Abstract
Parvovirus B19 (PB19) is a common infection among solid transplant recipients. Usually, it is asymptomatic, but sometimes it can become a real therapeutic challenge. We report a case of a kidney transplant recipient with relapsing pure red cell aplasia due to PB19 infection. Our patient was initially managed with standard treatment consisting of intravenous immunoglobulins and minimization of immunosuppressive treatment. However, when this approach became ineffective, conversion from tacrolimus to everolimus was done, with favorable results. This paper explores infection by PB19 in kidney transplant recipients and the potential benefits of a calcineurin inhibitor-free immunosuppression and the antiviral properties of mTOR inhibitors.
Keywords: Kidney transplantation, Anemia, Parvovirus B19, Immunoglobulin, mTOR inhibitor
Background
Human parvovirus B19 (PB19) is an Erythroparvovirus genus of the Parvoviridae family [1]. It has a high prevalence, with about 60–90% of the adult population having serologic positivity against the virus. It typically presents in adults as transient anemia with accompanying arthralgias and flu-like illness; nevertheless, most cases are asymptomatic [2]. PB19 is the third most common opportunistic viral infection within the first year of kidney transplantation. Immunocompromised patients can manifest more severe symptoms than immunocompetent individuals, often presenting with recurrent disease flares despite optimal therapy [3]. Frequent relapses are usually treated with intravenous immunoglobulin and reduction of immunosuppressive therapy. However, this reduction in immunosuppression potentially increases the risk of graft rejection [4]. We describe a recurrent case of pure red blood cell aplasia (PRCA) due to PB19 in a kidney transplant recipient (KTR) despite a reduction in immunosuppression and intravenous immunoglobulin treatment. Based on the potential antiviral properties of mTOR inhibitors [5], the patient was converted from tacrolimus to everolimus and has been asymptomatic ever since.
Case report
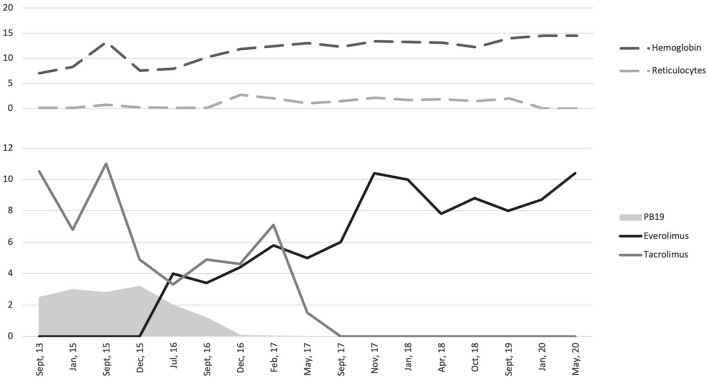
A 45-year-old woman with end-stage kidney disease due to autosomal dominant polycystic kidney disease received a living-donor kidney transplant in May 2013. Immunosuppressive induction consisted of basiliximab, tacrolimus, mycophenolic acid (MPA), and methylprednisolone. There were no surgical complications, and the creatinine level at discharge was 0.8 mg/dL. A month after transplantation, she presented with a two-day course of malaise and fever. Her physical examination was only remarkable for skin paleness, while urinalysis and kidney ultrasound were normal. Her complete blood count revealed normocytic anemia with a hemoglobin of 7.9 g/dL, normal platelet, and white blood cell count. She had no signs of active bleeding, and there was no evidence of intravascular hemolysis on lab tests (normal bilirubin, lactate dehydrogenase, and haptoglobin, with no schistocytes on peripheral blood smears). MPA induced anemia was suspected, its dose was reduced, darbepoetin was prescribed, and she was discharged home Fig. 1.
Fig. 1.
Relationship over time among parvovirus B19 (PB19) in the gray solid area, tacrolimus, and everolimus levels (light gray and black solid lines, respectively). In the top part of the image, we see that reticulocyte percentage and hemoglobin levels (light and dark gray dashed lines, respectively) increase and stabilize with time. **PB19 values are reported with scientific notation (106).
