Abstract
Background
Breast cancer (BC) has been increasing globally, though it is unclear whether the increases are seen across all age groups and regions and whether changes in rates can be primarily attributed to decreasing fertility rates. We investigated age-specific trends in BC incidence and mortality from 1990 to 2017, worldwide and by region, and evaluated whether incidence trends are explained by decreases in fertility.
Methods
We used country-level data to examine trends in BC incidence and mortality rates from 1990 to 2017 by region and age group. Linear mixed models were used to estimate age-specific rates from baseline models of year and were compared to fertility-adjusted models for incidence.
Results
The global BC mortality rate increased overall by 0.23% per year (95% CI=0.20, 0.25), with statistically significant increases in the under 50 and 70 and over age groups, and in 5 out of 7 regions. The global BC incidence rate increased overall by 1.44% per year (95% CI=1.42, 1.47), with statistically significant increases in all age groups, and in 6 out of 7 regions. After adjusting for fertility, the incidence annual percent change (APC) remained statistically significant (APC=0.84, 95% CI=0.81, 0.88), in all age groups, and in 6 of 7 regions.
Interpretation
The global increase in BC mortality is seen in most age groups and regions. The global increase in BC incidence is seen in all age groups and is highest in women under 50; increases remained in most regions even after considering declining fertility rates.
Funding
Breast Cancer Research Foundation and National Cancer Institute.
Keywords: Breast cancer, Incidence rate, Mortality rate, Global, Fertility
Research in context.
Evidence before this study
Breast cancer (BC) has been increasing globally, though it is unclear whether the increases are seen across all age groups and regions and whether the changes in rates can be primarily attributed to decreasing fertility rates. We searched PubMed using search terms “global” and “breast cancer” with different combinations of “incidence rate*”, “mortality rate*”, “trend*”, “fertility”, “parity”. We searched for primary research and review articles from January 1, 2000 to September 15, 2020, written in English, that examined global BC incidence and mortality trends and accounted for changes in fertility. Most studies focused on BC rates worldwide across all age groups and/or were focused on a specific region. We found few studies that investigated BC trends and accounted for fertility rates, none of which examined BC and fertility rates globally.
Added value of this study
We examined whether the increasing BC rates across the globe can be explained by changes in fertility rates. This study provides empirical evidence that BC incidence and mortality rates are significantly increasing globally, across age groups, regions, and age groups within regions, and that incidence trends cannot be explained by declines in fertility rates. These findings do not support the argument that declining fertility trends are responsible for increasing incidence trends and support the need for more research on environmental and extrinsic factors explaining these trends.
Implications of all the available evidence
The global increase in BC mortality is seen in most age groups and regions; the global increase in BC incidence is seen in all age groups and is highest in women under 50 years; the increases in incidence remained even after considering declining fertility rates. These temporal trends demand further investigation of environmental factors that have changed over the same time period.
Alt-text: Unlabelled box
1. Introduction
Breast cancer (BC) is the most common cancer and the leading cause of cancer death for women worldwide [1], but the burden of BC incidence and mortality varies by geographical region [2]. Multiple studies have found that BC incidence is highest in high income and high-middle income countries (HIC and HMIC, respectively) in Northern America, Australia/New Zealand, and regions of Europe – ranging from 85.8 to 91.6 cases per 100,000, whereas BC mortality rates are highest in regions of Africa and Oceania, which include mostly low-middle income and low income countries (LMIC and LIC, respectively) – ranging from 17.4 to 20.1 deaths per 100,000 [2], [3], [4], [5], [6].
The overall rates of BC incidence and mortality for the world population have continuously increased since registries began capturing data in 1990 [1,7], although trends vary by geographical region and by age group [2,8,9]. A study in 2020 found that BC incidence in women under 50 years increased in 20 of 44 populations across the globe from 1998–2012, most of which were in HIC, whereas BC incidence in women 50 years and older increased in 24 of 44 populations, mostly in countries undergoing socioeconomic transitions [6]. BC mortality rates have decreased over time in most HIC but remain high and are increasing in many LMIC and LIC [4].
