival of motor neuron 2 (SMN2) copy number or other markers limiting representativeness (score: ◊) [assessment of the non-exposed cohort was not applicable, and all studies were thus assigned a score (◊) for this criterion]; to ascertain comparability, we required details of the number of patients and distribution of age, sex, and SMA phenotype in the sample population (score for all four details: ◊◊; score for at least one detail: ◊); and to ascertain outcome, we required that resource use and/or costs were extracted from clinical charts or registries/databases containing physician-reported or administrative data [e.g., governmental population-based registries or claims databases] (score: ◊), a minimal follow-up of 1 month for prospective studies [given the frequency of care reported] (score: ◊), and that less than 25% of the total sample were lost to follow-up during the study period (score: ◊). Studies assigned the maximum score in all categories were judged to exhibit a low risk of bias.
Results
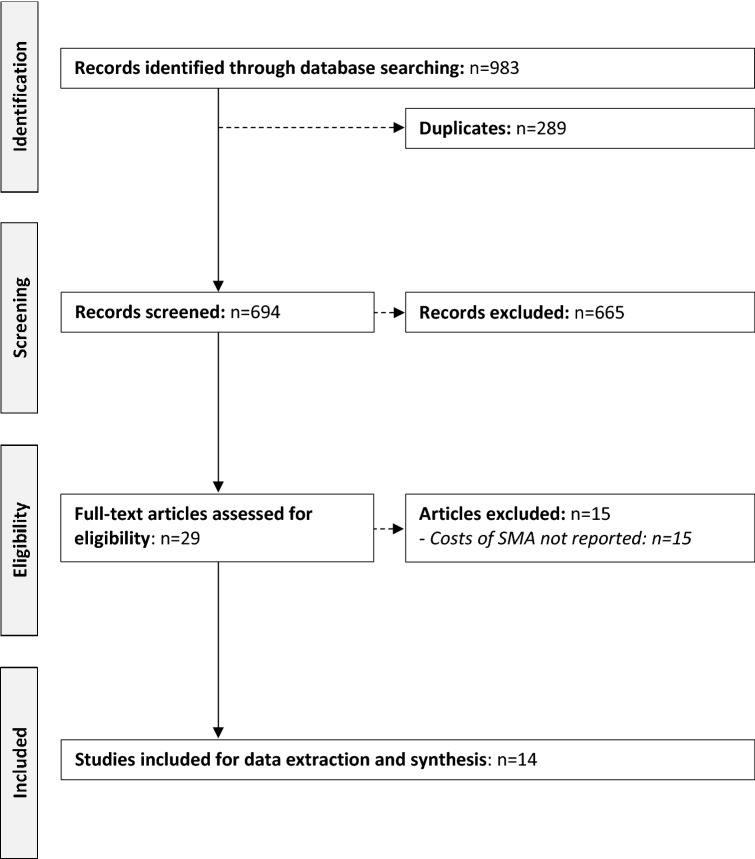
Our search strategy identified 983 publications (Fig. 1). Of these, 289 were duplicates, 665 were excluded following title and abstract screening, and 29 were selected for full-text review. Finally, 14 articles [13–26] were considered for data extraction and synthesis. The primary reason for article exclusion was lack of relevant cost data. One study [27] was not considered as it referenced cost estimates subsequently published in full by Tan et al. [21]. Summary details of the included publications are presented in Table 1. One of the included articles was written in Italian [22]. Identified estimates of costs of illness of SMA were converted to US dollars using the following rates: Pound sterling £1 = US$1.29156; Euro €1 = US$1.10090; and Swedish Krona SEK1 = US$0.10427 (obtained from Morningstar, Inc. [28] on 29 November, 2019).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of the selection process of the included publications. SMA spinal muscular atrophy
Table 1.
Characteristics of included studies
| Author (year) | Geographical setting(s) | Design | Data source(s) | Type of data | Study period | Patient population |
|---|---|---|---|---|---|---|
| Ali et al. (2019) [17] | UK | Retrospective, observational cohort study | Royal Stoke University Hospital | Paper notes and electronic clinical records | 2017–9 | 11 patients with SMA type I (45% male; median age: 2 years) treated with nusinersen |
| Armstrong et al. (2016) [19] | USA | Retrospective, observational cohort study | Military Health System Data Repository (MDR) | Defense Health Agency corporate healthcare data | 2003–12 | 239 patients with SMA (45% male; mean age NR) [phenotype NR] |
| Chambers et al. (2020) [25] | Australia | Cross-sectional, observational study; and retrospective, observational cohort study | Survey administrated to caregivers via two neuromuscular clinics and a patient support organization; the Sydney Children’s Hospital SMA clinic; and local unit costs | Self-reported data; and health service use and insurance data | 2016–17 | 40 patients with SMA (48% male; mean age: 9 years) [10% with SMA type I, 65% with SMA type II, and 25% with SMA type III] |
| Cardenas et al. (2019) [16] | USA | Retrospective, observational cohort study | Kids’ Inpatient Database (KID) part of the HCUP | Claims data | 2012 | 237 patients with SMA type I (43% male; mean age NR) |
| Darbà et al. (2019) [15] | Spain | Retrospective, observational cohort study | Spanish claims database Minimum Basic Data Set (Conjunto Mínimo Básico de Datos [CMBD]) | Claims data | 1997–2015 | 705 patients with SMA type I, SMA type III, progressive SMA, other SMA, or unspecified SMA (62% male; mean age: 37 years) [distribution of phenotypes NR] |
| Darbà (2020) [24] | Spain | Retrospective, observational cohort study | Public Data Analysis for Health Research and Innovation Program (PADRIS) database | Electronic clinical records | 2014–17 | 396 patients with SMA (53% male; mean age: 57 years) (6% with SMA type I, 29% with SMA type III, 43% with progressive SMA, 10% with other SMA, and 13% with unspecified SMA) |
| Droege et al. (2019) [18] | USA | Retrospective, observational cohort study | Symphony Health’s Integrated Dataverse (IDV) | Claims data | 2016–18 | 349 patients with SMA type I (44% male; mean age: 9 years) receiving conventional therapy; 45 patients with SMA type I (38% male; mean age: 12 years) receiving nusinersen; 5728 patients with SMA types II–IV (52% male; mean age: 31 years) receiving conventional therapy; and 404 patients with SMA types II–IV (49% male; mean age: 15 years) receiving nusinersen |
