Abstract
Purpose
The test with the highest diagnostic accuracy for diabetes insipidus is copeptin measurement after hypertonic saline infusion. However, the procedure is cumbersome and unpleasant due to rapid sodium increase. An oral stimulation test would be highly desirable. Macimorelin, an oral ghrelin agonist, is a newly approved diagnostic test for growth hormone (GH) deficiency, but its effects on copeptin/vasopressin are unknown and the effects on other pituitary hormones only scarcely investigated.
Methods
In this prospective, interventional, proof-of-concept study Copeptin and anterior pituitary hormones were measured in 28 healthy volunteers on two test days at baseline, 30, 45, 60, 90 and 120 min after a single dose of macimorelin (first visit: 0.5 mg/kg, second visit: 0.75 mg/kg).
Results
Baseline copeptin levels were 5.26 pmol/L [1.57, 6.81] and did not change after macimorelin intake (0.5 mg/kg: maximal median change 0.40 [− 0.49, 0.65] pmol/L, p = 0.442; 0.75 mg/kg: − 0.13 [− 0.45, 0.17] pmol/L, p = 0.442. Median GH levels increased from 3.67 mU/L with a maximal median change of 94.66 [IQR 56.5; 110.96] mU/L, p < 0.001. No effect was seen on cortisol, ACTH, LH and FSH levels. Prolactin (max. median change 100 [2.5; 146.5] mU/L, p = 0.004) and free thyroxine (fT4) (0.5 [0.2; 0.8] pmol/L, p < 0.001) increased, whereas TSH decreased (− 0.18 [− 0.22, − 0.09] mU/L, p < 0.001).
Conclusion
We confirm an increase of GH upon macimorelin in healthy volunteers. However, macimorelin did not stimulate copeptin and therefore does not provide an oral test alternative for the diagnosis of diabetes insipidus. Additionally, a stimulatory effect was seen for prolactin and fT4, but not for ACTH and gonadotropic hormones.
Registration
The trial was registered on ClinicalTrials.gov (NCT03844217) on February 18, 2019.
Keywords: Ghrelin agonist, Macimorelin, Copeptin, Pituitary hormones, Diabetes insipidus
Introduction
The diagnosis of pituitary hormone deficiencies is often cumbersome as an intravenous application of the stimulatory agent is mandatory [1]. In particular, the differential diagnosis of diabetes insipidus is challenging [2] and to date the test with the highest diagnostic accuracy is copeptin measurement after hypertonic saline infusion [3]. However, this test requires an intravenous application of the hypertonic saline solution, and close medical supervision throughout the test for tight control of plasma sodium levels. Moreover, it often causes discomfort in patients due to the rapid increase in plasma sodium levels. Recently, arginine infusion—well known as a growth hormone secretagogue (GHS)—was found to be a potent stimulator of the neurohypophysis and provides a new diagnostic tool in the differential diagnosis of central diabetes insipidus [4]. However, despite its better tolerance, this test still needs intravenous administration. An oral stimulation test would be easier to perform, cause less risks and discomfort to the patient, and would require fewer resources in clinical practice.
A single oral dose of macimorelin—a ghrelin receptor agonist—has been shown to stimulate GH-secretion and has proven as a well-tolerated, reproducible and safe diagnostic test for the diagnosis of adult growth hormone deficiency (AGHD) in comparison to the insulin tolerance test (ITT) and the GHRH-arginine test [5, 6]. Ghrelin has a stimulatory effect on copeptin/vasopressin both in vitro and in rodents [7–10]. Other GHS such as hexarelin [11] and arginine infusion [4] stimulate copeptin/vasopressin in humans. In analogy to this, we hypothesized that macimorelin would stimulate copeptin secretion from the posterior pituitary gland.
Not only the diagnostics of posterior pituitary hormone deficiencies are difficult, but also the assessment of anterior pituitary hormones can be challenging. Standard diagnostic tests of adrenal insufficiency are the ITT or cosyntropin test in cases with indeterminate morning cortisol levels to confirm the diagnosis of adrenocorticotropic hormone (ACTH) deficiency [12], and for this condition, there is no available oral stimulatory test. Growth hormone-secretagogues (GHS) are also known to stimulate the hypothalamo-pituitary-adrenal (HPA)-axis, and previous data have shown a stimulatory effect of hexarelin—a GHS, which is administered intravenously, on ACTH and cortisol in healthy volunteers [11]. We therefore hypothesized that macimorelin would stimulate also the HPA-axis and other hormones of the anterior pituitary gland apart from the known effect on GH.
Participants and methods
This study was conducted as a single-centre, open-label, prospective interventional proof-of-concept study in compliance with the ethical principles based on the Declaration of Helsinki and the applicable International Conference on Harmonization (ICH) guidelines on good clinical practice. Local ethic committees and Swissmedic approved the study protocol. Written informed consent was obtained from all study participants before inclusion. The study was preregistered on ClinicalTrials.gov (NCT03844217).
Study participants
Study participants had to be healthy and were recruited as volunteers via email at the University of Basel. Inclusion criteria were age between 18 and 60 years and no regular medication apart from hormonal contraception in females. Exclusion criteria were any known known medical history or underlying health condition, particularly any chronic renal or liver disease, body mass index (BMI) ≤ 18.5 kg/m2 or ≥ 40 kg/m2, pregnancy or breastfeeding, evidence of drinking disorders and diuresis (defined as polyuria > 50 mL/kg body weight/24 h and polydipsia > 3 l/24 h), a prolonged QT interval (QTc > 500 ms) or a known allergy towards macimorelin.
