Abstract
Objective
The primary aim of this study was to assess the histopathological criteria of neovascularisation following saphenofemoral high ligation with regard to the delineation of the pathophysiology of the process. The secondary aims were to describe the perivenous morphological changes and to present cost effective agents to histopathologically diagnose neovascularisation.
Methods
In a prospective study design, vein samples of consecutive patients with recurrent varicose veins in the groin undergoing surgery were collected. The samples were analysed by a vascular histopathologist with a light microscope using standard staining techniques.
Results
The study population comprised 35 patients, 24 of whom were female (69%). Histopathologically, 28 samples (80%) showed typical aspects of neovascularisation. The remaining seven specimens (20%) showed thickened residual veins. An irregular vascular network, increasing perivenous collagen and elastic fibres and perivenous lymph nodes were observed. Present venous valves were the main criterion for residual veins. A surprising finding was the presence of scar tissue in the views of reparative incomplete new valves. Standard staining agents were sufficient to make the diagnosis of neovascularisation in 73% of the samples and reduced the cost by 30% compared with the regular use of specific markers.
Conclusion
The histopathological analysis of operative specimens may clarify whether a varicose vein recurrence is the result of neovascularisation or some other cause. Although interesting for research, academic interest, and classification, this may be of very limited clinical relevance for the patient.
Keywords: Histopathology, Neovascularisation, Varicose veins
Highlights
-
•
Neovascularisation is a common cause of recurrent varicose veins.
-
•
Histopathology helps to clarify the underlying cause.
-
•
Scar tissue was found in the views of reparative incomplete valves.
Introduction
Neovascularisation has been defined as the presence of new veins situated at the site of the previous ligation of the great (or small) saphenous vein.1 This can result in clinical recurrence of varicose veins. In current practice, the diagnosis of recurrence is mostly proven by colour coded duplex ultrasound (DUS).1, 2, 3, 4, 5
Unfortunately, recurrent varicose veins are a common post-operative problem after high ligation. The most common causes are disease progression and neovascularisation, and less frequently technical or tactical errors.1, 2, 3, 4, 5 For neovascularisation a wide incidence ranging from 1.5% to 62% is reported in the literature.1,3, 4, 5,7,8 Neovascularisation in DUS is present in 25–94% of recurrent varicose veins.1,3, 4, 5
The validity of diagnosis by intra-operative macroscopic aspects or duplex sonographic identification of the cause of groin recurrence is limited.4,5 Histological examination of operative specimens helps to clarify the underlying cause of recurrence. According to the literature, the major microscopic criteria used for the diagnosis of neovascularisation are the presence of tortuosity of small vessels, scar tissue and an irregular vascular network, and the lack of vein valves.5, 6, 7
The aims of this study were to assess the microscopic criteria of neovascularisation following saphenofemoral high ligation with regard to the delineation of the pathophysiology of the process as well as to describe the perivenous morphological changes and to present cost effective procedures to histopathologically diagnose neovascularisation.
Patients and methods
Patients with clinically symptomatic recurrent varicose veins and previous great saphenous vein surgery were evaluated clinically as well as with DUS by an angiologist according to the current guidelines, and were directed to endovenous therapy or, if not eligible, to surgical treatment.1,5
Patients were identified retrospectively based on a prospective database between 1 October 2012 and 31 December 2017. All had undergone crossectomy and great saphenous stripping in the past. They underwent the re-operation at three different hospitals: Spital Frutigen, Switzerland (2012–2015), Spital Thun, Switzerland (2012–2015), and Klinik Hohmad, Switzerland (2015–2017). All patients signed written informed consent pre-operatively covering study participation.
Before operation, the varicose and perforating veins were marked by the surgeon with indelible ink on the patient's skin. Under general or regional anaesthesia, the groin was by choice re-opened via the pre-existing scar. After visualising the femoral artery as a landmark, the femoral vein was dissected, and the recurrent/neovascular veins were flush-ligated and divided. The veins were freed from the scar tissue as far as possible and then removed. Samples of these veins were taken and preserved in formalin and then sent to an experienced vascular histopathologist. Multiple slices of each sample were prepared and evaluated under the microscope. The samples were divided into two groups: Group A with obvious microscopic aspects of neovascularisation and Group B with microscopic aspects of a native vein.
For the analysis, the Olympus BX45 microscope (Olympus Corporation, Schweiz AG, Wallisellen, Switzerland) was used. The standard stains used were haematoxylin-eosin (HE) and elastica Van Gieson (EVG). Immunomarkers such as S100, Vimentin, and smooth muscle actin (SMA) were supplementarily used in selected cases to differentiate additional aspects (all staining procedures by Promed Laboratory, Marly, Switzerland).