Two weeks later, she presented worsened symptoms of fever, malaise, and tiredness. Her hemoglobin levels continued to decrease (7.2 g/dL), and fever persisted. The rest of her lab tests were normal, except for a low reticulocyte count (0.1%). A bone marrow aspirate revealed erythroid hypoplasia and was thus diagnosed with PRCA secondary to PB19. She was treated with intravenous immunoglobulin (IVIG) at 2 g/kg, and MPA was stopped with a good response (hemoglobin of 9.9 g/dL). However, for the next 22 months, PB19 serum viral load remained extremely high, with over a million units per milliliter, and in December 2014 and June 2015, she presented two relapses (defined as episodes of symptomatic anemia with low reticulocyte count). These episodes required new doses of IVIG and blood transfusions. In July 2015, a monthly prophylactic dose of IVIG was initiated in an attempt to control the infection. Despite this, viral loads remained over a million copies. Given the potential antiviral properties of mTOR inhibitors (mTORi), everolimus was initiated in December 2015 to allow the reduction of tacrolimus trough levels below 4 ng/mL. Two new relapses in April and September 2016 occurred while on prophylaxis. Such difficult management was attributed to immunosuppressive therapy, particularly tacrolimus. Everolimus titers were increased with the intention of complete withdrawal from tacrolimus, which was finally achieved six months later (June 2017). Monthly IVIG prophylaxis was kept until December 2017. Since September 2016, our patient has not had any new episodes of symptomatic anemia (through hemomglobin in the past two years of 12.2 g/dL), and although rt-PCR remained positive for PB19, viral loads progressively decreased (last 12 months mean of 1018 ± 533 UI/mL), and is currently undetectable (see table and figure) Table 1.
Table 1.
Comparison between Tacrolimus and Everolimus blood levels, with Parvovirus B19 (PB19) viral load
| Date | PB19 (UI/mL) | Everolimus (ng/mL) | Tacrolimus (ng/mL) | Hemoglobin (g/dL) | Reticulocytes (%) |
|---|---|---|---|---|---|
| Sept, 13 | > 1,000,000 | – | 10.5 | 7 | 0.1 |
| Jan, 15 | > 1,000,000 | – | 6.8 | 8.3 | 0.1 |
| Sept, 15 | > 1,000,000 | – | 11 | 13.2 | 0.7 |
| Dec, 15 | > 1,000,000 | – | 4.9 | 7.5 | 0.2 |
| Jul, 16 | > 1,000,000 | 4 | 3.3 | 7.9 | 0.1 |
| Sept, 16 | > 1,000,000 | 3.4 | 4.9 | 10.2 | 0.1 |
| Dec, 16 | 70,120 | 4.4 | 4.6 | 11.8 | 2.7 |
| Feb, 17 | 28,040 | 5.8 | 7.1 | 12.4 | 2 |
| May, 17 | 4100 | 5 | 1.5 | 13 | 1 |
| Sept, 17 | 2507 | 6 | – | 12.3 | 1.5 |
| Nov, 17 | 710 | 10.4 | – | 13.4 | 2.1 |
| Jan, 18 | 1200 | 10 | – | 13.2 | 1.7 |
| Apr, 18 | 803 | 7.8 | – | 13.1 | 1.8 |
| Oct, 18 | 1202 | 8.8 | – | 12.2 | 1.5 |
| Sept, 19 | 1042 | 8 | – | 14 | 2 |
| Jan, 20 | 308 | 8.7 | – | 14.5 | – |
| May, 20 | 0 | 10.4 | – | 14.5 | – |
A hyphen (-) was used to indicate the absence of data
Discussion
PB19 was discovered in 1975 [6]. This virus is known for causing aplastic crisis in patients with chronic hemolytic disorders. It is also responsible for erythema infectiosum in children, and, during pregnancy, it can cause hydrops fetalis or miscarriage. PB19 is usually asymptomatic in immunocompetent hosts, though it can occasionally present -as our patient initially did- with a flu-like illness [7].
Its tropism for P antigen explains the mechanism by which the virus causes anemia. This antigen is found primarily on human erythroid progenitor cells, on the surface of endothelial cells, on trophoblasts of placental tissue, myocytes, synovial cells, liver cells, and megakaryocytes PB19 infects cells and causes their destruction [2]. In the case of red blood cells, after replication within the erythrocyte, the liberation of mature virions results in lytic apoptosis of erythroid progenitors, causing intense viremia within a week of exposure to the virus. These events translate clinically as a normocytic normochromic aregenerative, erythropoietin-resistant anemia in 98.8% of cases, albeit it can also present with leucopenia or thrombopenia (37.5 and 21%, respectively) [8]. In our patient’s case, before the diagnosis of PCRA was made, we unsuccessfully treated the anemia with high dose darbepoetin (up to 100 mcg every week). The rest of her blood cells always remained within normal limits.
