Abstract
Urinary incontinence is one of the most common disorders especially in adult women. In this study, cellular and in-vivo analyses were performed on (3-glycidyloxypropyl) trimethoxysilane (GPTMS) and CaCl2 cross-linked alginate and gelatin hydrogels containing β-glycerophosphate and ascorbic acid to evaluate the regenerative potential as injectable compression agents for the treatment of urinary incontinence. The hydrogels were prepared with different percentages of components and were named as GA1 (7.2% w/v gelatin, 6% w/v sodium alginate, 0.5:1w/w GPTMS, CaCl2 1% (wt) sodium alginate, 50 μg/mL ascorbic acid, 1.5 mg/mL β-glycerophosphate), GA2 (10% w/v gelatin, 8.5% w/v sodium alginate, 0.5:1 w/w GPTMS, CaCl2 1% (wt) sodium alginate, 50 μg/mL ascorbic acid, 1.5 mg/mL β-glycerophosphate), and GA3 (10% (w/v) gelatin, 8.5% w/v sodium alginate, 1:1 w/w GPTMS, CaCl2 1% (wt) sodium alginate, 50 μg/mL ascorbic acid, 1.5 mg/mL β-glycerophosphate) hydrogels. The results of cell studies showed that although all three samples supported cell adhesion and survival, the cellular behavior of the GA2 sample was better than the other samples. Animal tests were performed on the optimal GA2 sample, which showed that this hydrogel repaired the misfunction tissue in a rat model within 4 weeks and the molecular layer thickness was reached the normal tissue after this duration. It seems that these hydrogels, especially GA2 sample containing 10% (w/v) gelatin, 8.5% (w/v) sodium alginate, 0.5:1 (w/w) GPTMS, CaCl2 1% (wt) sodium alginate, 50 μg/mL ascorbic acid, and 1.5 mg/mL β-glycerophosphate, can act as an injetable hydrogel for urinary incontinence treatment without the need for repeating the injection.
Keywords: Sodium alginate, Gelatin, Injectable hydrogels, GPTMS, Urinary incontinence
Introduction
Urinary incontinence or involuntary loss of urine is a prevalent problem in old women and even in young women after child birth (Ramseyer et al. 2010). This problem creates social and medical problems for patients especially for their psychological state (Heang et al. 2006). The proper relationship, normal anatomical, and physiological properties between the bladder, urethra, sphincter, pelvic floor, and nervous system result in urine continence. Any disorder in the function of these organs is associated with urinary incontinence (Lapitan and Cody 2016). Stress incontinence is the most prevalence urine incontinence problem and is defined as losing urine when coughing, laughing, sneezing or exercise; as a result of weak cushion supporting the base of the bladder and closing the urethra (Kirchin et al. 2012).
There are different methods for urinary incontinence treatment, among them injectable bulking agents, have attracted a lot of attentions (Elmelund et al. 2019). Injectable therapies are feasible and non-invasive procedure to replace these weak supporting tissue for the treatment of urinary incontinence and vesicoureteral reflux (Eui et al. 2005). Another benefit of injectable hydrogels is that they can be easily combined with bioactive agents or pharmaceutical agents, so accelerating the therapeutic process (Stevens et al. 2007). According to the above benefits, injectable bulking agent was selected for further investigations in this study.
Gelatin with excellent biocompatibility, biodegradability, low cost, and cell recognition sites is a good candidate for tissue regeneration applications (Luo et al. 2018; Namdarian et al. 2018). On the other hand, alginate is also a biodegradable and biocompatible with proper gelation, stability, and viscosity (Naghizadeh et al. 2018). The combination of gelatin and alginate was studied as injectable hydrogels with suitable properties for tissue engineering in different studies. In a recent study, Liu et al. (2020) designed therapeutic agents loaded gelatin/alginate injectable hydrogels in tissue engineering. Gelatin was used due to its cytocompatibility, the Arg–Gly–Asp (RGD) sequence, gelation properties, and proper cellular interactions; while alginate added to the composition owing to its low cost, biocompatibility, and ease of cross-linking. The prepared injectable hydrogels did not provoke side effects and accelerated the healing process. In another study, Yuan et al. (2017) investigated modified gelatin/alginate combination as injectable hydrogels for tissue engineering applications. Their results demonstrated cross-linked hydrogels had favorable mechanical, biocompatibility and controllable degradation ratio. Here, the combination of gelatin and alginate was chosen as the main components of the hydrogels according to the mentioned properties and cross-linking process was conducted by calcium chloride (CaCl2) and (3-glycidyloxypropyl) trimethoxysilane (GPTMS). CaCl2 acts as the cross-linker of the alginate and the calcium precursor of hydroxyapatite formation (Pilipenko et al. 2019; Yu et al. 2011). Silane-coupling cross-linking by GPTMS is achieved through the condensation reaction and Si–O–Si and Si–OH formation between gelatin, alginate, and GPTMS (Nouri-Felekori et al. 2019). GPTMS is a bioactive cross-linker that can enhance stability and mechanical properties of the hydrogel with no toxic effects on cell viability (Rasti et al. 2019; Sohrabi et al. 2020).
Ascorbic acid is a water soluble antioxidant and cofactor for collagen I and III synthesized by hydroxylation of proline and lysine (Maione-Silva et al. 2019). Furthermore, in an in-vivo study it was demonstrated that ascorbic acid can be useful in the improvement of urinary system function and urinary tract infections (Yousefichaijan et al. 2018). β-Glycerophosphate is an osteogenic supplement and can act as phosphorous source for hydroxyapatite mineralization (Wang and Stegemann 2010). When ascorbic acid is combined with β-glycerophosphate it synergistically induces an increase in mineralization by inducing neutral metalloproteinase addition in matrix vesicles and facilitating proteoglycans degradation and mineral precipitation (Wang et al. 2017). Although the use of injectable hydrogels is the least invasive treatment of urinary incontinence, the rapid absorption of hydrogels has limited their usage (Davis et al. 2013; Hurtado et al. 2008); therefore, the use of bioactive hydrogels improves healing process by forming an apatite compressive agent with a low rate of degradation, improving cellular interactions, stimulating host tissue nerves and muscles and as a result enhancing urinary sphincter complex function (Vardar et al. 2019; Wang et al. 2020).