Study drug
Macimorelin acetate is an oral ghrelin full receptor agonist, which was provided by Aeterna Zentaris GmbH. It has a molecular weight of 535.6 g/mole and its solubility in water is 300 mg/mL. Its structural formula has been published previously [13].
Study procedure
A study physician screened all participants in a screening visit. Eligibility criteria were checked, an electrocardiogram (ECG) to exclude long QT was conducted, a medical history questionnaire was obtained and a pregnancy test was performed in all women.
Study participants attended two study visits (V1 and V2) scheduled in the morning after an overnight fast. Participants were allowed to drink until midnight, alcohol was not allowed 24 h before the test started. At V1 the test was performed with a single oral dose of 0.5 mg/kg body weight macimorelin, which is the dose approved in the United States and Europe to diagnose AGHD [6]. The second test day V2 was scheduled at least one week apart using a single dose of 0.75 mg/kg body weight macimorelin. A peripheral intravenous catheter was placed 30 min before the baseline blood examination. Blood samples were drawn at baseline (0 min) and 30, 45, 60, 90 and 120 min after the intake of macimorelin. Vital signs were recorded at the same point of time when blood was drawn. An ECG to assess QTc-interval was performed at baseline and at 120 min of each study visit.
Laboratory assessment
All laboratory analyses were performed at the centre laboratory at University Hospital Basel immediately after collecting the samples. Copeptin levels were measured with a chemifluorescence sandwich immunoassay (BRAHMS CT-proAVP KRYPTOR, from BRAHMS GmbH, Hennigsdorf, Germany), with a lower detection limit of 0.4 pmol/L.
ACTH was quantified with chemiluminescence immunoassay (ACTH Immulite, Siemens Healthcare Diagnostics Products Ltd., Gwynedd, UK), with a reference range < 46.0 pg/mL after collecting the blood on ice. Cortisol, Prolactin, free thyroxine level (fT4), thyroid stimulating hormone (TSH), GH, luteinizing hormone (LH) and follicle stimulating hormone (FSH) were determined with an electrochemiluminescence immunoassay (ECLIA) (Cobas8000, Roche Diagnostics GmbH, Mannheim, Germany) and insulin like growth factor-1 (IGF-1) levels were measured with a chemiluminescence immunoassay (CLIA) (LIAISON XL, DiaSorin S.p.A., Saluggia, Italy).
Study endpoints
The predefined primary endpoint was the maximal change in copeptin levels upon the intake of a single oral dose of macimorelin 0.5 mg/kg body weight. Predefined secondary endpoints were the maximal change of copeptin levels upon intake of a single oral dose of macimorelin 0.75 mg/kg body weight, and—for each dose—the course of and maximal change in other anterior pituitary hormone levels (GH, ACTH, TSH, LH, FSH and prolactin), IGF-1, fT4, cortisol and the change from baseline to end of test in plasma sodium, plasma osmolality and QTc-interval. Further, clinical symptoms during the tests were assessed and documented.
Statistical analysis
Sample size was estimated in order to show an increase in copeptin level within two hours after the intake of a single oral-dose macimorelin. We used a resampling approach based on data of copeptin levels after arginine infusion from 50 healthy volunteers [4]. We assumed the absolute treatment effect of a single oral-dose macimorelin 0.5 mg/kg to be 0.5 pmol/L less than observed after arginine infusion. Hence, we expected a median change in copeptin of 2.3 pmol/L, as compared to 2.8 pmol/L. Sample sizes ni = 10–50 were examined by sampling with replacement 999 times ni data and testing them for a difference from zero at a significance level α of 0.05 using the Wilcoxon rank sum test. A sample size of 25 evaluable subjects would provide 90% power to reject the null hypothesis. Assuming a drop-out rate of 10% we aimed to recruit a total of 28 subjects.
Categorical variables are summarized by frequency (%) and continuous variables are summarized by mean (standard deviation; SD) in case of no obvious deviation from normal distribution or by median [interquartile range; IQR] otherwise. The maximum change in copeptin, the maximum changes in the other pituitary hormones and the changes in plasma sodium and osmolality after 0.5 mg/kg macimorelin and after 0.75 mg/kg macimorelin were each tested for a difference from zero using the sign test. The maximum change in copeptin was further compared for a difference between the two doses using the paired samples sign test. The within-subject differences in copeptin between the two doses are reported by median and IQR.
Since all these secondary analyses are exploratory, hypothesis-generating and not hypothesis-testing, no adjustment for multiple comparisons were made. P-values are not to be interpreted as confirmatory but as continuous measures that inform the generation of new hypotheses worthy of further investigation, in conjunction with the estimated effect sizes. In accordance with recent recommendations [14], the term “statistically significant” is not used.
All analyses were conducted using the statistical software package R (R Core Team, 2019), Version 3.6.0.
Results
Baseline characteristics
Twenty-eight healthy volunteers were included. They had a mean age of 22.4 years (SD 1.5), a median body mass index of 22.3 kg/m2 [IQR 21.5, 24.4] and 13 (46.4%) were male. A complete description of all baseline characteristics is provided in Table 1.