The study was approved by the Cantonal Ethics Committee for Research (KEK, Project-ID: 2017–02204), Bern, Switzerland. The microscope slides were blinded by a numeric code given by the KEK. Only the prepared slice was used for the evaluation.
Results
This observational study included 35 patients with a mean age of 61 years (range 41–80 years). Of these, 24 were female (69%). The primary operations had been performed 10–32 years earlier.
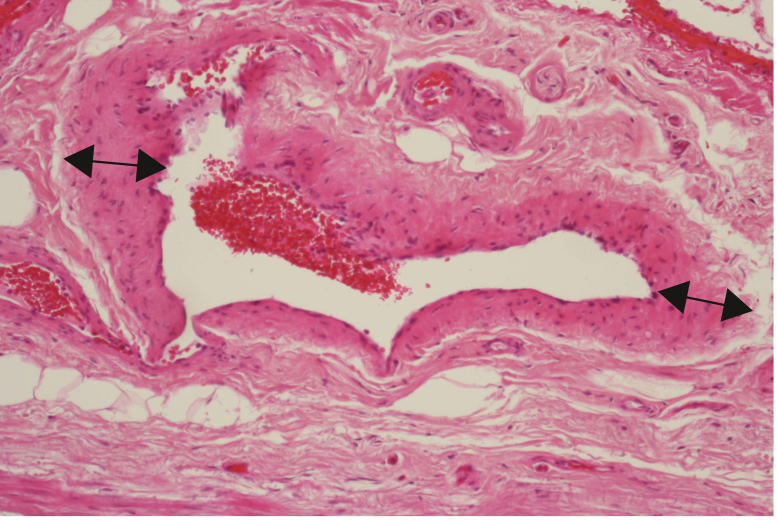
The cohort was split into two groups according to the histopathological diagnosis. Specimens from 28 patients (80% = Group A) showed morphologically typical aspects of neovascularisation (Table 1, Fig. 1). The remaining seven specimens (20% = Group B) showed residual veins with typical morphology of the venous wall texture (intima, media, adventitia, and sometimes vein valves) (Table 1).
Table 1.
Results of the study.
| Samples n = 35 |
||
|---|---|---|
| Neovascularisation n = 28 | Residual veins n = 7 | |
| Irregular vascular network | 14 (50) | 0 |
| Media hyperplasia | 20 (71) | 6 (86) |
| Collagen/elastic fibres | 28 (100) | 0 |
| Scar tissue | 28 (100) | 4 (57) |
| Vein valves | 0 | 2 (29) |
| Reparative incomplete valves | 15 (54) | 0 |
| Lymph nodes | 6 (21) | 0 |
| Nerve fibres perivenous | 3 (11) | 0 |
| Intramural nerve fibres | 0 | 3 (43) |
Data are presented as number (% refers to group) of patients.
Figure 1.
Typical aspects of neovascularisation with irregular thickness of the vein wall (↔ ) (HE, x200).
The standard stains HE and EVG were sufficient for a precise diagnosis in 27 cases. S100, SMA or Vimentin were used in eight cases (23%) to establish the diagnosis of neovascularisation or native vein. The S100 immunohistochemical stain helps to detect intramural and perivenous nerve fibres (Table 1). Using targeted resources including immune markers on a case by case basis in combination with a specialised vascular pathologist, the potential savings were estimated at 30% compared with regular analysis. Standard stains were sufficient to make the diagnosis of neovascularisation in 77% of the samples.
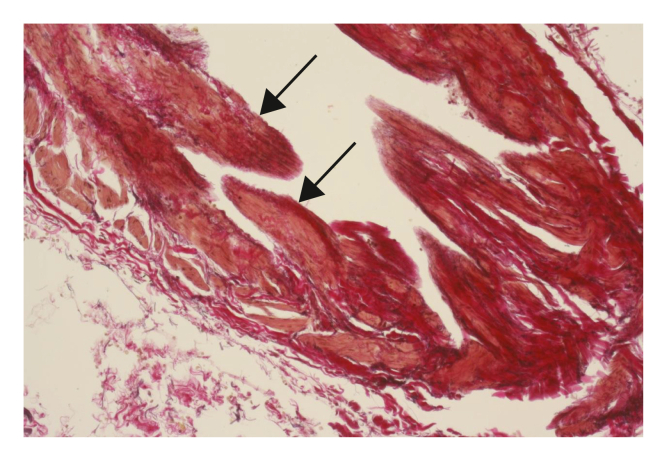
Neovascular veins showed no congenital valves, but more than half of the samples (54%) displayed reparative incomplete valves (Fig. 2).