In this work, we evaluated in-vitro cellular and in-vivo injection of ascorbic acid and β-glycerophosphate loaded gelatin/sodium alginate hydrogels in the urethral sphincter muscles for urinary incontinence treatment. Ascorbic acid stimulates the fibroblasts to produce more collagen and β-glycerophosphate as a bioactive agent provides the required bioactivity and forms hydroxyapatite-like bioactive compounds. Further studies have shown that hydroxyapatite-like bioactive compounds play an effective role in providing the required pressure. For this purpose, the gels were synthesized according to our previous study (Rezaei et al. 2020) and cell morphology and viability was studied in-vitro followed by histological investigations on the optimum hydrogel composition.
Materials and methods
Materials
Gelatin (Mw 40–50 kDa), (3-glycidyloxypropyl)trimethoxysilane (GPTMS, 1/07 g/mol), and glutaraldehyde (25%, d = 1.058 g/cm3) were purchased from Merck Co. Ltd. (Germany). Sodium alginate (viscosity 15–25 cP, 1% in H2O), ascorbic acid, β-glycerophosphate (solubility H2O:0.1 g/mL, clear, colorless), thiazolyl blue tetrazolium bromide (MTT, Mw 414.32 g/mol), and dimethyl sulfoxide (DMSO, 1×) were purchased from Sigma Co. Ltd. (USA). Ethanol (99.8%, Mw 46.07 g/mol), calcium chloride (CaCl2, Mw 110.98 g/mol), and phosphate buffer saline (PBS, pH 7.4) were purchased from Samchun Pure Chemicals CO. Ltd. (Korea). Dulbecco's modified eagle's medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin were purchased from Gibco-BRL, Life Technologies Co. Ltd. (NY). Botulinum-A toxin was purchased from Hugel Pharma (Korea). Medy-osmium tetroxide was purchased from Polyscience, Warmington Co. Ltd. (USA). All chemicals were utilized directly without further purification. All aqueous solutions were prepared with deionized (DI) water.
Preparation of hybrid hydrogels
Three different hydrogel compositions were prepared according to our previous study (Rezaei et al. 2020). Briefly, gelatin and sodium alginate solutions were dissolved in deionized water at 40 °C separately. After 30 min, the sodium alginate solution was added drop-wise to the gelatin solution and was stirred for one more hour at room temperature to homogenize the solution. GPTMS and calcium chloride was weighed according to the gel composition and were added to the solution under stirring condition followed by the addition of 50 μg/mL of ascorbic acid and 1.5 mg/mL of β-glycerophosphate after 2 h. The solution was stirred again and the calcium chloride (1% (wt) of sodium alginate) was added and stirred for 1 h. The prepared solutions were labeled as GA1, GA2, and GA3 according to the mentioned concentration in Table 1.
Table 1.
Samples compositions
| Code | Composition | |||||
|---|---|---|---|---|---|---|
| Gelatin (% W/V) | Sodium alginate (% W/V) | GPTMS (W/W) | CaCl2 (% wt sodium alginate) | Ascorbic acid (μg/mL) | β-Glycerophosphate (mg/mL) | |
| GA1 | 7.2 | 6 | 0/5:1 | 1 | 50 | 1.5 |
| GA2 | 10 | 8.5 | 0.5:1 | 1 | 50 | 1.5 |
| GA3 | 10 | 8.5 | 1:1 | 1 | 50 | 1.5 |
Characterization
Cell culture
To investigate cell-hydrogel interactions, cell culture was performed on the samples. For sterilization, hydrogels were washed with 0.1 M PBS and soaked in PBS solution containing penicillin–streptomycin for 4 h. After 4 h, the samples were taken out, washed with PBS solution three times and soaked in PBS solution for 4 h. Then, the samples were washed with PBS solution three times again followed by immersion in PBS solution for 24 h at 4 °C. After sterilization, 5 × 104 L929 fibroblast cells/well were seeded with the hydrogels and soaked in DMEM contained 15% (v/v) FBS and 100 mg/mL penicillin–streptomycin, and incubated in an incubator (37 °C, 5% CO2, 95% humidity) for 48 h. The culture medium was removed after 48 h and each sample was washed with PBS solution twice and fixed with 3% glutaraldehyde solution and 1% osmium tetroxide. Samples dehydration was performed by ascending concentrations of ethanol (30, 50, 70, 90, and 100%). Finally, the scaffolds were dried in air.
Cell morphology
For cell morphology study, the cultured samples after 48 h were coated by a gold thin layer and FE-SEM (MIRA3, TESCAN, Czechoslovakia) was used to observe the cell microstructure.
Cell viability
To evaluate the biocompatibility of the GA1, GA2, and GA3 samples, the MTT (3-{4,5-dimethylthiazol-2yl}-2,5-diphenyl-2H-tetrazolium bromide) test was performed after 24 and 72 h after cell culturing. For this purpose, the culture medium was removed, and 2 mL of MTT: culture medium (1:5) solution was added to each well-plate, and incubated for 4 h at 37 °C, 5% CO2, 95% humidity. After that, the medium was removed and 100 μL DMSO was added and the optical density of the solution was observed at 570 nm. The results are reported as a chart.
