Abstract
Extracellular vesicles (EVs) have emerged as important players in all aspects of cancer biology. Their function is mediated by their cargo and surface molecules including proteins, lipids, sugars and nucleic acids. RNA in particular is a key mediator of EV function both in normal and cancer cells. This statement is supported by several lines of evidence. First, cells do not always randomly load RNA in EVs, there seems to be a specific manner in which cells populate their EVs with certain RNA molecules. Moreover, cellular uptake of EV-RNA and the secondary compartmentalization of EV-RNA in recipient cells is widely reported, and these RNAs have an impact on all aspects of cancer growth and the anti-tumoral immune response. Additionally, EV-RNA seems to work through various mechanisms of action, highlighting the intricacies of EVs and their RNA cargo as prominent means of inter-cellular communication.
Keywords: Extracellular Vesicles, RNA, Cancer, Tumor microenvironment, microRNAs, non-coding RNAs
Introduction
In recent years, extracellular vesicles (EVs) have emerged as key drivers of cancer progression, a process not only characterized by the tumor cells, but also by the changes occurring to other cells of the tumor microenvironment and distant tissues. Frequently, the term exosome has been used to describe EVs derived from multivesicular bodies (MVBs), that are typically 30–150nm in diameter. Microvesicles on the other hand are usually described as larger vesicles up to 1000nm in size, which shed directly from plasma membrane. Large apoptotic bodies have also been reported as vesicles >1 micron in diameter mainly secreted by highly metastatic cancer cells (1). Since the nomenclature in the literature is inconsistent, guidelines from the International Society for Extracellular Vesicles (ISEV) have been published to normalize the terminology used, with the terms small EVs (sEVs) for EVs <200nm and large EVS (LEVs) for those >200nm (2). In this review, we will use the term EV to describe all cell-derived vesicles, unless specifically stated otherwise.
EVs express proteins from their parent cell on their lipid bilayer membrane, and they also contain various molecules within their lumen, including RNA (3). In the 1990s, EVs from B lymphocyte were shown to induce a T cell response, demonstrating that EVs were an important component of cell-cell communication (4). In the 2000s, the RNA within EVs was shown to be transferred to cells upon EV treatment, impacting signaling events within the recipient cell. These important findings have led to increased research in the role of EVs in cell-cell communication, particularly in understanding cancer progression. Notably, EV-delivered RNA has been uncovered as a key effector molecule in EV-driven cell signaling. Here, we provide an overview of the diverse roles of EV-derived RNA in the progression of cancer, highlighting the distinct mechanisms by which these RNA molecules can interact with cells following delivery by EVs. Understanding the role of EV-derived RNA in the growth and survival of tumors will provide targets for therapeutic interventions.
RNA loading into EVs
RNA is a major component in the complex cargo enclosed inside EVs to be delivered to recipient cells. But how does it get there? In recent years, numerous studies have put great efforts trying to solve this issue until some common loading mechanisms have been discovered. RNA-binding proteins (RBPs) revealed to be key players for RNA incorporation into EVs. By binding to the RNA, these proteins can regulate its loading into EVs and can contribute to its maintenance inside the EVs through the formation of stable complexes. Statello et al. highlighted the presence of 30 RBPs in exosomes postulating two possible paths for their function of RNA loading into the EVs. After processing, RNA can form complexes with RBPs (the so-called RNP-granules) that can enter into the luminal compartment of multivesicular bodies (MVB) through the creation of intermediate vesicles; these vesicles can be further processed inside MVB until their release as exosomes into the extracellular space. Alternatively, RNP-granules can exit the nucleus through the nucleus pore complex (NPC) and approach the MVB where they can be internalized due to the binding of specific receptors on the MVB external membrane. Once inside the MVB, RNP-granules are encapsulated into intraluminal vesicles which are secreted outside upon fusion of the MVB with the plasma membrane (5). However, RBPs could also negatively regulate RNA loading into small EVs in order to avoid losing functional information in the extracellular region (6). Other studies documented the involvement of members of the LC3 conjugation machinery or calcium binding proteins for selective loading of small RNAs in small EVs (7, 8). On the other hand, large EVs such as microvesicles seem to show different peculiarities about RNA loading. Argonaute-2 (Ago-2) has been correlated frequently with the packaging of RNA species in microvesicles. In a work by Lv and colleagues, Ago-2 showed to be relevant in facilitating miR-16 selective packaging in HeLa cells and in guiding its functions once delivered to the HEK293T cells where miR-16 overexpression caused reduction of Bcl-2 levels (9). The presence of Ago-2 in microvesicles has been also confirmed by Clancy et al. in a study conducted on tumor microvesicles (TMV). The authors demonstrated the existence of a molecular axis composed by exportin-5 and ARF-6 responsible for the loading of pre-micro RNA (miRNA) into TMVs through the activation of several GTPases. As a result of ARF6 activity, Ago-2 was found in TMVs together with other components of the RNA machinery processing suggesting that even immature RNAs can be loaded into EVs where they are subjected to further maturation processes before their conversion into mature miRNAs (10). Another mechanism of messenger RNAs (mRNAs) loading into microvesicles is the presence of a specific 25 nt-long sequence defined as “zip-code sequence”. When characterized by a CUGCC string and a miRNA binding site, this zip-code sequence leads to a significant enrichment of mRNAs in microvesicles of various cell types (11).
Even if numerous doubts are still expressed in the debate, interesting new evidences started to be published about the molecular mechanisms regulating RNAs sorting into EVs. The vast majority of studies in the field believe in a selective sorting of particular RNA species in EVs. But some reports tend to support a non-selective sorting model where non-specific factors like RNA concentration in the intracellular compartment might be responsible (12, 13)
The studies favoring a mechanism of preferential RNA sorting into EVs take into account multiple factors. In colorectal cancer (CRC), the mutational status of KRAS has been indicated as a strong driver for selective miRNA export and lncRNAs sorting into EVs (14). Indeed, EVs derived from mutant KRAS CRC cells exhibit distinct RNA profiles compared to the profiles of their parental cells suggesting that RNA levels in EVs don’t reflect their relative cellular abundance (15). Additionally, mutant KRAS and subsequent hyper activation of the MEK-ERK signaling pathway were linked with altered Ago-2 localization in MVBs and decreased expression in secreted exosomes together with significant changes in the amount of specific exosomal miRNAs (16). RBPs contribute to selective sorting by recruiting specific miRNAs to components of the endosome cellular machinery; this is the case for MEXC3, a ubiquitin E3 ligase that can interact with the adaptor AP-2 to mediate miR-451a specific sorting into exosomes (17). Another example is provided by YBX-1 which is able to shape the composition of small non-coding RNAs in exosomes by selectively enrich defined classes such as transfer RNA (tRNA), vaults RNA and Y-RNAs (18). In order to choose which miRNAs to transfer into EVs, some RBPs showed to recognize peculiar motifs on miRNA sequences called EXOmotifs. These motifs appeared to be largely expressed in enriched miRNAs into small EVs belonging to diverse cell types indicating a sort of shared mechanism of miRNA sorting (19, 20). Other authors described how particular post-translation modifications could also impact on RNA sorting. Koppers-Lalic et al demonstrated that 3’ end-uridylated miRNAs were enriched in B cells exosomes as opposed to their cellular counterparts which showed to have more 3’ end adenylation modifications (21). On the other hand, Van Balkom et al documented an increased amount of y-RNA and mRNA fragments (often associated with the endosome machinery) in endothelial exosomes compared to the cellular compartment implying that selective sorting of precise small RNA species could be even regulated by purging purposes (22).