Figure 2.
Reparative incomplete valves in neovascularisation probably induced by haemodynamic changes (→) (EVG, x400).
In six cases from Group A, lymph nodes were demonstrated in the adjacent tissue, and were seen exclusively in samples with neovascularisation (Table 1).
Discussion
A high proportion of neovascularisation was detected in this study (80%, Table 1); this is higher than reported in the literature.1, 2, 3, 4, 5,7,8 The major microscopic criteria of neovascularisation used in the literature (presence of scar tissue associated with an irregular vascular network, the lack of vein valves as well as the absence of intramural nerve fibres) could be proven in the present study. Additionally, the presence of perivenous collagen and elastic fibres is described, as well as reparative incomplete valves to diagnose neovascularisation. Perivenous lymph nodes and nerves represented the main perivenous morphologic changes.
A surprising finding in the present study was the presence of scar tissue in the views of reparative incomplete new valves. This was found in 54% of the samples with, and only with, proven neovascularisation. These were of course not congenital valves as in residual veins. The literature does not include any descriptions of such scar tissue forming valves in neovascular veins.5,6 However, these incomplete new valves did not fulfil the valve function in patients in the present study cohort, as indicated by the necessity for re-operation because of symptomatic recurrent varicose veins.
A limitation of this study is the prospective study design, in which there was no evaluation of the correlation between the pre-operative DUS and the histopathological findings. According to the requirements, the microscope slides had to be blinded using a numeric code. Unfortunately, this could not be connected with the operation report. All samples were evaluated and judged by only one specialised vascular pathologist. Overall, the results of this study are predominantly interesting for their scientific aspects. Therefore, the described cost effectiveness has a clear place in research. In daily practice this is achieved by not performing histopathology for pure classification reasons. Other limitations regard potential selection bias and a small sample size.
The histopathological analysis of operative specimens may clarify whether a recurrence is result of neovascularisation or not. Although scientifically interesting, this may not be of clinical relevance for the patient, unless a patient with a histologically proven neovascularisation is told to be more aware of another recurrence and could consult the doctor at an earlier stage of recurrence, this being easier to treat as being less advanced. However, this is speculation and lacks supporting data.
Acknowledgements
The authors would like to thank Dr. Jan Janzen MPhil, VascPath Switzerland for performing the histopathological sections and their description as well as for covering the ethical committee fee.
Conflict of interest
None.
Funding
None.
References
- 1.Wittens C., Davies A.H., Baekgaard N., Broholm R., Cavezzi A., Chastanet S. Editor's Choice – management of chronic venous disease: clinical practice guidelines of the European society for vascular surgery (ESVS) Eur J Vasc Endovasc Surg. 2015;49:712–714. doi: 10.1016/j.ejvs.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 2.De Maeseneer M.G.R. The role of postoperative neovascularisation in recurrence of varicose veins: from historical background to today's evidence. Acta Chir Belg. 2004;104:283–289. doi: 10.1080/00015458.2004.11679555. [DOI] [PubMed] [Google Scholar]
- 3.Kostas T., Ioannou C.V., Touloupakis E., Daskalaki E., Giannoukas A.D., Tsetis D. Recurrent varicose veins after surgery: a new appraisal of a common and complex problem in vascular surgery. Eur J Vasc Endovasc Surg. 2004;27:275–282. doi: 10.1016/j.ejvs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Van Rij A.M., Jones G.T., Hill G.B., Jiang P. Neovascularization and recurrent varicose veins: more histologic and ultrasound evidence. J Vasc Surg. 2004;40:296–302. doi: 10.1016/j.jvs.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Geier B., Mumme A., Hummel T., Marpe B., Stücker M., Asciutto G. Validity of duplex-ultrasound in identifying the cause of groin recurrence after varicose vein surgery. J Vasc Surg. 2009;49:968–972. doi: 10.1016/j.jvs.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 6.Glass G.M. Neovascularisation in recurrent sapheno-femoral incompetence of varicose veins: surgical anatomy and morphology. Phlebology. 1995;10:136–142. [Google Scholar]
- 7.Nyamekye I., Shephard N.A., Davies B., Heather B.P., Earnshaw J.J. Clinicopathological evidence that neovascularisation is a cause of recurrent varicose veins. Eur J Vasc Endovasc Surg. 1998;15:412–415. doi: 10.1016/s1078-5884(98)80202-5. [DOI] [PubMed] [Google Scholar]
- 8.Nesbitt C., Bedenis R., Bhattacharya V., Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus open surgery for great saphenous vein varices. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD005624.pub3. [DOI] [PubMed] [Google Scholar]