| Klug et al. (2016) [20] | Germany | Cross-sectional, observational study |
Survey administrated to patients identified via the German SMA patient registry; and local unit costs |
Self-reported data | 2013 | 189 patients with SMA (59% male; median age: 19 years) (6% with SMA type I, 39% with SMA type II, and 55% with SMA type III) |
| Lee et al. (2019) [14] | USA | Retrospective, observational cohort study | HCUP; and the Center for Health Information and Analysis | Claims data | 2005–13 | 229 patients with severe SMA (distribution of sex and mean age NR) [phenotype NR] |
| López-Bastida et al. (2017) [13] | Spain | Cross-sectional, observational study | Survey administrated to patients via national patient associations; and local unit costs | Self-reported data | 2015 | 81 patients with SMA (42% male; mean age: 7 years) [10% with SMA type I, 74% with SMA type II, and 16% with SMA type III] |
| Marcellusi et al. (2019) [22] | Italy | Cross-sectional, observational study | Survey administrated to patients via a national patient association; and local unit costs | Self-reported data | 2018 | 118 patients with SMA (41% male; mean age: 18 years) (23% with SMA type I, 48% with SMA type II, and 29% with SMA type III) |
| Peña-Longobardo et al. (2020) [26] | France; Germany; and UK | Cross-sectional, observational study | Survey administrated to caregivers via a patient organization/registry; and local unit costs | Self-reported data | 2015 | 27 French patients with SMA (41% male; mean age: 6 years) [19% with SMA type I, 48% with SMA type II, and 33% with SMA type III]; 25 German patients with SMA (28% male; mean age: 10 years) [44% with SMA type I, 48% with SMA type II, and 8% with SMA type III]; and 34 UK patients with SMA (50% male; mean age: 6 years) [21% with SMA type I, 59% with SMA type II, and 21% with SMA type III] |
| Tan et al. (2019) [21] | USA | Retrospective, observational cohort study | HealthCore Integrated Research Database | Claims data | 2006–16 | 341 patients with SMA (55% male; mean age: 45 years) [7% with SMA type I, 6% with SMA type II/III, and 87% with SMA type IV] |
| Zuluaga‑Sanchez et al. (2019) [23] | Sweden | Modeling study (CEA) | Clinical expert input; and local unit costs | Clinical and economic model input data | 2016 | Patients with SMA type I (45% male; mean age: 6 months) and SMA types II–IV (47% male, mean age: 4 years) [number of patients NA] |
CEA cost-effectiveness analysis, HCUP Healthcare Cost and Utilization Project, NA not applicable, NR not reported, SMA spinal muscular atrophy
In Which Geographical Settings Have Costs of Illness of SMA Been Studied?
Estimates of costs of illness of SMA were found for samples from a total of eight countries, namely Australia, France, Germany, Italy, Spain, Sweden, the UK, and the USA (Table 1). Five studies (36%) represented research of patients from the USA [14, 16, 18, 19, 21].
For Which Types of SMA Have Costs of Illness Been Estimated?
Nine (64%) studies reported estimates of costs of SMA type I [15–18, 20–23, 25], four (29%) of costs of SMA type II [13, 20, 22, 25], four (29%) of costs of SMA type III [15, 20, 22, 25], one (7%) of costs of SMA type IV [21], and one (7%) study of costs of progressive SMA, other SMA, and unspecified SMA [15]. Eleven (79%) studies did not explicitly disclose SMA type, or reported pooled results for more than one type of SMA (in some cases in addition to estimates stratified by SMA type, as reported above) [13–15, 18–21, 23–26]. Two studies (14%) estimated costs of “infantile SMA”, “childhood-onset SMA”, and/or “late-onset SMA” [21, 23], for the purpose of this review approximated as SMA type I, SMA type II/III, and SMA type IV, respectively.
What Types of Costs of Illness Have Been Estimated for Patients with SMA?
In total, nine (64%) studies estimated the per-patient annual direct medical cost of SMA [13, 18–24, 26], five (36%) the per-patient annual direct non-medical cost [13, 20, 22, 23, 26], four (29%) the per-patient annual indirect cost [20, 22, 23, 25], and six (43%) studies the per-patient annual total cost of illness [13, 20, 22, 23, 25, 26] (Table 1). Additionally, three (21%) studies estimated the per-patient cost per hospitalization of SMA [14–16], and one (7%) the per-patient annual hospitalization cost of SMA [17]. Finally, Chambers et al. [25] estimated the per-patient annual direct cost of SMA (comprising both medical and non-medical costs).
What Are the Known Costs of Illness of SMA?