Besides the widely reported presence of mRNA and miRNA in EVs, it is now clear that EVs carry an array of RNA species. Long non-coding (lncRNAs), circular (circRNAs), vault (vtRNAs) and piwi-interacting RNAs (piRNAs) are some examples of RNAs that can now be detected in EVs. The latest in RNA-sequencing technology has uncovered the complex transcriptome within EVs, and the presence of more RNA species has been elucidated (23). Whilst many studies show enrichment of specific RNA sequences in EV samples, others have found that EVs contain very little RNA; Chevillet et al for instance show that the average number of copies of miRNA in their EVs was roughly 1 for every 100 EVs (24), contrary to other reporting on the abundance of RNA in EVs. This study supports the possibility that a few exosomes may contain a few miRNA molecules (low occupancy/low miRNA concentration) or that one exosome might contain a high concentration of miRNAs (low occupancy/high miRNA concentration) (24). While the criteria of abundance of RNA in EVs are still unclear, and it is unknown what are the levels of EV-miRNAs able to affect functions in the recipient cell, this study provides the interesting scenario in which selective groups of exosomes (perhaps enriched with miRNAs according to the low occupancy/high miRNA concentration model) may still be able to deliver enough miRNA to the recipient cell to elicit a phenotypic response. Additionally, EV source, isolation and RNA detection techniques vary considerably, likely impacting the extent to which RNAs are detected in EVs.
RNA is mostly protected in EVs by RNase digestion suggesting that the majority of RNA detected in EV samples is encapsulated within the vesicles, rather than bound to the EV surface (25–27). On the other hand, much of the DNA associated with EVs is not protected following DNase treatment, perhaps pointing towards distant processes governing RNA and DNA association with EVs (28, 29).
EVs in the extracellular space and cellular uptake
In order for RNA-loaded EVs to impact cellular function in distant cells, the EVs must overcome biological barriers in the body whilst protecting their cargo. First, following secretion by the donor cell, EVs must remain intact within the interstitial fluid until they reach their target cell; further, EVs travelling to cells in distant tissues must penetrate the blood vessel endothelium and remain stable in the bloodstream. Upon reaching their target cell, the EV must then unload their RNA cargo so it becomes available to the cell.
EVs in the extracellular space
With the development of zebrafish embryos expressing CD63-pHluorin, Verweij et al. were able to live track single endogenous EVs. They saw EVs in tissue surrounding muscle segments and in the neural tube, moving irregularly and by what looks like Brownian motion, suggesting that they are mobile in the interstitial space (30). They also showed that EVs in the bloodstream were capable of penetrating the endothelium. These data are evidence of the ability of EVs to transit great distances and reach distant tissues. Tracking EVs following intravenous injection into mice provides information regarding the in vivo stability of the vesicles. Bala et al. used a miR-155 KO mouse model to study the half-life and biodistribution of miR-155 mimic loaded EVs. The results showed that 30 minutes after intravenous injection, plasmatic miR-155 levels in miR-155 KO mouse returned to baseline expression. In fact, around 90% of plasmatic miR-155 was no longer in circulation, but miR-155 was mostly detected in liver followed by adipose tissue, lung, muscle and kidney, revealing that EVs survive in the bloodstream and rapidly penetrate tissues (31).
The ability of EVs to penetrate tissues and enter the circulation not only reinforces their role in intercellular communication, but their presence, in the bloodstream in particular, makes them an interesting biomarker candidate. Numerous species of RNAs upregulated in cancer EVs compared with healthy counterparts are showing promise as potential novel diagnostic markers in cancer, such as miRNA (32), mRNA (33), lncRNA (34), circRNA (35) and even tRNA-derived small RNAs (36).
Cellular uptake of EVs
mRNA delivered by EVs to cells can be translated within an hour of EV treatment (37), and luciferin transferred to cells via EVs is catalyzed just minutes after EV treatment of the cell (26). Whilst these data show that EV cargo become available to cell soon after the addition of EVs to the cells, this does not tell us how the EV cargo escapes the EV lumen to become active within the receiving cell. Many studies have sought to gain a greater understanding of the machinery involved in EV uptake and processing to elucidate how EV cargo becomes active in the cell.
By labelling EVs with fluorescent probes, internalization of EVs by cells can be determined visually, with EVs appearing as punctate dots within cells (37, 38). Co-localization of EVs within endosomal compartments shows that EV internalization by cells occurs through endocytosis (39). EV uptake has been reported through various endocytic mechanisms, and previous reviews have provided overviews of these mechanisms (40, 41). Electron microscopy has revealed that following endocytic uptake, EVs still appear as intact vesicles within the endosome, meaning they did not fuse with the cell at the plasma membrane (42, 43); this however does not exclude the possibility that EV-cell fusion also occurs at the plasma membrane. The findings that EV cargo becomes functional shortly after EV uptake suggest a rapid unloading mechanism, however the precise apparatus used by cells to liberate EVs of their cargo remains unclear.
Role of EV RNA in cancer progression
Secretion of EVs is increased in cells under stress, such as those experiencing hypoxia, and it has been long known that cancer cells secrete EVs at a greater rate compared to their healthy counterparts (44). Increased EV secretion by the cancer cell is not simply a benign signature of the state of the cell, but these EVs in fact contribute to cancer progression through education of other cells within the tumor microenvironment and distant tissues. There is now an abundance of data to indicate that cancer EVs play key roles in numerous pro-tumorigenic processes (visualized in Figure 1, examples summarized in Table 1).
Figure 1. Roles of EV-RNA in cancer progression.