In-vivo studies
Animal study
The in-vivo experiments were conducted on the optimum prepared hydrogel (GA2). For this purpose, total nine female rats (weight 300–500 g) were selected for in-vivo studies. Anesthesia induction was performed by intra peritoneal injection of 10% ketamine 50 mg/kg and 2% xylazine at a dose of 10 mg/kg. The rats were placed in the supine position on the operating table. The abdominal surfaces of the mice were shaved and the genital area was disinfected. Botulinum-A toxin was selected to induce the urinary incontinence model and 10 μL were injected at the mid of the dorsal wall of the urethra. Animals were divided into three groups: (1) positive controls (rats without any disorders); (2) negative controls (rats with urethral incompetent without any treatment); and (3) GA2 groups (rats with urethral incompetent injected by GA2 hydrogels). After 3 days, 30 μL of freshly prepared GA2 hydrogel was injected into mid urethra of GA2 rat groups and the animals were kept in designated cages. All animal experiments were performed in accordance with National Institutes of Health animal care guidelines. After each week, some of the rats in each group were sacrificed and the healing process was evaluated.
Histopathological examination
4 weeks after the post injection, the rats were sacrificed and the urethra tissue was harvested. The harvested tissue was fixed by formaldehyde and dehydrated in ascending concentration of alcohols (70, 80, 96, and 100) for 1 h. In the clarification stage, the samples were clarified in two 1 h stages by xylazine followed by paraffin embedding for 2 h in an oven and 4 h soaking in fresh paraffin at the same condition. The molding process was performed in the special metal molds with molten paraffin. The prepared samples were kept in refrigerator at − 4 °C for 24 h. The samples were sectioned with a microtome with a thickness of 5 μm and stained with hematoxylin–eosin (H&E). The prepared samples were imaged using a light microscope (Carl Zeiss, Thornwood) with a digital camera.
Statistical analysis
All experiments were performed in fifth replicate. The results were given as mean ± standard error (SE). Statistical analysis was conducted by one-way ANOVA and Tukey’s test with significance reported when P < 0.05.
Results and discussion
Cellular responses
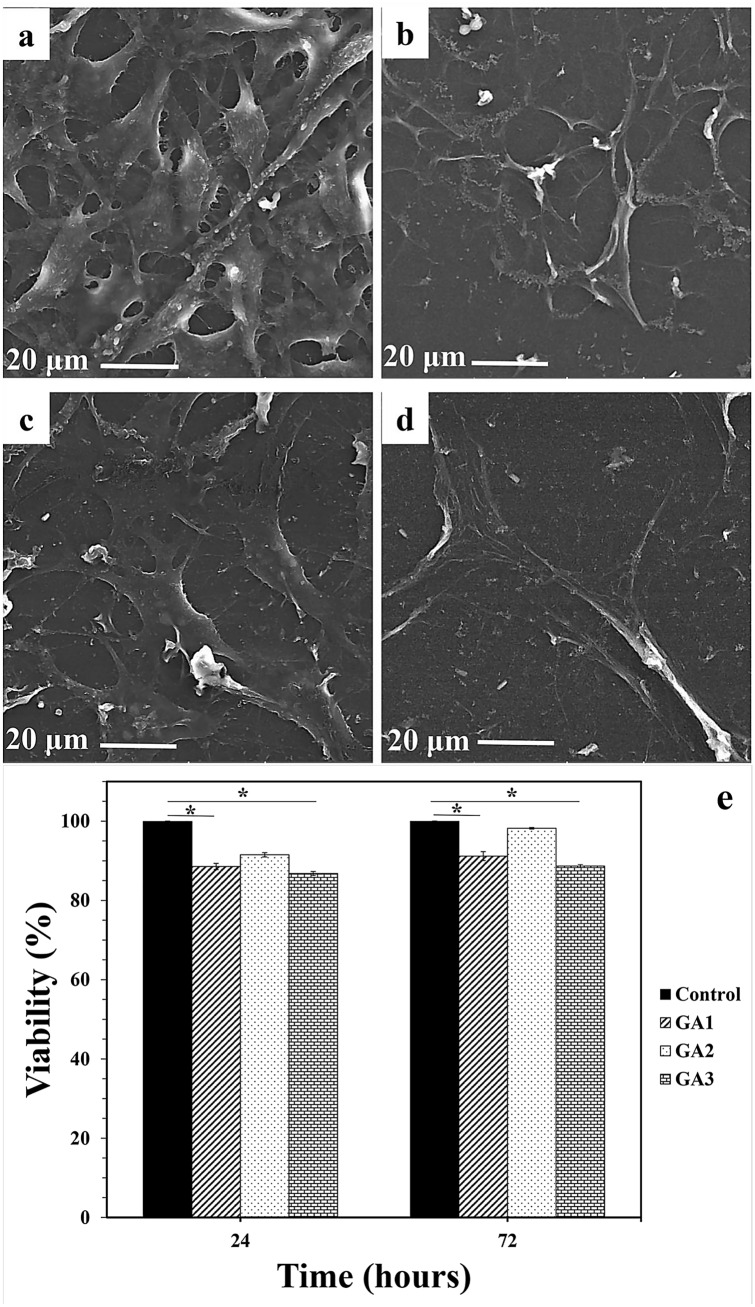
To study the cellular behavior of fabricated hydrogels (GA1, GA2, and GA3), the fibroblast cells were cultured on the surface of the samples for 48 h and then FE-SEM images were taken. The capability of biomaterial substrates to support cell adhesion, proliferation, and differentiation is an important factor in tissue engineering (Wen et al. 2014; Raisi et al. 2020). Cell adhesion is a dynamic process and is the first event that occurs when cells are in contact with biomaterials (Zhang et al. 2016; Movahedi et al. 2020). In 3D hydrogels, cells have the ability to migrate and interact with the extracellular matrix in three dimensions and the cells are more resistant to apoptosis, while in 2D cultured cells, the cells interactions are inhibited in two dimensions (Liao et al. 2009). The cell morphology on control sample, GA1, GA2, and GA3 hydrogels were demonstrated in Fig. 1a–d, respectively. As can be seen, the fibroblast cells were attached onto the surface of all hydrogels with filopodia formation; whereas it seemed GA2 provided more favorable environment for the cell adhesion and the whole surface was covered with attached cells in comparison to GA1 and GA2 hydrogels.
