Abstract
The restoration of normal functioning of damaged body tissues is one of the major objectives of tissue engineering. Scaffolds are generally used as artificial supports and as substrates for regenerating new tissues and should closely mimic natural extracellular matrix (ECM). The materials used for fabricating scaffolds must be biocompatible, non-cytotoxic and bioabsorbable/biodegradable. For this application, specifically biopolymers such as PLA, PGA, PTMC, PCL etc. satisfying the above criteria are promising materials. Poly(ε-caprolactone) (PCL) is one such potential candidate which can be blended with other materials forming blends, copolymers and composites with the essential physiochemical and mechanical properties as per the requirement. Nanofibrous scaffolds are fabricated by various techniques such as template synthesis, fiber drawing, phase separation, self-assembly, electrospinning etc. Among which electrospinning is the most popular and versatile technique. It is a clean, simple, tunable and viable technique for fabrication of polymer-based nanofibrous scaffolds. The design and fabrication of electrospun nanofibrous scaffolds are of intense research interest over the recent years. These scaffolds offer a unique architecture at nano-scale with desired porosity for selective movement of small molecules and form a suitable three-dimensional matrix similar to ECM. This review focuses on PCL synthesis, modifications, properties and scaffold fabrication techniques aiming at the targeted tissue engineering applications.
Keywords: Poly(ε-caprolactone), Electrospinning, Scaffolds, Nanofibrous substrate, Biocompatible, Tissue engineering
Introduction
Tissue engineering (TE) has emerged as a promising method in the field of regenerative medicine, which utilizes a three-dimensional (3D) artificial constructs called scaffolds for restoring the function of damaged tissues or organ. The scaffolds and cells are interconnected which attributes to transmission of biochemical signals for repairing the fully/partially damaged tissues during the tissue regeneration process. The imperative parameters which form the core of a scaffold preparation are cell type to be repaired, material used for scaffold and the growth factors to be combined which in turn effectively promotes the infiltration, adherence, migration and differentiation of cells on the surface of a scaffold (Roy et al. 2018; Norman and Desai 2005).
Recently, polymeric scaffolds are of research interest due to their ease of fabrication, feasibility in property modification, satisfactory formability and controlled biodegradability. Among various materials available such as synthetic, natural, biodegradable or non-biodegradable polymers, the preferred choice of material for scaffold depends on the targeted applications. Biodegradable polymers are of prime concern in the field of TE and few such polymers include polyesters such as poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL), poly (glycolic acid) (PGA) and poly(trimethylene carbonate) (PTMC). The composition, structure and arrangement of their constituents decide the final properties of the polymer. The properties such as chain length, crystallinity and extent of branching significantly influences the degradability of biodegradable polymers. The degradation behavior of a material used for scaffold fabrication has a pivotal role during tissue regeneration process. The rate of material degradation and tissue regeneration must be at the same pace because if the scaffold material degrades faster the connection between the cells/tissues will be lost, therefore, the healing process is retarded. On the other hand, the growth of tissues will be restricted if the material exhibits slow degradation rate (Dhandayuthapani et al. 2011; Malikmammadov et al. 2018).
The appropriate selection of material to function as an effective scaffold draws great attention towards synthetic biodegradable polymers, because the desired properties can be tailor made as per the applications. Amongst the widespread synthetic and biocompatible polymeric materials, poly(ε-caprolactone) is highly preferred due to its FDA approval and widespread usage as an implantable biomaterial in various biomedical applications. The characteristic features of PCL include cytocompatibility, biodegradability, non-toxicity, reasonable mechanical strength and tunable hydrophobicity. The surface properties of PCL can be modified to suit the TE applications by adapting suitable surface modification and blending strategies (Nazeer et al. 2019; Franca et al. 2016; Mondal et al. 2016). These modifications create active sites at the surface making PCL moderately hydrophilic in nature and thus improves cell affinity and cell/material interactions.
There are numerous techniques available for fabrication of scaffolds for TE applications. In contemporary period, practical application of nanofibers has been rising substantially due to their ability to meet demanding requirements for a specialized application. The design and fabrication of scaffold with all the required set of physiochemical properties is of immense concern and a great challenge in TE application. Tissue engineering scaffolds must manifest biocompatibility, desired topography, roughness and porosity. The desired pore size, shape and interconnection between the pores are essential morphological features which need to be controlled during scaffold fabrication (Ghasemi-Mobarakeh et al. 2008a, b; Cipitria et al. 2011). Electrospun scaffolds seem to accomplish the above traits in most of the applications (Rocco et al. 2014; Tian et al. 2012).
Electrospinning is one of the versatile and comparatively less expensive fabrication techniques to prepare nanofibrous scaffolds (Choi et al. 2008). The most fascinating properties of electrospun nanofibers over others include fibrillar morphology resembling the native ECM in the body, identical fiber diameter, ordered microstructure with high specific surface area, high porosity and varying topography/architecture that provides a favorable environment for cell adhesion and tissue growth (Cipitria et al. 2011). The microporous structure of nanofibers allow easy nutrient transport thereby providing a suitable environment for cell/cell and cell/material interactions while high surface area enables better cell adhesion (Cipitria et al. 2011; Abedalwafa et al. 2013; da Silva et al. 2010). Electrospinning is widely used because of its simplicity and numerous patterns of nanofibers that can be produced continuously. The authors group has extensively worked on electrospinning process for polymeric materials (Panda and Sahoo 1990, 2019; Naragund and Panda 2018) and now exploring the possibility of the same technique for scaffold preparation.
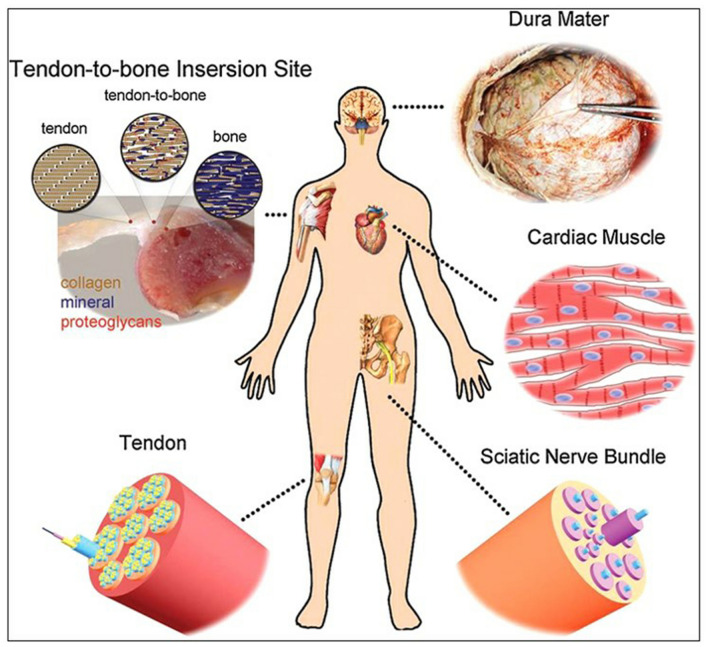
The TE scaffolds should contribute structural support for overall cellular activities under various environments. The distinct tissues such as heart, nerve and blood vessels in the human body exhibits particular ECM and anisotropic fibrous structures while skin and bone tissues are found to be more porous than other connective tissues. Hence, the design and development of a scaffold with appropriate geometry and desirable properties are a crucial factor for successful reconstruction of tissues (Jun et al. 2018). The selection of biomaterial and scaffold fabrication technique depends on the predetermined TE applications and should not have any antagonistic effect on native bioactivity of host cell. It is essential to construct a substrate that closely biomimic the natural tissues to restore the normal function of damaged tissues such as skin, bone, cartilage, vascular grafts etc. (Wang et al. 2013). The schematic representation of few types of tissues that can be regenerated in the human body by application of electrospun nanofibrous scaffolds is shown in Fig. 1. The scaffold should be designed and fabricated so that its surface structure and topography offer an appropriate binding site for cells (Gentile et al. 2017; Bhullar et al. 2017). The mechanical, physicochemical and biological properties are crucial for selecting scaffold materials in the field of TE. This review intends to summarize the recent research trends with respect to electrospun PCL-based scaffolds for various TE applications.
Fig. 1.
Application of electrospun scaffolds for tissue regeneration at various parts of human body. With permission Wang et al. (2013)
Synthesis of PCL
Polycaprolactone is a class of biodegradable aliphatic polyesters. It is a semi-crystalline polymer with high solubility at room temperature and it can be easily processed due to the low melting temperature and exceptional blend compatibility. Owing to its native biocompatibility and biodegradability, it is widely used as a synthetic biomaterial in various biomedical applications. Various methods have been adopted and reported in the literature to effectively synthesize PCL which yields better properties at affordable cost. Natta et al. (1934) were the first to report the thermal treatment of ε-caprolactone to synthesize PCL. The monomer ε-caprolactone is primarily subjected to ionic (both anionic and cationic) and metal catalyzed ring-opening polymerization (ROP) reactions to yield PCL. There are also few reported works focusing on the radical ring opening polymerization of 2–methylene–1,3–dioxepane (Won et al. 2008; Agarwal 2010) and on the condensation of 6–hydroxycaproic acid. Bailey et al. (1982), furthermore, suggested radical ring opening polymerization of cyclic ketene acetals as a synthetic route for PCL production. The enzymatic assisted synthesis using Candida Antarctica Lipase B immobilized on acrylic resin is also been reported (Mahapatro et al. 2004). After 20 days of reaction using lipase from Pseudomonas sp. at 45 °C ethyl 6–hydroxyhexanoate was polymerized with 82% yield and an average molecular weight of 5400 g/mol and a polydispersity index less than 2.26 (Dong et al. 1998).
ROP method gives a polymer with a higher molecular weight and a lower polydispersity index, as a result it is selected over other routes for PCL synthesis. The ROP method involves coordination/insertion mechanism for synthesizing PCL using metal complexes. Several transition metal catalysts based on zirconium, zinc, titanium and rare earth metals are reported. In particular, aluminum (III) isopropoxide and tin (II) 2–ethylhexanoate (Duda 1996) are widely used as catalysts. This method results in low polydispersity (close to 1.1) and very high molecular weight (up to 800,000 g/mol) of PCL. The physical, mechanical, thermal properties and duration of biodegradation depends on molecular weight, polydispersity index and degree of crystallinity. Hence, control of these parameters during the synthesis of PCL is crucial for many of the specific practical applications.
Davidson et al. (2006) reported the use of titanium complexes based on catechol ligands in ROP, polymer synthesized by this method showed narrow polydispersity index suggesting controlled polymerization. Braud et al. (1998) synthesized PCL oligomers without the addition of catalyst by polycondensation of 6–hydroxyhexanoic acid under vacuum removing the water formed during the reaction and pushing the equilibrium towards the formation of the polymer. The reaction could be completed in 6 h at a temperature that was gradually raised from 80 to 150 °C. Mingotaud et al. (1999) polymerized ε-caprolactone with different catalysts in organic solvents and in supercritical carbon dioxide. Rare earth metal-based catalysts are widely used due to their moderate acidity and non-toxicity. Scandium triflates with water and benzyl alcohol (initiators) were used to catalyze the ROP of ε-caprolactone. High molecular weight (Mn of 25,000 g/mol) polymer with low polydispersity (1.18) was obtained (Nomura et al. 2000). Figure 2 shows different methods of PCL synthesis. Besides synthesis of PCL homopolymers, research has been focused on the synthesis of several classes of copolymers, blends and composites to tailor the material properties for specific applications, including TE and drug delivery. The very next part of the review focuses on such modification aspects of PCL.
Fig. 2.
Different synthesis routes of PCL
Modification of PCL
PCL has provided an ample opportunity to explore beyond conventional materials and stands out to be the popular material for TE and drug delivery in recent years. PCL is widely used in its modified form with targeted features rather than in its pristine form to overcome the inherent limitations like high hydrophobicity, low cell adhesion, much slower degradation (extending from few months to years) which affects tissue replacement in the case of scaffolds, mechanical property limiting its application only to hard tissue engineering. The hydrophobic nature of PCL restricts cellular activities by virtue of which the tissue growth will be delayed on such surfaces. PCL backbone modifications by introduction of new functional groups have major implications on degradation behavior and reactivity. In the form of blends, copolymers, nanocomposites and as functionalized formulations PCL has significantly higher utility in comparison to its native form.
PCL-based composites
Incorporation of nanoparticles into the PCL matrix can virtually alter all the inherent properties of PCL and can broadly expand biomedical utility of the resultant PCL nanocomposites. The composite fibers give bead-free, porous structure to the nanofibrous mat than neat PCL during electrospinning process. There are various studies focusing on such PCL-based nanocomposites for TE and drug delivery applications. Substrates for TE applications require a highly porous and interconnected fibrous structure to ensure a conducive biological environment for cell attachment and proliferation as well as for tissue regrowth and nutrients flow. Nanofibrous scaffolds need to maintain their mechanical and structural integrity during in vitro and in vivo cell growth and tissue remodeling (Barnes et al. 2007). It is preferable to have a material with the elastic modulus close to that of target tissue to avoid any possible stress shielding effect. The need to synthesize such a scaffold material for wound healing, bone regeneration, drug delivery and regenerative medicine is highly in demand.
Rijal et al. (2018) prepared PCL/MgO and PCL/chitosan (CS)/MgO-based composite nanofibrous membranes through electrospinning process. The SEM micrographs showed that the pure PCL nanofibers were smooth and bead-free while PCL/MgO nanofibers exhibited rough surface. The presence of MgO in the nanofibers was confirmed by TEM images, which revealed that MgO nanoparticles were encapsulated within PCL nanofibers. From the tensile testing, it was observed that the ultimate tensile strength of pure PCL is 2.8 MPa while that of PCL/CS is 3.3 MPa, respectively. It was noticed that the young’s modulus increased with the increase in MgO concentration. When CS was added to pure PCL there was a decrease in the modulus from 21.6 to 6.8 MPa and there was no major change in the young’s modulus value with addition of MgO to PCL/CS blend. The average modulus values are comparable to the modulus of animal tissues such as human articular cartilage (1–10 MPa), bovine articular cartilage (2–7 MPa) (Silver et al. 2002) artery and vein (0.6–3.5 MPa), liver and kidney tissues (1–15 MPa) (Kim et al. 2012). The magnesium ions in the scaffolds were not toxic to the cells hence it is a suitable substrate for cell attachment (reported 75% of cell viability). The developed nanofibrous membranes in this study have the ability to mimic the physical structure and function of tissue ECM and thus have potential applications for many TE areas.
Sheng et al. (2014) reported studies on electrospun nanocomposite fiber mats of PCL with cellulose nanocrystals (CNCs), wherein the effect of CNC on surface hydrophilicity, thermal stability and mechanical properties of PCL has been studied. The hydrolysis of CNCs with sulfuric acid resulted in the deposition of SO32− anions on the surface of CNC as a result electrostatic charge density was increased during electrospinning leading to the formation of thin nanofibers due to extensive stretching of jet. The mechanical tests revealed that modulus and tensile strength of nanocomposite were improved due to reinforcing effect of CNC (to 10% loading) when compared to neat PCL mats. Since CNC is hydrophilic in nature the contact angle measurements of nanocomposites revealed that the surface wettability/hydrophilicity enhanced with the inclusion of CNC into PCL matrix. Sumitha et al. (2012) synthesized PCL/silver nanoparticles (AgNPs) composite nanofibers to study the effect of Ag NPs on the biological properties of scaffolds suitable for skin and bone TE applications. Addition of AgNPs could give antimicrobial property to the scaffold without compromising on the cell adhesion behavior. Neat PCL mats had fiber diameter in the range of 280–350 nm but PCL-Ag composite nanofibers showed a reduction in the fiber diameter (220–260 nm) with increasing AgNPs concentration. This reduction in the fiber diameter was due to the presence of AgNO3 salt in the electrospinning solution by virtue of which surface charge density of polymer jet increases which in turn leads to stretching of fibers. The antibacterial studies against S. aureus for pure PCL showed zero zone inhibition; whereas, composite scaffolds exhibited increase in inhibition zone with increase in AgNPs concentration. The cytotoxicity studies showed that both pure PCL and nano Ag-loaded composite as non-toxic. It was noticed from the cell culture studies that 0.2 mM nano Ag-loaded scaffolds possessed higher cell viability (98%) but further increase in concentration of nano Ag can become superficially toxic to stem cells.