Little is known about the major drivers of changing BC incidence and mortality rates across geographical regions and time, particularly for women under 50 years [6]. Differences in BC mortality rates across geographical regions might be driven by differential access to medical care, impacting timeliness of detection, treatment, as well as quality of treatment [10], [11], [12]. The increasing number of cancer cases, particularly common cancers such as BC, may in part be driven by increased lifespans around the world [13], [14], [15]. However, if cancer risk is also increasing in younger age groups, the increase in incidence cannot be fully explained by aging of the population. Differences in the incidence of BC might also be driven by lifestyle factors, such as delayed and reduced childbirth [2,5,16], that have been consistently associated with BC risk [17,18]. Yet, few studies have investigated the effects of childbearing behaviors, which vary geographically [19], on global BC rates over time. We recently conducted a study using historical data from the Connecticut Tumor Registry and found that the incidence rate of BC has been increasing in the state since at least 1935 for women of all ages, including women under 40 years, and these increases cannot be attributed to decreases in parity as the increase occurred long before the baby boom [20].
In this study, we compare nearly 30 years of data by world region (East Asia Pacific, Europe Central Asia, Latin America Caribbean, Middle East North Africa, North America, South Asia, Sub-Saharan Africa), to answer the following questions: 1.) Do changes in global BC incidence and mortality rates differ by age group and region, particularly in women under 50? 2.) Do changes in fertility rates over time explain trends in incidence?
2. Materials and methods
2.1. Data sources
We used publicly available and deidentified data from the Global Health Data Exchange (GHDx)[21] and World Bank[22]. As we only used country-specific rates, this study was exempt from ethical review and informed consent according to Code of Federal Regulations (45 CFR 46.101(b)). This study complies with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
The GHDx is a data catalog out of the Institute for Health Metrics Evaluation (IHME) and produces estimates of burden of diseases, including BC. GHDx estimates are created from a repository of input data, which includes data directly obtained from data holders and publications (i.e., administrative records, surveillance data, vital registration). BC data used in this study are compiled from 4117 data input sources for mortality, and 447 data input sources for incidence, including cancer registries and vital registries [21,23]. A list of citations for data input sources used for this study have been provided in eReference 1. Annual data on BC incidence and mortality rates were pulled for all available years, 1990 – 2017, by country. We conducted analyses on BC incidence and mortality rates using World Population age-standardized and age-specific rates. For our fertility variable, we used total fertility rates (TFR) from the World Bank. The World Bank collects data from international demographic data sources to create global indicators [22]. Fertility rates were pulled by country for years 1970 – 2007. We selected the period 1970 – 2007 to allow up to a 20-year lag for incidence data. A 10-year lag was used for overall (all ages) rates and for the under 50 and 50–69 age groups; a 20-year lag was used for the 70 and over age group.
2.2. Statistical analysis
We modeled secular trends in log-transformed country-level BC incidence and mortality rates using a linear mixed model with a random intercept and fixed linear time, or
where is the BC rate in year t for country, is fixed linear time (year), is TFR, and represents the random intercept for each country. We evaluated models stratified by country, age group (under 50 years, 50–69 years, and 70 and over years), and region, as well as by both age group and region. We included cross-product terms in models to test whether trends differed by age and region. We compared baseline models to models adjusted for country-level annual fertility rates using the Akaike information criterion (AIC). To assess trends, we calculated annual percent change (APC) globally, regionally, and by country. We performed a sensitivity analysis using different TFR lags, which included 5, 10, and 20 years. We also performed a sensitivity analysis evaluating incidence APCs in models further adjusted for percent of female survival to 65 years; we used data from the World Bank for years 1990–2017 [24]. All trend analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC). A P value of 0.05 for a 2-sided hypothesis test was considered statistically significant.