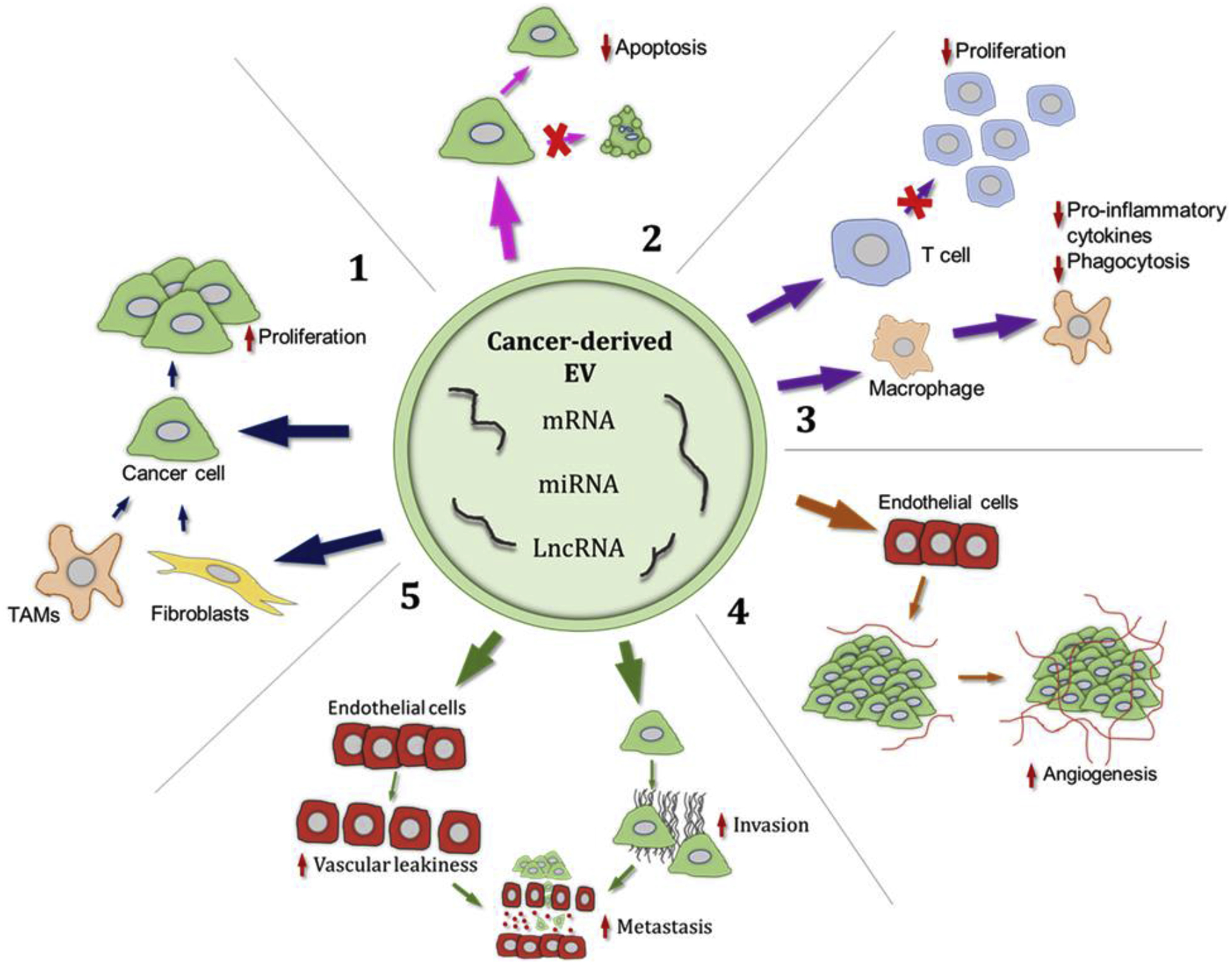
Cancer cell derived EVs carry an assortment of RNA, including messenger, micro and long non-coding RNAs. Upon delivery via EVs to recipient cells, these RNAs contribute to driving various phenotypic changes in cells of the tumor microenvironment and the cancer cells themselves, driving cancer progression. 1: Through direct action on cancer cells or indirectly via tumor associated macrophages and fibroblasts, EV-RNA induce proliferation in cancer cells. 2: Transfer of cancer EV-RNA to other cells leads to inhibition of apoptotic pathways in the recipient cell. 3: Cancer EV-RNAs, delivered to cells of the immune system, enable immune evasion for the tumor, by macrophage polarization and inhibiting T cell proliferation for example. 4: RNAs present in cancer EVs are capable of enhancing expression of angiogenic factors in endothelial cells, stimulating angiogenesis around the tumor. 5: Modulation of tight junction proteins in endothelial cells is also stimulated by cancer EV-RNAs, leading to vascular leakiness. The cancer EV-RNA effect on endothelial cells, along with increasing the migratory capability of cancer cells, both contribute to cancer cells metastasizing from the primary tumor. EV: extracellular vesicle, TAM: tumor associated macrophage
Table 1.
Examples of RNAs identified in EVs, and the RNA role in cancer progression.
| RNA species | RNA | Cancer of origin | Pro-tumoral Function | Reference |
|---|---|---|---|---|
| miRNA | miR-10b | Colorectal | Myofibroblast differentiation | (45) |
| miR-1246 | Breast | Increased viability, migration | (46) | |
| miR-25-3p | Liposarcoma | Pro-inflammatory cytokine production | (47) | |
| miR-222 | Pancreatic ductal adenocarcinoma | Proliferation | (48) | |
| miR-93-5p | Esophageal | Proliferation | (50) | |
| miR-1249-5p | Colorectal | Proliferation | (51) | |
| miR-93 | Hepatocellular carcinoma | Metastasis | (52) | |
| miR-99a-5p | Ovarian | Invasion, fibronectin and vitronectin upregulation | (53) | |
| miR-105 | Breast | Vascular leakiness | (54) | |
| miR-103 | Liver | Vascular leakiness | (55) | |
| miR-24-3p | Nasopharyngeal carcinoma | Downregulate T-cell proliferation | (77) | |
| miR-203 | Pancreatic | Dendritic cell dysfunction | (78) | |
| miR-21 | Ovarian | Apoptotic escape | (80) | |
| miR-122 | Breast | Metabolic reprogramming | (83) | |
| IncRNA | MALAT1 | Breast | Migration | (58) |
| HOTAIR | Bladder | Epithelial-mesenchymal transition | (60) | |
| ZFAS1 | Gastric | Cell cycle regulation | (61) | |
| RPPH1 | Colorectal | Macrophage polarisation | (62) | |
| lncRNA91H | Colorectal | Migration | (63) | |
| ZEB1-AS1 | Esophageal | Proliferation | (64) | |
| Linc-CCAT2 | Glioma | Angiogenesis | (70) | |
| POU3F3 | Glioma | Angiogenesis | (71) | |
| lncH19 | Liver | Angiogenesis | (72) | |
| TUC339 | Hepatocellular carcinoma | Pro-inflammatory cytokine production | (76) | |
| PVT1 | Colon | Apoptotic escape | (81) | |
| lncARSR | Renal carcinoma | Therapy resistance | (90) | |
| mRNA | hTERT | T-cell leukaemia | Inhibition of fibroblast senescence | (68) |
| MMP1 | Ovarian | Apoptosis of mesothelial cells | (86) |
Proliferation
For tumor cells to uncontrollably divide and grow, cancer cells must maintain growth-promoting signals that allow for entry into and progression through the cell cycle. Moreover, cancer cells can educate other cells within the tumor microenvironment to promote proliferative signaling, through secretion of RNA-loaded EVs.
EVs from colorectal cancer cells have been found to contain and transfer miR-10b to non-malignant fibroblasts to decrease PIK3CA levels. Downregulation of PI3KCA causes decreased fibroblast proliferation but stimulates the expression of TGF-β and SMα-actin, thus converting them into cancer-associated-fibroblasts that would promote cancer growth (45). Another miRNA, miR-1246, upregulated in breast cancer cells and loaded into EVs, can also drive cancer progression. Metastatic breast cancer cells transfer miR-1246 via EVs to non-malignant epithelial cells, downregulating cyclin-G2 (CCNG2), thus promote invasion in recipient cells (46). The vesicular transfer of miR-25–3p and miR-92a-3p from liposarcoma cells to tumor-associated macrophages (TAMs) promoted the secretion of pro-inflammatory cytokine IL-6 in a TLR7/8-dependent manner via the NF-kB pathway, which in turn promoted proliferation of the liposarcoma cells (47). Vesicular miR-222 is thought to be transmitted via EVs between pancreatic ductal adenocarcinoma cells to enhance cell invasion and proliferation. Transmission of miR-222 promotes proliferation of neighboring tumor cells by either directly regulating p27, or by activating AKT through inhibition of PPP2R2A expression, thereby inducing p27 phosphorylation, causing higher cytoplasmic levels of p27 (48).
