Abstract
Peritoneal dialysis (PD) is valuable for patients starting on renal replacement therapy because it preserves residual renal function, maintains hemodynamic stability, and affords higher quality of life than hemodialysis. Amyloid-related kidney disease is a rare condition and a cause of end-stage renal disease, the incidence of which appears to be rising in recent years. Hemoperitoneum is a common complication of PD. In some cases, it requires urgent treatment and careful monitoring for deterioration and potential complications. Although the kidney is a retroperitoneal organ, renal hemorrhage can cause bloody peritoneal dialysate. We encountered a rare case of amyloid light-chain amyloidosis where bilateral perirenal hematoma occurred shortly after initiation of PD. Amyloid angiopathy with increased blood vessel fragility and impaired vasoconstriction may promote bleeding. Therefore, hemoperitoneum in a patient on PD with disease causing fragile blood vessels, such as amyloidosis, should alert the physician to the possibility of underlying angiopathy.
Keywords: Amyloid light-chain amyloidosis, Bloody peritoneal dialysate, Hemoperitoneum, Peritoneal dialysis, Renal hemorrhage
Introduction
Amyloid light-chain (AL) amyloidosis is a heterogeneous group of diseases characterized by extracellular deposition of immunoglobulin light chains [1]. These amyloid fibrils can deposit in various locations, including the heart and kidneys, and cause organ failure [2]. Amyloid-related kidney disease is a rare condition and a cause of end-stage renal disease (ESRD), the incidence of which appears to be rising in recent years [3]. We are increasingly choosing renal replacement therapy (RRT) for patients with ESRD due to amyloidosis. Peritoneal dialysis (PD) is valuable for patients starting on RRT because it preserves residual renal function (RRF), maintains hemodynamic stability, and affords higher quality of life than hemodialysis (HD) [4]. Therefore, the number of cases of PD with underlying renal amyloidosis is expected to increase. Hemoperitoneum is a common complication of PD. Bloody peritoneal dialysate might be caused by the catheter itself, uremic bleeding, rupture of a renal cyst, retrograde menstrual bleeding, ovulation, retroperitoneal disease, or peritonitis [5]. Amyloidosis can be complicated by potentially life-threatening hemorrhage. We encountered a rare case of AL amyloidosis with bilateral perirenal hematoma that developed shortly after initiation of PD.
Case report
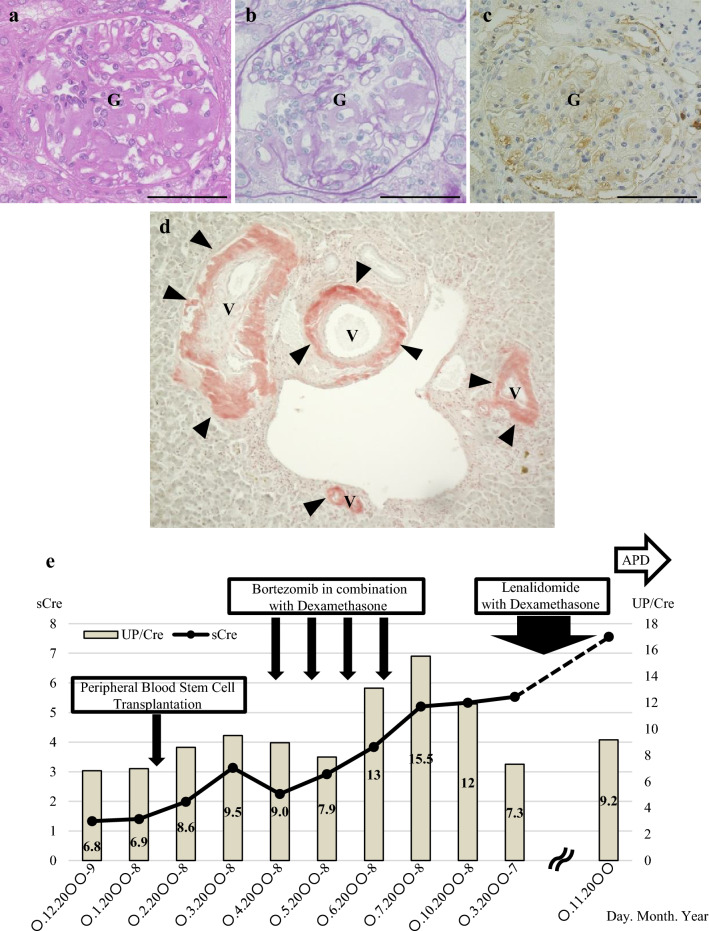
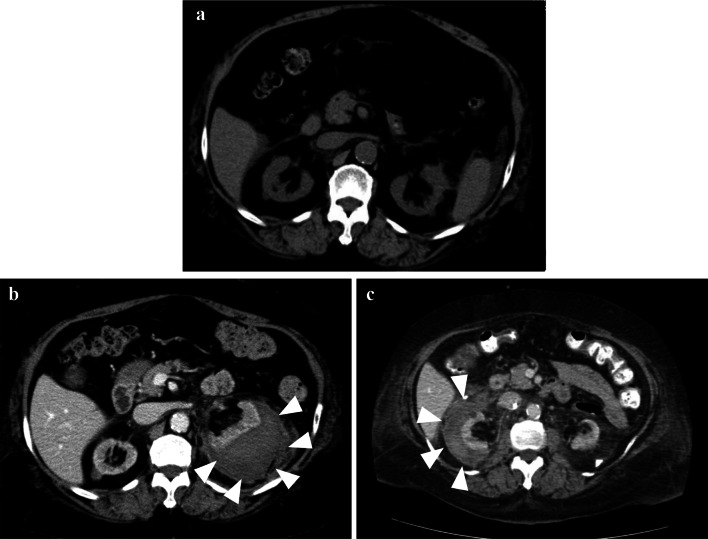