Fig. 1.
FE-SEM micrographs of cultured cells on control (a), GA1 (b), GA2 (c), and GA3 (d) hydrogels. Cell viability after 24 and 72 h after fibroblast culturing on all samples (e) (*P < 0.05, **P < 0.0001; ANOVA, all pairs were compared using Tukey’s test)
MTT test was also performed to evaluate the cell viability on all samples and the results were shown in Fig. 1e. All three hydrogels showed a high percentage of biocompatibility and cell viability. This is mainly due to the biocompatible gelatin and alginate main components of hydrogels (Shi et al. 2019; Uman et al. 2020). Also, the CaCl2 cross-linking of alginate facilitated the gelatin availability and improved cells interactions (Duan et al. 2013). On the other hand, collagenase is a cell secreted enzyme that degrades the extracellular matrix protein of collagen and thus leads to weakening the primary support structures. Ascorbic acid is a collagenase inhibitor and as a result of its addition to composition restricted collagen degradation and improved collagen synthesis (Oliva et al. 2019). It should be noted that β-glycerophosphate also acted as a biocompatible source of phosphate and regulated calcium phosphate growth activities and supported cells by releasing phosphate ions (Ding et al. 2015). In addition, the dual drug release affected synergically on cell growth and differentiation (Coelho and Fernandes 2000). Simultaneous β-glycerophosphate releasing along with ascorbic acid synergically improved cell adhesion and growth (Zhang et al. 2016). Furthermore, GPTMS resulted in interactions between both organic and inorganic components (Rasti et al. 2019). According to Shirosika et al. study (Shirosaki et al. 2015), the Si–OH and Si–O–Si groups in GPTMS create suitable regions for cell adhesion, as these bonds have the ability to adsorb cations such as Ca2+, which plays an effective role in biological processes such as cell adhesion. Therefore, the presence of GPTMS can be effective in proper cell-hydrogels interactions.
The same as cell morphology observations, although all three hydrogels had high biocompatibility, but GA2 sample showed higher cell viability than those of the GA1 and GA3 specimens. This may be due to the increased gelatin ratio of this sample compared to other samples. Gelatin is a major component of the extracellular matrix and interacts with the extracellular matrix environment, leading to further deposition of the extracellular matrix (Liao et al. 2009). Many hydrogels prevent long-term cell adhesion due to lack of cell receptors, while gelatin-contained hydrogels provide more cells ligands due to large number of glycine, proline, and hydroxyproline residues in gelatin structures (You et al. 2007). On the other hand, the reduction of low adhesion and cell viability in GA3 and GA1 compared to GA2 can be due to lower hydrophilicity of the two samples. Decreased water absorption would lead to reduced