2.3. Funding
This study was conducted using the resources of the Breast Cancer Research Foundation (Dr Terry) and National Cancer Institute (T32CA094061). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
3. Results
We analyzed BC and fertility data for 185 countries split into 7 regions, as classified by the World Bank, with the number of countries (n) in each region as follows: East Asia Pacific (n = 27), Europe Central Asia (n = 47), Latin America Caribbean (n = 33), Middle East North Africa (n = 20), North America (n = 2), South Asia (n = 8), and Sub-Saharan Africa (n = 48). Table 1 shows, by region, descriptive statistics of mean TFR, mean TFR by decade, average age-standardized incidence and mortality rates, and income classification. Sub-Saharan Africa had the highest mean TFR; Europe Central Asia and North America had the lowest mean TFR. All regions showed decreases in mean TFR over time, and all regions except North America had decreases in mean TFR each decade. North America and Europe Central Asia had the highest age-standardized incidence and mortality rates, averaged over 1990–2017; South Asia had the lowest incidence rate, and East Asia Pacific had the lowest mortality rate. There is a nearly three-fold difference in average incidence rates between high-income (North America and Europe Central Asia) and low-income (Sub-Saharan Africa) regions.
Table 1.
Descriptive statistics of world regions.
| Region Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | Countries (n) | Mean Total Fertility Ratea | Mean Total Fertility Rate by Decadea |
Average Age-Standardized Ratesb |
||||
| 1980–1989 | 1990–1999 | 2000–2007 | Incidence | Mortality | Income classificationc | |||
| East Asia & Pacific | 27 | 3.4 | 3.9 | 3.3 | 2.8 | 31.0 | 11.7 | Lower-Middle Income |
| Europe & Central Asia | 47 | 2 | 2.3 | 1.9 | 1.7 | 68.2 | 22.1 | High Income |
| Latin America & Caribbean | 33 | 3.2 | 3.7 | 3.1 | 2.6 | 38.8 | 17.4 | Upper-Middle Income |
| Middle East & North Africa | 20 | 4.2 | 5.5 | 4.0 | 3.0 | 32.3 | 15.7 | High Income |
| North America | 2 | 2 | 2.1 | 1.9 | 1.9 | 105.9 | 22.0 | High Income |
| South Asia | 8 | 4.7 | 5.7 | 4.6 | 3.6 | 21.5 | 15.0 | Lower-Middle Income |
| Sub-Saharan Africa | 48 | 5.9 | 6.4 | 5.8 | 5.3 | 24.4 | 18.6 | Low Income |
| Total | 185 | 3.7 | 4.3 | 3.6 | 3.2 | 16.9 | 43.8 | |
Mean Total Fertility Rate is the average fertility rate from 1980–2007, allowing for a 10-year lag, by region.
Age-standardized rates per 100,000, averaged over 1990–2017, by region.
Income classification represents the majority income group per region.
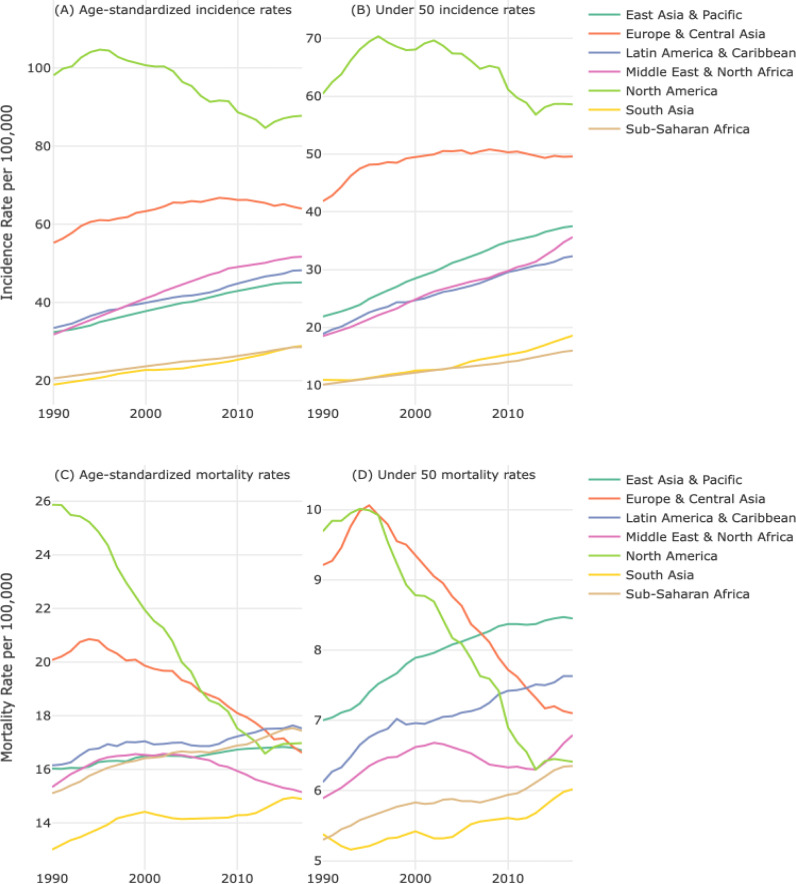
Fig. 1 shows the BC incidence and mortality rates over time, age-standardized (overall – includes all age groups) and for women under 50, by region. BC incidence rates have been increasing from 1990–2017 for all regions except North America, with rates increasing on average 1.6-fold overall and for women under 50. Mortality rates increased for 4 out of 7 regions, with rates increasing on average 1.1-fold overall and for women under 50.