Growth suppressor inhibition
Cancer cells must overcome factors that act to negatively regulate cell proliferation, such as tumor suppressors whose function it is control cell division, promote apoptosis, and suppress metastasis. In many tumors, tumor suppressor genes are mutated, leading to tumor development due to the removal of negative regulators of cell proliferation (49).
Tumor cells use extracellular vesicles in different ways and with different cargo to circumvent the negative regulation of cell proliferation. Esophageal cancer cells use extracellular vesicles to transfer miR-93–5p, which targets the tumor suppressor PTEN, in other esophageal cancer cells, thereby increasing cell proliferation of recipient cells (50).
Extracellular vesicles from TP53-deficient colorectal cancer cells have been found to transfer miRNAs miR-1249–5p, miR-6737–5p, and miR-6819–5p to nearby fibroblasts which in turn downregulated the expression of TP53 in the recipient fibroblasts as well as promoted their proliferation in vitro (51).
Invasion and metastasis
Cancer-cell derived EVs have been found to play crucial roles in multistep process of invasion and metastasis, through education of cells to increase matrix remodeling, and vascular permeability.
EVs from Hepatocellular Carcinoma have been found to be enriched in miR-93, which can directly target the metastasis suppressors CDKN1A, TP53INP1 and TIMP2, enabling invasion and metastasis (52). Another example are epithelial ovarian cancer EVs that are found to carry miR-99a-5p, which promote cell invasion by affecting neighboring peritoneal mesothelial cells through fibronectin and vitronectin upregulation (53). Metastatic breast cancer cells showed to transfer EVs containing miR-105 to endothelial cells in order to regulate expression of tight junction protein ZO-1, which in turn triggers the opening of cell wall junctions between endothelial cells, promoting vessel leakiness, and facilitating cancer cell extravasation, which stimulates metastasis to liver and brain (54). Another example of enhancing vascular permeability by targeting tight junction proteins in endothelial cells is miR-103, secreted by hepatoma cells to facilitate metastasis (55). Another miRNA involved in lymph node metastasis is miR-423–5p, which is enriched in the EVs of gastric cancer patients, that could be taken up by gastric cancer cells, thereby enhancing proliferation in vitro and in vivo by inhibiting suppressor of fused protein (SUFU) (56). miR-148a, delivered by EVs from glioblastoma cells may promote cancer cell proliferation and metastasis through targeting cell adhesion molecule 1 (CADM1) to activate the STAT3 pathway (57).
Cancer cells do not only make use of miRNAs to promote invasion and metastasis through the transfer of EVs from cancer cells to surrounding cells, but also transfer long noncoding RNAs, as well as mRNAs. One example is the long noncoding RNA MALAT1 (Metastasis-associated lung adenocarcinoma transcript 1) that has been found to be highly enriched in EVs from lung cancer patients and its expression level was associated with tumor stage and lymphatic metastasis (58). MALAT1 was also found in EVs of breast cancer patients, and associated with disease progression, suggesting a role in tumor growth and migration (59). Long intergenic noncoding RNA HOTAIR (HOX transcript antisense RNA) was found in EVs of urine of patients with urothelial bladder cancer (UBC), and loss of HOTAIR expression showed altered expression of epithelial-mesenchymal transition (EMT) genes such as SNAI1, TWIST1, ZEB1, ZO1, MMP1, LAMB3, and LAMC2, and reduced migration and invasion in vitro, suggesting an important role of HOTAIR in metastasis of bladder cancer (60). Another EV lncRNA found in gastric cancer (GC), ZFAS1, plays a role in metastasis by promoting proliferation and migration of GC cells by regulating cell cycle progression, apoptosis and EMT (61). Another lncRNA, RPPH1, a promoter of EMT was found in colorectal cancer cell-derived EVs, which interacts with TUBB3 to prevent its ubiquitination. RPPH1 is also shuttled into macrophages to promote M2 polarization, enhancing metastasis and proliferation of CRC cells (62). lncRNA91H in colorectal cancer cell-derived EVs enhance invasion and metastasis by modifying HNRNPK expression (63). EVs derived from esophageal cancer patients promote esophageal cancer progression by delivering long intergenic noncoding RNA ZEB1-AS1 to esophageal cancer cells to target miR-214, resulting in cell proliferation and metastasis (64).
Replicative immortality
Cancer cells use a variety of strategies to allow for an unlimited cell division. One of these strategies is the overexpression of telomerase, a reverse transcriptase that maintains telomere length during chromosome replication by synthesizing TTAGGG repeats at their ends (65). Telomerase is activated in human stem cells as well as reproductive cells (66), but remains inactive in most of somatic cells. Telomerase activity accounts for the immortality of 85–90% of human cancer cells (67). EVs from cancer cells have been found to contain mRNA of the transcript of the telomerase enzyme, hTERT, which is transported from the cancer cells to neighboring telomerase negative fibroblasts, generating an active enzyme that converts these fibroblasts into non-malignant, immortalized cells that showed increased cell proliferation, and protection from DNA damage as well as from apoptosis (68).
Angiogenesis
For cancer cells to proliferate and to metastasize, the supply of oxygen and nutrients to the cancer cells as well as disposal of waste products from the cells are of great importance. Furthermore, angiogenesis is controlled by activating as well as inhibiting molecules. While activating factors need to be upregulated in order to promote angiogenesis, negative regulators must be inactivated at the same time (69). EVs are used in different ways by cancer cells to mediate each step in the process of angiogenesis.
The intergenic long noncoding RNA CCAT2 (linc-CCAT2) was found to be overexpressed in the EVs of glioma cells, suggesting a prominent role in cancer progression. In vitro studies showed that overexpression of linc-CCAT2 in endothelial cells activated VEGFA and TGFβ, thereby promoting angiogenesis. It further caused Bcl-2 expression and inhibited the pro-apoptotic proteins Bax and caspase-3 expression, thereby decreasing apoptosis in the recipient endothelial cells, suggesting a new EV-mediated mechanism by which the vesicular transfer of linc-CCAT2 to endothelial cells could promote angiogenesis (70). Another study showed that the transfer of the long intergenic noncoding RNA POU3F3 from glioma cells to endothelial cells increased glioma angiogenesis by upregulating expression of bFGF, VEGFA, bFGFR, and Angio, key pro-angiogenesis factors in recipient cells (71).
CD90+ liver cancer cells have been sought to promote angiogenesis by the vesicular release of long noncoding RNA H19 (lncH19) to endothelial cells. In vitro experiments showed upregulation of VEGF production in endothelial cells, thus increasing the ability to arrange in vitro tubular-like structures, a typical angiogenic phenotype, and cell-to-cell adhesion (72).