water and nutrient transport, resulting in reduced cellular activity (Arabi et al. 2018). When the cells show better adhesion and growth on suitable hydrophilic surfaces their filopodia become more elongated (Mollaqasem et al. 2020; Zhang et al. 2016). High hydrophilicity of gelatin affects cell morphology by absorption of cellular fluids and leads to increased adhesion and cell viability in sample GA2 (Arabi et al. 2018). Ascorbic acid increases collagen type I secretion and, therefore, increases the collagen I/α2β2 integrin-mediated intracellular signaling; while on the other hands, phosphorous group of β-glycerophosphate activates extracellular related kinase signaling pathways and increases many osteogenic genes such as osteopontin gene and BMP2 besides phosphorate supply for hydroxyapatite deposition (Langenbach and Handschel 2013). The usage of dual ascorbic acid and β-glycerophosphate leads to neutral metalloproteinase increasing and may degrade proteoglycans for apatite formation and, therefore, accelerate mineralization (Wang et al. 2017). Therfore, according to the results GA2 was chosen as the optimum sample for furthure investigations.
In-vivo study
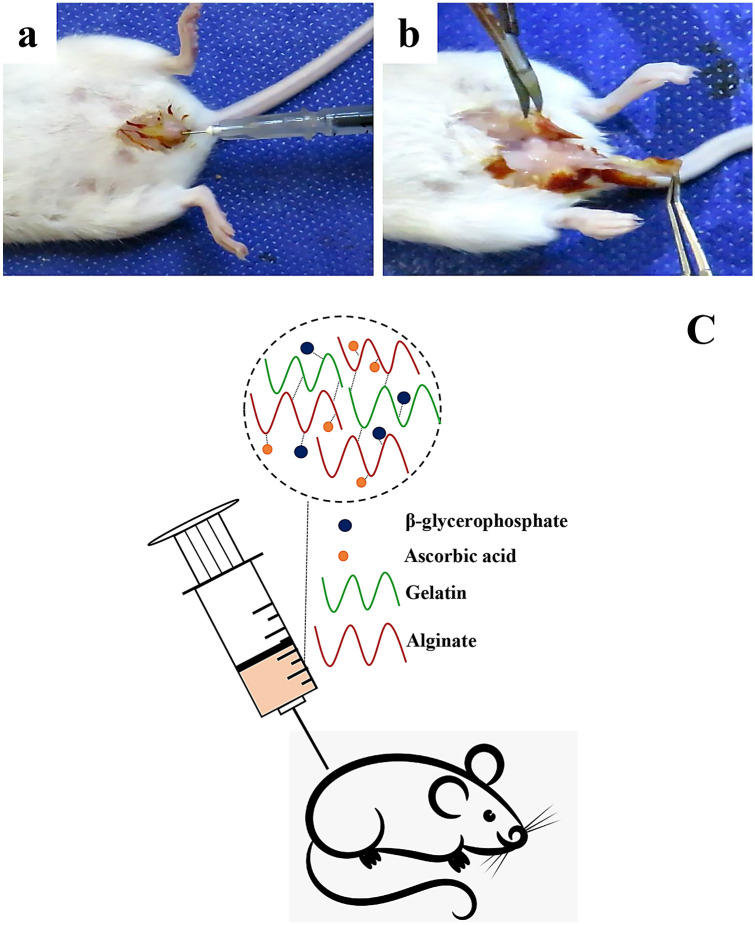
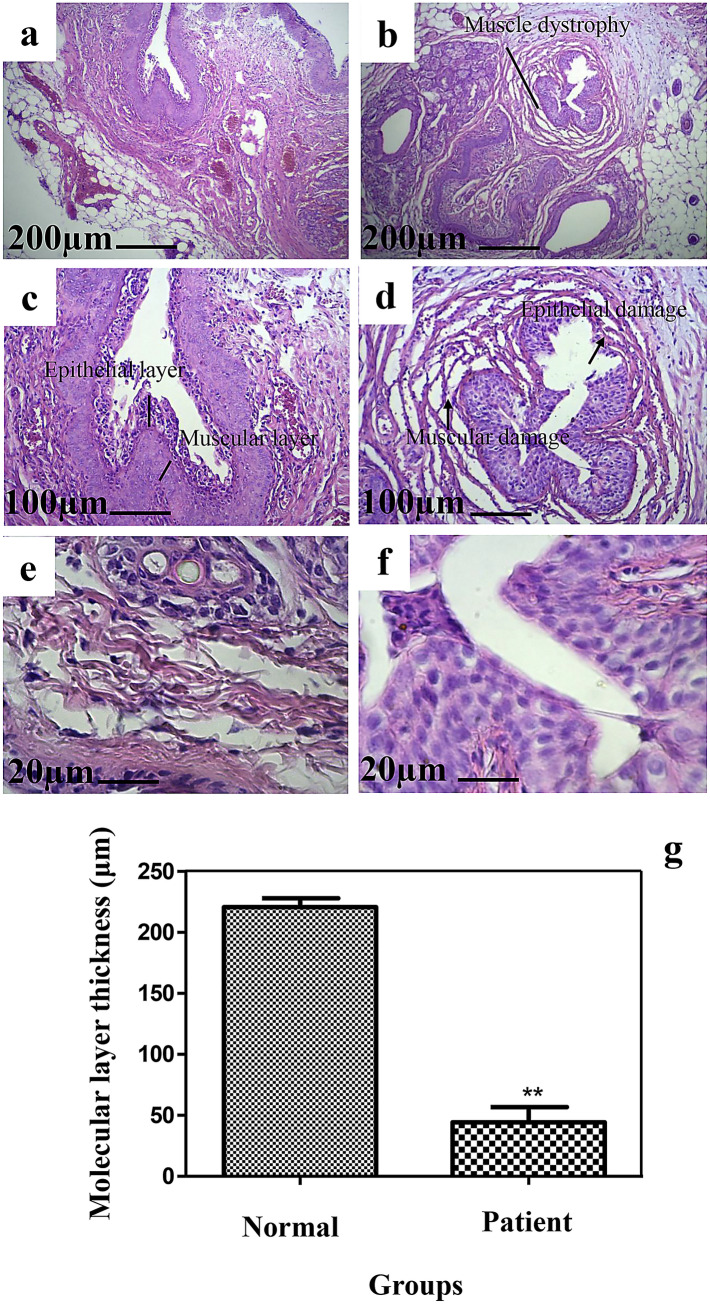
GA2 hydrogel injection was performed on rat animal models for in-vivo application (Fig. 2a) and the tissue was harvested and investigated after each time point (Fig. 2b). Schematic GA2 hydrogel injection is demonstrated in Fig. 2c. Histological analysis by H&E staining was performed at 2 weeks after incompetent urethral induction (Fig. 3b, d, e) and was compared with healthy tissue (Fig. 3a, c, e). The epithelial degradation and surrounding muscle deformation was observed and some epithelial cells were removed and apoptosed. Intervals and tissue rupture were observed between muscle layers. These disorders confirmed the property of urethral dysfunction modeling. The molecular layer thickness measurements demonstrated that the urethral layer thickness was decreased significantly after 2 weeks botulinum toxin-A injection (Fig. 3g). The layer thickness was 220.8 ± 7.295 μm in normal tissue and was decreased to 44.36 ± 12.31 μm after incompetent urethral induction. These results are in line with previous study (Takahashi et al. 2006) and demonstrated that Botulinum Toxin-A injection lowered the leak point pressure significantly with later shrinkage of smooth muscle and striated sphincters.
Fig. 2.
Ascorbic acid and β-glycerophosphate loaded gelatin/sodium alginate injection to induced urethral incontinence animal model (a) histological harvesting to investigate the hydrogel efficiently after 4 weeks (b). The schematic of ascorbic acid and β-glycerophosphate loaded gelatin/sodium alginate injection to a rat (c). The GA2 hydrogel contained CaCl2 and GPTMS cross-linked ascorbic acid and β-glycerophosphate loaded gelatin/sodium alginate hydrogel
Fig. 3.