Fig. 1.
Temporal trends in breast cancer incidence and mortality rates, age-standardized (all ages) and in women under 50 years, by world region.
(A) Age-standardized incidence rates (all ages), by region. (B) Incidence rates for women under 50, by region. (C) Age-standardized mortality rates (all ages), by region. (D) Mortality rates for women under 50, by region.
3.1. Mortality
The global APC for BC mortality increased by 0.23% (95% confidence interval (CI)=0.20, 0.25) per year since 1990 (Table 2). Age-stratified APCs were statistically significant and positive for two of the three age groups: under 50 (APC=0.09, 95% CI=0.04, 0.13) and 70 and over (APC=0.66, 95% CI=0.63, 0.70). Europe Central Asia (APC= −0.55, 95% CI=−0.60, −0.50) and North America (APC= −1.75, 95% CI=−1.86, −1.65) are the only regions in which mortality decreased over time; mortality rates increased in all other regions, ranging from 0.36% per year (95% CI=0.28, 0.44) in Middle East North Africa to 0.56% per year in East Asia Pacific (95% CI=0.51, 0.61), Latin American Caribbean (95% CI=0.52, 0.60) and Sub-Saharan Africa (95% CI=0.52, 0.60). North America has the largest change in mortality within each age group, with the largest change among women 50–69 years (APC=−2.26, 95% CI=−2.39, −2.13). The largest increase in mortality was observed in the 70 and over age group in South Asia (APC=0.95, 95% CI=0.80, 1.10). All regions had a statistically significant positive APC for under 50 mortality except Europe Central Asia and North America.
Table 2.
Annual percent change in breast cancer mortality rates, globally and by region and age group, 1990–2017.
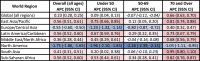 |
Notes: Annual percent change (APC) and 95% confidence interval (CI) estimated from a linear mixed model with a random intercept and fixed linear time. APCs in italics are not statistically significant. Positive APCs, which indicate increasing mortality over time, are in red (color shading darkens with increasing values); negative APCs, which indicate decreasing mortality over time, are in blue.
3.2. Incidence
Global BC incidence increased by 1.44% (95% CI=1.42, 1.47) per year since 1990 (Table 3). BC incidence has significantly increased in all age groups since 1990, ranging from 1.28% per year (50–69) to 1.55% per year (under 50). As for regional changes in incidence, all regions except North America have statistically significantly increased. Middle East North Africa had the largest per-year increase in overall incidence (APC=2.38, 95% CI=2.29, 2.47). North America is the only region with a decrease in overall incidence (APC=−0.62, 95% CI=−0.72, −0.53). Similar patterns were seen when stratified by region and age group. We found incidence trends were statistically significantly different by age groups within region.