Angiogenesis can also be promoted by the EV release of miRNAs by cancer cells. EVs released by monocytes showed high levels of miR-150, which was taken up by endothelial cells in vitro, resulting in the aberrant formation of capillary-like tubes, thus promoting a pro-angiogenic effect. MiR-150 is highly expressed in a variety of cancer types and in vivo tumor mouse model showed that the secretion of miR-150 from cancer cells into the circulation was increased, which promoted the abnormal generation of new vessels, thus significantly increasing angiogenesis (73).
Transcriptomic analysis of colorectal cancer cell-derived EVs revealed enrichment of mRNAs associated with M-phase cell cycle activities. Delivery of CRC cell-derived EVs to endothelial cells promotes proliferation of endothelial cells, suggesting angiogenic activity (74).
Immune system evasion
Two main strategies have been described to be used by cancer cells to evade immune destruction: circumventing immune recognition and promoting an immunosuppressive tumor microenvironment. Downregulation of cell-surface molecules, expression of inhibitory checkpoint molecules, as well as secretion of pro-inflammatory cytokines by cancers to stimulate non-tumor cells in the TME contribute to the escape from immune destruction (75). Cancer cell-derived EV-RNA has been shown to play a role in protecting tumors from the immune system.
The long intergenic noncoding RNA TUC339 is enriched in hepatocellular Carcinoma-derived EVs and transferred to macrophages; this lncRNA was shown to reduce pro-inflammatory cytokine production, decrease co-stimulatory molecule expression and to compromise phagocytosis (76).
EV miR-24–3p from nasopharyngeal carcinoma (NPC) cells was found to inhibit T-cell proliferation, both in vitro and in vivo, by downregulating FGF11 to regulate phosphorylation of ERK and STAT, thus decreasing the number and function of T-cells as a mechanism to evade immune destruction (77).
Another example of an EV miRNA that is used to manipulate immune cells in order to evade immune destruction is miR-203, secreted by pancreatic cancer cells. After treatment of dendritic cells with pancreatic cell-derived EVs, as well as miR-203 mimic, expression of TLR4 and the production of cytokines including TNF-a and IL-12, decreased, contributing to dysfunction of DCs (78).
Inhibition of apoptosis
For cancer cells to survive and proliferate, they must overcome apoptosis, a cellular program used to eliminate damaged cells or cells that display abnormalities, such as cancer cells. Cancer cells have many ways to circumvent programmed cell death, such as by increasing expression of anti-apoptotic regulators or survival signals, or by downregulating pro-apoptotic factors (79). Different mechanisms of evading programmed cell death via EVs have been reported.
EVs from ovarian serous carcinoma contain miR-21, a well-known oncogenic miRNA. miR-21 targets the pro-apoptotic protein Programed Cell Death 4 (PCDC4), thus contributing to the escape from apoptosis and thereby contributing to the malignancy of this type of cancer. Transferring miR-21 via EVs to recipient cells could promote oncogenic transformation of distant cells without direct contact (80).
The long non-coding RNA PVT1 was found in the EVs from metastatic colon cancer cells and has been correlated with c-Myc expression. Downregulation of PVT1 expression corresponded to suppression of c-Myc as well as inhibition of cell proliferation and increased apoptosis suggesting an anti-apoptotic role for PVT1 in colorectal cancer (81). Another long-noncoding RNA MALAT1 was found highly expressed in EVs derived from Non-Small Cell Lung Cancer (NSCLC) patients and was found to have a preventive role against apoptosis in lung cancer cell lines (58).
Energy metabolism reprogramming
Non-malignant, normal cells use oxidative phosphorylation inside the mitochondria to produce energy in form of ATP. Cancer cells, on the other hand, can reprogram the energy metabolism to produce energy through aerobic glycolysis in the cytoplasm (82).
Cancer cells have developed different strategies, including the use of EVs, to reprogram their energy metabolism. One example is the release of miR-122 from EVs of breast cancer cells into non-tumor cell in the pre-metastatic niche, where it causes suppression of glucose uptake by downregulation of the glycolytic enzyme pyruvate kinase (PKM). Modifying glucose utilization by recipient cells is used to reprogram systemic energy metabolism to support cancer growth in vivo and in vitro (83).
Diverse mechanisms of action of EV-RNA
EVs from cancer cells and the surrounding tumor microenvironment have been shown to drive many cancer processes through the delivery of RNA species to recipient cells. Not only does EV-RNA impact many facets of cancer progression, as well as other physiological and pathological processes, but the RNA can also interact with the recipient cell following EV delivery through a multitude of mechanisms summarized in Figure 2. The host of mechanisms described here reveals the variety of functional RNA species that EVs carry, and adds layers of complexity to the manner of EV cargo transfer to a cell.
Figure 2. Mechanisms of action for EV-RNA and EV-DNA.
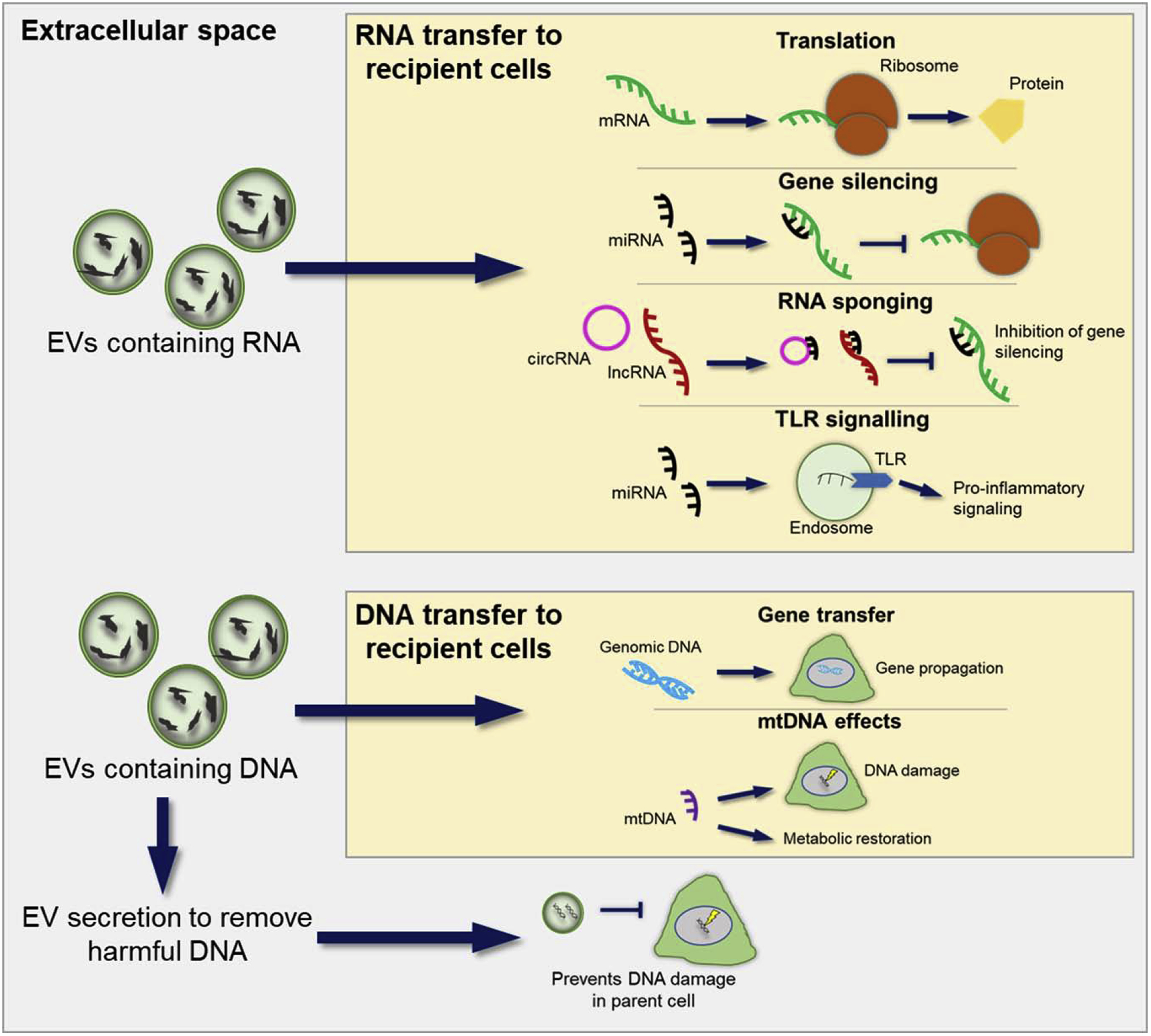