The patient was a 62-year-old woman who had been diagnosed with AL (primary) amyloidosis (λ immunoglobulin light chains) at the age of 53 years by renal biopsy (Left kidney). The histological findings at the time of the renal biopsy are shown in Fig. 1. Significant amyloid deposits were present in the vascular walls (Fig. 1d). We performed bone marrow biopsy to rule out multiple myeloma. Clonal plasma cells in bone marrow were less than 10%. Serum M protein was less than 3 g/dL and bone scintigraphy showed no bone lesions. Therefore, multiple myeloma was excluded. She underwent autologous stem cell transplantation followed by treatment with bortezomib–dexamethasone (Fig. 1e). However, her renal function gradually worsened. When AL amyloidosis was diagnosed, her renal function had already deteriorated (eGFR: 35 mL/min/1.73 m2). She had mild hypertension but no diabetes mellitus. We attributed the decline in renal function to an effect of AL amyloidosis. We considered that the persistent severe proteinuria due to AL amyloidosis resulted in progression to CKD. Eight years after the diagnosis of AL amyloidosis, she was started on PD because of ESRD. She did not undergo chemotherapy after induction of PD because the serum difference in involved and uninvolved free light chains (dFLC) was 13 mg/L, indicating a VGPR (very good partial response) at that time. At initiation of PD, her blood pressure was 131/82 mmHg, pulse was 97/min, body temperature was 36.6 °C, body height was 1.52 m, and body weight was 94.8 kg (body mass index, 41 kg/m2). Her chief concern was fatigue. The laboratory data at initiation of PD are summarized in Table 1. Automated PD (low-calcium peritoneal dialysis solution with 1.5% dextrose; Baxter, Deerfield, IL, USA) was performed and the patient did well in the outpatient setting. The APD prescription was dwell volume of 2400 mL/m2, 2.2 h/cycle, and 3 cycles/session, which provides 7200 mL of total volume and 8 h per session. She was taking some oral medications, including an antihypertensive drug, a diuretic, active vitamin D, a statin, and a xanthine oxidase reductase inhibitor. She had also previously had an ischemic stroke and was taking prophylactic low-dose aspirin. She did not develop perirenal hematoma between the diagnosis of AL amyloidosis and the initiation of PD (Fig. 2a). Six months after starting PD, she developed a perirenal hematoma that was diagnosed by contrast-enhanced computed tomography (Fig. 2b, left kidney). We confirmed bloody peritoneal dialysate (PD effluent; red blood cells > 100 per high-power field), left lumbar back pain, and severe anemia (hemoglobin decreased from 10.5 g/dL to 7.3 g/dL). The symptoms improved with conservative treatment, so PD was continued. One year after the first perirenal hematoma was detected, another with hemoperitoneum was seen on the contralateral side (Fig. 2c). She was admitted to hospital where she received a blood transfusion for hypotension. Aspirin was discontinued. However, her residual renal function deteriorated because of severe anemia (hemoglobin decreased from 10.3 g/dL to 6.9 g/dL), so PD was discontinued (Fig. 3). The laboratory data for the renal hemorrhage event on the contralateral side are summarized in Table 2.
Fig. 1.