Table 1.
Baseline characteristics are indicated as mean (standard deviation), median [inter-quartile range] or frequency (%) as appropriate
| n | 28 |
| Male, n (%) | 13 (46.4%) |
| Age, years (SD) | 22.4 (1.5) |
| Alcohol consumption (glassweek) | 2.0 [1.0, 3.0] |
| Current smoker, n (%) | 0 (0%) |
| BMI (kg/m2) | 22.3 [21.5, 24.4] |
| Oral contraceptive pill, n (%) | 5 (33.3%) |
| Plasma sodium (mmol/L) | 141 [140, 142] |
| Plasma osmolality (mmol/kg) | 289.5 [288, 292] |
Percentages for oral contraceptive pill refer to female participants
Effect of macimorelin on copeptin levels and plasma osmolality
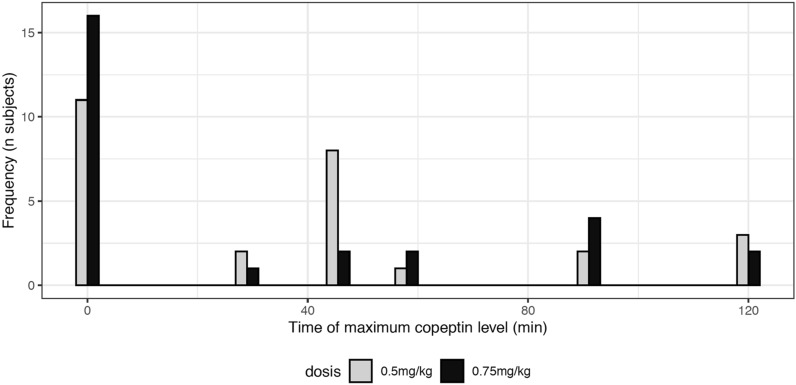
Median [IQR] baseline copeptin levels were 5.26 [1.57, 6.81] pmol/L and 4.25 [2.04, 6.29] pmol/L for the 0.5 mg/kg and 0.75 mg/kg body weight doses, respectively. We found no evidence for an increase in copeptin for either dose (Fig. 1, Table 2). The maximal changes in copeptin levels were not different from zero: 0.40 [− 0.49, 0.65] pmol/L (p = 0.442) and − 0.13 [− 0.45, 0.17] pmol/L (p = 0.442), accordingly. For 11 and 17 subjects, respectively, all copeptin values after intake of macimorelin were even lower or equal to the respective baseline value. For those participants reaching their maximum level after the intake of macimorelin, the peak was most often seen after 45 min for the dose of 0.5 mg/kg and at one of the later time points for the dose of 0.75 mg/kg (Fig. 2, Table 2). The within-subject difference in maximal copeptin change between 0.75 mg/kg and 0.5 mg/kg macimorelin dose was not different from zero: − 0.21 [− 1.02, 0.42] pmol/L (p = 0.199). Plasma sodium levels or plasma osmolality slightly decreased (Table 2).
Fig. 1.
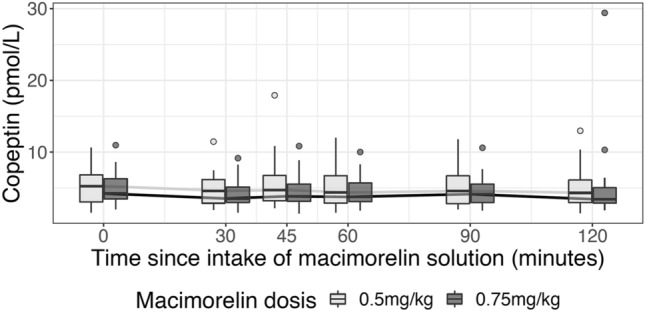
Time course of copeptin after a single oral stimulation with macimorelin 0.5 mg/kg and 0.75 mg/kg
Table 2.