An overview of the mechanisms by which RNA and DNA, delivered by EVs, can interact with recipient cells. mRNA carried by EVs can be translated upon delivery to a cell. Delivered miRNAs can repress expression of their targets, whereas delivered circRNAs and lncRNAs carry miRNA seed regions, competing with miRNA targets for binding of the miRNAs. EV-miRNAs have been shown to be ligands for receptors, for example, miR-21 was shown to activate TLR8, increasing pro-inflammatory signaling (94). EVs also carry both genomic and mtDNA. Gene propagation from cell to cell can occur via EVs. mtDNA delivered by EVs to cells can restore metabolic activity in the cell. DNA damage in cells receiving mtDNA from EVs can also occur. Some DNA, harmful to the cell, has been shown to be secreted in EVs, preventing DNA damage in the parent cell, demonstrating a role for EVs in ridding the cell of harmful material. EV: extracellular vesicle, mRNA: messenger RNA, miRNA: micro RNA, circRNA: circular RNA, lncRNA: long non-coding RNA, TLR: toll-like receptor, mtDNA: mitochondrial DNA.
Translation
EVs transport mRNA to recipient cells to directly contribute to the protein signature of that cell. These mRNAs are translated in the donor cells. Cells manipulated to express mRNA for reporter proteins such as luciferase or GFP will load these mRNAs into EVs, and following delivery of these EVs to cells not expressing these transcripts, translated GFP/luciferase proteins could be detected, therefore showing that EVs can transfer translatable mRNA between cells (3, 84). Furthermore, EVs can promote phenotypic change in other cells through the transfer of mRNA. Ragni et al found that MSC-derived EVs carried IL-10 mRNA, and that upon treatment of kidney tubular cells with these EVs, these cells would then express both IL-10 mRNA and protein (85). MMP1 is a protein linked to poor prognosis in ovarian cancer patients, its mRNA can be transferred to mesothelial cells from ovarian cancer cells via EVs and undergo translation (86).
Gene silencing
The typical role of miRNA in post-transcriptional regulation of gene expression is widely reported in studies of EV-delivered miRNA. For example, EVs from metastatic breast cancer cells contain mir-105, this mir can target binding sites on the 3’ UTR of the tight junction protein ZO-1, resulting in reduced expression of the ZO-1 gene (87). Downregulation of such a protein in a metastatic cancer setting increases vascular leakiness. Pathogens can also hijack EV loading, including the RNA cargo. EBV miRNAs for instance are present in EVs from EBV infected B cells, these miRNAs can repress EBV targets in recipient cells following EV treatment (88).
RNA sponging
Long non-coding RNAs (lncRNAs) are large RNA molecules which can recognise complementary sequences but are not translated into proteins. EVs are enriched in lncRNAs which contain miRNA seed regions (89), indicating an ability to abrogate miRNA induced gene repression through “sponging” of the miRNA, thereby making the miRNAs unavailable to their targets. In sunitinib resistant renal carcinoma cells, lncARSR binds miRs 34 and 449, facilitating c-Met expression; this lncRNA could also be transferred to sunitinib sensitive cells by EVs, desensitising the cells (90), suggesting lncRNAs delivered by EVs can sponge miRNAs.
circRNAs are formed by back-splicing of mRNA that links the two ends of the molecule. circRNA can also act as a sponge for miRNAs by carrying a competing miRNA binding site. CDR1as is a circRNA which can bind miR-7, inhibiting miR-7 activity and leading to increased levels of miR-7 target genes (91). CDR1as expression in EVs and its miR-7 sponging activity in recipient cells has also been reported (92).
Receptor activation
Besides the most commonly described RNA functions, single stranded RNAs are also known to act as ligands for Toll-like receptors (TLRs). Single stranded RNA from HIV can induce pro-inflammatory responses in T-cells and macrophages through TLR7 and TLR8 activation (93). It was later shown that mir-21 and mir-29a, delivered by cancer EVs, could act as ligands for TLR8, triggering a pro-inflammatory response in macrophages, leading to tumour growth and metastasis (94). This alternate mechanism of action of EV-miRNA underlines the complexity of RNA handover from EV to cell. Unlike the aforementioned RNA mechanisms of action, receptor activation by EV miRNAs requires these molecules to be available to TLR ligand binding sites in the endosome lumen.
Role for EV-DNA?
As well as the assortment of RNA species detected in EVs, a number of studies have also detected DNA, including mitochondrial DNA (mtDNA) in their EVs. In patients with metastatic breast cancer, EVs in the blood were found to contain mtDNA (29). Furthermore, this study showed that mtDNA from cancer-associated fibroblast (CAF) EVs could rescue metabolically dormant cells through delivery of mtDNA, restoring oxidative phosphorylation. Another study reported DNA damage in cells treated with mtDNA-containing EVs derived from irradiated fibroblasts (95). The mechanisms by which mtDNA is loaded in to EVs is unclear, but also highlights the intricacies involved in cargo transfer between cells via EVs.
Nuclear DNA is also known to be carried by EVs. Oncogenes can be transferred to cells via apoptotic bodies resulting in the induction of a pro-tumorigenic phenotype, revealing a potential role for EVs in the propagation of oncogenes (96). Epstein-Barr virus (EBV) infected cells produce apoptotic bodies containing EBV DNA, the following internalization of these apoptotic bodies by cells, EBV genes could be expressed, suggesting that transferred DNA can be transcribed and translated (97). Interestingly, Takahashi et al reported a role for EVs in removing DNA from DNA damaged cells to prevent cell death (98). Rather than their utility as vehicles, transferring molecules from one cell to another, here the authors describe a mechanism by which EVs are used to remove harmful components from cells.