Histological analysis by H&E staining of healthy tissue (a, c, e) and the tissue 2 weeks after incompetent urethral induction (b, d, e). The molecular layer thickness measurements of healthy tissue and the tissue after 2 weeks Botulinum Toxin-A injection (g) (*P < 0.05, **P < 0.0001; ANOVA, all pairs were compared using Tukey’s test)
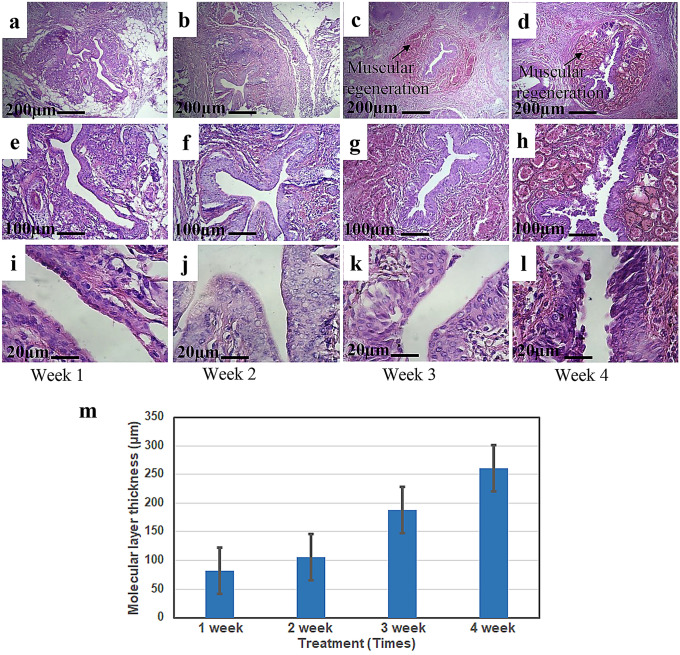
The healing process of incompetent urethral induction was followed for 4 weeks after GA2 hydrogel injections and the histological observations are shown in Fig. 4a–l. The epithelial and muscle tissue damage was treated within a few weeks after GA2 treatment. According to the achieved results, although the healing process was not significant in the first and second weeks; however, the healing process especially in the epithelial layer was accelerated during the third and fourth weeks. In addition, the apoptosis epithelial cells were removed and the tissue was improved over the time and new cells were replaced. These results proved the effectiveness of GA2 gel in the induced urethral incontinence animal model after 4 weeks. No inflammatory reaction was observed after injection of hydrogels. Localized delivery of β-glycerophosphate and ascorbic acid to the affected site was safer, more effective, and stimulated the cells to accelerate the healing process (Yan et al. 2018). After 4 weeks of GA2 hydrogel injection a thick muscle layer arrangement was developed with a circular muscle layer that performed the urinary sphincter function and prevented the urine loss. These observations are consistent with the previous studies of functional tissue formation (Kim et al. 2011).
Fig. 4.
Histological analysis by H&E staining within 4 weeks after GA2 sample injections (a–l). The molecular layer thickness measurements of injured tissue during 4 weeks (m) (*P < 0.05, **P < 0.0001; ANOVA, all pairs were compared using Tukey’s test)
The molecular layer thickness was measured within these 4 weeks and the results are demonstrated in Fig. 4m. According to the measurements, the molecular layer was increased during the time and reached to the normal layer thickness after 4 weeks.
Previously used injectable hydrogels acted as a temporarily compression agent and needed to be re-injected after a while (Vardar et al. 2019). Considering the formation of muscle and epithelial tissue after 4 weeks, it can be concluded that the GA2 hydrogel induced the formation of functional muscle tissue and there was no need to repeat injection process. In addition, GA2 hydrogels inhibited un-functional scar tissue formation. The released ascorbic acid could acclerate the reduced collagen synthesis as a consequence of urethral incontinence induction (Mangır et al. 2015). According to our previously evaluated biodegradability assessments by immersion of GA2 sample for 33 days in phosphate buffer saline solution, there was a loss of about 82.453 ± 5.071% of its weight (Rezaei et al. 2020). Therefore, it seems that the hydrogels can be degraded at a suitable time after tissue repair after approximately 4 weeks. Biodegraded hydrogel adsorption can be performed by foreign body giant cells or hydrolytic degradation by body fluids, and enzymatically by various surrounding enzymes (Oh et al. 2015). According to the cellular animal model evaluations, it seems that GA2 hydrogel can be effective in the treatment of urinary incontinence.
The results presented in this article are the early stages of in-vitro cellular and in-vivo studies. Further studies on the use of different percentages of calcium chloride cross-linker and its effect on the properties of hydrogels, loading of fat cells into hydrogels, qualitative and quantitative analyses of degradation products with tests such as NMR, the expression of collagen genes in-vitro due to the interaction of fibroblasts with hydrogels, the loading of growth factors such as fibroblast growth factor into hydrogels and the loading of stem cells into hydrogels are some of the studies that should be addressed in the future. The aim of this study was to clarify the effectiveness of this gel in the treatment of urinary incontinence.
Conclusion
Crosslinked gelatin/sodium alginate hydrogels with different concentration of components were prepared and loaded with β-glycerophosphate and ascorbic acid to treat urinary incontinence. Cellular tests showed high support of GA2 hydrogels with higher gelatin concentration of cell adhesion and viability. In general, the GA2 sample with higher gelatin ratio than GA1 and a lower cross-linker ratio than GA3 exhibited better properties as an injectable compression agent. Animal tests also revealed that the lost epithelial and muscle tissue was gradually repaired after 4 weeks of GA2 injection and there was no evidence of scar tissue formation or inflammation responses in animal model. It seems that this hydrogel can act as a compressive injectable bulking agent in urinary incontinence treatment.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
This chapter does not contain any studies with human participants performed by any of the authors.
Statement on the welfare of animals
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This research was performed as a PhD thesis and all ethical issues are approved by Islamic Azad University-Science and Research Branch.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hessam Rezaei, Email: Rezaei_bme@yahoo.com.
Azadeh Asefnejad, Email: Asefnejad@srbiau.ac.ir.
Morteza Daliri-Joupari, Email: Daliri@nigeb.ac.ir.
Sedigheh Joughehdoust, Email: S.joughehdoust@maastrichtuniversity.nl.