Table 3.
Annual percent change in breast cancer incidence rates, globally and by region and age group, 1990–2017, unadjusted and adjusted for country-level fertility rates.
 |
Notes: Annual percent change (APC) and 95% confidence interval (CI) estimated from a linear mixed model with a random intercept and fixed linear time. APCs in italics are not statistically significant. Positive APCs, which indicate increasing incidence over time, are in red (color shading darkens with increasing values); negative APCs, which indicate decreasing incidence mortality over time, are in blue.
After adjusting for country-level fertility trends, the global APC was reduced but remained positive and statistically significant (APCadjusted=0.84, 95% CI=0.81, 0.88). This was also true for all age groups: under 50 (APCadjusted=0.72, 95% CI=0.65, 0.79), 50–69 years (APCadjusted=0.77, 95% CI=0.72, 0.82), and 70 and over (APCadjusted=1.02, 95% CI=0.97, 1.07). Positive APCs remained across regions after adjusting for fertility but with some variation in the direction of change – e.g., lower after adjustment but still positive: Middle East North Africa (APC changed from 2.38% to 0.28%), and higher after adjustment: South Asia (APC changed from 1.72% to 2.24%). Positive APCs also remained after adjusting for fertility trends for age groups within region.
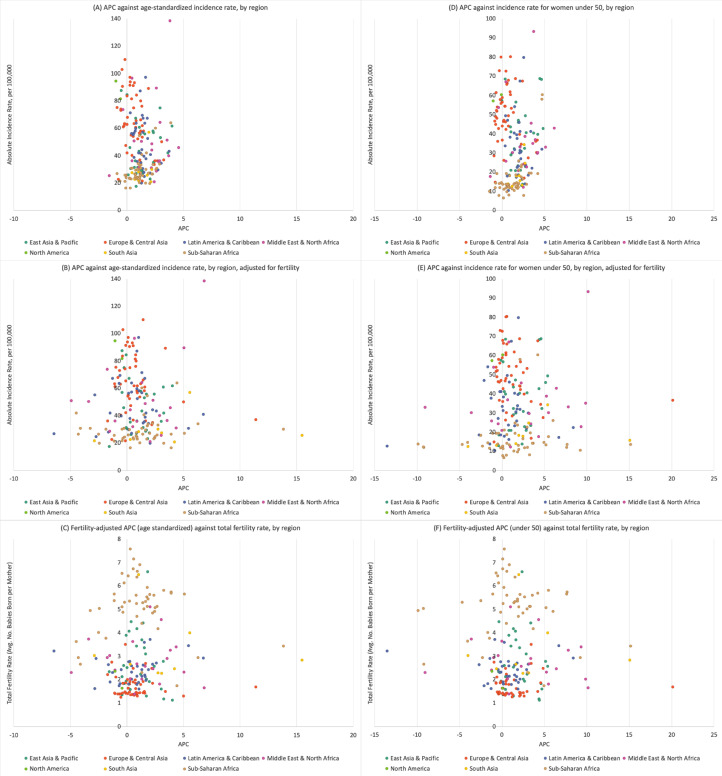
Fig. 2 shows overall and under 50 APCs, before and after fertility adjustment, compared against 2017 incidence rates and 2007 fertility rates for every country, grouped by region. The under 50 incidence rates ranged from under 10 cases to over 90 cases per 100,000. The APC ranged from −1% to 6% (Burundi, APC = −1.4; Libya, APC = 6.2), with the majority of countries having an APC between >0% and 5%. After adjusting for fertility, the APC ranged from −13% to over 20%. Though some countries shifted to a negative APC, the majority of countries had a positive APC after fertility adjustment. In each panel of Fig. 2, overall findings are consistent after adjustment for fertility across each region. eFig. 1 shows the patterns of BC incidence under 50 and fertility rates from 1990 (averaged over 1990–1994) to 2017 (averaged over 2013–2017) by region. Each region shows a decline in fertility, though not every region has an increase in incidence rate; North America and South Asia show a decrease in incidence from 1990 to 2017. In the bottom panel of eFig. 1, we show the 1990 to 2017 change in under 50 fertility and incidence rates for the countries with the highest and lowest APC, prior to fertility adjustment, for their region.