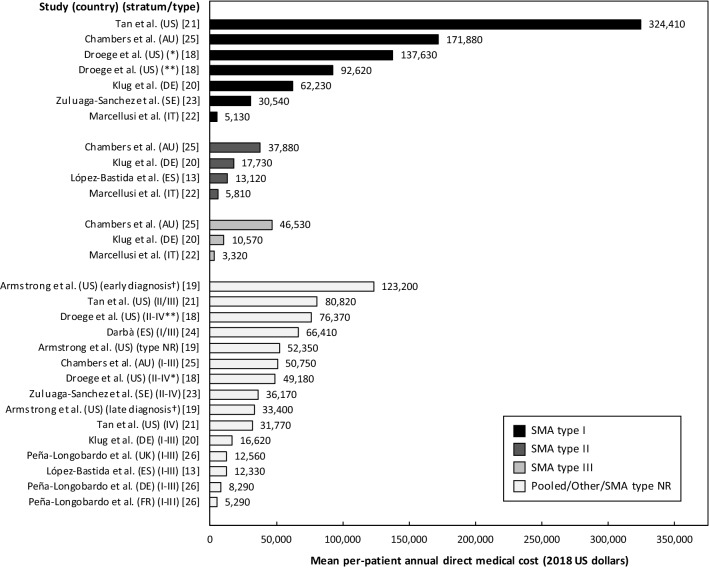
Costs of illness of SMA reported by the included studies are summarized in Table 2. Estimates of the mean per-patient annual direct medical cost of illness (excluding nusinersen-related costs), also illustrated in Fig. 2, were between $3320 for SMA type III in Italy [22] and $324,410 for SMA type I in the USA [21]. When accounting for the cost of nusinersen, the per-patient annual direct medical cost was estimated at $1,000,280 (SMA type I) and $1,109,060 (SMA types II–IV) in the USA [18]. As shown in Table 3, there was variability across studies concerning resource categories included in identified estimates of the direct medical cost of illness. Deducting items typically considered non-medical resources (i.e., non-medical travel costs, respite care, and costs associated with vehicle and home modifications) from the estimate of the per-patient annual direct cost of illness reported by Chambers et al. [25] yields an inferred estimate of the per-patient annual direct medical cost of illness in Australia of $171,880 for SMA type I, $37,880 for SMA type II, and $46,530 for SMA type III.
Table 2.
Costs of illness of spinal muscular atrophy (SMA) reported by included studies
| Author (year) | Estimated cost categories | Perspective of analysis | Currency; year of valuation | Estimated costs [in 2018 US dollarsa] | Risk of biasb |
|---|---|---|---|---|---|
| Ali et al. (2019) [17] | Per-patient annual hospitalization cost (excluding the cost of nusinersen and its administration) | Healthcare/payer | Pound sterling (£); year NRc |
Mean per-patient annual hospitalization cost • SMA type I (n = 11): £99,960 [$129,100] |
Selection: ◊◊◊◊ Comparability: ◊◊ Outcome: ◊◊◊ |
| Armstrong et al. (2016) [19] | Per-patient annual direct medical cost | Healthcare/payer | US dollar ($); year NRd |
Mean per-patient annual direct medical cost • Pooled sample, SMA type NR (n = 239): $47,860 [$52,350] • Diagnosis ≤ 1 year of age (n = 45): $112,640 [$123,200] • Diagnosis > 1 year of age (n = 194): $30,540 [$33,400] |
Selection: ◊◊◊◊ Comparability: ◊ (age and phenotype NR) Outcome: ◊◊◊ |
| Chambers et al. (2020) [25] | Per-patient annual direct cost (medical and non-medical); per-patient annual indirect cost (i.e., production losses for caregivers estimated using the HCA, as well as informal care costs); and per-patient annual total cost of illness | Societal; caregiver; and patient | US dollar ($); 2017 |
Mean per-patient annual direct cost • SMA types I–III (n = 40): $80,560 [$82,250] • SMA type I (n = 4): $185,250 [$189,760] • SMA type II (n = 26): $77,780 [$79,670] • SMA type III (n = 10): $52,220 [$53,490] Mean per-patient annual indirect cost • SMA types I–III (n = 40): $63,150 [$64,680] • SMA type I (n = 4): $44,100 [$45,170] • SMA type II (n = 26): $73,130 [$74,910] • SMA type III (n = 10): $42,770 [$43,810] Mean per-patient annual total cost of illness • SMA types I–III (n = 40): $143,710 [$147,200] • SMA type I (n = 4): $229,350 [$234,930] • SMA type II (n = 26): $150,910 [$154,580] • SMA type III (n = 10): $94,980 [$97,300] |
Selection: ◊◊◊ (uncertain diagnosis) Comparability: ◊◊ Outcome: ◊◊ (self-reported data) |
| Cardenas et al. (2019) [16] | Per-patient cost per hospitalization (i.e., total hospital costs derived from total charges) | Healthcare/payer | US dollar ($); 2012 |
Mean per-patient cost per hospitalization • SMA type I (n = 237): €50,190 [$54,890] |
Selection: ◊◊◊◊ Comparability: ◊ (age NR) Outcome: ◊◊◊ |
| Darbà et al. (2019) [15] | Per-patient cost per hospitalization (i.e., total hospital costs) | Healthcare/payer | Euro (€); 2015 |
Mean per-patient cost per hospitalization • Pooled sample (n = 705): €6270 [$7150] • SMA type I (n = NR): €9420 [$10,740] • SMA type III (n = NR): €5770 [$6580] • Progressive SMA (n = NR): €5750 [$6560] • Other SMA (n = NR): €5380 [$6130] • Unspecified SMA (n = NR): €5290 [$6,030] |
Selection: ◊◊◊◊ Comparability: ◊ (uncertain classification of phenotypes; distribution of phenotypes NR) Outcome: ◊◊◊ |
| Darbà (2020) [24] | Per-patient annual direct medical cost | Healthcare/payer | Euro (€); 2013 |
Mean per-patient annual direct medical cost • SMA type I, SMA type III, progressive SMA, other SMA, and unspecified SMA (n = 396): €58,610 [$66,410] |
Selection: ◊◊◊◊ Comparability: ◊ (uncertain classification of phenotypes) Outcome: ◊◊◊ |
| Droege et al. (2019) [18] | Per-patient annual direct medical cost | Healthcare/payer | US dollar ($); 2018 |
Mean per-patient annual direct medical cost • SMA type I, conventional therapy (n = 349): $137,630 • SMA type I, nusinersen (n = 45): $1,000,280 ($92,620 excluding treatment cost) • SMA types II–IV, conventional therapy (n = 5728): $49,180 • SMA types II–IV, nusinersen (n = 404): $1,109,060 ($76,370 excluding treatment cost) |
Selection: ◊◊◊◊ Comparability: ◊ (uncertain phenotype definition) Outcome: ◊◊◊ |
| Klug et al. (2016) [20] | Per-patient annual direct medical cost; per-patient annual direct non-medical cost (including informal care costs); per-patient annual indirect cost (i.e., production losses for the patient and one caregiver estimated using the HCA); and per-patient annual total cost of illness | Societal | Euro (€); 2013 |