References
- Arabi N, Zamanian A, Rashvand SN, Ghorbani F. The tunable porous structure of gelatin-bioglass nanocomposite scaffolds for bone tissue engineering applications: physicochemical, mechanical, and in-vitro properties. Macromol Mater Eng. 2018;303:1700539. doi: 10.1002/mame.201700539. [DOI] [Google Scholar]
- Coelho MJ, Fernandes MH. Human bone cell cultures in biocompatibility testing Part II: effect of ascorbic acid, β-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials. 2000;21:1095–1102. doi: 10.1016/S0142-9612(99)00192-1. [DOI] [PubMed] [Google Scholar]
- Davis NF, Kheradmand F, Creagh T. Injectable biomaterials for the treatment of stress urinary incontinence: their potential and pitfalls as urethral bulking agents. Int Urogynecol J. 2013;24:913–919. doi: 10.1007/s00192-012-2011-9. [DOI] [PubMed] [Google Scholar]
- Ding G-J, Zhu Y-J, Qi C, et al. Amorphous calcium phosphate nanowires prepared using beta-glycerophosphate disodium salt as an organic phosphate source by a microwave-assisted hydrothermal method and adsorption of heavy metals in water treatment. RSC Adv. 2015;5:40154–40162. doi: 10.1039/C5RA04624F. [DOI] [Google Scholar]
- Duan B, Hockaday LA, Kang KH. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101:1255–1264. doi: 10.1002/jbm.a.34420.3D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmelund M, Sokol ER, Karram MM, Dmochowski R, Klarskov N. Patient characteristics that may influence the effect of urethral injection therapy for females stress urinary incontinence. J Urol. 2019;202:125–131. doi: 10.1097/JU.0000000000000176. [DOI] [PubMed] [Google Scholar]
- Eui RC, Kang SW, Kim BS. Poly (lactic-co-glycolic acid) microspheres as a potential bulking agent for urological injection therapy: preliminary results. J Biomed Mater Res Part B Appl Biomater. 2005;72:166–172. doi: 10.1002/jbm.b.30138. [DOI] [PubMed] [Google Scholar]
- Heang S, Youl J, Ho S, Sup S, Hong S, Ho J. PCL microparticle-dispersed PLGA solution as a potential injectable urethral bulking agent. Biomaterials. 2006;27:1936–1944. doi: 10.1016/j.biomaterials.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Hurtado EA, McCrery RJ, Appell RA. Complications of ethylene vinyl alcohol copolymer as an intraurethral bulking agent in men with stress urinary incontinence. Urology. 2008;71:662–665. doi: 10.1016/j.urology.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Kim IG, Oh SH, Lee JY, Lee JY, Lee JY. Bioactive porous beads as an injectable urethral bulking agent: in vivo animal study for the treatment of urinary incontinence. Tissue Eng-Part A. 2011;17:1527–1535. doi: 10.1089/ten.tea.2010.0600. [DOI] [PubMed] [Google Scholar]
- Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012;2012:2–4. doi: 10.1002/14651858.CD003881.pub3. [DOI] [PubMed] [Google Scholar]
- Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapitan MCM, Cody JD. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2016;2:1–17. doi: 10.1002/14651858.CD002912.pub6. [DOI] [PubMed] [Google Scholar]
- Liao H, Zhang H, Chen W. Differential physical, rheological, and biological properties of rapid in situ gelable hydrogels composed of oxidized alginate and gelatin derived from marine or porcine sources. J Mater Sci Mater Med. 2009;20:1263–1271. doi: 10.1007/s10856-009-3694-4. [DOI] [PubMed] [Google Scholar]
- Liu B, Li J, Lei X, et al. Cell-loaded injectable gelatin/alginate/LAPONITE® nanocomposite hydrogel promotes bone healing in a critical-size rat calvarial defect model. RSC Adv. 2020;10:25652–25661. doi: 10.1039/d0ra03040f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Li Y, Qin X, Wa Q. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mater Des. 2018;146:12–19. doi: 10.1016/j.matdes.2018.03.002. [DOI] [Google Scholar]
- Maione-Silva L, de Castro EG, Nascimento TL, et al. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci Rep. 2019;9:1–14. doi: 10.1038/s41598-018-36682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangır N, Bullock AJ, Roman S, Osman N, Chapple C, Macneil S. Production of ascorbic acid releasing biomaterials for pelvic floor repair. Acta Biomater. 2015;29:188–197. doi: 10.1016/j.actbio.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaqasem VK, Asefnejad A, Nourani MR, Goodarzi V, Kalaee MR. Incorporation of graphene oxide and calcium phosphate in the PCL/PHBV core-shell nanofibers as bone tissue scaffold. J Appl Polym Sci. 2020;138:e49797. doi: 10.1002/app.49797. [DOI] [Google Scholar]
- Movahedi M, Asefnejad A, Rafienia M, Khorasani MT. Potential of novel electro-spun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int J Biolog Macromol. 2020;146:627–637. doi: 10.1016/j.ijbiomac.2019.11.233. [DOI] [PubMed] [Google Scholar]
- Naghizadeh Z, Karkhaneh A, Khojasteh A. Self-crosslinking effect of chitosan and gelatin on alginate based hydrogels: injectable in situ forming scaffolds. Mater Sci Eng C. 2018;89:256–264. doi: 10.1016/j.msec.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Namdarian P, Zamanian A, Asefnejad A, Saeidifar M. Evaluation of freeze-dry chitosan-gelatin scaffolds with olibanum microspheres containing dexamethasone for bone tissue engineering. Polymer Korea. 2018;42(6):982–993. doi: 10.7317/pk.2018.42.6.982. [DOI] [Google Scholar]
- Nouri-Felekori M, Khakbiz M, Nezafati N, Mohammadi J, Eslaminejad MB. Comparative analysis and properties evaluation of gelatin microspheres crosslinked with glutaraldehyde and 3-glycidoxypropyltrimethoxysilane as drug delivery systems for the antibiotic vancomycin. Int J Pharm. 2019;557:208–220. doi: 10.1016/j.ijpharm.2018.12.054. [DOI] [PubMed] [Google Scholar]
- Oh SH, Bae JW, Kang JG, et al. Dual growth factor-loaded in situ gel-forming bulking agent: passive and bioactive effects for the treatment of urinary incontinence. J Mater Sci Mater Med. 2015;26:33. doi: 10.1007/s10856-014-5365-3. [DOI] [PubMed] [Google Scholar]