Conclusion
Increasing experimental evidence supports the role of EVs in cancer biology. Initially considered simply as “trash bins” of the cell to get rid of waste products and/or toxic compounds, EVs have emerged as key mediators of cell-to-cell communication within the tumor microenvironment, orchestrating different hallmarks of the malignant phenotype. Several questions still need to be addressed in this field. First, it is unclear how a cargo RNA released in the cytosol of the recipient cell can reach a receptor located in the endosomal compartment (such as TLR8) or in the mitochondrion. Perhaps active transporters are responsible for the transfer of such RNAs from the cytosol to different intracellular compartments, or a specific “fusion” mechanism between the EV membrane on one side and the plasma membrane and endosomal membrane on the recipient cell side occur, resulting in the direct transfer from the EV lumen to the intra-endosomal compartment. In this scenario, the presence of the EV-delivered RNA in the cellular cytosol could be the result of a “leakage” from the endosomal compartment to the cytosol of the recipient cell. Secondly, while several studies have focused on the role of EV-microRNAs, it has been reported that microRNAs are not the only (and possibly not even the most represented) type of RNA in EV cargo. Therefore, the role of other non-coding RNAs delivered by EVs should be better clarified. Recently, Albanese at el. Reported that microRNAs are only minimally present in EVs and their delivery to recipient cells is very inefficient (99). This very rigorously conducted study is in contrast with many other reports in support of the role of EV-microRNAs in shaping the biology of the tumor microenvironment. Different methods of EV isolation/preparation, different concentrations of EVs used for functional studies and different EV administration modalities could affect the outcome of these experiments and could explain the different conclusions of these studies, but certainly additional experimental evidence is warranted to address this important point. Another open question is whether DNA is actively released by small extracellular vesicles (100). EV heterogeneity could explain some of the contrasting results on this topic, however more studies are necessary to fully address how extracellular DNA is transferred from one cell to another.
A greater understanding of the different properties of EV-RNA (from its loading into EVs, to its biodistribution, uptake and mechanism of action in the recipient cells) will enhance our comprehension of the biology of cancer. This will lead to the development of new strategies to restore a physiological inter-cellular communication, and therefore to the development of new therapeutics, improving the clinical outcome of cancer patients.
Acknowledgments
We apologize with the colleagues whose work has not been cited due to space limitations. Dr. Fabbri is the recipient of a Pablove Accelerator award. Work in Dr. Fabbri’s lab is supported by the National Institutes of Health (NIH/NCI) grant 5P30CA071789-20. Work in Dr. Fabbri’s lab is supported by the NIH/NCI grants R01CA215753 and R01CA219024.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicts of interest to declare.
References
- 1.Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6(13):11327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Statello L, Maugeri M, Garre E, Nawaz M, Wahlgren J, Papadimitriou A, et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One. 2018;13(4):e0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Boza J, Boeckx A, Lion M, Dequiedt F, Struman I. hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cell Mol Life Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagiwara K, Katsuda T, Gailhouste L, Kosaka N, Ochiya T. Commitment of Annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett. 2015;589(24 Pt B):4071–8. [DOI] [PubMed] [Google Scholar]
- 8.Leidal AM, Huang HH, Marsh T, Solvik T, Zhang D, Ye J, et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. 2020;22(2):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lv Z, Wei Y, Wang D, Zhang CY, Zen K, Li L. Argonaute 2 in cell-secreted microvesicles guides the function of secreted miRNAs in recipient cells. PLoS One. 2014;9(7):e103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clancy JW, Zhang Y, Sheehan C, D’Souza-Schorey C. An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat Cell Biol. 2019;21(7):856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Ströbel T, Erkan EP, et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tosar JP, Gámbaro F, Sanguinetti J, Bonilla B, Witwer KW, Cayota A. Assessment of small RNA sorting into different extracellular fractions revealed by high-throughput sequencing of breast cell lines. Nucleic Acids Res. 2015;43(11):5601–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gámbaro F, Li Calzi M, Fagúndez P, Costa B, Greif G, Mallick E, et al. Stable tRNA halves can be sorted into extracellular vesicles and delivered to recipient cells in a concentration-dependent manner. RNA Biol. 2019:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinger SA, Cha DJ, Franklin JL, Higginbotham JN, Dou Y, Ping J, et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell Rep. 2018;25(3):715–25.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, et al. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, et al. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016;15(5):978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu P, Li H, Li N, Singh RN, Bishop CE, Chen X, et al. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. PLoS One. 2017;12(10):e0185992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R, et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A. 2017;114(43):E8987–E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanathan S, Shenoda BB, Lin Z, Alexander GM, Huppert A, Sacan A, et al. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J Extracell Vesicles. 2019;8(1):1650595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, et al. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17(3):799–808. [DOI] [PubMed] [Google Scholar]
- 21.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–58. [DOI] [PubMed] [Google Scholar]
- 22.van Balkom BW, Eisele AS, Pegtel DM, Bervoets S, Verhaar MC. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4:26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turchinovich A, Drapkina O, Tonevitsky A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front Immunol. 2019;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42(11):7290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna). 2010;117(1):1–4. [DOI] [PubMed] [Google Scholar]
- 29.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114(43):E9066–E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, et al. Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Dev Cell. 2019;48(4):573–89.e4. [DOI] [PubMed] [Google Scholar]
- 31.Bala S, Csak T, Momen-Heravi F, Lippai D, Kodys K, Catalano D, et al. Biodistribution and function of extracellular miRNA-155 in mice. Sci Rep. 2015;5:10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhavan B, Yue S, Galli U, Rana S, Gross W, Müller M, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136(11):2616–27. [DOI] [PubMed] [Google Scholar]
- 33.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin LY, Yang L, Zeng Q, Wang L, Chen ML, Zhao ZH, et al. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan S, Gou Q, Pu W, Guo C, Yang Y, Wu K, et al. Circular RNA F-circEA produced from EML4-ALK fusion gene as a novel liquid biopsy biomarker for non-small cell lung cancer. Cell Res. 2018;28(6):693–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6:7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96. [DOI] [PubMed] [Google Scholar]
- 39.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–66. [DOI] [PubMed] [Google Scholar]
- 40.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: Mechanisms of Uptake. J Circ Biomark. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288(24):17713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–801. [DOI] [PubMed] [Google Scholar]
- 45.Dai G, Yao X, Zhang Y, Gu J, Geng Y, Xue F, et al. Colorectal cancer cell-derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bulletin du cancer. 2018;105(4):336–49. [DOI] [PubMed] [Google Scholar]
- 46.Li XJ, Ren ZJ, Tang JH, Yu Q. Exosomal MicroRNA MiR-1246 Promotes Cell Proliferation, Invasion and Drug Resistance by Targeting CCNG2 in Breast Cancer. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;44(5):1741–8. [DOI] [PubMed] [Google Scholar]