Pathological findings on a renal biopsy. a Hematoxylin–eosin stain, 400 × . b Periodic acid–Schiff stain, 400 × . c λ-light-chain stain, 400 × . d Direct fast scarlet stain, 100 × . e Clinical course during treatment of AL amyloidosis. APD automated peritoneal dialysis, G glomerulus, sCre serum creatinine level, UP/Cre urine protein/creatinine ratio, V vessel. Bar = 50 μm
Table 1.
The laboratory data at initiation of PD
| < Complete blood count > | < Biochemistry > | ||
| WBC | 8811/μL | TP | 6.3 g/dL |
| RBC | 329 × 104/μL | ALB | 3.4 g/dL |
| Hb | 9.5 g/dL | Glb | 2.9 g/dL |
| Ht | 30.4% | AST | 9 U/L |
| PLT | 20.0 × 104/μL | ALT | 8 U/L |
| LD | 249 U/L | ||
| < Coagulation test > | γGT | 11 U/L | |
| PT-INR | 0.94 | ALP | 127 IU/L |
| APTT | 24.6 s | Cre | 9.14 mg/dL |
| BUN | 68 mg/dL | ||
| < Venous blood gas analysis > | UA | 6.9 mg/dL | |
| pH | 7.343 | CRP | 0.34 mg/dL |
| pCO2 | 35.2 mmHg | Fe | 90 µg/dL |
| pO2 | 28.9 mmHg | Ferritin | 121 ng/mL |
| HCO3 | 18.7 mmol/L | Na | 138 mEq/L |
| Base excess | − 6.3 mmol/L | K | 4.5 mEq/L |
| Cl | 104 mEq/L | ||
| Ca | 9.5 mg/dL | ||
| IP | 6.4 mg/dL | ||
| Mg | 2.1 mg/dL |
Alb albumin, ALP alkaline phosphatase, ALT alanine aminotransferase, APTT activated partial thromboplastin time, AST aspartate aminotransferase, BUN blood urea nitrogen, Ca calcium, Cl chloride, Cre creatinine, CRP C-reactive protein, Fe iron, γGT γ-glutamyl transpeptidase, Glb globulin, Hb hemoglobin, HCO3− bicarbonate ions, Ht hematocrit, IP phosphate, K potassium, LDH lactate dehydrogenase, Mg magnesium, Na sodium, pCO2 partial pressure of carbon dioxide, PLT platelets, pO2 partial pressure of oxygen, PT-INR Prothrombin Time-International Normalized Ratio, RBC red blood cells, TP total protein, UA uric acid, WBC white blood cells
Fig. 2.
a Abdominal computed tomography (CT) scans showing before hemorrhage. b Abdominal contrast-enhanced CT scans showing the first hemorrhage (left kidney) and c second hemorrhage (right kidney)
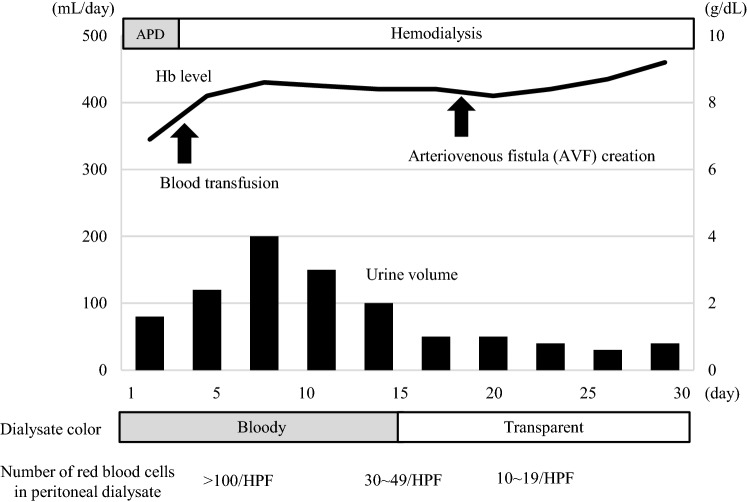
Fig. 3.
Clinical course during the second admission. Peritoneal dialysis was temporarily discontinued and hemodialysis was started on the second hospital day. The bloody peritoneal dialysate gradually thinned and became light but the patient became anuric during the hospital stay. Therefore, peritoneal dialysis was not resumed and hemodialysis was continued thereafter. Hb hemoglobin, HPF high-power field
Table 2.