An electrospun PCL/hydroxyapatite (HA) nanocomposite substrate with better cell adhesion and differentiation aiming at bone TE applications was reported by Li et al. (2018). The inclusion of HA into PCL matrix did not alter the pore size and contact angle of the resulting mats. The pure PCL mats exhibited uniform, continuous and smooth surface but the addition of HA nanoparticles yielded rough surface on electrospun nanofibers. The nanocomposites possessed higher elongation when compared to pure PCL. The higher HA content in the nanofibers led to more selective cell adherence site providing higher surface area and roughness which promotes enhanced rate of cell proliferation when compared to pure PCL. In another study by Chong et al. (2015), PCL/nano HA composites for TE applications, the addition of nHA resulted in bead-less fibers without agglomerates but thicker than those of neat PCL nanofibers.
Dutta et al. (2019) reported PCL/cellulose nanocrystals (CNC) composites for bone tissue engineering application with enhanced osteogenesis. Wherein the hydrophobic nature of PCL was addressed using CNC extracted from rice husk biomass. In the study, the synergistic effect of PCL/CNC composites in terms of cell viability, mechanical strength and osteogenesis induction was evaluated. A significant enhancement in mechanical properties was reported for the fabricated scaffolds indicating the strong interactions between PCL and CNCs. In addition, the cell viability was higher in the presence of nanofibers indicating their non-cytotoxicity and biocompatibility.
Zadehnajar et al. (2020a, b) studied the cell response characteristics of MWCNTs incorporated PCL/gelatin nanocomposite scaffolds. The presence of MWCNTs in the solution improved fiber stretching during electrospinning by virtue of which fiber diameter was reduced. The surface wettability of PCL/gelatin scaffolds was also improved upon the addition of MWCNTs. The nanocomposites possessed higher tensile strength when compared to blend nanofibers, this was attributed to the orientation of CNTs in the direction of fiber during processing. Both the blend and nanocomposite scaffolds offer a suitable support for cell adhesion but enhanced cell proliferation was reported in nanocomposites. The improved cell response in nanocomposites was due to more adequate distribution of fiber diameter and surface wettability mimicking the natural ECM. The cell viability studies depicted that nanocomposites offer a suitable cell recognition site for adherence and proliferation and hence enhanced viability than blended nanofibers. Sara et al. (2016) studied electrospun PCL/layered double hydroxide (LDH) nanocomposite scaffolds for adipogenic differentiation of adipose-derived mesenchymal stem cells. The PCL nanofibers were found to be uniform and bead-free but after incorporating LDH into PCL matrix there was a reduction in average fiber diameter of composite nanofibers. It was noticed that LDH addition resulted in the improvement of tensile strength, elongation-at-break, hydrophilicity and rate of degradation. Thus, the nanocomposite scaffolds with high porosity (94%) encouraged cell proliferation and adhesion thereby serving as an appropriate substrate for soft tissue engineering application. PCL/nano diamond (ND)-based nanocomposite fibers for wound dressing were prepared and characterized for assessing the cytocompatibility by Houshyar et al. (2019). The resultant scaffold showed excellent cellular activities without any cytotoxicity effect. The nanocomposites exhibited significantly less affinity to Staphylococcus aureus bacteria with increasing ND concentration. The outcomes represented that scaffolds likely offer desired properties for wound healing applications thus enhancing epithelial cell proliferation and limiting the microbial growth on nanofibrous substrate. Thus, PCL-based composites offer many advantages than neat PCL for TE applications in terms of fabrication, desired property achievement and end use/service requirements.
PCL-based blends and copolymers
Broad spectrum of PCL compatibility with a wide range of other polymers makes it a better candidate to be modified by blending. Natural biocompatible and biodegradable polymer such as gelatin can be blended into PCL to achieve combined benefit of both the polymer, overcoming their individual limitations. Cellular affinity, mechanical property and degradation rate can be tailor-made by such blending. Kim et al. (2010) reported the mechanical properties and cell proliferation behavior of PCL and cross-linked PCL/gelatin blend nanofibers. The PCL/gelatin nanofibers crosslinked with genipin exhibited better structural integrity compared to non-crosslinked nanofibers. With the increase in gelatin concentration hydrophilicity and the fiber diameter increased; whereas, with the increase in genipin concentration the extent of crosslinking increased in the nanofibers. The neat PCL nanofibers showed lesser modulus and tensile strength when compared to PCL/gelatin and cross-linked nanofibers. It was claimed that PCL/gelatin nanofibers maintain the essential mechanical properties and can function as a suitable scaffold material for muscle TE applications.
Adeli-sardou et al. (2019) evaluated the role of PCL/gelatin blend electrospun nanofibers containing lawsone (2–hydroxy–1,4–naphthoquinone) for skin tissue regeneration. The prepared scaffolds showed increased cell attachment and proliferation along with prolonged release of lawsone over a period of 20 days. A 1% lawsone-loaded scaffold displayed an in-vivo highest impact on wound healing activity by increasing re-epithelialization of the wound. These electrospun nanofibers have exceptional features to act as wound dressing patch. The scaffold with high surface area/volume ratio is desired to acquire better cell proliferation, adhesion, growth, migration and differentiation. Nanofibrous scaffolds with interconnected pores can provide such environment and hence make them excellent choice for TE applications. Ebrahimi et al. (2014) prepared electrospun PCL homopolymer and PCL-PEG-PCL triblock copolymer-based nanofibers for TE applications. PEG copolymerization with PCL renders hydrophilicity, biocompatibility, non-toxicity, non-antigenic and non-immunogenic characteristics. Such properties enhance resistance to bacterial cell adhesion and reduce protein adsorption. With increases in molecular weight of PEG the fiber diameter decreased but bead size increased. The copolymer-based nanofibers showed smooth fiber formation with low molecular weight PEG. It is evident that polymer composition plays a significant role in designing the fiber morphology for TE applications.
Electrospun PCL/gelatin blend nanofibrous scaffold for TE applications were reported by Gautam et al. (2014). It was noticed that the concentration of both PCL and gelatin influenced the fiber diameter and morphology. Smooth, uniform and bead-free nanofibers were obtained with less gelatin content in the electrospinning solution. With the increase in gelatin concentration, more beads were seen in the nanofibrous mats. The increase in fiber diameter from 291 to 1173 nm was attributed to the decrease in gelatin content. Since gelatin influences the surface charge density of polymer jet during electrospinning the progressive changes in the fiber diameter and morphology was observed. The MTT assay confirmed the biocompatibility and non-cytotoxic nature of the scaffold materials. The cell culture studies showed that the prepared scaffolds were adequate substrates for cell adhesion and proliferation when compared to neat PCL scaffolds. The cell morphology studies of PCL/gelatin scaffolds showed that after 24 h of cell seeding, a small round-shaped cell which was adhered started to stretch after 48 h and was spindle shape by the end of 72 h as shown in Fig. 3. Thus, the modified form of PCL is a promising material for TE applications.
Fig. 3.
PCL/gelatin scaffolds showing L929 fibroblast cell seeding after 24 h (a), (b) and (c) at × 500, × 2500 and × 5000 magnification. With permission Gautam et al. (2014)
In TE, development of closely biomimetic nanofibrous scaffolds is a major technological challenge. Mahoney et al. (2016) developed electrospun nanofibers of PCL/depolymerized chitosan blend for use as a scaffold in regenerating tracheal tissue. The SEM studies revealed that adding chitosan into PCL matrix resulted in reduced fiber diameter. The ultimate tensile strength remained unaffected and it was very similar to pure PCL. No significant difference between cytotoxicity levels was observed between blend and pristine PCL scaffolds. Consequently, the prepared nanofibrous scaffolds were considered safe, with sufficient structural integrity and which may serve as a probable candidate for tracheobronchial TE. The electrospun PCL/zein/gum arabic blend scaffold for skin TE was reported by Rad et al. (2018). Gum arabic and zein polymers were used as a polysaccharide and protein component of the scaffold and PCL polymer for strength, elasticity and time setting of scaffold degradability. The presence of gum arabic increased the hydrophilicity, cell viability and offered antibacterial property to the scaffolds. Porosity in scaffolds was more than 80% which was desirable for cell infiltration. Tensile strength of 1.4–3.0 MPa and an elongation of 19.1–44.1%, anticipated for skin TE application, were claimed in the study.
Elastin and collagen are the two most abundant proteins present in the human body, and with uses as biomaterials offer fascinating properties to the scaffolds. The optimal combination of PCL, collagen and elastin allows tunable mechanical and physicochemical properties. Aguirre-Chagala et al. (2017) fabricated PCL/collagen/elastin nanofibers by electrospinning which were considered useful as potential material for scaffolds in TE applications. Kim and Kim (2018) fabricated a novel nano topographical patterned fibrous scaffold based on PCL/collagen blend that can be used as multifunctional biomaterial for rapid wound healing. The pore size and porosity of the scaffold was tailored based on the required biomedical application. The scaffold with specific nanopatterns showed superior biocompatibility both in-vivo and in-vitro. It was found that nanofibrous scaffolds that were structurally/morphologically very similar to natural ECM could be in demand for TE applications. Yao et al. (2017) developed a porous 3D nanofibrous PCL/PLA-based blend scaffolds with smooth surface morphology for osteogenic proliferation. From the mechanical testing it was noticed that pure PCL nanofibers showed higher strength in wet conditions than in dry condition; whereas, the stress–strain behavior of PCL/PLA blend nanofibers was identical under wet and dry conditions. The cell culture studies revealed that the cell viability of both pure and blend scaffolds was very similar; whereas, cell growth was enhanced in blend scaffolds due to PLA content in the composition. Both pure and blend 3D scaffolds were good substrate for cell attachment and there were no major changes in the morphology of the adhered cells. The variation of PLA content in the blend did not affect the hydrophobicity of scaffolds.
Plasma-treated PCL
Plasma treatment is a convenient technique to modify the specific surface properties such as surface wettability, surface chemistry and biofunctionality without affecting bulk properties of the biomaterials. The cell-material interaction would be improved by plasma treatment due to the concentration of polar groups on the surface. Once the polymer surface is subjected to plasma treatment, surface energy of the polymer is enhanced. When PCL-based nanofibrous scaffolds are activated by plasma, the active species like ions, electrons and free radicals bombard the exposed surface causing bond scission of the polymeric backbone, by virtue of which free radicals are formed on the scaffold surface. These free radicals further facilitate grafting and cross-linking reactions. Thus, the polymer surface with enriched polar groups and improved hydrophilicity promotes cell adherence and cell/material interaction. The functional groups added onto the scaffold’s surface by plasma treatment primarily depends on the type of working gas used viz., air, O2, N2, Ar, He or NH3. The parameter like plasma exposure time and power greatly affects the degree of wettability on the scaffold surface which is a prime requirement of TE substrates (Asadian et al. 2020; Ghobeira et al. 2017).
Several research groups have studied the effect of plasma treatment on electrospun PCL scaffolds. Safaeijavan et al. (2014) modified the surface of electrospun PCL using air plasma technique. Wherein surface modification introduced carboxyl groups onto PCL surface and then gelatin was covalently grafted onto the scaffold surface to create cell recognition sites and the resulting substrate was more suitable for fibroblast cell proliferation when compared to pure PCL scaffolds. The contact angle was 132° and 0° for neat PCL and surface-treated PCL scaffolds, respectively. Likewise, Atyabi et al. (2016) compared the effect of non-thermal (oxygen) plasma and helium cold plasma surface modification techniques on the biological properties of PCL nanofibers. The SEM images confirmed that the fibre diameter of electrospun mats were unchanged even after plasma treatment. The cold plasma treatment added CH functional groups on the surface of neat PCL and hence the variation in the wettability of non-thermal and cold plasma-treated PCL mats was observed. The contact angle was 122° > 20° > 0° for PCL > cold atmospheric plasma-treated PCL > non-thermal plasma-treated PCL. The cell culture studies revealed that cold plasma-modified PCL nanofibers served as an appropriate substrate for fibroblast cell differentiation when compared to other two scaffolds.
In another study, the electrospun PCL scaffolds were subjected to low pressure RF discharge plasma (acrylic acid and oxygen) treatment. The plasma-treated scaffolds exhibited improved surface wettability and higher oxygen content when compared to untreated PCL scaffolds. The cells proliferation was better on low pressure RF discharge plasma-modified PCL scaffolds in comparison with pure PCL scaffolds (Ko et al. 2015). Furthermore, Asadian et al. (2017) reported the effect of pre and post electrospinning plasma treatments on morphology and surface hydrophilicity of electrospun PCL nanofibers. The morphological study revealed that untreated electrospun mats had dense beads while bead-free and smooth mats were obtained when PCL solution was treated for 5 min with atmospheric pressure plasma jet (APPJ) prior to electrospinning. The contact angle of nanofibrous scaffolds decreased in the following order 55° < 64° < 95° < 127° for combined plasma-treated, dielectric barrier discharge (DBD) plasma-treated after electrospinning, APPJ treated before electrospinning and untreated scaffolds, respectively. The double plasma-treated PCL nanofibers showed improved hydrophilicity and even spreading of cells on scaffolds when compared to APPJ alone and DBD alone modified scaffolds.
Shafei et al. (2017) synthesized electrically conductive PCL scaffolds for TE applications. SEM images revealed that there was no significant difference in the fibre diameter of neat PCL, polypyrrole (PPy)-coated PCL and oxygen plasma-treated PCL scaffolds. The higher oxygen content on plasma-treated scaffolds was confirmed by XPS studies and this clearly represents the presence of polar groups on the surface of plasma-treated PCL scaffolds. Contact angle measurement of 103°, 135° and 0° for neat PCL, PPy-coated PCL and plasma-treated PCL scaffolds suggest improvement of hydrophilicity. The PPy-coated PCL was subjected to oxygen plasma treatment to make scaffolds hydrophilic and the cell culture studies revealed that the scaffolds were non-toxic and plasma-treated scaffolds resulted in a greater number of fibroblast cell adherence and differentiation when compared to untreated scaffolds.
Yan et al. (2013) described the influence of NH3 and O2 plasma treatment on the surface characteristics of aligned and randomly oriented PCL nanofibrous mats. The NH3 and O2 plasma treatment introduced both nitrogen and oxygen containing functional groups on the scaffold surface. The existence of polar groups was well-established by XPS analysis and results revealed that oxygen content increased from 9 to 18% for plasma-treated randomly oriented PCL mats and meshes. Plasma-treated electrospun mats displayed the contact angle of 0° indicating improved surface hydrophilicity. The osteoblast cell proliferation was improved in the direction of fibre orientation for plasma-treated mats. In conclusion, a thorough understanding of the PCL and its modification has a vast potential to be claimed as the ultimate biomaterial for TE applications.