Summary table of outcomes according to macimorelin dose
| Dose (mg/kg) | Baseline | Maximal changes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Minimum | First quartile | Median | Third quartile | Maximum | N | Minimum | First quartile | Median | Third quartile | Maximum | P value | ||
| Copeptin (pmol/L) | 0.5 | 27 | 1.57 | 3.07 | 5.26 | 6.815 | 10.62 | 27 | − 1.17 | − 0.50 | 0.40 | 0.65 | 14.66 | 0.442 |
| Copeptin (pmol/L) | 0.75 | 27 | 2.04 | 3.48 | 4.25 | 6.295 | 10.97 | 27 | − 1.61 | − 0.45 | − 0.13 | 0.18 | 21.36 | 0.442 |
| GH (mlU/L) | 0.5 | 28 | 0.16 | 0.435 | 3.665 | 13.325 | 40.9 | 28 | − 3.51 | 56.50 | 94.67 | 110.96 | 137.81 | < 0.001 |
| GH (mlU/L) | 0.75 | 27 | 0.15 | 0.31 | 1.6 | 17.25 | 58.4 | 25 | 6.43 | 80.40 | 101.10 | 128.20 | 147.69 | < 0.001 |
| IGF-1 (nmol/L) | 0.5 | 28 | 19.7 | 25.975 | 30.95 | 38.625 | 45 | 28 | − 1.60 | − 0.30 | 0.65 | 2.33 | 5.10 | 0.185 |
| IGF-1 (nmol/L) | 0.75 | 27 | 21.9 | 26.05 | 30.1 | 38.35 | 43 | 27 | − 0.80 | − 0.10 | 0.40 | 1.10 | 3.60 | 0.442 |
| fT4 (pmol/L) | 0.5 | 28 | 11.1 | 14 | 15.45 | 16.25 | 17.6 | 28 | − 1.00 | 0.20 | 0.50 | 0.80 | 1.50 | < 0.001 |
| fT4 (pmol/L) | 0.75 | 27 | 9.5 | 13.6 | 14.8 | 16.5 | 18.3 | 27 | − 0.30 | 0.40 | 0.60 | 0.90 | 1.50 | < 0.001 |
| TSH (mlU/L) | 0.5 | 28 | 0.762 | 1.19 | 1.565 | 2.095 | 4.77 | 28 | − 0.77 | − 0.22 | − 0.18 | − 0.09 | 0.21 | < 0.001 |
| TSH (mlU/L) | 0.75 | 27 | 0.685 | 1.405 | 1.72 | 2.33 | 4.25 | 27 | − 0.97 | − 0.32 | − 0.17 | − 0.07 | 0.10 | < 0.001 |
| Prolactin (mU/L) | 0.5 | 28 | 136 | 245 | 303 | 417.25 | 675 | 28 | − 214.00 | 2.50 | 100.00 | 146.50 | 339.00 | 0.004 |
| Prolactin (mU/L) | 0.75 | 27 | 131 | 236 | 312 | 429.5 | 694 | 27 | − 113.00 | 12.50 | 101.00 | 309.50 | 640.00 | < 0.001 |
| Cortisol (nmol/L) | 0.5 | 28 | 237 | 341.25 | 369 | 427.75 | 706 | 28 | − 153.00 | − 66.75 | − 16.00 | 90.25 | 179.00 | 0.572 |
| Cortisol (nmol/L) | 0.75 | 27 | 174 | 307.5 | 379 | 429 | 766 | 27 | − 157.00 | − 58.00 | − 14.00 | 55.50 | 346.00 | 0.701 |
| ACTH (ng/L) | 0.5 | 27 | 5 | 14.15 | 19.9 | 26.55 | 66 | 26 | − 24.40 | − 3.55 | 1.65 | 9.58 | 121.60 | 0.169 |
| ACTH (ng/L) | 0.75 | 27 | 6.5 | 14.05 | 18.6 | 24.85 | 56.8 | 27 | − 13.30 | − 0.85 | 2.10 | 8.60 | 102.40 | 0.248 |
| LH (IU/L) | 0.5 | 28 | 0.3 | 3.85 | 7.2 | 10.15 | 23.9 | 27 | − 2.60 | − 1.05 | − 0.30 | 0.90 | 12.30 | 0.701 |
| LH (IU/L) | 0.75 | 27 | 0.3 | 3.9 | 5.1 | 6.95 | 16 | 26 | − 2.70 | − 0.60 | − 0.25 | 1.23 | 6.30 | 0.557 |
| FSH (IU/L) | 0.5 | 28 | 0.3 | 3.225 | 4.75 | 5.475 | 8.3 | 27 | − 0.40 | − 0.10 | 0.00 | 0.20 | 1.40 | 0.442 |
| FSH (IU/L) | 0.75 | 27 | 0.3 | 2.5 | 3.8 | 5 | 8 | 26 | − 0.50 | − 0.10 | 0.00 | 0.20 | 0.50 | 0.327 |
| Plasma sodium (mmol/l) | 0.5 | 28 | 137 | 140 | 141 | 142 | 144 | 28 | − 4.00 | − 2.00 | − 1.00 | 0.25 | 3.00 | 0.013 |
| Plasma sodium (mmol/l) | 0.75 | 27 | 138 | 139.5 | 141 | 141.5 | 145 | 27 | − 4.00 | − 2.50 | − 2.00 | 0.00 | 5.00 | 0.006 |
| Plasma osmolality (mmol/kg) | 0.5 | 28 | 283 | 288 | 289.5 | 292 | 295 | 28 | − 8.00 | − 3.00 | − 0.50 | 1.00 | 5.00 | 0.572 |
| Plasma osmolality (mmol/kg) | 0.75 | 27 | 284 | 288 | 290 | 292.5 | 299 | 27 | − 9.00 | − 3.00 | − 2.00 | 0.00 | 7.00 | 0.002 |
Fig. 2.
Maximum copeptin times, i.e. the measurement time point at which copeptin levels were maximal. Time zero indicates the baseline, i.e. before intake of macimorelin
Effect of macimorelin on growth hormone levels
Upon the intake of each single dose of macimorelin GH levels increased significantly (Fig. 3a, Table 2). The median [IQR] maximal change from baseline was 94.7 [56.5, 111.0] mU/L (p < 0.001) under the dose of 0.5 mg/kg body weight, and 101.1 [80.4, 128.2] mU/L (p < 0.001) under the dose of 0.75 mg/kg body weight. The peak was most often seen 45 min after the intake for either dose. However, we observed no change in IGF-1 levels upon intake of either dose (Fig. 3b, Table 2).