Mean per-patient annual direct medical cost • SMA types I–III (n = 189): €14,340 [$16,620] • SMA type I (n = 12): €53,710 [$62,230] • SMA type II (n = 73): €15,310 [$17,730] • SMA type III (n = 104): €9130 [$10,570] Mean per-patient annual direct non-medical cost • SMA types I–III (n = 189): €40,380 [$46,790] • SMA type I (n = 12): €45,960 [$53,520] • SMA type II (n = 73): €58,610 [$67,910] • SMA type III (n = 104): €26,940 [$31,220] Mean per-patient annual indirect cost • SMA types I–III (n = 189): €15,850 [$18,360] • SMA type I (n = 12): €8140 [$9440] • SMA type II (n = 73): €16,360 [$18,950] • SMA type III (n = 104): €16,380 [$18,980] Mean per-patient annual total cost of illness • SMA types I–III (n = 189): €70,570 [$81,770] • SMA type I (n = 12): €107,810 [$124,920] • SMA type II (n = 73): €90,270 [$104,600] • SMA type III (n = 104): €52,440 [$60,770] |
Selection: ◊◊◊◊ Comparability: ◊◊ Outcome: ◊◊ (self-reported data) |
| Lee et al. (2019) [14] | Per-patient cost per hospitalization (i.e., total hospital costs derived from total charges) | Healthcare/payer | US dollar ($); 2017 |
Mean per-patient cost per hospitalization • Pooled sample, SMA type NR (n = 229): $104,200 [$106,740] • Tracheostomy, SMA type NR (n = 99): $115,250 [$118,070] • No tracheostomy, SMA type NR (n = 130): $89,120 [$91,290] |
Selection: ◊◊◊◊ Comparability: ◊ (age, sex, and phenotype NR) Outcome: ◊◊◊ |
| López-Bastida et al. (2017) [13] | Per-patient annual direct medical cost; per-patient annual direct non-medical cost (including informal care costs); and per-patient annual total cost of illness | Societal | Euro (€); 2014 |
Mean per-patient annual direct medical cost • SMA types I–III (n = 81): €10,880 [$12,330] • SMA type II (n = 60): €11,580 [$13,120] Mean per-patient annual direct non-medical cost • SMA types I–III (n = 81): €22,840 [$25,880] • SMA type II (n = 60): €26,090 [$29,560] Mean per-patient annual total cost of illness • SMA types I–III (n = 81): €33,720 [$38,210] • SMA type II (n = 60): €37,670 [$42,690] |
Selection: ◊◊◊ (uncertain diagnosis) Comparability: ◊◊ Outcome: ◊◊ (self-reported data) |
| Marcellusi et al. (2019) [22] | Per-patient annual direct medical cost; per-patient annual direct non-medical cost; per-patient annual indirect cost (i.e., production losses for the patient and one caregiver estimated using the HCA, as well as costs associated with paid informal care); and per-patient annual total cost of illness | Societal | Euro (€); 2017 |
Mean per-patient annual direct medical cost • SMA type I (n = 27): €4630 [$5130] • SMA type II (n = 57): €5240 [$5810] • SMA type III (n = 34): €3000 [$3320] Mean per-patient annual direct non-medical cost • SMA type I (n = 27): €18,200 [$20,150] • SMA type II (n = 57): €14,910 [$16,510] • SMA type III (n = 34): €4990 [$5530] Mean per-patient annual indirect cost • SMA type I (n = 27): €13,050 [$14,460] • SMA type II (n = 57): €10,730 [$11,880] • SMA type III (n = 34): €9520 [$10,550] Mean per-patient annual total cost of illness • SMA type I (n = 27): €35,680 [$39,520] • SMA type II (n = 57): €30,410 [$33,680] • SMA type III (n = 34): €16,070 [$17,790] |
Selection: ◊◊◊ (uncertain diagnosis) Comparability: ◊◊ Outcome: ◊◊ (self-reported data) |
| Peña-Longobardo et al. (2020) [26] | Per-patient annual direct medical cost; per-patient annual direct non-medical cost (including informal care costs); and per-patient annual total cost of illness | Societal | Euro (€); 2014 |
Mean per-patient annual direct medical cost • France, SMA types I–III (n = 27): €4670 [$5290] • Germany, SMA types I–III (n = 25): €7310 [$8,290] • UK, SMA types I–III (n = 34): €11,080 [$12,560] Mean per-patient annual direct non-medical cost • France, SMA types I–III (n = 27): €27,370 [$31,020] • Germany, SMA types I–III (n = 25): €44,670 [$50,620] • UK, SMA types I–III (n = 34): €43,210 [$48,970] Mean per-patient annual total cost of illness • France, SMA type I–-III (n = 27): €32,040 [$36,310] • Germany, SMA types I–III (n = 25): €51,980 [$58,910] • UK, SMA types I–III (n = 34): €54,300 [$61,530] |
Selection: ◊◊◊◊ Comparability: ◊◊ Outcome: ◊◊ (self-reported data) |
| Tan et al. (2019) [21] | Per-patient annual direct medical cost | Healthcare/payer | US dollar ($); 2015 |
Mean per-patient annual direct medical cost • SMA type I (n = 23): $306,200 [$324,410] • SMA types II–III (n = 23): $76,280 [$80,820] • SMA type IV (n = 296): $29,990 [$31,770] |
Selection: ◊◊◊◊ Comparability: ◊◊ Outcome: ◊◊◊ |
| Zuluaga‑Sanchez et al. (2019) [23] | Per-patient annual direct medical cost; per-patient annual direct non-medical cost; per-patient annual indirect cost (i.e., production losses for one caregiver estimated using the HCA); and per-patient annual total cost of illness | Societal | Swedish krona (SEK); 2016 |
Mean per-patient annual direct medical cost • SMA type I (year 1): SEK 339,980 [$36,790] • SMA type I (year 2): SEK 224,340 [$24,280] • SMA types II–IV (year 1): SEK 299,970 [$32,460] • SMA types II–IV (year 2): SEK 368,460 [$39,870] Mean per-patient annual direct non-medical cost • SMA type I (year 1): SEK 1,264,190 [$136,800] • SMA type I (year 2): SEK 1,263,940 [$136,770] • SMA types II–IV (year 1): SEK 1,011,360 [$109,440] • SMA types II–IV (year 2): SEK 1,011,480 [$109,450] Mean per-patient annual indirect cost • SMA type I (year 1 and 2): SEK 523,850 [$56,690] • SMA types II–IV (year 1): SEK 261,920 [$28,340] • SMA types II–IV (year 2): SEK 130,960 [$14,170] Mean per-patient annual total cost of illness • SMA type I (year 1): SEK 2,128,020 [$230,270] • SMA type I (year 2): SEK 2,012,130 [$217,730] • SMA types II–IV (year 1): SEK 1,573,250 [$170,240] • SMA types II–IV (year 2): SEK 1,510,900 [$163,490] |
Selection: ◊◊◊◊ Comparability: ◊◊ Outcome: ◊◊ (clinical expert input) |
Note: Costs were rounded to nearest ten. Details of resources included in estimates of per-patient annual direct medical costs are presented in Table 3