- Oliva F, Maffulli N, Gissi C, et al. Combined ascorbic acid and T3 produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: a proof of concept study. J Orthop Surg Res. 2019;14:1–10. doi: 10.1186/s13018-019-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko N, Gonçalves OH, Bona E, et al. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr Polym. 2019;223:115035. doi: 10.1016/j.carbpol.2019.115035. [DOI] [PubMed] [Google Scholar]
- Raisi A, Asefnejad A, Shahali M, Doozandeh Z, Kamyab Moghadas B, Saber-Samandari S, Khandan A. A soft tissue fabricated using freeze-drying technique with carboxymethyl chitosan and nanoparticles for promoting effects on wound healing. J Nanoanal. 2020;7:262–274. doi: 10.22034/JNA.2022.680836. [DOI] [Google Scholar]
- Ramseyer P, Micol LA, Engelhardt EM, Osterheld MC, Hubbell JA, Frey P. In vivo study of an injectable poly(acrylonitrile)-based hydrogel paste as a bulking agent for the treatment of urinary incontinence. Biomaterials. 2010;31:4613–4619. doi: 10.1016/j.biomaterials.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Rasti M, Hesaraki S, Nezafati N. Effects of GPTMS concentration and powder to liquid ratio on the flowability and biodegradation behaviors of 45S5 bioglass/tragacanth bioactive composite pastes. J Appl Polym Sci. 2019;136:17–22. doi: 10.1002/app.47604. [DOI] [Google Scholar]
- Rezaei H, Asefnejad A, Daliri-Joupari M, Joughehdoust S. The physicochemical and mechanical investigation of siloxane modified gelatin/sodium alginate injectable hydrogels loaded by ascorbic acid and β-glycerophosphate. Mater Today Commun. 2020;2020:101914. doi: 10.1016/j.mtcomm.2020.101914. [DOI] [Google Scholar]
- Shi J, Chen S, Wang L, et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J Biomed Mater Res Part B Appl Biomater. 2019;107:2040–2049. doi: 10.1002/jbm.b.34295. [DOI] [PubMed] [Google Scholar]
- Shirosaki Y, Okamoto K, Hayakawa S, Osaka A, Asano T. Preparation of porous chitosan–siloxane hybrids coated with hydroxyapatite particles. Biomed Res Int. 2015;2015:1–6. doi: 10.1155/2015/392940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi M, Yekta BE, Rezaie H, et al. Enhancing mechanical properties and biological performances of injectable bioactive glass by gelatin and chitosan for bone small defect repair. Biomedicines. 2020;8:1–19. doi: 10.3390/biomedicines8120616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Yang Y, Mohandas A, Stucker B, Nguyen KT. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater. 2007;85(2):573–582. doi: 10.1002/jbm.b.30962. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Chen Q, Ogushi T, et al. Periurethral injection of sustained release basic fibroblast growth factor improves sphincteric contractility of the rat urethra denervated by botulinum-A toxin. J Urol. 2006;176:819–823. doi: 10.1016/j.juro.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Uman S, Dhand A, Burdick JA. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J Appl Polym Sci. 2020;137:1–20. doi: 10.1002/app.48668. [DOI] [Google Scholar]
- Vardar E, Vythilingam G, Pinnagoda K, et al. A bioactive injectable bulking material; a potential therapeutic approach for stress urinary incontinence. Biomaterials. 2019;206:41–48. doi: 10.1016/j.biomaterials.2019.03.030. [DOI] [PubMed] [Google Scholar]
- Wang L, Stegemann JP. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31:3976–3985. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cao X, Zhang Y. A novel bioactive osteogenesis scaffold delivers ascorbic acid, β-glycerophosphate, and dexamethasone in vivo to promote bone regeneration. Oncotarget. 2017;8:31612–31625. doi: 10.18632/oncotarget.15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Duan M, Rahman M, et al. Use of bioactive extracellular matrix fragments as a urethral bulking agent to treat stress urinary incontinence. Acta Biomater. 2020;117:156–166. doi: 10.1016/j.actbio.2020.09.049. [DOI] [PubMed] [Google Scholar]
- Wen C, Lu L, Li X. Mechanically robust gelatin-alginate IPN hydrogels by a combination of enzymatic and ionic crosslinking approaches. Macromol Mater Eng. 2014;299:504–513. doi: 10.1002/mame.201300274. [DOI] [Google Scholar]
- Yan H, Zhong L, Jiang Y, et al. Controlled release of insulin-like growth factor 1 enhances urethral sphincter function and histological structure in the treatment of female stress urinary incontinence in a rat model. BJU Int. 2018;121:301–312. doi: 10.1111/bju.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You SJ, Ahn WS, Jang HS, Il Kang M (2007) Preparation and characterization of gelatin-poly(vinyl alcohol) hydrogels for three-dimensional cell culture. J Ind Eng Chem 13(1):116–120
- Yousefichaijan P, Goudarzi AA, Rezagholizamenjany M, et al. Efficacy of ascorbic acid supplementation in relief of symptoms due to febrile upper urinary tract infection in children, a clinical trial and hospital based study. Arch Pediatr Infect Dis. 2018;6:4–9. doi: 10.5812/pedinfect.57071. [DOI] [Google Scholar]
- Yu B, Poologasundarampillai G, Turdean-Ionescu C, Smith ME, Jones JR. A new calcium source for bioactive sol–gel hybrids. Bioceram Dev Appl. 2011;1:1–3. doi: 10.4303/bda/d110178. [DOI] [Google Scholar]
- Yuan L, Wu Y, Gu QS, El-Hamshary H, El-Newehy M, Mo X. Injectable photo crosslinked enhanced double-network hydrogels from modified sodium alginate and gelatin. Int J Biol Macromol. 2017;96:569–577. doi: 10.1016/j.ijbiomac.2016.12.058. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li M, Gao L, et al. Effect of dexamethasone, β-glycerophosphate, OGP and BMP2 in TiO2 nanotubes on differentiation of MSCs. Mater Technol. 2016;2016:1–10. doi: 10.1080/10667857.2016.1152655. [DOI] [Google Scholar]