- 47.Casadei L, Calore F, Creighton CJ, Guescini M, Batte K, Iwenofu OH, et al. Exosome-Derived miR-25–3p and miR-92a-3p Stimulate Liposarcoma Progression. Cancer research. 2017;77(14):3846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Tao Y, Wang X, Jiang P, Li J, Peng M, et al. Tumor-Secreted Exosomal miR-222 Promotes Tumor Progression via Regulating P27 Expression and Re-Localization in Pancreatic Cancer. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018;51(2):610–29. [DOI] [PubMed] [Google Scholar]
- 49.Sun W, Yang J. Functional mechanisms for human tumor suppressors. Journal of Cancer. 2010;1:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu MX, Liao J, Xie M, Gao ZK, Wang XH, Zhang Y, et al. miR-93–5p Transferred by Exosomes Promotes the Proliferation of Esophageal Cancer Cells via Intercellular Communication by Targeting PTEN. Biomedical and environmental sciences : BES. 2018;31(3):171–85. [DOI] [PubMed] [Google Scholar]
- 51.Yoshii S, Hayashi Y, Iijima H, Inoue T, Kimura K, Sakatani A, et al. Exosomal microRNAs derived from colon cancer cells promote tumor progression by suppressing fibroblast TP53 expression. Cancer science. 2019;110(8):2396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue X, Wang X, Zhao Y, Hu R, Qin L. Exosomal miR-93 promotes proliferation and invasion in hepatocellular carcinoma by directly inhibiting TIMP2/TP53INP1/CDKN1A. Biochemical and biophysical research communications. 2018;502(4):515–21. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura A, Sawada K, Nakamura K, Kinose Y, Nakatsuka E, Kobayashi M, et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC cancer. 2018;18(1):1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer cell. 2014;25(4):501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fang J-H, Zhang Z-J, Shang L-R, Luo Y-W, Lin Y-F, Yuan Y, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology (Baltimore, Md). 2018;68(4):1459–75. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Fu H, Wang B, Zhang X, Mao J, Li X, et al. Exosomal miR-423–5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Molecular carcinogenesis. 2018;57(9):1223–36. [DOI] [PubMed] [Google Scholar]
- 57.Cai Q, Zhu A, Gong L. Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bulletin du cancer. 2018;105(7–8):643–51. [DOI] [PubMed] [Google Scholar]
- 58.Zhang R, Xia Y, Wang Z, Zheng J, Chen Y, Li X, et al. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490(2):406–14. [DOI] [PubMed] [Google Scholar]
- 59.Zhang P, Zhou H, Lu K, Lu Y, Wang Y, Feng T. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. Onco Targets Ther. 2018;11:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, et al. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS One. 2016;11(1):e0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143(6):991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang Z-X, Liu H-S, Wang F-W, Xiong L, Zhou C, Hu T, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell death & disease. 2019;10(11):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao T, Liu X, He B, Nie Z, Zhu C, Zhang P, et al. Exosomal lncRNA 91H is associated with poor development in colorectal cancer by modifying HNRNPK expression. Cancer Cell Int. 2018;18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y-G, Zhou M-W, Bai L, Han R-Y, Lv K, Wang Z. Extracellular vesicles promote esophageal cancer progression by delivering lncZEB1-AS1 between cells. European review for medical and pharmacological sciences. 2018;22(9):2662–70. [DOI] [PubMed] [Google Scholar]
- 65.Bryan TM, Cech TR. Telomerase and the maintenance of chromosome ends. Current Opinion in Cell Biology. 1999;11(3):318–24. [DOI] [PubMed] [Google Scholar]
- 66.Mantell LL, Greider CW. Telomerase activity in germline and embryonic cells of Xenopus. The EMBO journal. 1994;13(13):3211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhaene K, van Marck E, Parwaresch R. Telomeres, telomerase and cancer: an up-date. Virchows Archiv : an international journal of pathology. 2000;437(1):1–16. [DOI] [PubMed] [Google Scholar]
- 68.Gutkin A, Uziel O, Beery E, Nordenberg J, Pinchasi M, Goldvaser H, et al. Tumor cells derived exosomes contain hTERT mRNA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget. 2016;7(37):59173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denekamp J. Review article: angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. The British journal of radiology. 1993;66(783):181–96. [DOI] [PubMed] [Google Scholar]
- 70.Lang H-L, Hu G-W, Zhang B, Kuang W, Chen Y, Wu L, et al. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncology reports. 2017;38(2):785–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang H-L, Hu G-W, Chen Y, Liu Y, Tu W, Lu Y-M, et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. European review for medical and pharmacological sciences. 2017;21(5):959–72. [PubMed] [Google Scholar]
- 72.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Zhang Y, Liu Y, Dai X, Li W, Cai X, et al. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem. 2013;288(32):23586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong BS, Cho J-H, Kim H, Choi E-J, Rho S, Kim J, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC genomics. 2009;10:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development. 2018;32(19–20):1267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Lei Y, Wu M, Li N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA TUC339. International journal of molecular sciences. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye S-B, Zhang H, Cai T-T, Liu Y-N, Ni J-J, He J, et al. Exosomal miR-24–3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. The Journal of pathology. 2016;240(3):329–40. [DOI] [PubMed] [Google Scholar]
- 78.Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cellular immunology. 2014;292(1–2):65–9. [DOI] [PubMed] [Google Scholar]
- 79.Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends in cell biology. 2013;23(12):620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cappellesso R, Tinazzi A, Giurici T, Simonato F, Guzzardo V, Ventura L, et al. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer cytopathology. 2014;122(9):685–93. [DOI] [PubMed] [Google Scholar]
- 81.Guo K, Yao J, Yu Q, Li Z, Huang H, Cheng J, et al. The expression pattern of long non-coding RNA PVT1 in tumor tissues and in extracellular vesicles of colorectal cancer correlates with cancer progression. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39(4):1010428317699122. [DOI] [PubMed] [Google Scholar]
- 82.WARBURG O. On the origin of cancer cells. Science (New York, NY). 1956;123(3191):309–14. [DOI] [PubMed] [Google Scholar]
- 83.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nature cell biology. 2015;17(2):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–8. [DOI] [PubMed] [Google Scholar]
- 85.Ragni E, Banfi F, Barilani M, Cherubini A, Parazzi V, Larghi P, et al. Extracellular Vesicle-Shuttled mRNA in Mesenchymal Stem Cell Communication. Stem Cells. 2017;35(4):1093–105. [DOI] [PubMed] [Google Scholar]
- 86.Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahadi A, Brennan S, Kennedy PJ, Hutvagner G, Tran N. Long non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomes. Sci Rep. 2016;6:24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29(5):653–68. [DOI] [PubMed] [Google Scholar]
- 91.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–9. [DOI] [PubMed] [Google Scholar]
- 94.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ariyoshi K, Miura T, Kasai K, Fujishima Y, Nakata A, Yoshida M. Radiation-Induced Bystander Effect is Mediated by Mitochondrial DNA in Exosome-Like Vesicles. Sci Rep. 2019;9(1):9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bergsmedh A, Szeles A, Henriksson M, Bratt A, Folkman MJ, Spetz AL, et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A. 2001;98(11):6407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holmgren L, Szeles A, Rajnavölgyi E, Folkman J, Klein G, Ernberg I, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999;93(11):3956–63. [PubMed] [Google Scholar]
- 98.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Albanese M, Chen AY-F, Hüls C, Gärtner K, Tagawa T, Keppler OT, et al. Micro RNAs are minor constituents of extracellular vesicles and are hardly delivered to target cells. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428–45.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]