Immunocompetent individuals can generate antibodies against the virus within ten days of infection [9]. Even though seroprevalence of PB19 in KTRs is similar to that of the general population (about 60%), all transplant recipients are at risk of severe symptomatic infection [10], mainly due to their inability to generate an adequate immunological response as a result of immunosuppression. Egbuna et al. found a non-significant association between erythropoietin-resistant anemia and hemoglobin values below 10 g/dL in KTRs. This association may justify screening for PB19 in erythropoietin-resistant anemia transplant patients [10]. The incidence of symptomatic infection is highest during the first year after transplantation, precisely when immunosuppression is strongest, with most cases presenting within three months of the procedure [9]. Some of the risk factors associated with PB19 infection are transplants from donors after circulatory death and the use of tacrolimus or anti-thymocyte globulin as immunosuppressant agents [11]. Our patient’s first symptoms appeared four weeks after transplantation, and tacrolimus was part of her maintenance immunosuppressive therapy. However, anti-thymocyte globulin was not used.
The data on whether PB19 infection by itself entails a higher risk of graft rejection is not clear. A study suggests that the virus's intrarenal presence might be related to rejection risk, but the sample was small, and these results have not been replicated [12]. In our case, despite persistent high viral loads, the patient's renal function remained intact.
According to current guidelines, standard therapy against PB19 infection consists of a total dose of 2 g/kg of IVIG in 5 divided doses [4]. This management is usually successful in acute symptomatic patients. However, early kidney transplant recipients often relapse after the effect of IVIG wears off [13]. This is what happened to our patient. We saw she responded well to IVIG therapy but relapsed frequently when the IVIG effect disappeared. In such cases, new courses of IVIG and reduction of immunosuppression are needed to control the infection and avoid recurrences, with the latter entailing an increased risk of graft rejection. There is some PB19 infection control evidence after minimizing calcineurin inhibitors (CNIs) dose [14]. Despite the reduction of tacrolimus and prophylactic maintenance therapy with IVIG (2 g/kg every 3–6 weeks), relapses continued to occur.
It has been shown that solid organ transplant recipients can benefit from the antiviral properties of mTORi. These drugs can increase memory T cell’s efficacy, functionality, and ability to inhibit viral cell growth. Multiple studies indicate that mTORi is useful in preventing infection by cytomegalovirus or BK virus, or that they may play a part in treating Epstein Barr Virus post-transplant lymphoproliferative disease, and Herpes virus-related Kaposi sarcoma [5].
The standard immunosuppressive kidney transplantation regimens are usually a combination of prednisone and a CNI together with either an mTORi or MPA [15]. Both CNIs and mTORi are prescribed by adjusting for trough drug levels. When a CNI is used alone, it is dosed to achieve trough drug levels around 10 ng/mL, but when used in combination with an mTORi, the sum of both should seek those same 10 ng/mL. Treatment with mTORi without a CNI is not a standard given that at full dosage, they have shown to produce important side effects [16]. However, it can be used instead of CNIs in exceptional cases such as active neoplasia at reduced doses (4—6 ng/mL) or close to full dosage (10 ng/mL) in difficult to treat viral infections if tolerated [17]. In this case, the novelty is that it has never been introduced to control a PB19 infection.
Few reported cases mention the use of mTORi as part of maintenance immunosuppressive therapy in solid organ transplant recipients with symptomatic PB19 infection. In the few reported cases, the mTORi is either removed first after being suspected of being responsible for the anemia or used concomitantly with a CNI. Cases usually resolve with either the use of IVIG or a reduction in CNI dose [14].
In our case, both monthly prophylactic treatment with IVIG, and minimization of immunosuppression (MPA removal and tacrolimus dose reduction) failed to control PB19 infection. Given that further reduction in immunosuppression was not feasible, everolimus was started to allow for further tacrolimus reduction. Despite this, she presented a new relapse with viral loads of PB19 around 70,000 UI/mL. Finally, tacrolimus was withdrawn entirely. This modification allowed her to be relapse-free and without new needs for IVIG or blood transfusions to this day.
In conclusion, immunosuppressive therapy minimization -particularly CNIs- is a fundamental aspect of PB19 infection management. The concern is that this minimization could increase the risk of graft rejection. The conversion from a CNI to an mTORi could be an interesting approach in difficult-to-manage cases similar to ours. With this strategy, we can maintain immunosuppression and minimize the risk of rejection. Further studies are needed to establish whether this approach can be applied to other relapsing PB19 infection cases.
Author contributions
D.R., N.E. and I.R. identified the importance of the case and decided to report it. D.R., E.H., F.D, acquired the data from the electronic health records. D.R., E.C, J.J.B. and I.R. made the figures and drafted and revised the paper. All authors have revised the drafts and approved the final one.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The information regarding this case has been consistent with the World Medical Association Declaration of Helsinki.
Informed consent to participate
Written informed consent was obtained from the patient for anonymized information to be published in this article.