HCA human capital approach, NR not reported
aAdjusted for inflation to 2018 values using country-specific consumer price index data from the World Bank and subsequently converted to US dollars
bAssessed with the Newcastle–Ottawa Scale. Maximum score: ◊◊◊◊ for selection, ◊◊ for comparability, and ◊◊◊ for outcome (see the Methods section for details)
cIn the absence of data, year of valuation was assumed to be 2018
dIn the absence of data, year of valuation was assumed to be 2012
Fig. 2.
Mean per-patient annual direct medical cost of spinal muscular atrophy (SMA). AU Australia, DE Germany, ES Spain, FR France, IT Italy, NR not reported, US USA. Subgroups from Droege et al. [18]: (*) Conventional therapy, (**) nusinersen (excluding the mean treatment cost of nusinersen of $907,660 and $1,032,690 per patient and year for SMA type I and types II–IV, respectively). Subgroups from Armstrong et al. [19]: (†) Early diagnosis (≤ 1 year of age) and late diagnosis (> 1 year of age); SMA type not reported. Estimates from Zuluaga-Sanchez et al. [23] are presented as averages of costs for year 1 and year 2. The sample from Darbà [24] includes progressive SMA, other SMA, and unspecified SMA. Costs from Chambers et al. [25] were derived by deducting items typically considered non-medical resources from the reported estimate of the per-patient annual direct cost of illness. Costs were adjusted for inflation to 2018 values using country-specific consumer price index data from the World Bank and subsequently converted to US dollars
Table 3.
Resources included in identified estimates of the per-patient annual direct medical cost of spinal muscular atrophy
| Author (year) | Inpatient care | Outpatient care | Emergency care | Prescription drugs | OTC drugs | Medical devices and aids | Co-payments |
|---|---|---|---|---|---|---|---|
| Armstrong et al. (2016) [19] | × | × | NR | × | |||
| Chambers et al. (2020) [25] | × | × | × | × | × | × | × |
| Darbà (2020) [24] | × | × | × | ||||
| Droege et al. (2019) [18] | × | × | NR | × | |||
| Klug et al. (2016) [20] | × | × | NR | × | × | ||
| López-Bastida et al. (2017) [13] | × | × | × | × | × | ||
| Marcellusi et al. (2019) [22] | × | × | × | × | |||
| Peña-Longobardo et al. (2020) [26] | × | × | × | × | NR | × | |
| Tan et al. (2019) [21] | × | × | × | × | |||
| Zuluaga-Sanchez et al. (2019) [23] | × | × | × | × | × |
NR not reported (i.e., that the information was not provided, but that the specific resource category could have been included in the estimation, for example as part of higher level cost categories), OTC over-the-counter
Three studies [14–16] examined the per-patient cost per hospitalization of SMA (Table 2). Cardenas et al. [16] estimated the mean per-patient cost per hospitalization (derived from total hospital charges) at $54,890 for 237 US patients (43% male, distribution of age not reported) with SMA type I. The second study, Darbà and Marsa, estimated the mean per-patient cost per hospitalization (including costs associated with medical staff, equipment, and resources per tariffs from the Spanish Ministry of Health) at $7150 for a sample of 705 Spanish patients (62% male, mean age: 37 years) with different types of SMA (as reported in Table 1), and at $10,740, $6580, $6560, $6130, and $6030 for SMA type I, SMA type III, progressive SMA, other SMA, and unspecified SMA, respectively (number of patients not reported by SMA type) [15]. Finally, Lee Jr et al. [14] estimated the mean per-patient cost per hospitalization (derived from total hospital charges) at $106,740 for 229 US patients with “severe” SMA (defined as diagnosis during the first year of life, and/or diagnosis for SMA and tracheostomy during the first 3 years of life). For patients with and without tracheostomy, the cost was $118,070 and $91,290, respectively [14]. Different from the per-patient cost per hospitalization of SMA, Ali et al. [17] estimated the mean per-patient annual hospitalization cost of illness for 11 UK patients with SMA type I treated with nusinersen at $129,100 (excluding the cost of nusinersen and its administration).
The mean per-patient direct non-medical cost of SMA was estimated at between $25,880 and $136,800 (Table 2). The lowest estimate was derived from a sample of 81 Spanish patients (SMA types I–III) and included informal care costs (based on the number of hours devoted to informal care recorded using the recall method, estimated at 4 hours per day on average, valued using the proxy good method, i.e., at a shadow price of a market substitute), as well as disease-related costs for personal assistants, travel expenses, legal advice, modifications/investments to the house or car, and other expenses [13]. The highest estimate was based on clinical expert input of the expected duration of personal assistance per day (estimated at 12 hours, on average) and transportation costs for patients with SMA type I in a Swedish setting [23]. Additionally, Marcellusi et al. [22] estimated the mean per-patient total cost of car and home modifications and paid informal care in Italy at $20,150 for SMA type I, $16,510 for SMA type II, and $5530 for SMA type III. Summating costs of items typically considered direct non-medical resources from Chambers et al. [25] (i.e., non-medical travel, respite care, vehicle and home modifications, and informal care) yields an inferred estimate of the per-patient annual direct non-medical cost of illness in Australia at $46,830 for SMA type I, $76,500 for SMA type II, and $20,050 for SMA type III.