Laboratory data after the second perirenal hemorrhage
| < Complete blood count > | < Biochemistry > | ||
| WBC | 9820/μL | TP | 5.1 g/dL |
| RBC | 238 × 104/μL | ALB | 1.3 g/dL |
| Hb | 6.9 g/dL | Glb | 3.8 g/dL |
| Ht | 22.3% | AST | 11 U/L |
| PLT | 26.3 × 104/μL | ALT | 8 U/L |
| LD | 315 U/L | ||
| < Coagulation test > | γGT | 8 U/L | |
| PT-INR | 1.05 | ALP | 172 IU/L |
| APTT | 26.8 s | Cre | 14.91 mg/dL |
| BUN | 38 mg/dL | ||
| < Peritoneal dialysis fluid > | UA | 7.9 mg/dL | |
| Red blood cell | > 100/HPF | CRP | 9.3 mg/dL |
| White blood cell | 1–4/HPF | Fe | 17 µg/dL |
| Mesothelial cell | < 1/HPF | Ferritin | 121 ng/mL |
| Bacteria | (−) | Na | 140 mEq/L |
| K | 4.9 mEq/L | ||
| < Peritoneal dialysate culture > | No bacteria | Cl | 101 mEq/L |
| Ca | 8.8 mg/dL | ||
| < Peritoneal dialysate cytology > | Class II | IP | 6.7 mg/dL |
| Mg | 2.2 mg/dL |
Alb albumin, ALP alkaline phosphatase, ALT alanine aminotransferase, APTT activated partial thromboplastin time, AST aspartate aminotransferase, BUN blood urea nitrogen, Ca calcium, Cl chloride, Cre creatinine, CRP C-reactive protein, Fe iron, γGT γ-glutamyl transpeptidase, Glb globulin, Hb hemoglobin, Ht hematocrit, IP phosphate, K potassium, LDH lactate dehydrogenase, Mg magnesium, Na sodium, PLT platelets, PT-INR Prothrombin Time-International Normalized Ratio, RBC red blood cells, TP total protein, UA uric acid, WBC white blood cells
Discussion
We encountered a rare case of bilateral perirenal hematoma shortly after initiation of PD. Hemoperitoneum in patients on PD is often attributed to intraperitoneal abdominal pathology, mechanical or gynecological [6]. On rare occasions, hemoperitoneum might result from pathology in the retroperitoneum, often of renal origin. Potential causes of bloody dialysate include rupture of a cyst in patients with polycystic kidney disease [7], acquired cystic disease [8], and renal tumor. Normally, the kidney is a retroperitoneal organ and is not expected to be involved in intra-abdominal bleeding. Few cases of retroperitoneal bleeding have been described. A possible explanation is adhesion between the wall of a cyst and the peritoneum because of their anatomical proximity and inflammation secondary to intracystic hemorrhage [9]. Our patient had developed multiple acquired cysts adjacent to the peritoneum in both kidneys. Therefore, we considered rupture of renal cysts and aspirin to have contributed to her severe perirenal hematoma.
These findings raise the question of why bilateral renal hematoma developed in such a short period of time. A previous report described confirmation of spontaneous or peri-interventional hemorrhage in approximately one-third (28%) of patients with systemic amyloidosis during the course of the disease [10]. In patients with AL amyloidosis, the most important pathogenetic factors are acquired hemostatic abnormalities, including coagulation factor deficiencies, hyperfibrinolysis, and platelet dysfunction [11]. It has been reported that analysis of TAT complexes, fibrinogen, and PIC can be used to differentiate localized AL amyloidosis from systemic amyloidosis [12]. We did not check TAT complexes and PIC, but FDP, ATIII, α2-PI, fibrinogen, and bleeding time were all normal (Table 3). Because our patient had renal-limited AL amyloidosis, not systemic AL amyloidosis, we speculated that amyloid angiopathy with increased blood vessel fragility and impaired vasoconstriction might have caused the perirenal hematoma. In this case, we had found significant amyloid deposition in the vessel walls on a previous renal biopsy (Fig. 1d). Although direct confirmation was not possible, vascular fragility due to amyloidosis might have contributed to rupture of a renal cysts. Therefore, patients with AL amyloidosis on PD should be carefully assessed for hemoperitoneum by abdominal computed tomography.
Table 3.
Coagulation test results
| < Coagulation test > | |
| PT-INR | 0.87 |
| APTT | 23.1 s |
| FDP | 3.2 μg/mL |
| ATIII | 95.6% |
| α2-PI | 94.0% |
| Fib | 205 mg/dL |
| Bleeding time | 2.5 min |
APTT activated partial thromboplastin time, ATIII antithrombin III, α2PI α2 plasmin inhibitor, FDP fibrin/fibrinogen degradation products, Fib fibrinogen, PT-INR Prothrombin Time-International Normalized Ratio
The molecular mechanism by which AL amyloidosis causes bilateral perirenal hematoma via cyst rupture has not been elucidated in detail. However, serum levels of angiogenic cytokines, such as angiopoietin-1, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and angiogenin, have been reported to be higher in patients with AL amyloidosis [13]. Angiogenesis has recently been implicated in the growth of renal cysts in polycystic kidney disease [14]. Therefore, renal cysts might be prone to rupture in AL amyloidosis, which is characterized by fragile blood vessels. Further study is needed in terms of basic research.