Informed consent to publish
Written informed consent was obtained from the patient for anonymized information to be published in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virus taxonomy update The International Committee on Taxonomy of Viruses. Arch Virol. 1993;133(3–4):491–5. [PubMed] [Google Scholar]
- 2.Kelly HA, Siebert D, Hammond R, Leydon J, Kiely P, Maskill W. The age-specific prevalence of human parvovirus immunity in Victoria, Australia compared with other parts of the world. Epidemiol Infect. 2000;124(3):449–57. doi: 10.1017/s0950268899003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertoni E, Rosati A, Zanazzi M, Azzi A, Zakrzewfka K, Guidi S, Salvadori M. Severe aplastic anaemia due to B19 parvovirus infection in renal transplant recipient. Nephrol Dial Transplant. 1995;10(8):1462–3. [PubMed] [Google Scholar]
- 4.Liefeldt L, Buhl M, Schweickert B, Engelmann E, Sezer O, Laschinski P, Preuschof L, Neumayer HH. Eradication of parvovirus B19 infection after renal transplantation requires reduction of immunosuppression and high-dose immunoglobulin therapy. Nephrol Dial Transplant. 2002;17(10):1840–2. doi: 10.1093/ndt/17.10.1840. [DOI] [PubMed] [Google Scholar]
- 5.Cervera C, Cofan F, Hernandez C, Soy D, Marcos MA, Sanclemente G, Bodro M, Moreno A, Diekmann F, Campistol JM, Oppenheimer F. Effect of mammalian target of rapamycin inhibitors on cytomegalovirus infection in kidney transplant recipients receiving polyclonal antilymphocyte globulins: a propensity score-matching analysis. Transpl Int. 2016;29(11):1216–1225. doi: 10.1111/tri.12848. [DOI] [PubMed] [Google Scholar]
- 6.Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1(7898):72–3. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- 7.Siegl G, Bates RC, Berns KI, Carter BJ, Kelly DC, Kurstak E, Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- 8.Brodin-Sartorius A, Mekki Y, Bloquel B, Rabant M, Legendre C. Infection par le Parvovirus B19 après transplantation rénale. Nephrol Ther. 2012;8(1):5–12. doi: 10.1016/j.nephro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Neild G, Anderson M, Hawes S, Colvin BT. Parvovirus infection after renal transplant. Lancet. 1986;2(8517):1226–7. doi: 10.1016/s0140-6736(86)92245-2. [DOI] [PubMed] [Google Scholar]
- 10.Khameneh ZR, Sepehrvand N, Sohrabi V, Ghasemzadeh N. The seroprevalence of Parvovirus B19 among kidney transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2014;25(1):16–21. doi: 10.4103/1319-2442.124465. [DOI] [PubMed] [Google Scholar]
- 11.Baek CH, Kim H, Yang WS, Han DJ, Park SK. Risk factors and long-term outcomes of parvovirus B19 infection in kidney transplant patients. Transpl Infect Dis. 2017;19(5). 10.1111/tid.12754. [DOI] [PubMed]
- 12.Barzon L, Murer L, Pacenti M, Biasolo MA, Della Vella M, Benetti E, Zanon GF, Palù G. Investigation of intrarenal viral infections in kidney transplant recipients unveils an association between parvovirus B19 and chronic allograft injury. J Infect Dis. 2009;199(3):372–80. doi: 10.1086/596053. [DOI] [PubMed] [Google Scholar]
- 13.Gosset C, Viglietti D, Hue K, Antoine C, Glotz D, Pillebout E. How many times can parvovirus B19-related anemia recur in solid organ transplant recipients? Transpl Infect Dis. 2012;14(5):E64–70. doi: 10.1111/j.1399-3062.2012.00773.x. [DOI] [PubMed] [Google Scholar]
- 14.Beckhoff A, Steffen I, Sandoz P, Hirsch HH, Schaub S. Relapsing severe anaemia due to primary parvovirus B19 infection after renal transplantation: a case report and review of the literature. Nephrol Dial Transplant. 2007;22(12):3660–3. doi: 10.1093/ndt/gfm531. [DOI] [PubMed] [Google Scholar]
- 15.Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y, Chadban S, Oppenheimer F, Sommerer C, Oberbauer R, Watarai Y, Legendre C, Citterio F, Henry M, Srinivas TR, Luo WL, Marti A, Bernhardt P, Vincenti F, Investigators TRANSFORM, Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation, J Am Soc Nephrol. 2018;29(7):1979–91. 10.1681/ASN.2018010009. [DOI] [PMC free article] [PubMed]
- 16.Grinyó JM, Cruzado JM. Mycophenolate mofetil and sirolimus combination in renal transplantation. Am J Transplant. 2006;6(9):1991–9. doi: 10.1111/j.1600-6143.2006.01398.x. [DOI] [PubMed] [Google Scholar]
- 17.de Fijter JW. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation. 2017;101(1):45–55. doi: 10.1097/TP.0000000000001447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.