Four studies [20, 22, 23, 25] reported indirect costs of SMA. Klug et al. [20] estimated the mean per-patient annual indirect cost of SMA, quantified as disease-related production losses due to patient and caregiver absenteeism and presenteeism from work, at between $9440 (SMA type I) and $18,980 (SMA type III) in Germany. Corresponding costs for Italy and Australia (excluding costs associated with informal care to facilitate comparison) were estimated at between $8940 (SMA type III) and $14,230 (SMA type I) [22], and $16,210 (SMA type I) and $40,200 (SMA type II) [25], respectively. Finally, Zuluaga‑Sanchez et al. [23] estimated the mean per-patient annual indirect cost of SMA (quantified as production losses for one caregiver) in a Swedish setting at between $14,170 (SMA types II–IV) and $56,690 (SMA type I) based on clinical expert input (assuming that one caregiver is fully absent from work for SMA type I, and partially absent for SMA types II–IV).
The mean per-patient annual total cost of illness of SMA, including direct medical, direct non-medical, and indirect costs, was estimated at between $97,300 (SMA type III) and $234,930 (SMA type I) in Australia [25], $60,770 (SMA type III) and $124,920 (SMA type I) in Germany [20], $17,790 (SMA type III) and $39,520 (SMA type I) in Italy [22], $38,210 (SMA types I–III) and $42,690 (SMA type II) in Spain [13], and $163,490 (SMA types II–IV) and $230,270 (SMA type I) in Sweden [23]. Peña-Longobardo et al. [26] estimated the mean per-patient annual total cost of illness of SMA, comprising direct medical and non-medical costs (including informal care, but not indirect costs) for SMA types I–III at $36,310 in France, $58,910 in Germany, and $61,530 in the UK. Additional details of identified costs are presented in Table 2.
Risk of Bias
Two (14%) studies [17, 21] were judged to exhibit a low risk of bias as assessed using the NOS (Table 2). Reasons for a risk of bias included uncertain representativeness owing to the lack of details concerning confirmation of diagnosis of SMA [13, 22, 25], limited comparability owing to inadequate description of the distribution of age, sex, and/or SMA phenotype in the studied samples, or uncertain classification of SMA phenotypes [14–16, 18, 19, 24], and self- or clinical expert reported data [13, 20, 22, 23, 25, 26].
Discussion
The objective of this study was to conduct a systematic review of costs of illness of SMA globally. We found the current body of evidence of the per-patient annual direct medical cost of SMA to be subject to considerable heterogeneity between individual studies (Tables 2 and 3). Reasons for this variability concern differences regarding (1) the scope of included medical resources (as used or consumed because of SMA), (2) measured quantities of included resources, and/or (3) prices/unit costs of included resources used in the calculation. For example, in their assessment of the direct medical cost of SMA, Chambers et al. [25] included inpatient care, outpatient care, and emergency care, prescription and over-the-counter drugs, as well as costs associated with medical devices and aids and co-payments , whereas Darbà only considered the first three of these categories [24]. Moreover, there may be non-trivial differences between studies also within defined resource categories (e.g., including all outpatient visits vs visits to a selected set of practitioners). For these reasons, cost estimates are typically not directly comparable between studies, in particular if conducted in different geographical settings. That being said, the range in direct medical costs identified as part of this review was still somewhat surprising.
Because of differences in costing methodologies and healthcare systems, and considering the broad range in estimates identified as part of this review, it is not straightforward to directly compare costs of SMA with those of other diseases. Interestingly, Armstrong et al. [19] also studied a cohort of individuals without SMA in the USA. The authors found that the mean per-patient annual direct medical cost of SMA was about 2600% greater than estimates for those without the disease. In addition, Cardenas et al. [16] estimated the mean per-patient cost per hospitalization for SMA type I to be around 850% higher than for patients without any complex chronic conditions in the USA. Although not directly applicable to other settings, these data should help readers interpret and contextualize the magnitude of these cost components of the total burden of illness of SMA. Moreover, the high variability in costs between studies also characterized estimates within samples. Indeed, in some cases, such as Armstrong et al. [19], a small proportion of patients (about 2%) had costs equal to or exceeding $1,000,000 (and about 13% equal to or exceeding $500,000) per year. These findings highlight the importance of deriving estimates from adequately powered studies for a meaningful inference in diseases with a heterogeneous presentation, such as SMA.
Outcomes of our review revealed that costs were also markedly different across SMA phenotypes. In particular, the per-patient annual direct medical cost of SMA type I was notably higher than that of the other types of the disease. This is not unexpected given the complete dependency on 24-h care and ventilation in these children if they survive the first year of life, at least prior to the institution of disease-modifying treatments.
We identified two studies investigating costs in relation to nusinersen (excluding the cost of nusinersen and its administration). Ali et al. [17] estimated the per-patient annual hospitalization cost at $129,100 in a sample of 11 patients treated with nusinersen in the UK. Droege et al. [18] found nusinersen compared with conventional therapy to be associated with lower per-patient annual direct medical costs for SMA type I ($92,620 vs $137,630), but not SMA type II-III ($76,370 vs $49,180), and estimated the mean per-patient annual cost of nusinersen at $907,660 for SMA type I and $1,032,690 for SMA types II–IV in the USA. These findings show that although nusinersen may be associated with lower resource utilization for some patients, at the group or population level, overall resource use and associated costs remain high, even after excluding the cost of the treatment.