PCL-reinforced hydrogels
Usage of hydrogels for tissue engineering scaffolds arose due to their close resemblance to natural ECM. Hydrogels can absorb large amounts of water, improving biocompatibility over bulk polymers and can provide a suitable porous environment through which cells can efficiently migrate and proliferate. However, poor mechanical properties can impede their direct application. To overcome this drawback meshes of nanofibers could be embedded in their matrix to form a biomimetic composite structure (Butcher et al. 2014). PCL is chosen as the fiber material in most cases due to its biological inertness, strong mechanical response and potential to integrate biofunctional motifs in structure to fine-tune the cell behaviour.
Strange et al. (2014) reported fabrication of three-dimensional PCL electrospun fibre-hydrogel composites for intervertebral disc regeneration, which are prone to damage and incapable of natural healing. Thick, randomly aligned electrospun PCL fibre structures infiltrated with alginate was used as structural tissue scaffolds. The composites found to exhibit large strains-to-failure and were mechanically robust than pure hydrogels even with a very small volume fraction of fibres, closely mimicking the composite structure of natural tissue. Electrospun PCL fibre network provided structural cues to cells and offered improved mechanical strength, while hydrogels provided a three-dimensional environment for nutrient transport. The mechanical strength was fine-tuned by varying the volume fraction and concentration of the constituent materials. Mechanical reinforcement of urinary bladder matrix by electrospun PCL nanofibers was studied by Ghafari et al. (2017). They fabricated hybrid hydrogel scaffolds with well-defined biomimetic architecture of better mechanical properties for tissue engineering applications. The ECM gel was taken from rat bladder and the electrospun PCL nanofibers were embedded within the gel to form hybrid hydrogel. The nanofiber-reinforced scaffolds displayed additional structural integrity and mechanical stability. Thus the concept of nanofiber-reinforced hydrogel can be used as promising material in bladder tissue or other load-bearing soft tissue engineering.
Heart valve-related complaints are the major causes of death globally. Although prosthetic valves are generally used to treat this, existing prosthetic grafts cannot grow with the patient age while maintaining normal valve hemodynamic and mechanical properties. Tissue engineering can be a possible solution to this dispute through use of patients own cells and biodegradable scaffolds. Tissue-engineered heart valves can generate autologous heart valve constructs that retains the capability to grow and remodel over time. The scaffolds prepared from hybrid hydrogel made of methacrylated hyaluronic acid/methacrylated gelatin reinforced by electrospun poly(glycerol sebacate) (PGS)/PCL fiber is used by Eslami et al. (2014) for heart valve tissue engineering application. Most hydrogel scaffolds, in spite of their similarity to heart valve tissue, are not mechanically fit for the dynamic stresses of the heart valve microenvironment. Hence, they are generally reinforced by electrospun nanofibers or microfibers. Compared to hydrogel or electrospun scaffolds alone, combined structure provides a more appropriate three-dimensional assembly for heart valve tissue engineering. The ECM mimicking hydrogel component of the composite scaffold retains cells within the structure, while the elastomeric electrospun fibers provide porous structure to mimic the cellular environment, support tissue growth and contributes adequate mechanical strength similar to that of natural heart valve tissue. In another study by Reddy et al. (2014), PCL/oligomer hydrogel of bisphenol A ethoxylated dimethacrylate (BPAEDMA) scaffolds for cardiac tissue engineering was evaluated. The PCL/BPAEDMA composite nanofibrous scaffolds displayed essential guidance for cell organization, adhesion, proliferation, survival and normal functioning of cardiac cells to clinically beneficial level. Cardiac cells proliferation found to increase upon addition of BPAEDMA to PCL scaffolds due to the balanced hydrophilicity and helped to decrease the modulus of PCL from 39.68 to 3.55 MPa, appropriate for cardiac tissue engineering.
A nanocomposite hydrogel reinforced with coaxial PCL/gelatin nanofibers was successfully developed by Kai et al (2012). The nanofiber-reinforced hydrogels exhibited increased moduli and compressive strength than pristine gelatin hydrogels and the modulus of the composite hydrogel found the increase with increasing nanofiber concentration. In another study by Shelke et al. (2016), the composite scaffold comprised of electrospun PCL nanofiber coated with sodium alginate was reported for neural tissue engineering application. The nanofiber lattice included in the hydrogel scaffold provided the required tensile strength and helped to retain suture thread on the nerve graft. Sodium alginate used in the study controlled the matrix hydrophilicity, material stiffness and controlled release of biological molecules. These composite scaffolds supported superior cell adhesion, proliferation, viability and neurogenic differentiation than PCL nanofibers alone, making it suitable for peripheral nerve tissue regeneration. Though electrospun PCL fiber matrices provide mechanical properties ideal for tissue regeneration, they lack the optimal hydrophilicity to promote cell adhesion. In addition, their slow degradation behavior makes PCL less anticipated for transient biomedical applications. To overcome these limitations they can be embedded in suitable hydrogels, which in turn also helps in overcoming the poor mechanical performance of hydrogels.
Characteristic properties of PCL scaffolds
Artificial organs and TE materials face a dual challenge of satisfying the biological and mechanical properties of the tissues they intend to replace. The important properties of a scaffold material include non-toxicity, non-antigenicity, non-immunogenicity, biocompatibility, desired hydrophilicity and bioresorbable characteristics with tunable mechanical strength (Siddiqui et al. 2018). For cells to infiltrate, proliferate and differentiate, the scaffold’s morphology also plays a crucial role. The scaffold’s with desired pore size (> 20 µm) and surface roughness (5–50 µm) is very much essential for the diffusion of nutrients in any TE application (Abedalwafa et al. 2013). The electrospun PCL nanofibers are thus an attractive material for TE applications because it can fulfill all the desired properties of a scaffold upon suitable modifications.
The most fundamental feature a material should possess while designing and developing a scaffold is to maintain structural integrity until the new tissue is proliferated. To perform well, the scaffold has to retain its mechanical strength from the point of its implantation to the regeneration of tissues and complete healing. The electrospun PCL nanofibrous scaffolds with higher porosity exhibits lesser tensile strength and young’s modulus when compared to the bulk PCL material (Abedalwafa et al. 2013; Siddiqui et al. 2018; Bolaina-Lorenzo et al. 2017), hence suitable strategies like multiple layer stacking and integration are required. The literature reveals that the degradation time taken by PCL is 2–4 years (Pitt et al. 1981). The CH2 moieties in the repeating unit of PCL makes it hydrophobic and leads to slower degradation when compared to other polyesters (Abedalwafa et al. 2013). This can be suitably addressed by the surface modification/plasma treatment or by blending it with suitable hydrophilic compounds or by copolymerizing it with appropriate reactive monomeric moieties (Sanchez-Gonzalez et al. 2018). The delayed degradation of PCL is advantageous under sluggish healing conditions as it provide adequate mechanical strength until the tissues are regenerated and healed. The chemical composition of a material, molecular weight and crystallinity also decide the rate at which water diffuses into the polymer chains and causes the bonds scission (Perale and Hilborn 2016). However, degradation and healing rates should synchronize for better results.
Bioresorption is another requirement of an ideal scaffold, the process of removal/decay of material from implanted biomaterial after the intended function without any enduring side effects is highly preferred. The aliphatic ester linkages existing in PCL are readily available to react with water molecules and undergo hydrolysis (Brugmans et al. 2015). The biodegradation mechanism of PCL in human body is through hydrolysis of ester linkages, by virtue of which low molecular weight compounds like caproic acid is eventually formed which can safely elude in vivo (Lam et al. 2008; Diaz et al. 2014). All these interesting properties of electrospun PCL, makes it a more appropriate material in the field of regenerative medicine for diverse TE applications.
Techniques for PCL-based scaffold fabrication
The cells and tissues in human body are well-defined and ordered into three-dimensional architecture. To engineer these natural functional tissues, scaffolds have to be fabricated by suitable methodology to enable cell spreading and guide their growth into three-dimensional space. The fabrication of 3D scaffolds with high surface area, appropriate pore size and pores interconnectivity depends on processing method. Since these are very crucial parameters for designing a TE scaffold, the selection of suitable preparation method is very essential. Table 1 represents different methods used for non-nanofibrous scaffold fabrication with their merits and demerits.
Table 1.
Techniques used for non-nanofibrous scaffold fabrication
| Method | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Gas foaming |
Free from organic solvents Operates at less temperature |
Inadequate pore interconnectivity Low mechanical strength |
Khang (2017) |
| Rapid prototyping |
Ease of attaining desired fiber architecture Controlled geometry, porosity and pore size Custom-made and accurate pore structure No need of supporting material |
High temperature requirement Expensive equipment |
Khang (2017) |
| Injectable gel |
Ease of incorporating functional bioactive compounds Intricate scaffolds can be fabricated |
– | Khang (2017) |
| Solvent casting |
Simple, easy and cost effective scaffold fabrication Does not demand sophisticated equipment |
Toxic solvents usage More time is required for solvent evaporation Scaffolds might be toxic due to presence of solvent traces |
Subia et al. (2010) |
| Particulate-leaching technique |
Process is easy to carry out Scaffolds with precise pore size can be achieved Requires lesser polymer content to fabricate Pore size (~ 500 μm) and 94–95% of porosity |
It is difficult to control inter-pore spacing’s and pore shape | Subia et al. (2010) |
| Fiber mesh | Higher surface area | Scaffolds possess less dimensional stability | Subia et al. (2010) |
There are several other reported techniques such as fiber bonding, melt molding, membrane lamination and high internal phase emulsion for fabricating TE scaffolds which are not widely practiced in recent times. The variants of rapid prototyping such as fused deposition modeling (FDM), solid-free form, 3D printing, selective laser ablation (SLA), selective laser sintering (SLS) and stereolithography techniques are of current focus to prepare well architect TE scaffolds. Table 2 depicts the various methods of nanofiber-based scaffold fabrication with their advantages and limitations (Ramakrishna et al. 2005).
Table 2.
Different methods of nanofiber-based scaffold fabrication
| Processing Technique | Processability | Fibre diameter | Advantages | Disadvantages |
|---|---|---|---|---|
| Template synthesis | Easy | Around 100 nm | Fibers with varied diameters can be easily made using suitable template | Difficult to produce long continuous nanofibers |
| Fiber drawing | Easy | 2–100 nm | Minimum equipment requirement | Discontinuous process |
| Self-assembly | Difficult | Around 100 nm |
Suitable for small nanofibers Good control over pore size, percent porosity and fiber diameter |
Expensive material Intricate process Complex design parameters |
| Phase separation | Easy | 50–500 nm | Consistent property |
Suitable only for selected polymers Difficult to control scaffold morphology |
| Electrospinning | Easy | 3–1000 nm |
Continuous process and gives nanofibers of higher L/D ratio Precise control over fiber diameter, percent porosity and pore size |
Jet instability |
Electrospinning process
Electrospinning process with the precise control of process parameters has gained popularity among rest of the techniques in recent times to produce nanofiber-based scaffolds. The principle of electrospinning is to apply high voltage/electric field to draw micron to submicron sized diameter of fibers from polymer melt or solution through an orifice. During electrospinning, high voltage will be applied to polymeric solution/melt to create an electrified jet at the capillary tip and there exists a potential difference between liquid and grounded collector. The Taylor cone formation at the tip of capillary is driven by the strong electrostatic repulsion between like charges in the liquid and electrostatic attraction between the oppositely charged liquid and collector. Once the applied voltage reaches a threshold value and overcomes the surface tension of the liquid, a fiber jet will be ejected from the Taylor cone. At this point, the ejected jet will be drawn and stretched to obtain nanofibers. The solvent evaporates while jet travels through air prior to its deposition on the collector (Mkhabelal and Ray 2014). Tissue function depends on fiber alignment, diameter and nanostructure, all these parameters can be controlled easily in electrospinning technique and substrate can be produced with desired hierarchy for various TE applications (Cipitria et al. 2011; Abedalwafa et al. 2013; da Silva et al. 2010).
Coaxial electrospinning process
Co axial electrospinning/co-electrospinning is an alteration of conventional electrospinning process wherein more than two polymer solutions are electropsun concurrently to form core/shell-structured nanofibers. Biphasic nanofibers can be prepared using coaxial electrospinning, which allows the fabrication of new class of nanomaterials with unique architectures and a synergistic combination of properties. A wide range of electrospinnable and non-spinnable material combinations like polymer/inorganic, inorganic/inorganic and polymer/polymer nanofibers can be coaxially electropsun to engineer new class of materials at nanoscale. Coelectrospinning applies electric field to a plastic syringe with two compartments containing different polymer solutions (core and shell) by virtue of which polymer jet becomes stretched and forms a Taylor cone at the tip of the syringe and subsequently nanofibers are collected. The bending instability experienced by the droplet is similar to conventional electrospinning. The spinnerets used in coaxial electrospinning share a common axis to create coated/hollow/multichannel nanofibers. This fiber structure provides desired topography for various TE applications (Yarin 2011; Qin 2017).
Parameters affecting the electrospinning process
During electrospinning process the morphology and diameter of nanofibers are greatly influenced by solution properties, processing variables and environmental conditions. These parameters can be controlled to obtain a well-defined nanofibrous structure that offers excellent scaffold properties and desirable atmosphere for cell communication. Table 3 summarizes the effect of electrospinning parameters on final properties of nanofibers. Both polymer solution and melt can be used as starting material for electrospinning; however, solution is more commonly used. Plasma-treated solution can be used to obtain desired surface functionality.
Table 3.
Electrospinning process parameters
| Parameters | Remarks | Reference | |
|---|---|---|---|
| Solution properties | Concentration | When the concentration is very low electrospinning becomes difficult whereas at higher concentration spherical shaped beads become spindle-like and forms uniform fiber. Depends on molecular weight of the polymer | Ramakrishna et al. (2005) |
| Molecular weight | Polymer of low molecular weight form beads rather than fibers and high molecular weight form fibers of larger diameter. Smooth nanofibers are obtained with moderate molecular weight | Robb and Lennox (2011) | |
| Surface tension | Charge of the polymer solution influences the fiber jet formation. Surface tension in turn reduces the surface area per unit mass of the fluid | Ramakrishna et al. (2005); Christanti and Walker (2001) | |
| Viscosity | Highly viscous solutions are difficult to spin while very less viscous solutions lead to the formation of beaded nanofibers | Subbiah et al. (2005) | |
| Solvent volatility | Very slow rate of solvent evaporation leads to thin film formation of polymer solution rather than fibers | Ramakrishna et al. (2005) | |
| Conductivity | Lesser dielectric constant of the solvent leads to a solution of lower conductivity. With the increase in the conductivity of the solution fiber diameter decreases | Robb and Lennox (2011) | |
| Processing variables | Applied voltage | When polymer solution is exposed to very high voltage, a narrow and less stable Taylor cone is formed due to higher amount of charges in the solution leading to jet acceleration and drawing more solution from the needle tip | Ramakrishna et al. (2005) |
| Tip to collector distance (TCD) | If TCD is very small then interconnected fiber mesh is formed due to lesser time available for solvent evaporation hence optimal TCD should be selected for proper solvent removal along with uniform stretching during its flight time | Ramakrishna et al. (2005) | |
| Flow rate/feed rate | When the feed rate is high the fibers are formed with larger diameter and residual solvent leads to the formation of webs whereas lower feed rate is essential which in turn provides more time for solvent evaporation | Ramakrishna et al. (2005) | |
| Solution temperature | It decreases the viscosity of the polymer solution and increases the evaporation rate of the solvent during processing | Ramakrishna et al. (2005) | |
| Environmental conditions | Surrounding temperature | Increase in temperature leads to faster solvent evaporation with the reduction in fiber diameter | Robb and Lennox (2011) |
| Relative humidity | Very high RH causes water condensation on the fiber surface and thus leads to circular pore formation whose depth varies with RH. Similarly low RH causes volatile solvent to evaporate faster | Ramakrishna et al. (2005) | |
| Pressure | Below atmospheric pressure the jet formed is unstable and at very low pressure there is direct discharge of charges and hence electrospinning is not possible | Ramakrishna et al. (2005) | |
Applications of PCL in tissue engineering
There are varieties of natural and synthetic polymers available for making nanofibrous TE scaffolds. Out of those, PCL is highly preferred due to its bioresorbable nature, essential physical and chemical properties appropriate for TE applications. Electrospun scaffolds are of major interest because they possess a well-defined architecture with controlled fiber diameter, pore dimensions and topography that promotes cell growth. Figure 4 shows various stages involved in scaffold assisted TE approach (Asadian et al. 2020). There are various research reports investigating electrospun PCL-based scaffolds for the tissue regeneration in cardiac (Reddy et al. 2014), bone (Hiep et al. 2017), nerve (Barbarisi et al. 2015), skin (Zarekhalili et al. 2017; Chaudhari et al. 2016; Ranjbar-Mohammadi and Bahrami 2015; Venugopal et al. 2005a, b) and drug delivery applications (Mohammadi et al. 2016; Ahmed et al. 2016). In the upcoming sections few significant applications of PCL scaffolds are discussed.