Fig. 3.

Time course of GH and IGF-1 for each macimorelin dose
Effect of macimorelin on the pituitary-adrenal axis
Median ACTH levels at baseline were 19.9 [IQR 14.15, 26.55] pg/mL and 18.60 [14.05, 24.85] pg/mL for the 0.5 mg/kg and 0.75 mg/kg body weight doses, respectively. We found no evidence for an increase in ACTH levels for either dose (Fig. 4a, Table 2). The maximal changes in ACTH levels were not different from zero: 1.65 [− 3.55, 9.58] pg/mL (p = 0.169) and 2.10 [− 0.85; 8.60] pg/mL (p = 0.248), accordingly. Similarly, median baseline cortisol levels of 237 [341; 428] nmol/L did not change with a maximal median change of − 16.00 [− 66.75; 90.25] nmol/L (p = 0.572) after 0.5 mg/kg and − 14.00 [− 58.00, 55.50] nmol/L (p = 0.701) after 0.75 mg/kg macimorelin, respectively (Fig. 4b, Table 2).
Fig. 4.
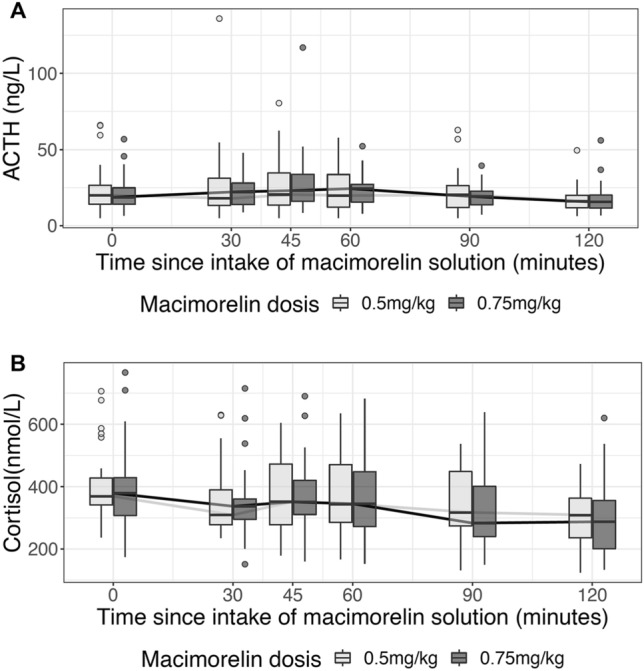
Time course of ACTH and cortisol for each macimorelin dose
Effect of macimorelin on other anterior pituitary hormones and free thyroxine levels
Median TSH levels decreased from 1.56 [IQR 1.19, 2.09] mU/L with a maximal median change of − 0.18 [− 0.22, − 0.09] mU/L (p < 0.001) after the dose of 0.5 mg/kg and a maximal median change of − 0.17 [− 0.32, − 0.07] mU/L (p < 0.001) after 0.75 mg/kg, respectively (Fig. 5a, Table 2). In contrast fT4 levels increased from a median at baseline of fT4 15.45 [14, 16.25] pmol/L with a maximal median change of 0.5 [0.2, 0.8] pmol/L, (p < 0.001) under the dose of 0.5 mg/kg and a maximal median change of 0.6 [0.4, 0.9] pmol/L (p < 0.001) under 0.75 mg/kg, accordingly (Fig. 5b, Table 2).
Fig. 5.

Time course of TSH, fT4, prolactin and gonadotropic hormones for each macimorelin dose
Prolactin levels increased at both doses from baseline levels 303 [245, 417] mU/L with a maximal median change of 100 [2.5, 146.5] mU/L (p = 0.004) after the dose of 0.5 mg/kg macimorelin. The maximal median change in prolactin levels after the higher dose of 0.75 mg/kg macimorelin accounted for 101 [13, 310] mU/L (p < 0.001) (Fig. 5c, Table 2). There was no change in LH or FSH levels in all study participants (Fig. 5d, e, Table 2) and also a subgroup analysis of men only did not reveal changes in LH or FSH levels.
Safety and tolerability
There were no severe adverse events occurring during this study, the general tolerability was high and the recorded adverse events were mild: dizziness (4%), dysgeusia (4%), fatigue (18%) and headache (4%). Table 3 provides a full description of all adverse events during the study sequence including causality.
Table 3.
Number of adverse effects occurring during the study sequence
| Adverse effect | Dose (mg/kg) | Patients with event | Total patients | % affected patients |
|---|---|---|---|---|
| Diarrhea | 0.5 | 0 | 28 | 0 |
| Diarrhea | 0.75 | 0 | 28 | 0 |
| Dizzinessb | 0.5 | 1 | 28 | 4 |
| Dizzinessb | 0.75 | 1 | 28 | 4 |
| Dysgeusiaa | 0.5 | 1 | 28 | 4 |
| Dysgeusia | 0.75 | 0 | 28 | 0 |
| Fatigueb | 0.5 | 5 | 28 | 18 |
| Fatigueb | 0.75 | 3 | 28 | 11 |
| Headacheb | 0.5 | 1 | 28 | 4 |
| Headacheb | 0.75 | 1 | 28 | 4 |
| Others | 0.5 | 0 | 28 | 0 |
| Others | 0.75 | 0 | 28 | 0 |
aCausality of dysgeusia was probable
bAll other adverse effects were assessed as unlikely to be related to the study drug macimorelin
The most relevant safety concern about macimorelin is QT-prolongation [13]. We noticed no QT prolongation during this study. The dose of 0.5 mg/kg macimorelin did not relevantly change median QTc interval from baseline 405 [288, 411] ms to 412 [356; 422] ms after 120 min. Neither did the higher dose of 0.75 mg/kg macimorelin (QTc-intervalbaseline 396 [391, 414] ms to QTc-interval120 min 403 [391, 421] ms).