In articles reporting estimates of the per-patient annual total direct cost of illness of SMA (comprising direct medical and non-medical costs), direct non-medical costs (including informal care costs, if available) were found to make up a considerable proportion of total direct costs. In fact, across included studies, this cost category accounted for an average of 68% of the per-patient annual total direct cost, between 21% (SMA type I, Australia) and 86% (SMA types I–III, Germany) [13, 20, 22, 23, 25, 26]. These findings may be compared with corresponding estimates for other serious neuromuscular diseases, such as Duchenne muscular dystrophy, where direct non-medical costs comprised between 47% and 73% of total direct costs in Germany, Italy, the UK, and the USA [29]. Time devoted to informal caregiving in SMA also varied between studies, settings, and phenotypes, from 9 hours per day on average in France [26], to 4 hours per day on average in Spain [13], 4–15 hours in Germany [20, 26], 10–12 h in Sweden [23], and 13 hours in the UK [26] (data not reported by Chambers et al. [25]). However, because of different methods, direct comparison of these estimates should be made with caution. Nonetheless, these findings underscore the substantial burden of informal caregiving in SMA.
Identified estimates of production losses and associated costs of SMA varied substantially across studies and settings. Considering the relative magnitude of this cost category noted in this review, and the range in estimates, future study of indirect costs in SMA appears warranted to increase the understanding of the total economic burden of the disease from the perspective of society.
The outcomes of this review have several implications for health policy and future research. First, up-to-date cost estimates are lacking for most settings. This implies that further cost research will be necessary to map out the health economic context of SMA in most countries. Second, given the very high variability in identified costs, it is not readily apparent how the current evidence base should best inform economic evaluations. This also concerns the lack of longitudinal cost data in this indication to enable assessment of long-term outcomes. Third, our review shows that in most settings, little is known of costs associated with specific treatments, including nusinersen. This constitutes an important topic for future research, especially considering the growing battery of novel high-cost therapies in this indication. Last, several studies included in this review were judged to be subject to a risk of bias, mainly relating to incomplete reporting, documentation, and/or stratification, which serve as a reminder of the importance of providing sufficient details for meaningful interpretation and contextualization of outcomes from cost studies in SMA. Indeed, averaging costs across SMA types (in particular SMA type I with other types of the disease) would be expected to render pooled estimates of ambiguous magnitude and unclear external validity (as illustrated in Fig. 2).
There are two main limitations to this literature review. First, we did not systematically search for relevant gray literature, which means that some estimates of costs of illness of SMA might not have been identified. However, given the absence of thorough peer review and critical appraisal of most literature published outside the traditional scientific/academic distribution channels, such as journals, the importance of this limitation for the overall interpretation of the review results is expected to be minor. Second, the NOS assesses aspects of quality and bias of non-randomized studies, not specifically cost research. Therefore, some studies that were assigned a perfect score rating based on the NOS might still be subject to non-trivial limitations and bias (relating to e.g., the relevance of included medical and non-medical resources, and valuation methods). In this regard, it is also worth noting that for some cost types (e.g., informal care costs), primary data collection (via e.g., surveys) is likely necessary (despite being associated with potential bias), as this type of information would not be expected to be available from administrative or disease-specific registries, or clinical charts. Additionally, as expected given the generic nature of the instrument, aspects of some criteria of the NOS (e.g., disease-specific requirements and thresholds) are defined by the researcher, which means that there is a degree of subjectivity in the application of the scale and assessment of the risk of bias. Finally, it is important to keep in mind that there is no universal classification of costs of illness. Informal care costs, for example, can be considered a direct non-medical or an indirect cost of illness. In this study, we extracted and reported all identified costs of illness of SMA separately (in Table 2), but also synthesized them into a set of pre-defined cost categories, in which we considered the informal care cost of illness a direct non-medical cost under the assumption that the assistance/aid otherwise would have to be provided by paid professionals.
Conclusions
We show that the current body of evidence of costs of SMA is generally scarce and characterized by considerable heterogeneity across geographical settings and disease phenotypes. Our review provides data pertaining to the economic impact of SMA, which is of particular relevance in light of emerging treatments and ongoing research in this field, and underscores the substantial unmet medical need in this patient population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Karolinska Institute. No funding was received for the preparation of this article.
Conflict of interest
Erik Landfeldt is an employee of ICON plc (Stockholm, Sweden), outside the submitted work. Astrid Pechmann reports receiving compensation for presentations and training activities from Biogen, and research funding from Biogen and AveXis, outside the submitted work. Thomas Sejersen reports receiving honoraria for lectures and participation in advisory board meetings from Biogen, AveXis, and Roche, outside the submitted work. Hugh J. McMillan has participated in an advisory board for AveXis, and reports research funding from Roche, outside the submitted work. Hanns Lochmüller has no conflicts of interest that are directly relevant to the content of this article
Availability of Data and Material
All data analyzed as part of this study are included in this published article (and its supplementary information files).