Fig. 4.
Various stages involved in scaffold assisted TE approach (
Source: Asadian et al. (2020))
PCL for bone tissue engineering
Bone tissue engineering (BTE) is the reassuring pathway to grow a novel implantable bone substitutes like bone grafts to treat bone disorders. The bone disorders or defect include loss of bone due to infections, injuries, trauma etc. Thus, bone regeneration through BTE is crucial to restore the normal functioning of joints. The tendons and ligaments are dense connective tissues which are comprised of collagen, helps in mobility and balancing of tissues in the human body (Chong et al. 2015; Awad et al. 2014). PCL is a slow degrading biomaterial which can ensure adequate mechanical strength until the bone tissues are regenerated and healed subsequently. There are various studies on the synthesis and evaluation of PCL-based blends and nanocomposites to fabricate potential bone grafts. For instance, Chong et al. (2015) studied PCL and PCL/nano-hydroxyapatite (PCL/nHA)-based nanofibrous scaffolds and reported well distribution of nHA particles in PCL matrix rendering the desired morphology to nanocomposites. The PCL/nHA scaffolds are more hydrophilic than pure PCL because of presence of OH bonds in nHA. The nanofibrous substrates with high specific surface area, small fiber diameter and porosity are more appropriate for bone tissue regeneration because osteoblasts are anchorage dependent cells. In addition, there are various studies on the modification of PCL scaffolds to augment their degradation rate so as to suit bone repair rate.
Binulal et al. (2012) evaluated PCL/nG (gelatin nanoparticles) scaffolds for possible bone TE application. The PCL/nG scaffolds showed improved cell adhesion with efficient restoration. Though broken fibers were observed after 2–4 weeks of degradation, morphology was unaffected, as the gelatin nanoparticles were embedded in the core of nanofiber. The cells were well-distributed on PCL/nG scaffolds and cell viability was higher on PCL/nG when compared to pure PCL scaffolds. The other research group Remya et al. (2013) working on PCL/(polycaprolactone/polyethyleneglycol/polycaprolactone) (CEC)/nHA composite stated that the prepared scaffolds were non-cytotoxic and showed improved surface wettability attributing to enhanced cell viability and proliferation among other scaffolds. The spindle-shaped morphology of the cells was observed on PCL/CEC/nHA scaffolds. The porosity, in the following order, was 92%, 80% and 80% on PCL, PCL/CEC and PCL/CEC/nHA scaffolds. The tensile strength of PCL, PCL/ nHAP and PCL/CEC scaffolds was 7.3 MPa, 8.5 MPa and 10.1 MPa, in the order given. The ultimate tensile strength of 12 MPa was exhibited by PCL/CEC/nHAP composite scaffold. There was a drop in the tensile strength of all the scaffolds after PBS treatment for 90 days. The respective values decreased by 58%, 83%, 36% and 75% for the corresponding PCL, PCL/CEC, PCL/nHAP and PCL/CEC/nHAP scaffolds.
The effect of gelatin inclusion and its concentration on cell proliferation and degradation rate of PCL nanofibrous scaffolds was evaluated by Binulal et al. (2014). The rate of biodegradation increased with increasing gelatin concentration for PCL/gelatin scaffolds. The cell proliferation was more on 30 and 40%(wt) gelatin-loaded composite scaffolds than on PCL/50%(wt) gelatin scaffold. The cell attachment and distribution were improved on PCL/gelatin scaffolds compared to pure PCL scaffolds.
In addition, the structure of the scaffold may influence the degradation behavior of PCL. Pattanashetti et al. (2019) fabricated single-layered PCL, poly (vinyl alcohol): sodium alginate (P:S), double-layered PCL/P:S and triple-layered PCL/P:S/PCL-based scaffolds. The in-vitro biodegradation studies revealed that for all the scaffolds the rate of degradation decreased. From the tensile test data, it was observed that PCL/P:S/PCL and PCL/P:S scaffolds showed lesser stress when compared to cross-linked PCL/P:S/PCL hybrid and PCL/P:S scaffolds, respectively. The tensile strength and young's modulus of PCL/P:S and cross-linked PCL/P:S scaffolds were decreased but PCL/P:S/PCL scaffolds possessed increase in stress values when compared to neat PCL scaffolds. From MTT assay it was observed that all the scaffolds were non-toxic in nature, in addition well-supported the cell proliferation. With increasing time, cell count also increased and there was no evidence of cell death reported. The percentage of cell proliferation (for 96 h) was 79%, 95%, 91%, 95% and 98% for their respective neat PCL, PCL/P:S, PCL/P:S/PCL, cross-linked PCL/P:S and cross-linked PCL/P:S/PCL scaffolds.
There are few studies demonstrating the improved cell responses for modified PCL scaffolds. Naghashzargar et al. (2015) studied the effect of electrospun PCL and poly(3–hydroxybutyrate) (P3HB) nanofiber coating on silk fibroin (SF) yarn for tendon and ligament TE applications. The cell viability was found to be more in hybrid scaffolds when compared to SF yarns but there was no major difference in viability between SF/PCL and SF/P3HB. While the tensile tests revealed that SF yarns undergoes deformation easily but hybrid scaffolds (SF/ES-PCL and SF/ES-P3HB) exhibit less extension and improved maximum load-bearing capability. Sharifi et al. (2018) investigated the biological performance of PCL/chitosan and PCL/carboxymethyl chitosan (CMC)-based blend scaffolds. After 48 h of culture period, the cell viability of PCL/10% chitosan and PCL/10% CMC scaffolds was higher than pure PCL scaffolds and all the scaffolds were confirmed to be biocompatible and non-toxic. Causa et al. (2006) prepared PCL/hydroxyapatite (HA)-based composite scaffolds to study the effect of HA particles on cellular interaction of scaffolds. It was identified that PCL/13% HA scaffold allows improved cell differentiation when compared to other scaffolds and this serves as a potential substrate for bone tissue regeneration. The elastic modulus was 8 MPa, 21.43 MPa and 27.90 MPa, for each respective, neat PCL, PCL/13% HA and PCL/20% HA, respectively. Similarly, the ultimate tensile strength was 0.93 MPa, 2.19 MPa and 1.33 MPa for neat PCL, PCL/13% HA and PCL/20% HA.
PCL for cartilage tissue engineering
Cartilage is a connective tissue found in many parts of the body but it is softer and much more flexible than bone. The main function is to ease the mechanical stress transmission with minimal friction. Most common defects in cartilage tissues are generated due to osteochondritis, aging and trauma. Cartilage is much more difficult to selfheal after injury because of its low regenerative capability. TE provides one of the most promising routes to repair damaged cartilage tissues by seeding chondrogenic cells onto scaffolds that are biodegradable and biocompatible in nature. The bioactive molecules such as simvastatin and kartogenin are more commonly used with various synthetic and natural polymers to produce a suitable substrate for cartilage tissue regeneration (Huang et al. 2019).
It is very hard to design a perfect scaffold for repairing the cartilage tissues. Recently a research group described the effect of incorporating bioactive molecules into electrospun nanofibers on biological performance of scaffolds. Silva et al. (2020) studied the suitability of kartogenin (KGN-loaded poly(glycerol sebacate) (PGS)/PCL core–shell nanofibrous scaffolds for cartilage TE applications. The core–shell structure of PGS/PCL scaffold is shown in Fig. 5. The in-vitro release studies showed that after 21 days, 0.32 µg KGN/mg of scaffold for PGS-KGN/PCL and 1.11 µg of KGN/mg of scaffold for PCL-KGN was released, respectively. The cell culture studies revealed that KGN-loaded scaffolds had better cell adhesion and growth when compared to PGS/PCL and neat PCL scaffolds. The cell count and proliferation were higher on PGS-KGN/PCL compared to other scaffolds. The mechanical properties of scaffolds were influenced by fiber alignment. The elastic modulus increased from 4.02 MPa (non-aligned) to 9.90 MPa (aligned) for PCL scaffolds similarly 5.06 MPa (non-aligned) to 11.78 MPa (aligned) for PGS/PCL scaffolds. There were no significant changes observed in the structure and mechanical properties of PCL-KGN and PGS-KGN/PCL scaffolds. The percent cell viability of PCL/collagen electrospun scaffolds were reported to be higher when compared to neat PCL scaffolds by Munir and Callanan (2018) and the scaffolds showed moderate biodegradation rate. From SEM, it was observed that nanofibers had porous structure with varying pore size. The PCL/collagen scaffolds exhibited a drop in pore size with increasing collagen content from 0.2–0.8% viz., 101, 73 and 30 µm was the pore size corresponding to 0.2%, 0.4% and 0.8% collagen. The blend scaffolds showed an increment in their young’s modulus with increasing collagen content when compared to collagen and PCL scaffolds.
Fig. 5.
TEM images of a PCL scaffold and b Core–shell structure of PGS/PCL scaffold. With permission Silva et al. (2020)
Similarly, the PCL/gelatin/MWNTs scaffolds were found to be more bioactive and rate of degradation of PCL/gelatin was 22.2% while that of PCL/gelatin/MWNTs was 0.33%. While the porosity of PCL/gelatin and PCL/gelatin/MWNTs scaffolds was 83.05% and 81.7%, respectively. The tensile strength of PCL/gelatin and PCL/gelatin/MWNTs was 0.98 MPa and 2.92 MPa, respectively. The PCL/gelatin/MWNTs-based scaffolds were non-toxic and showed prominently higher cell viability when compared to PCL/gelatin. This was due to reactive functional groups of gelatin and MWNTs, which offered the most adequate surface wettability for cartilage tissue regeneration (Zadehnajar et al. 2020a, b).
The surface of electrospun PCL can be modified by chemical treatment, surface coating and plasma treatment, so that the modified surface provides desired environment for tissue regeneration (Cipitria et al. 2011). These strategies have been successfully used by researchers to achieve the desirable surface topology. Alemi et al. (2019) used cold atmospheric plasma to alter surface characteristics of electrospun PCL/chitosan and PCL/carboxymethyl chitosan (CMC) scaffolds for enhancing the cell adhesion and proliferation. The helium plasma-treated PCL/CMC scaffolds provided adequate surface for cell attachment and differentiation. The cells were spread throughout PCL/CMC scaffold, this was attributed to improved hydrophilicity of plasma-treated samples when compared to untreated scaffolds. The scaffolds were found to be biocompatible and living cells were distributed throughout the surface. The cell viability was more on plasma-treated PCL/CMC scaffolds than on other scaffolds. The effect of phytochemicals-loaded PCL scaffold was studied by Venugopal et al. (2019) for bone and cartilage TE applications. It showed enhanced extracellular matrix production and DNA content. The SEM images (Fig. 6) of cell cultured scaffolds showed a spindle-shaped cells attached to the substrate but it was observed that cell proliferation was higher and cell viability was 90% on PCL/phytochemical-loaded scaffolds with even distribution than on PCL scaffolds. Phytochemical-loaded nanofibers, however, showed faster biodegradation than control nanofibers.
Fig. 6.
SEM micrographs comparing cell morphology on PCL-based scaffolds with and without phytochemicals. With permission Venugopal et al. (2019)
PCL for neural tissue engineering
Design and development of efficient scaffold for restoring neural functioning in human body are very challenging. The neurodegenerative diseases and traumatic injuries to central nervous system (CNS) and peripheral nervous system (PNS) cause severe and permanent damage. The nerve cells do not recover on their own and hence the regeneration of CNS and PNS is a crucial factor in nerve TE. Polymer-based scaffold materials are of broader concern in nerve TE due to their unique inherent properties. Conducting polymers are of research focus as electrical properties of neural scaffold should mimic neural tissue signaling to neurons and ease the reconstruction of neural connections. The electrospun polymer scaffolds are widely used because of their preferred topographical nanoscale features which suits for nerve cell regeneration (Boni et al. 2018).
Bolaina-Lorenzo et al. (2017) studied suitability of PCL/chitosan blend scaffolds for peripheral nerve TE. Due to lack of cell recognition sites on pure PCL scaffolds, it showed a less cell viability of 60%; whereas, chitosan blended scaffolds showed more than 80%. The Schwann cells showed better adhesion and proliferation on PCL/5% chitosan-loaded scaffold due to higher wettability/active sites at the surface of the scaffold. Biofunctionalization/chemical treatments help in promoting cell–cell and cell-material interactions of electrospun scaffolds. PCL modified by hydrolysing (H-PCL), adding matrigel (B-PCL) and matrigel-functionalized PCL (F-PCL) is reported by Ghasemi-Mobarakeh et al. (2010). The H-PCL scaffolds exhibited higher cell proliferation when compared to PCL scaffolds due to increased surface wettability. The rate of cell growth was the same for B-PCL and PCL nanofibrous scaffolds because the surface wettability of B-PCL remained unchanged after blending, but the cell proliferation of F-PCL scaffolds was higher than all the remaining scaffolds.
The salient features of neural tissue regeneration scaffold, include flexibility, stability, biochemical-electrochemical signalling, fibre alignment, which should function as delivery vehicle (Siddiqui et al. 2018). The research investigations manifest that neurotic cells grow in a direction along-side of the fibre axis thus the comparison between cell culture studies of aligned and randomly oriented PCL/polypyrrole (PPy) nanofibers revealed that aligned nanofibers had higher cell migration than randomly oriented ones (Xie et al. 2009). In a similar study, the aligned and random PCL/gelatin scaffolds to support neurite outgrowth was studied by Ghasemi-Mobarakeh et al. (2008a, b). Figure 7 represents SEM images of aligned and random 70/30 PCL/gelatin scaffolds. The 70/30 PCL/gelatin scaffold subjected to cell culture studies revealed that cells were grown in the direction parallel to that of fiber orientation and was distributed more evenly over the PCL/gelatin substrate than on pure PCL substrate. It was observed that gelatin content influenced cell viability and better cell proliferation was on aligned nanofibers than on random nanofibers.
Fig. 7.