Discussion
Our study has the following main findings. First, we found no evidence for a stimulatory effect of macimorelin 0.5 mg/kg or 0.75 mg/kg body weight on copeptin levels in healthy volunteers. Second, we confirm a stimulatory effect of macimorelin on GH levels in healthy volunteers. Third, we observed a stimulatory effect on prolactin and fT4, but not on ACTH nor on gonadotropic hormones.
The differential diagnosis of diabetes insipidus is important, as an incorrect diagnosis could result in an inadequate treatment and lead to serious consequences for the affected patients [2]. Copeptin measurement after hypertonic saline infusion is currently the test with the highest diagnostic accuracy for diabetes insipidus [3]. However, the test procedure is cumbersome as tight sodium monitoring is mandatory and discomfort as well as side effects occur often [1]. Ghrelin is known to potently stimulate vasopressin secretion in vitro [7, 8, 10] and in vivo in rodents [9]. Hexarelin a GHS was found to stimulate vasopressin in humans [15]. And so did arginine, another GHS, which was previously used to diagnose AGHD [16]. Arginine infusion has proven as a non-osmotic stimulation test for patients with diabetes insipidus with a comparable accuracy to the hypertonic saline infusion but a better tolerance [4]. Nevertheless, it still needs an intravenous application, making it an elaborative test procedure. By analogy to its GH-stimulating properties, an oral stimulation test with a single dose of macimorelin would be highly attractive to assess for the possibility of diabetes insipidus.
However, neither the standard dose of macimorelin 0.5 mg/kg used in AGHD testing nor 0.75 mg/kg did increase copeptin levels during the test sequence of 120 min. Copeptin levels were highest at baseline in many participants. For those reaching their maximum of copeptin levels after the intake of macimorelin, the peak was seen around 45 min after intake for the dose of 0.5 mg/kg, which correlated with the peak in GH levels. Our data are in line with a previous study investigating the effect of an acryl ghrelin infusion on thirst and copeptin levels in eight hypopituitary patients [17]. Also in this study no stimulatory effect was seen on vasopressin/copeptin levels.
We can only speculate why macimorelin in the investigated doses does not stimulate copeptin in contrast to arginine. In fact, we postulate a different pathway by which arginine stimulates the pituitary cells in contrast to macimorelin. A lack of effect due to insufficient bioavailability of macimorelin seems less probable for the following reasons. Firstly, because of the above mentioned study of Vestergaard et al. [17] in which also the intravenously administered ghrelin lacked to stimulate copeptin levels in humans. And secondly because macimorelin presented increased oral bioavailability when compared with other GHS [18].
One could argue that the absorption of macimorelin in our study was insufficient and higher doses are needed to successfully stimulate the release of copeptin. We did not measure plasma concentrations of the study drug, but previous studies found a single oral dose of macimorelin to induce a strong dose-dependent increase in GH levels lasting 120 min and peak plasma concentrations of the study drug were reached between 50 and 75 min [18, 19]. As the behaviour of GH stimulation in our study correlates well with former studies, we feel confident that macimorelin has been absorbed efficiently. At the time when this study was designed, we had based our hypothesis on the fact that the greatest GH-increases after macimorelin administration was observed with doses ranging between 0.5 and 1.0 mg/kg as we had considered a dose-response effect of macrimorelin on the GH receptors in the brain and possibly on copeptin secretion. However, our present study refuted that hypothesis that there was a dose–response effect of macimorelin on copeptin secretion. More recently, Klaus et al. [20] reaffirmed the fact that macrimorelin dose range between 0.5 and 1.0 mg/kg provided the best dose range to induce GH secretion and transient increases in ACTH, cortisol, and prolactin levels, which were not macimorelin dose-related. However, whether even higher doses of macimorelin > 0.75 mg/kg are needed to observe increases in copeptin levels remains unclear. The use of supra-physiological doses of macimorelin up to 2 mg/kg body weight has been reported to be safe with no significant adverse events [13, 20] and therefore further evaluation with higher doses might be interesting. We conclude that neither the standard dose of 0.5 mg/kg body weight nor the higher dose of 0.75 mg/kg macimorelin have stimulatory effects on copeptin and therefore cannot be recommended to be used for the differential diagnosis of diabetes insipidus.
Macimorelin acetate effectively stimulates endogenous GH secretion in healthy volunteers [19] and has only recently proven as an accurate, well-tolerated and safe diagnostic test for patients with AGHD [6]. Our data confirm the stimulatory effect of macimorelin on GH levels and are in line with a previously published dose-escalation study in healthy volunteers [19], where doses of 0.05 mg/kg up to 0.5 mg/kg stimulated GH secretion. The maximal change of GH after the dose of 0.5 mg/kg was similar in our study compared to the mentioned study [19]. As reported earlier, we confirm that the stimulatory effect of macimorelin is limited to GH without any stimulation of IGF-1 levels [5]. This is probably explained by the longer half-life of IGF-1 levels compared to half-life of GH levels [21].