Authors’ Contributions
Erik Landfeldt conceptualized and designed the study, conducted the literature review and the analysis, led the interpretation of findings, drafted the manuscript, critically reviewed the manuscript for important intellectual content, and approved the final manuscript version as submitted. Astrid Pechmann conducted the literature review, interpreted the findings, critically reviewed the manuscript for important intellectual content, and approved the final manuscript version as submitted. Hugh J. McMillan interpreted the findings, critically reviewed the manuscript for important intellectual content, and approved the final manuscript version as submitted. Hanns Lochmüller interpreted the findings, critically reviewed the manuscript for important intellectual content, and approved the final manuscript version as submitted. Thomas Sejersen conceptualized and designed the study, interpreted the findings, critically reviewed the manuscript for important intellectual content, and approved the final manuscript version as submitted.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
References
- 1.Verhaart IEC, Robertson A, Leary R, McMacken G, Konig K, Kirschner J, et al. A multi-source approach to determine SMA incidence and research ready population. J Neurol. 2017;264(7):1465–1473. doi: 10.1007/s00415-017-8549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogino S, Wilson RB, Gold B. New insights on the evolution of the SMN1 and SMN2 region: simulation and meta-analysis for allele and haplotype frequency calculations. Eur J Hum Genet. 2004;12(12):1015–1023. doi: 10.1038/sj.ejhg.5201288. [DOI] [PubMed] [Google Scholar]
- 3.Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy: a literature review. Orphanet J Rare Dis. 2017;12(1):124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817. doi: 10.1212/wnl.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–891. doi: 10.1002/ana.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 7.Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy: new phenotypes, new challenges, new implications for care. J Neuromuscul Dis. 2020;7(1):1–13. doi: 10.3233/jnd-190424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Landfeldt E, Zethraeus N, Lindgren P. Standardized questionnaire for the measurement, valuation, and estimation of costs of informal care based on the opportunity cost and proxy good method. Appl Health Econ Health Policy. 2019;17(1):15–24. doi: 10.1007/s40258-018-0418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannesson M. The concept of cost in the economic evaluation of health care: a theoretical inquiry. Int J Technol Assess Health Care. 1994;10(4):675–682. doi: 10.1017/S0266462300008254. [DOI] [PubMed] [Google Scholar]
- 11.Wells G, Shea B, O’Connell J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Oct 2019.
- 12.World Health Organization. International classification of diseases for mortality and morbidity statistics. 10th Revision. 2020. https://icd.who.int/browse10/2019/en. Accessed 30 Sep 2020.
- 13.Lopez-Bastida J, Pena-Longobardo LM, Aranda-Reneo I, Tizzano E, Sefton M, Oliva-Moreno J. Social/economic costs and health-related quality of life in patients with spinal muscular atrophy (SMA) in Spain. Orphanet J Rare Dis. 2017;12(1):141. doi: 10.1186/s13023-017-0695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Jr, Franca UL, Graham RJ, McManus ML. Pre-nusinersen hospitalization costs of children with spinal muscular atrophy. Pediatr Neurol. 2019;92:3–5. doi: 10.1016/j.pediatrneurol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Darba J, Marsa A. Patient characteristics and hospitalisation costs of spinal muscular atrophy in Spain: a retrospective multicentre database analysis. BMJ Open. 2019;9(11):e031271. doi: 10.1136/bmjopen-2019-031271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardenas J, Menier M, Heitzer MD, Sproule DM. High healthcare resource use in hospitalized patients with a diagnosis of spinal muscular atrophy type 1 (SMA1): retrospective analysis of the Kid’s Inpatient Database (KID) Pharmacoecon Open. 2019;3(2):205–213. doi: 10.1007/s41669-018-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali I, Gilchrist F, Carroll WD, Alexander J, Clayton S, Willis T, et al. Healthcare utilisation in SMA type 1 patients treated with nusinersen. Arch Dis Child. 2019;104:A71–A72. doi: 10.1136/archdischild-2019-rcpch.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Droege M, Sproule D, Arjunji R, Gauthier-Loiselle M, Cloutier M, Dabbous O. Economic burden of spinal muscular atrophy in the United States: a contemporary assessment. J Med Econ. 2020;23(1):73–79. doi: 10.1080/13696998.2019.1646263. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong EP, Malone DC, Yeh WS, Dahl GJ, Lee RL, Sicignano N. The economic burden of spinal muscular atrophy. J Med Econ. 2016;19(8):822–826. doi: 10.1080/13696998.2016.1198355. [DOI] [PubMed] [Google Scholar]
- 20.Klug C, Schreiber-Katz O, Thiele S, Schorling E, Zowe J, Reilich P, et al. Disease burden of spinal muscular atrophy in Germany. Orphanet J Rare Dis. 2016;1(1):58. doi: 10.1186/s13023-016-0424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan H, Gu T, Chen E, Punekar R, Shieh PB. Healthcare utilization, costs of care, and mortality among patients with spinal muscular atrophy. J Health Econ Outcomes Res. 2019;6(3):185–195. doi: 10.36469/63185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcellusi A, Bini C, Casiraghi J, D’Ambrosio F, Rotundo MA, et al. Cost of illness of spinal muscular atrophy (SMA) in Italy. Glob Reg Health Technol Assess. 2019 doi: 10.1177/2284240319867662. [DOI] [Google Scholar]
- 23.Zuluaga-Sanchez S, Teynor M, Knight C, Thompson R, Lundqvist T, Ekelund M, et al. Cost effectiveness of nusinersen in the treatment of patients with infantile-onset and later-onset spinal muscular atrophy in Sweden. Pharmacoeconomics. 2019;37(6):845–865. doi: 10.1007/s40273-019-00769-6. [DOI] [PubMed] [Google Scholar]
- 24.Darba J. Direct medical costs of spinal muscular atrophy in the Catalonia region: a population-based analysis. Clin Drug Invest. 2020;40(4):335–341. doi: 10.1007/s40261-020-00897-4. [DOI] [PubMed] [Google Scholar]
- 25.Chambers GM, Settumba SN, Carey KA, Cairns A, Menezes MP, Ryan M, et al. Prenusinersen economic and health-related quality of life burden of spinal muscular atrophy. Neurology. 2020;95(1):e1–10. doi: 10.1212/WNL.0000000000009715. [DOI] [PubMed] [Google Scholar]
- 26.Peña-Longobardo LM, Aranda-Reneo I, Oliva-Moreno J, Litzkendorf S, Durand-Zaleski I, Tizzano E, et al. The economic impact and health-related quality of life of spinal muscular atrophy: an analysis across Europe. Int J Environ Res Public Health. 2020;17(16):5640. doi: 10.3390/ijerph17165640.PMID:32764338;PMCID:PMC7459726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malone DC, Dean R, Arjunji R, Jensen I, Cyr P, Miller B, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Market Access Health Policy. 2019;7(1):1601484. doi: 10.1080/20016689.2019.1601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morningstar, Inc. 2019. https://www.morningstar.com. Accessed 29 Nov 2019.
- 29.Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014;83(6):529–536. doi: 10.1212/wnl.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.