SEM images showing random and aligned PCL/gelatin nanofibers of 70/30 composition. With permission Ghasemi-Mobarakeh et al. (2008a, b)
Several studies reported on modification of biodegradation rate and surface characteristics of PCL scaffolds by blending or by composite making. The cell adhesion, migration and proliferation were reported to be higher on PCL/hyaluronic acid (HA) composite scaffolds than on PCL scaffolds, this was due to reduced fibre diameter with increasing HA content. Higher the HA content in the scaffolds, higher is the rate of degradation of scaffold material (Entekhabi et al. 2016). The nerve stem cells (NSC) were found to be more adhered (90%) on collagen scaffolds but PCL/collagen composite scaffolds supported and favored the migration of NSCs with better differentiation (80%) when compared to pure PCL (58%) and pure collagen (38%) scaffolds. It was observed that composite scaffolds degraded at a faster rate than pure PCL and cell adhesion and proliferation was more on aligned fibers (94% adhesion and 71% proliferation) than on randomly (73% adhesion and 52% proliferation) oriented fibers (Hackett et al. 2010). Similarly, the hydrophilic blend of PCL/chitosan substrate offers a suitable surface for cell attachment and migration than does neat PCL. The cell proliferation was approximately 48% higher on PCL/chitosan scaffolds than on PCL scaffolds, which is mainly due to amino groups of chitosan in blend nanofibers by virtue of which spindle-shaped Schwann cells were adhered and distributed. The Schwann cells showed a flat morphology on PCL scaffolds (Prabhakaran et al. 2008). Gupta et al. (2009) prepared PCL/gelatin-based nanofibrous nerve implants and studied the effect of fibre orientation on nerve cell regeneration. Cell proliferation was higher in blend scaffolds than on pure PCL scaffolds which was due to improved surface wettability of PCL/gelatin scaffolds. The morphological analysis confirmed spindle-shaped cells on blend scaffolds with uniform distribution and good proliferation but flat, round-shaped cells with lesser count was observed on neat PCL scaffolds.
The electrical properties of PCL can be modified by blending it with organic and inorganic nanoparticles. Saderi et al. (2018) evaluated the effect of incorporating gold nanoparticles (Au NPs) on electrospun PCL/chitosan scaffolds for Schwann cell (SC) culture. The studies showed that AuNPs modified the conductivity of electrospun scaffolds which was one of the most essential properties for a substrate to be used in nerve TE applications. The cell cultured electrospun mats showed that both PCL/1.5% chitosan and PCL/1.5% chitosan/AuNPs supported cell adhesion and growth but the presence of gold NPs enhanced the rate of proliferation of SCs on respective scaffold. The electrically conductive PCL/chitosan/AuNPs scaffolds were reported to be non-toxic and possessed an ideal surface for nerve TE. Heidari et al. (2019) studied the influence of graphene oxide on cell adhesion properties of PCL/gelatin nanofibers for nerve cell regeneration. The biodegradation order was PCL/gelatin/graphene oxide > PCL/gelatin > PCL. The FESEM image (Fig. 8) of neural cell culture on scaffolds indicated a spindle-shaped cells on both PCL/gelatin and PCL/gelatin/graphene oxide scaffolds after 3 days. The PCL/gelatin/graphene oxide scaffolds exhibited higher cell growth after 7 days which was due to the electrical conductivity of graphene oxide, and encouraged the nerve cells to adhere on substrate and proliferate.
Fig. 8.
EESEM images of PCL/gelatin/graphene oxide scaffolds showing a Adherence of PC12 cells after 2 days and b Adherence of PC12 cells after 7 days. With permission Heidari et al. (2019)
PCL for skin tissue engineering
Skin grafting is the conventional technique used to restore native tissue after a trauma. However, availability of sufficient healthy skin and the additional health risks allied with the procedure pose serious complications. In such situations skin regeneration scaffolds are basically used to treat very deep skin injuries like cuts or burns which in turn help in restoring the damaged tissues. The main aim of skin TE is to reconstruct skin and to restore the functions of all the three layers of skin. The research reports on the preparation of scaffolds using novel biomaterials for skin regeneration or wound healing applications are discussed below.
The scaffolds used for skin TE application should be porous in nature. Rad et al. (2019) studied the effect of adding C. officinalis extract into electrospun PCL/zein/Gum Arabic (GA) scaffolds for skin regeneration. The porosity of electrospun mat was in the range of 78–83% with pore size of 3.5–4.2 µm. It was reported that PCL/zein/GA scaffolds are more appropriate substrate with enhanced cell proliferation when compared to pure PCL scaffolds. Further improvement in the growth of cells was observed in scaffolds containing C. officinalis extract. In another study, the cell adhesion and multiplication were found to be higher and uniform on PCL/collagen substrate with spindle-shaped cell morphology when compared to neat PCL. This was attributed to unique surface topography and improved wettability of PCL/collagen nanofibrous mats (Sharif et al. 2018). Gomes et al. (2017) studied the suitability of binary (chitosan (CS)/PCL, CS/gelatin (GEL) and PCL/GEL) and tertiary blend (CS/PCL/GEL) systems for skin regeneration. The CS containing scaffolds were remarkably hydrophilic in nature with 0° contact angle, whereas, PCL blended scaffolds possessed a balanced hydrophilic nature with contact angle of 58°. It was noticed from the cell culture studies that the scaffolds without CS functions as a good substrate for cell adhesion. The order of cell adhesion (50–100%) was as follows, PCL/GEL > CS/GEL > CS/PCL/GEL > CS/PCL. The morphological analysis of cell culture samples revealed that the entire scaffold is covered with cells and spindle-shaped cell growth occurred on mats. CS imparts antimicrobial, anti-inflammatory nature to the blend and supports in re-epithelialization of skin but results in low cell adhesion, however, the drawback is balanced by presence of gelatin.
The structure of the scaffold and its composition influences skin tissue reconstruction. It was noticed that apart from surface functionality, surface topography is also one of the prominent factors to promote cell proliferation in any TE scaffolds. Zahedi et al. (2019) prepared PCL/chitosan and a natural herb encapsulated drug eluting core–shell nanofibrous scaffold for wound healing and skin regeneration. The composition of the nanofiber was PEO/aloevera as the core and PCL/chitosan/keratin as the shell material. The presence of aloevera promotes cell adhesion, infiltration and growth. The cell viability was more than 80% for the core–shell structured nanofibers and they support uniform spreading of cells due to non-cytotoxic nature of the scaffolds. Likewise, Zarekhalili et al. (2017) synthesized PVA/gum tragacanth (GT)/PCL hybrid nanofibers using a two-nozzle electrospinning technique for skin TE applications. The presence of PVA and GT in the hybrid nanofibers makes the scaffolds hydrophilic in nature. When compared to neat PCL scaffolds, cell adhesion and proliferation was more prominent on PVA/GT scaffolds which was attributed to hydrophilicity and distinct biological features. The cells were distributed uniformly across the nanofibrous mat with better cell attachment and growth. The percentage of cell viability of hybrid nanofibers was 89.90%, 110.80% and 107.2% after their respective 3, 5 and 7 days. The role of surface coating to achieve structural and surface compatibility of electrospun PCL for skin regeneration was evaluated by Ghosal et al. (2017). The hydrophilicity of the electrospun mat was of the order of collagen-coated PCL > TiO2—PCL > PCL. The collagen-coated PCL scaffolds showed higher cell viability than neat PCL, but the cell count was less in TiO2-loaded PCL scaffolds.
The flexibility to incorporate bioactive components like nanoparticles, growth factors, anti-inflammatory agents, antimicrobials and wound healing drugs into the nanofibers is an added advantage to promote faster healing of skin. Salehi et al. (2020) reported use of PCL/gelatin electrospun nanofibrous mat containing bioactive agent cinnamon for wound healing applications. Anti-inflammatory and antibacterial action of cinnamon showed better wound closure efficacy compared to pristine PCL/gelatin. Non-woven electrospun PCL scaffolds as a physical barrier can impede the penetration of microbes preventing infections. Smart and controlled release of active molecule in spatial and time-based fashion is very crucial for the successful repair of damaged skin (Joseph et al. 2019). Hence the right combination of materials helps in imparting desired properties to the scaffold for intended application. The fundamental challenges are integration with host tissue and maintaining the thickness, texture of dermal, epidermal and hypodermal layers of the skin substitute closely mimicking the native skin of the patient.
PCL for cardiac tissue engineering
The heart consists of a special kind of muscle tissue called myocardium or cardiac tissue which is essential for cardiac cycles. The scaffolds for use to restore the functioning of cardiac tissue should be biocompatible and biodegradable thereby mimicking the native myocardium with desired mechanical properties (Rai et al. 2015). The flexibility of electrospinning process provides fabrication of scaffolds with essential properties (conductive, mechanical and biodegradable) that are appropriate for cardiac TE application (Hekmati and Norouzi 2017). The mechanical properties of native myocardium ECM is found to be 3–15 kPa of tensile stress, 22–90% of tensile strain and 0.02–0.5 MPa of Young’s modulus, respectively (Hekmati and Norouzi 2017; Chen et al. 2008). An ideal electropsun scaffold for cardiac TE should possess the characteristic features like conductivity, active sites for cell recognition, desired surface wettability and mechanical properties (Kitsara et al. 2017).
The cardiovascular diseases like myocardial infarction (MI) and heart failure causes death, therefore, cardiac patches are engineered to re-establish the structure and functioning of injured tissues. These patches are capable of replenishing the cellular activities after MI. An ideal electrospun cardiac patch exhibits interconnected porous structure with varying pore dimensions essential for cell migration, proliferation, vascularization along with nutrient and oxygen exchange. In order to improvise the bioactivity of cardiac patches, they are biofunctionalized with growth factors (Rai et al. 2015; Hekmati and Norouzi 2017).
Cardiac patch is generally fabricated by electrospinning of poly(glycerol sebacate) (PGS)/PCL. SEM images revealed uniform bead-less fibers with 0.4–5.2 µm sized pores and 62% of porosity. When compared to neat PCL and PGS fibres, the PGS/PCL blend fibers exhibited higher stiffness and slower degradation rate than PGS fibers. The cell culture studies revealed that vascular endothelial growth factor (VEGF)-functionalized PGS/PCL scaffolds showed better cell adhesion, growth and differentiation of C2C12, rat cardiac progenitor cells (rCPCs) and rat aortic endothelial cells (rAoECs) than non-functionalized PGS/PCL scaffolds. Thus functionalized scaffolds can function as an ideal candidate for cardiac patch (Rai et al. 2015). Similarly, smooth electrospun PCL and PCL/PGS blend nanofibers were evaluated for cardiac tissue engineering application. It was observed that with the inclusion of PGS into PCL matrix, the average fiber diameter of blend nanofibers increased but their corresponding mechanical properties decreased. The PCL/PGS nanofibers were found to be more hydrophilic than neat PCL nanofibrous mats. The faster degradation behavior is observed for pure PGS fibers in comparison with slightly cross-linked PGS fibers. The PCL/PGSMXL (cross-linked scaffold) substrate showed favorable surface for the survival of ST2 cells (Vogt et al. 2019).
The cardiac progenitor cells are tissue specific in nature and are capable of promoting myocardium tissue differentiation on polymeric scaffolds. In line with this, PCL/poly glycolic acid (PGA) nanofibers were evaluated for adherence and growth of cardiac progenitor cells on both pure and blend scaffolds by Aghdam et al. (2014). It was observed that with increasing PGA content in blend composition, the hydrophilicity, porosity and mechanical properties of the blend scaffolds was improved. The pure PCL and PGA electrospun mats exhibited 81% and 90% of porosity, respectively. From the cell culture studies it was observed that multinucleated myocyte cells were adhered and differentiated well on PCL/PGA scaffolds than on pure PCL scaffolds but beyond 50% of PGA content, the proliferation of cardiac progenitor cells reduced.
The fabrication of conductive scaffolds for TE application is essential for functionalizing the nano-networks to engineer tissue growth. Few nanomaterials like graphene, CNTs, reduced graphene oxide etc. are incorporated into polymer matrix to create nanocomposites which are capable of providing conductive environment for culturing cells. This electro-conductive networks are fascinated for cardiac cell culturing. For instance, Hitscherich et al. (2018) studied the effect of graphene inclusion on cardiac cell growth. The electrical stimulation offered by uniformly dispersed graphene nanoparticles in PCL matrix attributed to improve volume conductivity of composite scaffolds and cardio myocytes cell’s adherence with spontaneous contraction of cells. The composite scaffolds were non-toxic in nature. In another study, the effect of chemical treatment, electrical stimulation and inclusion of CNTs on cardiac cell differentiation was evaluated by Crowder et al. (2013). From the cell culture studies it was observed that hMSCs proliferated well on PCL/CNT composite scaffold, electrically stimulated PCL scaffold than on azacytidine-treated PCL scaffolds. The typical elongated cardio myocytes cell morphology was observed on composite scaffold without any electrical stimulation.
Conductive scaffolds found to demonstrate ideal performance during cardiac tissue regeneration, because the adhesion, proliferation and differentiation of any specific cell (especially cardiac cells) is a function of surface properties which in turn is controlled by electrical stimulus. However, these surface properties are controlled by surface charges by virtue of which cell recognition sites on the electropsun scaffolds are also controlled (Kitsara et al. 2017). Few studies have reported on the appropriate combination of biodegradable synthetic/natural polymer for fabrication of heart valves. For instance, electropsun PCL/PLLA scaffolds was used to fabricate tri-leaflet heart valve structure (Fig. 9). Various PCL/PLLA combinations were evaluated for their biomechanical properties. It was observed that after seeding cardiac stem cells on scaffold, the metabolic activities were enhanced gradually with increasing PLLA concentration in the blend samples. With dynamic cell seeding technique, valvular interstitial cells penetrated into the pores of the scaffold. When compared to pure PLLA, pure PCL was found to be stiffer possessing better mechanical properties. The order of stiffness is shown to vary as PLLA < mixtures < blends < PCL. Thus, PCL/PLLA blend system functions as an ideal scaffold for heart valve TE in comparison with their individual constituents (Hasan et al. 2018).
Fig. 9.
Schematic representation showing the tri-leaflet structured heart valve made out of PCL/PLLA. With permission Hasan et al. (2018)
Pore size is one of the most unique feature of tissue engineered scaffold, 10 µm is the required pore size in a scaffold. A research group of Jana et al. (2019) reports the effect of polymer concentration (14%, 16% and 18% PCL) on the properties of electropsun scaffold designed for heart valve TE. From SEM images it was observed that the fibers were homogeneous, bead-free and cylindrical. The pore size of the scaffolds was found to be 20.19 µm, 27.43 µm and 27.14 µm corresponding to 14%, 16% and 18% PCL concentrations. Similarly, Young’s modulus was 3.41 MPa, 7.88 MPa and 8.91 MPa for 14%, 16% and 18% PCL scaffolds. The higher tensile properties of 16% and 18% PCL scaffold led to the reduction of cellular activities. It was observed that 14% PCL scaffold exhibited adequate pore size and tensile properties essential for cell attachment and proliferation, hence it was found to be a suitable scaffold for heart valve TE application.