We have described our experience in a case of bilateral perirenal hematoma as a result of cyst rupture following initiation of PD in a patient with AL amyloidosis. Patients with AL amyloidosis have acquired hemostatic abnormalities and blood vessel fragility. Therefore, perirenal hemorrhage should be strongly suspected in patients with AL amyloidosis who are on PD and admitted for acute abdomen and hemoperitoneum. Thorough examination is required in such patients.
Acknowledgements
The authors would like to thank the patient and her family for their contribution to this report. The authors also thank ThinkSCIENCE, Japan, for medical writing support.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
Ethical approval for publication was not required by Kawasaki Medical School.
Informed consent
We obtained written informed consent from the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glenner GG. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts) N Engl J Med. 1980;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 2.Fuah KW, Lim CTS. Renal-limited AL amyloidosis—a diagnostic and management dilemma. BMC Nephrol. 2018;19(1):307. doi: 10.1186/s12882-018-1118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidd J, Carl DE. Renal amyloidosis. Curr Probl Cancer. 2016;40(5–6):209–219. doi: 10.1016/j.currproblcancer.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol. 2011;6(2):447–456. doi: 10.2215/CJN.07920910. [DOI] [PubMed] [Google Scholar]
- 5.Harnett JD, Gill D, Corbett L, Parfrey PS, Gault H. Recurrent hemoperitoneum in women receiving continuous ambulatory peritoneal dialysis. Ann Intern Med. 1987;107(3):341–343. doi: 10.7326/0003-4819-107-2-341. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg A, Bernardini J, Piraino BM, Johnston JR, Perlmutter JA. Hemoperitoneum complicating chronic peritoneal dialysis: single-center experience and literature review. Am J Kidney Dis. 1992;19(3):252–256. doi: 10.1016/s0272-6386(13)80006-6. [DOI] [PubMed] [Google Scholar]
- 7.Bagon JA. Haemoperitoneum originating in renal cyst in a patient with ADPKD not treated by dialysis. Nephrol Dial Transpl. 2000;15(2):251–253. doi: 10.1093/ndt/15.2.251. [DOI] [PubMed] [Google Scholar]
- 8.Levine E, Slusher SL, Grantham JJ, Wetzel LH. Natural history of acquired renal cystic disease in dialysis patients: a prospective longitudinal CT study. AJR Am J Roentgenol. 1991;156(3):501–506. doi: 10.2214/ajr.156.3.1899744. [DOI] [PubMed] [Google Scholar]
- 9.Borras M, Valdivielso JM, Egido R, de Vera PV, Bordalba JR, Fernandez E. Haemoperitoneum caused by bilateral renal cyst rupture in an ACKD peritoneal dialysis patient. Nephrol Dial Transpl. 2006;21(3):789–791. doi: 10.1093/ndt/gfi298. [DOI] [PubMed] [Google Scholar]
- 10.Mumford AD, O'Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454–460. doi: 10.1046/j.1365-2141.2000.02183.x. [DOI] [PubMed] [Google Scholar]
- 11.Sucker C, Hetzel GR, Grabensee B, Stockschlaeder M, Scharf RE. Amyloidosis and bleeding: pathophysiology, diagnosis, and therapy. Am J Kidney Dis. 2006;47(6):947–955. doi: 10.1053/j.ajkd.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Suga N, Miura N, Kitagawa W, Morita H, Banno S, Imai H. Differential diagnosis of localized and systemic amyloidosis based on coagulation and fibrinolysis parameters. Amyloid. 2012;19(2):61–65. doi: 10.3109/13506129.2012.663425. [DOI] [PubMed] [Google Scholar]
- 13.Kastritis E, Roussou M, Michael M, Gavriatopoulou M, Michalis E, Migkou M, et al. High levels of serum angiogenic growth factors in patients with AL amyloidosis: comparisons with normal individuals and multiple myeloma patients. Br J Haematol. 2010;150(5):587–591. doi: 10.1111/j.1365-2141.2010.08288.x. [DOI] [PubMed] [Google Scholar]
- 14.Reed BY, Masoumi A, Elhassan E, McFann K, Cadnapaphornchai MA, Maahs DM, et al. Angiogenic growth factors correlate with disease severity in young patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;79(1):128–134. doi: 10.1038/ki.2010.355. [DOI] [PMC free article] [PubMed] [Google Scholar]