We also confirm the high tolerability and safety profile of macimorelin. The study drug was well tolerated and no severe adverse events occurred. A QT-prolongation of about 11 ms has been described during the development of macimorelin [13]. One single participant of a former study [6] had an asymptomatic QT-prolongation. However, in this case there was described a co-medication with citalopram, which is known to cause QT prolongation. We noticed no prolongation of the QT-interval in our study. Our data are therefore reassuring concerning the safety and tolerability profile of macimorelin used in this new diagnostic test for AGHD.
The reference standard for diagnosing ACTH deficiency is the ITT, an elaborate and for some patients an unpleasant and risky test procedure due to the induced hypoglycemia. Therefore, also for adrenal insufficiency, an easier to perform, less risky and orally administered stimulation test would be highly attractive. Besides the already discussed stimulation on GH, GHS also stimulate other anterior pituitary hormones such as prolactin, ACTH and cortisol. Hexarelin as one of the most potent GHS has been found to stimulate the HPA-axis in 15 healthy young males [11]. However, Hexarelin did not prove as a useful test for the evaluation of ACTH/cortisol reserve [15] as it could not consistently discriminate between patients with pituitary insufficiency from healthy controls as it did not stimulate cortisol to a level, which could rule out adrenal insufficiency [15]. The effects of macimorelin on the HPA-axis have been investigated only in few healthy volunteers (n = 9 and n = 6) with doses of 0.5 mg/kg body weight showing inconsistent responses of ACTH and cortisol [19, 20]. In our study including a larger sample size, we observed no increase neither in cortisol nor in ACTH level, but cortisol levels rather decreased following the circadian rhythm. Macimorelin in the investigated doses therefore cannot be recommended as a test for diagnosing adrenal insufficiency.
In contrast, macimorelin stimulated prolactin levels with an increase with peak values around 45 min, correlating with GH-peaks. This is in agreement with previous studies [11].
GHS-receptors are also found in the thyroid gland [22]. In rats, thyroxine (T4) and TSH levels decreased upon central injection of ghrelin [23, 24]. In healthy volunteers, no effect of macimorelin was found on TSH levels [20]. Interestingly and in contrast to this study, we observed a significant decrease in TSH levels, consistent with the findings in animal studies, and vice versa an increase in fT4 levels, thus leading to a constellation towards “hyperthyroidism”. To the best of our knowledge, this has not yet been described and is a novel and physiopathologically interesting finding. No stimulatory effect was found on LH and FSH levels.
Our study has several strengths and limitations. Strengths are that it is the first study investigating the effects of macimorelin on posterior pituitary hormones, particularly copeptin/vasopressin. Furthermore, we investigated our hypothesis with a concise study design, sample size consideration and sound methodology. As a limitation, we did not use a placebo controlled study design, as this was a proof-of concept study. However, by confirming the results in GH stimulation, we have a positive control and are confident that our results can be considered as valid and reliable. A further limitation is that IGF-1 levels were measured by LIAISON XL, as it is known that this assay can be affected by IGF-binding proteins.
To conclude, we herein confirm the potent stimulatory effect of macimorelin on GH release in healthy volunteers. However, macimorelin did not stimulate copeptin levels and therefore cannnot be used as a simple oral test for the differential diagnosis of diabetes insipidus. Additionally, macimorelin also exerted a stimulatory effect on prolactin and thyroid hormones, but not for cortisol, ACTH or gonadotropic hormones.
Acknowledgements
We thank all healthy volunteers for their participation in the study, our study nurses Cemile Bathelt, Joyce Santos de Jesus and Nina Hutter for excellent support and thank Aeterna Zentaris GmbH Frankfurt for donating the IMP macimorelin.
Author contributions
All authors contributed to the study conception. SU and SL recruited the study participants. Statistical analysis were performed by DV. SU, SL, JD, BS, DV, NA, KY, ARO and MCC were involved in the interpretation of the data and in writing the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Universität Basel (Universitätsbibliothek Basel). The study was funded by a Grant of the Swiss National Foundation 320030_182753 to MCC. SU received a grant from the “Schweizerische Gesellschaft für Endokrinologie und Diabetologie”. Aeterna Zentaris provided the IMP macimorelin but was not involved in the study design nor analysis of the data. There was no other commercial support for this study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
Nicola Ammer is an employee of Aeterna Zentaris GmbH Frankfurt and Antonio Ribeiro-Oliveira an employee of Ipsen Bioscience in Cambridge/Massachusetts. All other authors have nothing to disclose in relation to this work.