PCL for vascular tissue engineering
Tissue engineering is one of most promising strategy to engineer a blood vessel with small diameter to restore the normal functioning of vascular system. Accordingly, the material used for synthesizing scaffolds for vascular tissue regeneration play a vital role, offering good cell and blood compatibility, desirable mechanical strength, biodegradability etc. (Li et al. 2020). The bio-inert nature of PCL limits its TE application. This property can be modified by blending PCL with various bioactive molecules/natural polymers/biopolymers etc. For instance, Li et al. (2020) prepared PCL/keratin-based electropsun mats for vascular TE application. From SEM images it was observed that both pure PCL and PCL/keratin mats were uniform, defect free, and porous in nature with required interconnected pore structure which mimics the native ECM. PCL is hydrophobic in nature, while PCL/keratin composite scaffolds are hydrophilic. With increasing keratin content, the surface wettability of the composite was also improved. The amine and carboxyl groups of keratin are responsible for this behavior and it was found that PCL/keratin bio-composite mats favored the adhesion of cells and their proliferation. The PCL/keratin and nitric oxide generating scaffolds supported the proliferation of human umbilical vein endothelial cells (HUVECs) but suppressed the growth of human umbilical smooth muscle cells (HUASMC). The scaffolds were found to be non-toxic in nature thereby promoting cellular activities and extended blood clotting time.
Likewise, Fu et al. (2014) synthesized gelatin/PCL and collagen/poly (l–lactic acid–co–ε–caprolactone) (PLCL)-based electropsun vascular scaffolds. SEM images confirmed the formation of bead-free homogeneous fibers. Both PCL and PLCL are hydrophobic in nature but after blending them with gelatin and collagen, the resultant hybrid scaffolds are extremely hydrophilic in nature. Both the scaffolds were found to be non-toxic in nature but collagen/PLCL scaffolds form relatively homogeneous vessel-like tissue rather gelatin/PCL scaffold. Both the scaffolds showed the required mechanical properties. During cell culture studies, spindle-shaped cell morphology was observed on collagen/PLCL scaffolds and hence it served as an efficient scaffold material for cardiovascular tissue regeneration.
The biomaterials used to design an efficient vascular graft should possess desirable physical and biomechanical features. Electropsun PCL/collagen (5–75%) nanofiber was studied by Bertram et al. (2017) for vascular TE application. SEM images revealed that the pure and blend fibers were randomly oriented and average fiber diameter of pure PCL mats was 274 nm while that of PCL/5% collagen was 191 nm, respectively. From the cell culture studies it was observed that, there were no significant changes in the rate of proliferation with increasing collagen content in the blend composition. There were more cells surviving on PCL/5% and 25% collagen in comparison with other scaffolds. The T17b murine embryonic endothelial progenitor cells (eEPCs) proliferated well on PCL/25% Collagen and hence this scaffold functions as an efficient substrate for regenerating vascular tissue.
Another research group reports blending of PCL with collagen to alter the surface wettability to improve the adherence of coronary artery smooth muscle cells. It was observed that PCL/collagen was more hydrophilic than PCL scaffolds hence PCL/collagen scaffolds promoted the attachment and proliferation of cells. Both the scaffolds were found to be non-toxic in nature. SEM observation showed a normal phenotype-shaped cells distributed uniformly on PCL/collagen scaffold. This was attributed due to the presence of amide group in collagen (Venugopal et al. 2005a, b). The electropsun PCL scaffolds can be further subjected to surface modification by coating gelatin and poly(dopamine) (PDA) to improve the cell affinity. The average fiber diameter was slightly enhanced after coating with gelatin and PDA. The water contact angle significantly changed for both the scaffolds but the cell survival was more on PDA-coated PCL rather than gelatin-coated PCL. Further, PDA-coated scaffolds were treated with few non-adherent materials like PTFE, PDMS, PE and silicone rubber to evaluate the viability of human umbilical vein endothelial cells (HUVECs) on the modified scaffolds. The substrates displayed 8.8, 7.5, 2.7 and 7.5 times for the corresponding PTFE, PDMS, PE and silicone rubber. The creation of ad-layer is an effective approach for designing vascular TE scaffolds (Ku and Park 2010).
Coaxial electrospinning can concurrently electropsun two polymer solutions using a unique spinneret with coaxial capillaries. Thus fabricated nanofibers exhibit core/shell morphology with synergistic property and performs as a functional scaffold for various TE applications. There are several studies reporting the fabrication of PCL-based core–shell structured vascular grafts. Duan et al. (2016) evaluated PCL/collagen-based core–shell nanofibers for vascular TE. It was observed that the scaffolds were non-toxic in nature and showed affinity towards cells. The cross-linked scaffolds possessed improved mechanical properties. The heparinized scaffolds functions as a potential substrate for the proliferation of vascular cells. Coimbra et al. (2017) prepared PCL/functionalized gelatin (GeMA)-based electropsun core/shell scaffolds for vascular TE. From SEM images it was observed that in comparison with spun fibers, the cross-linked fibers were dense enough and hence resulted in reduced porous structure. The surface wettability of PCL/GeMA fibers was gradually enhanced with increasing GEMA content. In vitro cell culture studies revealed the biocompatibility of both pure PCL and PCL/GeMA fibers which attributes to survival of cells on the core/shell substrate.
The core/shell nanofibers were additionally functionalized with growth factors to improve the bioactivity of the resulting scaffold. Co-axially electropsun PCL/GEL, PCL/GEL/SF and PCL/GEL/SF/VEGF scaffolds were evaluated as vascular grafts. Smooth fibers with interconnected pores were observed in SEM micrographs. The contact angle variation was in the following trend PCL/GEL > PCL/GEL/SF/VEGF (150 ng) > PCL/GEL/SF/VEGF (250 ng) > PCL/GEL/SF. The tensile strength/Young’s modulus was 3/30.2 MPa, 3.3/33.7 MPa, 2.8/34.9 MPa and 2.6/32.4 MPa for their corresponding PCL/GEL, PCL/GEL/SF, PCL/GEL/SF/VEGF (150 ng) and PCL/GEL/SF/VEGF (250 ng). From cell culture studies it was observed that PCL/GEL/SF/VEGF (250 ng) scaffolds secreted more collagen when compared to other scaffolds. All the scaffolds are biocompatible and demonstrated that, vascular proteins are expressed in differentiated MSCs and they are suitable for regenerating vascular tissue. However, PCL/GEL/SF/VEGF (250 ng) scaffold with desired mechanical properties and surface wettability was an ideal substrate for vascular grafts (Ezhilarasu et al. 2019).
Future perspective
The future of tissue engineering is filled with huge potential and promise to fulfill the multitude medical needs. This approach can narrow down the critical gap between the rising number of patients on the waiting list for organ transplantation. Organ failure or tissue loss/damage is one of the most disturbing and expensive issue in health care sector. Tissue engineering can be a viable solution to the above problem if properly addressed, as it combines the suitable material properties and cell transplantation to develop auxiliary tissues and/or support endogenous regeneration. However, the challenges like bulk production at large scale, regulatory approval, clinical validation and affordable cost, development of neo-tissues and neo-organs and limited market penetration are still the bottle-necks in the field. The collective advances in clinical progresses and commercialization indicates that the technology will reach increasing numbers of patients in the years to come.
Summary
The engineering of tissues on a suitable polymeric scaffold emerged as a pertinent methodology to restore the damaged tissues and organs in recent times. PCL-based nanofibrous scaffolds produced by electrospinning technique are attractive due to their close resemblance to the structural characteristics of natural extracellular matrix. High plasticity, ductility, optimal porosity, pore size and pore interconnectivity of PCL electrospun scaffold has given a way to fabricate successful functional material for TE. Tissue engineering faces a challenge of satisfying the desired physicochemical, biological and mechanical properties of the tissues they intend to replace. Nontoxicity, non-antigenicity, non-immunogenicity, biocompatibility, cytocompatibility, hemocompatibility, bioresorption, adequate cell adhesion and proliferation, antimicrobial nature, desired hydrophilicity and tunable mechanical strength are the properties expected by an ideal scaffold material, unfortunately all these properties cannot be found in a single material. There comes the role of functionalization, material blending, composite making, multi-material stacking/integration which can be easily accommodated with PCL so as to make it suitable for targeted applications. However, numerous clinical trials and in vitro and in vivo testing is required to judge the suitability of material. The tunable functionalities, outstanding structural and mechanical properties offer exceptional versatility to electrospun PCL-based scaffolds, making them highly preferred choice for varied type of tissue engineering applications in current scenario.
Acknowledgements
One of the authors, Sowmya B. would like to thank Council of Scientific and Industrial Research (CSIR), New Delhi for providing research fellowship [CSIR-GATE-JRF, File no: 31/GATE/03(13)/2018-EMR-I].
Declarations
Conflict of interest
Author, Sowmya B declares that she has no conflict of interest. Author, A. B. Hemavathi declares that she has no conflict of interest. Author, P. K. Panda declares that he has no conflict of interest.
Ethical statement
This review article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abedalwafa M, Wang F, Wang L, Li C. Biodegradable poly-ε-caprolactone (PCL) for tissue engineering applications: a review. Rev Adv Mater Sci. 2013;34:123–140. [Google Scholar]
- Adeli-Sardou M, Yaghoobi MM, Torkzadeh-Mahani M, Dodel M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int J Biol Macromol. 2019;124:478–491. doi: 10.1016/j.ijbiomac.2018.11.237. [DOI] [PubMed] [Google Scholar]
- Agarwal S. Chemistry, chances and limitations of the radical ring-opening polymerization of cyclic ketene acetals for the synthesis of degradable polyesters. Polym Chem. 2010;1:953–964. doi: 10.1039/c0py00040j. [DOI] [Google Scholar]
- Aghdam RM, Shakhesi S, Najarian S, Mohammadi MM, Ahmadi Tafti SH, Mirzadeh H. Fabrication of a nanofibrous scaffold for the in vitro culture of cardiac progenitor cells for myocardial regeneration. Int J Polym Mater Polym Biomater. 2014;63:229–239. doi: 10.1080/00914037.2013.800983. [DOI] [Google Scholar]
- Aguirre-Chagala YE, Altuzar V, Leon-Sarabia E, Tinoco-Magana JC, Yanez-Limon JM, Mendoza-Barrera C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater Sci Eng C. 2017;76:897–907. doi: 10.1016/j.msec.2017.03.118. [DOI] [PubMed] [Google Scholar]
- Ahmed SM, Ahmed H, Tian C, Tu Q, Guo Y, Wang J. Whey protein concentrate doped electrospun poly(epsilon-caprolactone) fibers for antibiotic release improvement. Colloids Surf B Biointerfaces. 2016;143:371–381. doi: 10.1016/j.colsurfb.2016.03.059. [DOI] [PubMed] [Google Scholar]
- Alemi PS, Atyabi SA, Sharifi F, Mohamadali M, Irani S, Bakhshi H, Atyabi SM. Synergistic effect of pressure cold atmospheric plasma and carboxymethyl chitosan to mesenchymal stem cell differentiation on PCL/CMC nanofibers for cartilage tissue engineering. Polym Adv Technol. 2019;30:1356–1364. doi: 10.1002/pat.4568. [DOI] [Google Scholar]
- Asadian M, Grande S, Morent R, Nikiforov A, Declercq H, Geyter ND. Effects of pre- and post-electrospinning plasma treatments on electrospun PCL nanofibers to improve cell interactions. J Phys Conf Ser. 2017;841:1–6. doi: 10.1088/1742-6596/841/1/012018. [DOI] [Google Scholar]
- Asadian M, Chan KV, Norouzi M, Grande S, Cools P, Morent R, Geyter ND. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials. 2020;10:119–182. doi: 10.3390/nano10010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atyabi SM, Sharifi F, Irani S, Zandi M, Mivehchi H, Nagheh Z. Cell attachment and viability study of PCL nano-fiber modified by cold atmospheric plasma. Cell Biochem Biophys. 2016;74:181–190. doi: 10.1007/s12013-015-0718-1. [DOI] [PubMed] [Google Scholar]
- Awad HA, O’Keefe RJ, Lee CH, Mao JJ. Bone tissue engineering: Clinical challenges and emergent advances in orthopedic and craniofacial surgery. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. New York: Elsevier Science; 2014. pp. 1733–1743. [Google Scholar]
- Bailey WJ, Ni Z, Wu SR. Synthesis of poly-ε-caprolactone via a free radical mechanism. Free radical ring-opening polymerization of 2-methylene-1,3-dioxepane. J Polym Sci Polym Chem Ed. 1982;20:3021–3030. doi: 10.1002/pol.1982.170201101. [DOI] [Google Scholar]
- Barbarisi M, Marino G, Armenia E, Vincenzo Q, Rosso F, Porcelli M, Barbarisi A. Use of polycaprolactone (PCL) as scaffolds for the regeneration of nerve tissue. J Biomed Mater Res Part A. 2015;103:1755–1760. doi: 10.1002/jbm.a.35318. [DOI] [PubMed] [Google Scholar]
- Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59:1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Bertram U, Steiner D, Poppitz B, Dippold D, Kohn K, Beier JP, Detsch R, Boccaccini AR, Schubert DW, Horch RE, Arkudas A. Vascular tissue engineering: effects of integrating collagen into a PCL based nanofiber material. Biomed Res Int. 2017;2017:1–11. doi: 10.1155/2017/9616939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar SK, Rana D, Lekesiz H, Bedeloglu AC, Ko J, Cho Y, Aytac Z, Uyar T, Jun M, Ramalingam M. Design and fabrication of auxetic PCL nanofiber membranes for biomedical applications. Mater Sci Eng C. 2017;81:334–340. doi: 10.1016/j.msec.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Binulal NS, Natarajan A, Menon D, Bhaskaran VK, Mony U, Nair SV. Gelatin nanoparticles loaded poly(ε-caprolactone) nanofibrous semi-synthetic scaffolds for bone tissue engineering. Biomed Mater. 2012;7:065001–65016. doi: 10.1088/1748-6041/7/6/065001. [DOI] [PubMed] [Google Scholar]
- Binulal NS, Natarajan A, Menon D, Bhaskaran VK, Mony U, Nair SV. PCL-gelatin composite nanofibers electrospun using diluted acetic acid-ethyl acetate solvent system for stem cell-based bone tissue engineering. J Biomater Sci Polym Ed. 2014;25:325–340. doi: 10.1080/09205063.2013.859872. [DOI] [PubMed] [Google Scholar]
- Bolaina-Lorenzo E, Martinez-Ramos C, Monleon-Pradas M, Herrera-Kao W, Cauich-Rodriguez JV, Cervantes-Uc JM. Electrospun polycaprolactone/chitosan scaffolds for nerve tissue engineering: Physicochemical characterization and Schwann cell biocompatibility. Biomed Mater. 2017;12:015008–15019. doi: 10.1088/1748-605X/12/1/015008. [DOI] [PubMed] [Google Scholar]
- Boni R, Ali A, Shavandi A, Clarkson AN. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci. 2018;25:1–21. doi: 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braud C, Atlan A, Ducos C, Vert M. Capillary zone electrophoresis in normal or reverse polarity separation modes for the analysis of hydroxy acid oligomers in neutral phosphate buffer. J Chromatogr B Biomed. 1998;706:73–82. doi: 10.1016/S0378-4347(97)00468-4. [DOI] [PubMed] [Google Scholar]
- Brugmans MCP, Sontjens SHM, Cox MAJ, Nandakumar A, Bosman AW, Mes T, Janssen HM, Bouten CVC, Baaijens FPT, Driessen-Mol A. Hydrolytic and oxidative degradation of electrospun supramolecular biomaterials: In vitro degradation pathways. Acta Biomater. 2015;27:21–31. doi: 10.1016/j.actbio.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Butcher AL, Offeddu GS, Oyen ML. Nanofibrous hydrogel composites as mechanically robust tissue engineering scaffolds. Trends Biotechnol. 2014;32:564–570. doi: 10.1016/j.tibtech.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Causa F, Netti PA, Ambrosio L, Ciapetti G, Baldini N, Pagani S, Martini D, Giunti A. Poly-ε-caprolactone/hydroxyapatite composites for bone regeneration: In vitro characterization and human osteoblast response. J Biomed Mater Res Part A. 2006;76:151–162. doi: 10.1002/jbm.a.30528. [DOI] [PubMed] [Google Scholar]
- Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, Singh SR, Pillai SR. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17:1974–2005. doi: 10.3390/ijms17121974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Chong LH, Hassan MI, Sultana N (2015) Electrospun polycaprolactone (PCL) and PCL/nano-hydroxyapatite (PCL/nHA) based nanofibers for bone tissue engineering application. 10th Asian Control Conf (ASCC) 1–4. 10.1109/ASCC.2015.7244569.