Ethical approval
This study was conducted in compliance with the ethical principles based on the Declaration of Helsinki and the applicable International Conference on Harmonization (ICH) guidelines on good clinical practice. Local ethic committees and Swissmedic approved the study protocol. Written informed consent was obtained from all study participants before inclusion. The study was preregistered on ClinicalTrials.gov (NCT03844217).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Christ-Crain M. Diabetes insipidus—new concepts for diagnosis. Neuroendocrinology. 2020;110(9–10):859–867. doi: 10.1159/000505548. [DOI] [PubMed] [Google Scholar]
- 2.Christ-Crain M, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54. doi: 10.1038/s41572-019-0103-2. [DOI] [PubMed] [Google Scholar]
- 3.Fenske W, et al. A copeptin-based approach in the diagnosis of diabetes insipidus. N Engl J Med. 2018;379(5):428–439. doi: 10.1056/NEJMoa1803760. [DOI] [PubMed] [Google Scholar]
- 4.Winzeler B, et al. Arginine-stimulated copeptin measurements in the differential diagnosis of diabetes insipidus: a prospective diagnostic study. Lancet. 2019;394(10198):587–595. doi: 10.1016/S0140-6736(19)31255-3. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JM, et al. Macimorelin (AEZS-130)-stimulated growth hormone (GH) test: validation of a novel oral stimulation test for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2013;98(6):2422–2429. doi: 10.1210/jc.2013-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia JM, et al. Macimorelin as a diagnostic test for adult GH deficiency. J Clin Endocrinol Metab. 2018;103(8):3083–3093. doi: 10.1210/jc.2018-00665. [DOI] [PubMed] [Google Scholar]
- 7.Korbonits M, et al. The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol. 1999;11(7):521–528. doi: 10.1046/j.1365-2826.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 8.Nemoto T, et al. The effects of ghrelin/GHSs on AVP mRNA expression and release in cultured hypothalamic cells in rats. Peptides. 2011;32(6):1281–1288. doi: 10.1016/j.peptides.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaki S, et al. Role of ghrelin in the regulation of vasopressin release in conscious rats. Endocrinology. 2002;143(5):1589–1593. doi: 10.1210/endo.143.5.8804. [DOI] [PubMed] [Google Scholar]
- 10.Galfi M, et al. Ghrelin-induced enhancement of vasopressin and oxytocin secretion in rat neurohypophyseal cell cultures. J Mol Neurosci. 2016;60(4):525–530. doi: 10.1007/s12031-016-0850-4. [DOI] [PubMed] [Google Scholar]
- 11.Korbonits M, et al. The growth hormone secretagogue hexarelin stimulates the hypothalamo-pituitary-adrenal axis via arginine vasopressin. J Clin Endocrinol Metab. 1999;84(7):2489–2495. doi: 10.1210/jcem.84.7.5811. [DOI] [PubMed] [Google Scholar]
- 12.Bancos I, et al. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216–226. doi: 10.1016/S2213-8587(14)70142-1. [DOI] [PubMed] [Google Scholar]
- 13.Aeterna Zentaris (2019) Investigator’s Brochure Macimorelin (AEZS-130) Edition no. 7. Charleston, SC (USA), Frankfurt am Main (Germany)
- 14.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond "p < 0.005". Am Stat. 2019;73(Suppl 1):1–19. doi: 10.1080/00031305.2019.1583913. [DOI] [Google Scholar]
- 15.Korbonits M, et al. Hexarelin as a test of pituitary reserve in patients with pituitary disease. Clin Endocrinol. 1999;51(3):369–375. doi: 10.1046/j.1365-2265.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- 16.Growth Hormone Research S Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990–3993. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 17.Vestergaard ET, et al. Acute intravenous acyl ghrelin infusion induces thirst but does not affect sodium excretion: two randomized, double-blind, placebo-controlled crossover studies in hypopituitary patients. Eur J Endocrinol. 2019;181(1):23–30. doi: 10.1530/EJE-19-0027. [DOI] [PubMed] [Google Scholar]
- 18.Broglio F, et al. EP1572: a novel peptido-mimetic GH secretagogue with potent and selective GH-releasing activity in man. J Endocrinol Invest. 2002;25(8):RC26–RC28. doi: 10.1007/BF03345096. [DOI] [PubMed] [Google Scholar]
- 19.Piccoli F, et al. Pharmacokinetics and pharmacodynamic effects of an oral ghrelin agonist in healthy subjects. J Clin Endocrinol Metab. 2007;92(5):1814–1820. doi: 10.1210/jc.2006-2160. [DOI] [PubMed] [Google Scholar]
- 20.Klaus B, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macimorelin in healthy adults: results of a single-dose, randomized controlled study. Growth Horm IGF Res. 2020;52:101321. doi: 10.1016/j.ghir.2020.101321. [DOI] [PubMed] [Google Scholar]
- 21.Peacey SR, Shalet SM. Insulin-like growth factor 1 measurement in diagnosis and management of acromegaly. Ann Clin Biochem. 2001;38(Pt 4):297–303. doi: 10.1258/0004563011900678. [DOI] [PubMed] [Google Scholar]
- 22.Poher AL, Tschop MH, Muller TD. Ghrelin regulation of glucose metabolism. Peptides. 2018;100:236–242. doi: 10.1016/j.peptides.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kordi F, Khazali H. The effect of ghrelin and estradiol on mean concentration of thyroid hormones. Int J Endocrinol Metab. 2015;13(1):e17988. doi: 10.5812/ijem.17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoudi F, et al. The effect of central injection of ghrelin and bombesin on mean plasma thyroid hormones concentration. Iran J Pharm Res. 2011;10(3):627–632. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.