- Christanti Y, Walker LM. Surface tension driven jet break up of strain-hardening polymer solutions. J Nonnewton Fluid Mech. 2001;100:9–26. doi: 10.1016/S0377-0257(01)00135-5. [DOI] [Google Scholar]
- Cipitria A, Skelton A, Dargaville TR, Dalton PD, Hutmacher DW. Design, fabrication and characterization of PCL electrospun scaffolds: a review. J Mater Chem. 2011;21:9419–9453. doi: 10.1039/c0jm04502k. [DOI] [Google Scholar]
- Coimbra P, Santos P, Alves P, Miguel SP, Carvalho MP, de Sa KD, Correia L, Ferreira P. Coaxial electrospun PCL/Gelatin-MA fibers as scaffolds for vascular tissue engineering. Colloids Surf b Biointerfaces. 2017;159:7–15. doi: 10.1016/j.colsurfb.2017.07.065. [DOI] [PubMed] [Google Scholar]
- Crowder SW, Liang Y, Rath R, Park AM, Maltais S, Pintauro PN, Hofmeister W, Lim CC, Wang X, Sung HJ. Poly(-caprolactone) carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells. Nanomedicine. 2013;8:1763–1776. doi: 10.2217/nnm.12.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MLA, Martins A, Costa-Pinto AR, Costa P, Faria S, Gomes M, Reis RL, Neves NM. Cartilage tissue engineering using electrospun pcl nanofiber meshes and MSCs. Biomacromol. 2010;11:3228–3236. doi: 10.1021/bm100476r. [DOI] [PubMed] [Google Scholar]
- Davidson MG, Jones MD, Lunn MD, Mahon MF. Synthesis and X-ray structures of new titanium (IV) aryloxides and their exploitation for the ring opening polymerization of ε-caprolactone. Inorg Chem. 2006;45:2282–2287. doi: 10.1021/ic051708n. [DOI] [PubMed] [Google Scholar]
- Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:1–19. doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- Diaz E, Sandonis I, Valle MB. In vitro degradation of poly(caprolactone)/nHA composites. J Nanomater. 2014;2014:1–8. doi: 10.1155/2014/802435. [DOI] [Google Scholar]
- Dong H, Wang H, Cao S, Shen J. Lipase-catalyzed polymerization of lactones and linear hydroxyesters. Biotechnol Lett. 1998;20:905–908. doi: 10.1023/A:1005441707356. [DOI] [Google Scholar]
- Duan N, Geng X, Ye L, Zhang A, Feng Z, Guo L, Gu Y. A vascular tissue engineering scaffold with core-shell structured nano-fibers formed by coaxial electrospinning and its biocompatibility evaluation. Biomed Mater. 2016;11:035007–035018. doi: 10.1088/1748-6041/11/3/035007. [DOI] [PubMed] [Google Scholar]
- Duda A. Polymerization of ε-caprolactone initiated by aluminum isopropoxide carried out in the presence of alcohols and diols: kinetics and mechanism. Macromolecules. 1996;29:1399–1406. doi: 10.1021/ma951442b. [DOI] [Google Scholar]
- Dutta SD, Patel DK, Seo YR, Park CW, Lee SH, Kim JW, Kim J, Seonwoo H, Lim KT. In vitro biocompatibility of electrospun poly (ε-caprolactone)/cellulose nanocrystals-nanofibers for tissue engineering. J Nanomater. 2019;2019:1–11. doi: 10.1155/2019/2061545. [DOI] [Google Scholar]
- Ebrahimi O, Rahmati-Yamchi M, Davaran S. Preparation and characterization of novel electrospun poly(ε-caprolactone)-based nanofibrous scaffolds. Artif Cells Nanomed Biotechnol. 2014;44:504–509. doi: 10.3109/21691401.2014.965310. [DOI] [PubMed] [Google Scholar]
- Entekhabi E, Nazarpak MH, Moztarzadeh F, Sadeghi A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater Sci Eng C. 2016;69:380–387. doi: 10.1016/j.msec.2016.06.078. [DOI] [PubMed] [Google Scholar]
- Eslami M, Vrana NE, Zorlutuna P, Sant S, Jung S, Masoumi N, Khavari-Nejad RA, Javadi G, Khademhosseini A. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J Biomater Appl. 2014;29:399–410. doi: 10.1177/2F0885328214530589. [DOI] [PubMed] [Google Scholar]
- Ezhilarasu H, Sadiq A, Ratheesh G, Sridhar S, Ramakrishna S, Mohd MH, Rahim YMM, Jose R, Reddy VJ. Functionalized core/shell nanofibers for the differentiation of mesenchymal stem cells for vascular tissue engineering. Nanomedicine. 2019;14:201–214. doi: 10.2217/nnm-2018-0271. [DOI] [PubMed] [Google Scholar]
- Franca DC, Bezerra EB, de Souza Morais DD, Araujo EM, Wellen RMR. Hydrolytic and thermal degradation of PCL and PCL/bentonite compounds. Mater Res. 2016;19:618–627. doi: 10.1590/1980-5373-MR-2015-0797. [DOI] [Google Scholar]
- Fu W, Liu Z, Feng B, Hu R, He X, Wang H, Yin M, Huang H, Zhang H, Wang W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int J Nanomedicine. 2014;9:2335–2344. doi: 10.2147/2FIJN.S61375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S, Chou CF, Dinda AK, Potdar PD, Mishra NC. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater Sci Eng C. 2014;34:402–409. doi: 10.1016/j.msec.2013.09.043. [DOI] [PubMed] [Google Scholar]
- Gentile P, McColgan-Bannon K, Gianone NC, Sefat F, Dalgarno K, Ferreira AM. Biosynthetic PCL-graft-collagen bulk material for tissue engineering applications. Materials. 2017;10:1–17. doi: 10.3390/ma10070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafari AM, Rajabi-Zeleti S, Naji M, Ghanian MH, Baharvand H. Mechanical reinforcement of urinary bladder matrix by electrospun polycaprolactone nanofibers. Sci Iran. 2017;24:3476–3480. doi: 10.24200/sci.2017.4418. [DOI] [Google Scholar]
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Ghasemi-Mobarakeh L, Morshed M, Karbalaei K, Fesharaki M, Nasr-Esfahani MH, Baharvand H. Electrospun poly (ε-caprolactone) nanofiber mat as extracellular matrix. Cell J. 2008;10:179–184. [Google Scholar]
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Bio-functionalized PCL nanofibrous scaffolds for nerve tissue engineering. Mater Sci Eng C. 2010;30:1129–1136. doi: 10.1016/j.msec.2010.06.004. [DOI] [Google Scholar]
- Ghobeira R, Geyter ND, Morent R. Plasma surface functionalization of biodegradable electrospun scaffolds for tissue engineering applications. In: Rohman G, editor. Biodegradable polymers: recent developments and new perspectives. Croatia: IAPC Publishing; 2017. pp. 191–236. [Google Scholar]
- Ghosal K, Manakhov A, Zajickova L, Thomas S. Structural and surface compatibility study of modified electrospun poly(ε-caprolactone) (PCL) composites for skin tissue engineering. AAPS Pharm Sci Tech. 2017;18:72–81. doi: 10.1208/s12249-016-0500-8. [DOI] [PubMed] [Google Scholar]
- Gomes S, Rodrigues G, Martins G, Henriques C, Silva JC. Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. Int J Biol Macromol. 2017;102:1174–1185. doi: 10.1016/j.ijbiomac.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Gupta D, Venugopal J, Prabhakaran MP, Dev VRG, Low S, Choon AT, Ramakrishna S. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater. 2009;5:2560–2569. doi: 10.1016/j.actbio.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Hackett JM, Dang TNT, Tsai EC, Cao X. Electrospun biocomposite polycaprolactone/collagen tubes as scaffolds for neural stem cell differentiation. Materials. 2010;3:3714–3728. doi: 10.3390/ma3063714. [DOI] [Google Scholar]
- Hasan A, Soliman S, El Hajj F, Tseng YT, Yalcin HC, Marei HE. Fabrication and in vitro characterization of a tissue engineered PCL-PLLA heart valve. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-26452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari M, Bahrami SH, Ranjbar-Mohammadi M, Milan PB. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater Sci Eng C. 2019;103:109768–109777. doi: 10.1016/j.msec.2019.109768. [DOI] [PubMed] [Google Scholar]
- Hekmati AH, Norouzi M. Electrospun scaffolds for cardiac tissue engineering. In: Uyar T, Kny E, editors. Electrospun materials for tissue engineering and biomedical applications: research, design and commercialization. UK: Woodhead Publishing; 2017. pp. 289–297. [Google Scholar]
- Hiep NT, Khon HC, Hai ND, Byong-Taek L, Toi VV, Hung LT. Biocompatibility of PCL/PLGA-BCP porous scaffold for bone tissue engineering applications. J Biomater Sci Polym Ed. 2017;28:864–878. doi: 10.1080/09205063.2017.1311821. [DOI] [PubMed] [Google Scholar]
- Hitscherich P, Aphale A, Gordan R, Whitaker R, Singh P, Lai-Hua X, Patra P, Lee EJ. Electroactive graphene composite scaffolds for cardiac tissue engineering. J Biomed Mater Res Part A. 2018;106:2923–2933. doi: 10.1002/jbm.a.36481. [DOI] [PubMed] [Google Scholar]
- Houshyar S, Kumar GS, Rifai A, Tran N, Nayak R, Shanks RA, Padhye R, Fox K, Bhattacharyya A. Nanodiamond/poly-ε-caprolactone nanofibrous scaffold for wound management. Mater Sci Eng C. 2019;100:378–387. doi: 10.1016/j.msec.2019.02.110. [DOI] [PubMed] [Google Scholar]
- Huang H, Xu H, Zhang J. Current tissue engineering approaches for cartilage regeneration. In: Nikolopoulos DD, Safos GK, Dimitrios K, editors. Cartilage tissue engineering and regeneration techniques. UK: IntechOpen; 2019. pp. 1–20. [Google Scholar]
- Jana S, Bhagia A, Lerman A. Optimization of polycaprolactone fibrous scaffold for heart valve tissue engineering. Biomed Mater. 2019;14:065014–065030. doi: 10.1088/1748-605X/ab3d24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Augustine R, Kalarikkal N, Thomas S, Seantier B, Grohens Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater Today Commun. 2019;19:319–335. doi: 10.1016/j.mtcomm.2019.02.009. [DOI] [Google Scholar]
- Jun I, Han HS, Edwards JR, Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19:745–759. doi: 10.3390/ijms19030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai D, Prabhakaran MP, Stahl B, Eblenkamp M, Wintermantel E, Ramakrishna S. Mechanical properties and in vitro behavior of nanofiber-hydrogel composites for tissue engineering applications. Nanotechnology. 2012;23:095705. doi: 10.1088/0957-4484/23/9/095705. [DOI] [PubMed] [Google Scholar]
- Khang G. Handbook of intelligent scaffolds for tissue engineering and regenerative medicine. 2. Singapore: Pan Stanford Publishing; 2017. [Google Scholar]
- Kim JI, Kim CS. Harnessing nanotopography of PCL/collagen nanocomposite membrane and changes in cell morphology coordinated with wound healing activity. Mater Sci Eng C. 2018;91:824–837. doi: 10.1016/j.msec.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Kim MS, Jun I, Shin YM, Jang W, Kim SI, Shin H. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol Biosci. 2010;10:91–100. doi: 10.1002/mabi.200900168. [DOI] [PubMed] [Google Scholar]
- Kim HN, Kang D-H, Kim MS, Jiao A, Kim D-H, Suh K-Y. Patterning methods for polymers in cell and tissue engineering. Ann Biomed Eng. 2012;40:1339–1355. doi: 10.1007/s10439-012-0510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsara M, Agbulut O, Kontziampasis D, Chen Y, Menasche P. Fibers for hearts: a critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017;48:20–40. doi: 10.1016/j.actbio.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Ko YM, Choi DY, Jung SC, Kim BH. Characteristics of plasma treated electrospun polycaprolactone (PCL) nanofiber scaffold for bone tissue engineering. J Nanosci Nanotechnol. 2015;15:192–195. doi: 10.1166/jnn.2015.8372. [DOI] [PubMed] [Google Scholar]
- Ku SH, Park CB. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31:9431–9437. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Lam CXF, Savalani MM, Teoh SH, Hutmacher DW. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomed Mater. 2008;3:034108–34123. doi: 10.1088/1748-6041/3/3/034108. [DOI] [PubMed] [Google Scholar]
- Li H, Huang C, Jin X, Ke Q. An electrospun poly(ε-caprolactone) nanocomposite fibrous mat with a high content of hydroxyapatite to promote cell infiltration. RSC Adv. 2018;8:25228–25235. doi: 10.1039/c8ra02059k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wang Y, Jin X, Dou J, Han X, Wan X, Yuan J, Shen J. Fabrication of PCL/keratin composite scaffolds for vascular tissue engineering with catalytic generation of nitric oxide potential. J Mater Chem B. 2020;8:6092–6099. doi: 10.1039/d0tb00857e. [DOI] [PubMed] [Google Scholar]
- Mahapatro A, Kumar A, Gross RA. Mild, solvent-free ω-hydroxy acid polycondensations catalyzed by candida antarctica lipase B. Biomacromol. 2004;5:62–68. doi: 10.1021/bm0342382. [DOI] [PubMed] [Google Scholar]
- Mahoney C, Conklin D, Waterman J, Bhattarai N. Electrospun nanofibers of poly (ε-caprolactone)/depolymerized chitosan for respiratory tissue engineering applications. J Biomater Sci Polym Ed. 2016;27:611–625. doi: 10.1080/09205063.2016.1144454. [DOI] [PubMed] [Google Scholar]
- Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. 2018;29:863–893. doi: 10.1080/09205063.2017.1394711. [DOI] [PubMed] [Google Scholar]
- Mingotaud A-F, Cansell F, Gilbert N, Soum A. Cationic and anionic ring-opening polymerization in supercritical CO2 preliminary results. Polymer J. 1999;31:406–410. doi: 10.1295/polymj.31.406. [DOI] [Google Scholar]
- Mkhabelal VJ, Ray SS. Poly(ε-caprolactone) nanocomposite scaffolds for tissue engineering: a brief overview. J Nanosci Nanotechnol. 2014;14:535–545. doi: 10.1166/jnn.2014.9055. [DOI] [PubMed] [Google Scholar]
- Mohammadi MR, Rabbani S, Bahrami SH, Joghataei MT, Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers. Mater Sci Eng C. 2016;69:1183–1191. doi: 10.1016/j.msec.2016.08.032. [DOI] [PubMed] [Google Scholar]
- Mondal D, Griffith M, Venkatraman SS. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. Int J Polym Mater Polym Biomater. 2016;65:255–265. doi: 10.1080/00914037.2015.1103241. [DOI] [Google Scholar]
- Munir N, Callanan A. Novel phase separated polycaprolactone/collagen scaffolds for cartilage tissue engineering. Biomed Mater. 2018;13:051001–51012. doi: 10.1088/1748-605X/aac91f. [DOI] [PubMed] [Google Scholar]
- Naghashzargar E, Fare S, Catto V, Bertoldi S, Semnani D, Karbasi S, Tanzi MC. Nano/micro hybrid scaffold of PCL or P3Hb nanofibers combined with silk fibroin for tendon and ligament tissue engineering. J Appl Biomater Funct Mater. 2015;13:156–168. doi: 10.5301/jabfm.5000216. [DOI] [PubMed] [Google Scholar]
- Naragund VS, Panda PK. Electrospinning of polyacrylonitrile nanofiber membrane for bacteria removal. J Mater Sci Appl. 2018;4:68–74. [Google Scholar]
- Natta FJV, Hill JW, Carothers WH. Studies of polymerization and ring formation. XXIII. 1 ε-caprolactone and its polymers. J Am Chem Soc. 1934;56:455–457. doi: 10.1021/ja01317a053. [DOI] [Google Scholar]
- Nazeer MA, Yilgor E, Yilgor I. Electrospun polycaprolactone/silk fibroin nanofibrous bioactive scaffolds for tissue engineering applications. Polymer. 2019;168:86–94. doi: 10.1016/j.polymer.2019.02.023. [DOI] [Google Scholar]
- Nomura N, Taira A, Tomioka T, Okada M. Catalytic approach for cationic living polymerization: Sc(OTf)3-catalyzed ring-opening polymerization of lactones. Macromolecules. 2000;33:1497–1499. doi: 10.1021/ma991580r. [DOI] [Google Scholar]
- Norman JJ, Desai TA. Control of cellular organization in three dimensions using a microfabricated polydimethylsiloxane-collagen composite tissue scaffold. Tissue Eng. 2005;11:378–387. doi: 10.1089/ten.2005.11.378. [DOI] [PubMed] [Google Scholar]
- Panda PK, Sahoo B. Synthesis and applications of electrospun nanofibers-a review. In: Navani NK, Sinha S, Govil JN, editors. Nanotechnology, fundamental and applications. 1. Studiun Press LLC Publishing; 1990. pp. 399–416. [Google Scholar]
- Panda PK, Sahoo B. Water purification by removal of pathogens using electrospun polymer nanofiber membranes: a review. J Mater Sci Appl. 2019;5:1–8. [Google Scholar]
- Pattanashetti NA, Achari DD, Torvi AI, Doddamani RV, Kariduraganavar MY. Multilayer electrospinning of PCL and PVA: NaAlg nanofibres for bone tissue engineering. Materialia. 2019 doi: 10.2139/ssrn.3484669. [DOI] [Google Scholar]
- Perale G, Hilborn J. Bioresorbable polymers for biomedical applications: From fundamentals to translational medicine. Cambridge: Woodhead Publishing; 2016. [Google Scholar]
- Pitt CG, Chasalow FI, Hibionada YM, Klimas DM, Schindler A. Aliphatic polyesters I. The degradation of poly(ϵ-caprolactone) in vivo. J Appl Polym Sci. 1981;26:3779–3787. doi: 10.1002/app.1981.070261124. [DOI] [Google Scholar]
- Prabhakaran MP, Venugopal JR, Chyan TT, Hai LB, Chan CK, Lim AY, Ramakrishna S. Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Eng Part A. 2008;14:1787–1797. doi: 10.1089/ten.tea.2007.0393. [DOI] [PubMed] [Google Scholar]
- Qin X. Coaxial electrospinning of nanofibers. In: Afshari M, editor. Electrospun nanofibers. UK: Woodhead Publishing; 2017. pp. 41–71. [Google Scholar]
- Rad ZP, Mokhtari J, Abbasi M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater Sci Eng C. 2018;93:356–366. doi: 10.1016/j.msec.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Rad ZP, Mokhtari J, Abbasi M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int J Biol Macromol. 2019;135:530–543. doi: 10.1016/j.ijbiomac.2019.05.204. [DOI] [PubMed] [Google Scholar]
- Rai R, Tallawi M, Frati C, Falco A, Gervasi A, Quaini F, Roether JA, Hochburger T, Schuhert DW, Seik L, Barbani N, Lazzeri L, Rosellini E, Boccaccini AR. Bioactive electrospun fibers of Poly(glycerol sebacate) and Poly(ε-caprolactone) for cardiac patch application. Adv Healthc Mater. 2015;4:2012–2025. doi: 10.1002/adhm.201500154. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S, Fujihara K, Teo WE, Lim TC, Ma Z. An introduction to electrospinning and nanofibers. Singapore: World Scientific; 2005. [Google Scholar]
- Ranjbar-Mohammadi M, Bahrami SH. Development of nanofibrous scaffolds containing gum tragacanth/poly (ε-caprolactone) for application as skin scaffolds. Mater Sci Eng C. 2015;48:71–79. doi: 10.1016/j.msec.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Reddy CS, Venugopal JR, Ramakrishna S, Zussman E. Polycaprolactone/oligomer compound scaffolds for cardiac tissue engineering. J Biomed Mater Res A. 2014;102:3713–3725. doi: 10.1002/jbm.a.35045. [DOI] [PubMed] [Google Scholar]
- Remya KR, Joseph J, Mani S, John A, Varma HK, Ramesh P. Nanohydroxyapatite incorporated electrospun polycaprolactone/polycaprolactone-polyethyleneglycol-polycaprolactone blend scaffold for bone tissue engineering applications. J Biomed Nanotechnol. 2013;9:1483–1494. doi: 10.1166/jbn.2013.1640. [DOI] [PubMed] [Google Scholar]
- Rijal NP, Adhikari U, Khanal S, Pai D, Sankar J. Magnesium oxide-poly (ε-caprolactone)-chitosan based composite nano fiber for tissue engineering applications. Mater Sci Eng B. 2018;228:18–27. doi: 10.1016/j.mseb.2017.11.006. [DOI] [Google Scholar]
- Robb B, Lennox B. The electrospinning process, conditions & control. In: Bosworth LA, Downes S, editors. Electrospinning for tissue regeneration. UK: Woodhead Publishing; 2011. pp. 51–66. [Google Scholar]
- Rocco KA, Maxfield MW, Best CA, Dean EW, Breuer CK. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng Part B Rev. 2014;20:628–640. doi: 10.1089/ten.teb.2014.0123. [DOI] [PubMed] [Google Scholar]
- Roy T, Maity PP, Rameshbabu AP, Das B, John A, Dutta A, Ghorai SK, Chattopadhyay S, Dhara S. Core-shell nanofibrous scaffold based on polycaprolactone-silk fibroin emulsion electrospinning for tissue engineering applications. Bioengineering. 2018;5:68–85. doi: 10.3390/bioengineering5030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saderi N, Rajabi M, Akbari B, Firouzi M, Hassannejad Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J Mater Sci Mater Med. 2018;29:134–144. doi: 10.1007/s10856-018-6144-3. [DOI] [PubMed] [Google Scholar]
- Safaeijavan R, Soleimani M, Divsalar A, Eidi A. Biological behavior study of gelatin coated PCL nanofiberous electrospun scaffolds using fibroblasts. J Paramed Sci. 2014;5:67–73. doi: 10.22037/jps.v5i1.5467. [DOI] [Google Scholar]
- Salehi M, Niyakan M, Ehterami A, Haghi-Daredeh S, Nazarnezhad S, Abbaszadeh-Goudarzi G, Vaez A, Hashemi SF, Rezaei N, Mousavi SR. Porous electrospun poly(ε-caprolactone)/gelatin nanofibrous mat containing cinnamon for wound healing application: in vitro and in vivo study. Biomed Eng Lett. 2020;10:149–161. doi: 10.1007/s13534-019-00138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gonzalez S, Diban N, Urtiaga A. Hydrolytic degradation and mechanical stability of poly(ε-caprolactone)/reduced graphene oxide membranes as scaffolds for in vitro neural tissue regeneration. Membranes. 2018;8:12–26. doi: 10.3390/membranes8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S, Shavandi M, Ahangari G, Shokrolahi F. Electrospun layered double hydroxide/poly(ε-caprolactone) nanocomposite scaffolds for adipogenic differentiation of adipose-derived mesenchymal stem cells. Appl Clay Sci. 2016;128:52–63. doi: 10.1016/j.clay.2016.04.004. [DOI] [Google Scholar]
- Shafei S, Foroughi J, Chen Z, Wong CS, Naebe M. Short oxygen plasma treatment leading to long-term hydrophilicity of conductive PCL-PPy nanofiber scaffolds. Polymers. 2017;9:614–628. doi: 10.3390/polym9110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S, Ai J, Azami M, Verdi J, Atlasi MA, Shirian S, Samadikuchaksaraei A. Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J Biomed Mater Res Part B Appl Biomater. 2018;106:1578–1586. doi: 10.1002/jbm.b.33966. [DOI] [PubMed] [Google Scholar]
- Sharifi F, Atyabi SM, Norouzian D, Zandi M, Irani S, Bakhshi H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int J Biol Macromol. 2018;115:243–248. doi: 10.1016/j.ijbiomac.2018.04.045. [DOI] [PubMed] [Google Scholar]
- Shelke NB, Lee P, Anderson M, Mistry N, Nagarale RK, Ma XM, Yu X, Kumbar SG. Neural tissue engineering: nanofiber-hydrogel based composite scaffolds. Polym Adv Technol. 2016;27:42–51. doi: 10.1002/pat.3594. [DOI] [Google Scholar]
- Sheng L, Jiang R, Zhu Y. Electrospun cellulose nanocrystals/polycaprolactone nanocomposite fiber mats. J Macromol Sci b: Physics. 2014;53:820–828. doi: 10.1080/00222348.2013.861311. [DOI] [Google Scholar]
- Siddiqui N, Asawa S, Birru B, Baadhe R, Rao S. PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol. 2018;60:506–532. doi: 10.1007/s12033-018-0084-5. [DOI] [PubMed] [Google Scholar]
- Silva JC, Udangawa RN, Chen J, Mancinelli CD, Garrudo FFF, Mikael PE, Cabral JMS, Ferreira FC, Linhardt RJ. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater Sci Eng C. 2020;107:110291–110303. doi: 10.1016/j.msec.2019.110291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver FH, Bradica G, Tria A. Elastic energy storage in human articular cartilage: estimation of the elastic modulus for type II collagen and changes associated with osteoarthritis. Matrix Biol. 2002;21:129–137. doi: 10.1016/S0945-053X(01)00195-0. [DOI] [PubMed] [Google Scholar]
- Strange DG, Tonsomboon K, Oyen ML. Mechanical behaviour of electrospun fibre-reinforced hydrogels. J Mater Sci Mater Med. 2014;25:681–690. doi: 10.1007/s10856-013-5123-y. [DOI] [PubMed] [Google Scholar]
- Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96:557–569. doi: 10.1002/app.21481. [DOI] [Google Scholar]
- Subia B, Kundu J, Kundu SC. Biomaterial scaffold fabrication techniques for potential tissue engineering applications. In: Eberli D, editor. Tissue engineering. Europe: InTech Publisher; 2010. pp. 141–157. [Google Scholar]
- Sumitha MS, Shalumon KT, Sreeja VN, Jayakumar R, Nair SV, Menon D. Biocompatible and antibacterial nanofibrous poly(ε-caprolactone)-nanosilver composite scaffolds for tissue engineering applications. J Macromol Sci Part A Pure Appl Chem. 2012;49:131–138. doi: 10.1080/10601325.2012.642208. [DOI] [Google Scholar]
- Tian H, Tang Z, Zhuang X, Chen X, Jing X. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci. 2012;37:237–280. doi: 10.1016/j.progpolymsci.2011.06.004. [DOI] [Google Scholar]
- Venugopal J, Zhang YZ, Ramakrishna S. Fabrication of modified and functionalized polycaprolactone nanofibre scaffolds for vascular tissue engineering. Nanotechnology. 2005;16:2138–2142. doi: 10.1088/0957-4484/16/10/028. [DOI] [PubMed] [Google Scholar]
- Venugopal J, Ma LL, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11:847–854. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- Venugopal E, Sahanand KS, Bhattacharyya A, Rajendran S. Electrospun PCL nanofibers blended with Wattakaka volubilis active phytochemicals for bone and cartilage tissue engineering. Nanomed Nanotechnol Biol Med. 2019;21:102044–102054. doi: 10.1016/j.nano.2019.102044. [DOI] [PubMed] [Google Scholar]
- Vogt L, Rivera LR, Liverani L, Piegat A, El Fray M, Boccaccini AR. Poly(ε-caprolactone)/poly(glycerol sebacate) electrospun scaffolds for cardiac tissue engineering using benign solvents. Mater Sci Eng C. 2019;103:109712–109725. doi: 10.1016/j.msec.2019.04.091. [DOI] [PubMed] [Google Scholar]
- Wang X, Ding B, Li B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today. 2013;16:229–241. doi: 10.1016/j.mattod.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won BSK, Ee KL, Ae WB, Im HK. Precipitation polymerization of 2-methylene-1,3-dioxepane in supercritical carbon dioxide. Polym J. 2008;40:332–338. doi: 10.1295/polymj.PJ2007095. [DOI] [Google Scholar]
- Xie J, MacEwcm MR, Willerth SM, Li X, Moran DW, Sakiyama-Elbert SE, Xia Y. Conductive core-sheath nanofibers and their potential application in neural tissue engineering. Adv Funct Mater. 2009;19:2312–2318. doi: 10.1002/adfm.200801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Jones J, Yuan X, Xu X, Sheng J, Lee JCM, Ma G, Yu Q. Plasma treatment of random and aligned electrospun PCL nanofibers. J Med Biol Eng. 2013;33:171–178. doi: 10.5405/jmbe.1072. [DOI] [Google Scholar]
- Yao Q, Cosme JGL, Xu T, Miszuk JM, Picciani PHS, Fong H, Sun H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarin AL. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym Adv Technol. 2011;22:310–317. doi: 10.1002/pat.1781. [DOI] [Google Scholar]
- Zadehnajar P, Akbari B, Karbasi S, Mirmusavi MH. Preparation and characterization of poly ε-caprolactone-gelatin/multi-walled carbon nanotubes electrospun scaffolds for cartilage tissue engineering applications. Int J Polym Mater Polym Biomater. 2020;69:326–337. doi: 10.1080/00914037.2018.1563088. [DOI] [Google Scholar]
- Zadehnajar P, Karbasi S, Akbari B, Ghasemi L. Incorporation of multi-walled carbon nanotubes into electrospun PCL/gelatin scaffold: The influence on the physical, chemical and thermal properties and cell response for tissue engineering. Mater Technol. 2020;35:39–49. doi: 10.1080/10667857.2019.1651539. [DOI] [Google Scholar]
- Zahedi E, Esmaeili A, Eslahi N, Shokrgozar MA, Simchi A. Fabrication and characterization of core-shell electrospun fibrous mats containing medicinal herbs for wound healing and skin tissue engineering. Mar Drugs. 2019;17:1–13. doi: 10.3390/md17010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarekhalili Z, Bahrami SH, Ranjbar-Mohammadi M, Milan PB. Fabrication and characterization of PVA/Gum tragacanth/PCL hybrid nanofibrous scaffolds for skin substitutes. Int J Biol Macromol. 2017;94:679–690. doi: 10.1016/j.ijbiomac.2016.10.042. [DOI] [PubMed] [Google Scholar]