Fig. 2.
Annual percent change in breast cancer incidence compared against absolute breast cancer incidence rate in 2017 and total fertility rate in 2007 for each country, grouped by world region, overall (age-standardized) and in women under 50.
APC = annual percent change. Y-axis: incidence rate (per 100,000) in 2017; X-axis: APC from 1990 to 2017. The three left panels, (A), (B) and (C), show the APC, incidence rates and fertility rates for overall age; the three right panels, (D), (E), and (F), show the APC, incidence rates, and fertility rates for women under 50 years. The top panels, (A) and (D), show APCs of the time-trend, base model and incidence rates; the middle panels, (B) and (E), show APCs from the fertility-adjusted model and incidence rates; and the bottom panels (C) and (F), show APCs from the fertility-adjusted model and total fertility rates.
Of the 185 countries analyzed, 152 countries had a statistically significant positive APC for overall incidence before adjusting for fertility; after fertility adjustment, 97 countries had a significant positive APC (eTable 1). For incidence in women under 50, 147 of the 185 countries had a statistically significant positive APC before adjusting for fertility; after fertility adjustment, 106 countries had a significant positive APC.
Our sensitivity analysis had consistent results regardless of lag-time (eTable 2). Further, the direction (positive or negative) and statistical significance of regional APCs did not change after further adjustment for percent female survival to 65, except for Middle East North Africa (eTable 3).
4. Discussion
Using 27 years of incidence and mortality data from 185 countries, we found that BC mortality rates have been increasing at a statistically significant rate for women under 50 and 70 and over, and in every world region, with the exception of Europe Central Asia and North America. We also found that incidence rates for every age group and every world region, other than North America, have been statistically significantly increasing since 1990, even after adjusting for fertility rates. The global increase in BC incidence is seen in all age groups, and is highest in women under 50 years, and therefore cannot be fully explained by increases in life expectancy; the increases remained, with few exceptions, after considering declining fertility rates.
To our knowledge, this is the only study to examine global BC rates by age group, region, and age groups within regions, as well as to adjust for fertility. Our findings are consistent with previous studies that have found significant increases in global BC mortality and incidence rates [1,7], with variations in rates by region and age group, particularly for mortality rates [8,25]. Torre et al. found BC mortality was higher in HIC and HMIC when evaluating rates for 2003–2007, but we found that this is no longer always the case when we evaluated mortality rates for 1990–2017. Our data suggest Sub-Saharan Africa (LIC) now has higher mortality rates than Europe Central Asia and North America (HIC) and support recent findings on global disparities in BC mortality rates [6,25]. The decrease in BC mortality in North America and Europe Central Asia is likely due to population-level BC screening (which has been shown to increase BC incidence but decrease BC mortality), early diagnosis, and advances in BC treatment [26]. Conversely, the increasing BC mortality in the other five regions may be due to a lack of population screening programs and thus late-stage diagnosis, and barriers to treatment and cancer care [27].
This study also corroborates findings that LMIC and LIC countries have experienced a faster increase in BC incidence in recent decades [1], which is consistent with the World Health Organization (WHO) prediction that LIC and LMIC will make up 70% of increased cancer burden by 2030 [28]. In testing the hypothesis that the declining fertility rate is a driver of increased BC rates, we found BC incidence rates statistically significantly increased in all age-specific strata and nearly all region-specific strata, regardless of fertility. The APC remained positive and statistically significant in 6 regions, and in 15 out of 21 age-region-specific groups, with the exception of Europe Central Asia (under 50), Middle East North Africa (under 50, 50–69), and North America (all age groups). This is notable considering fertility rates for each region have been declining throughout the time period. We even saw an increase in APC after adjusting for fertility in some regions and countries, such as in South Asia (overall, under 50, 50–69). These findings support that the declining fertility rates do not explain the increasing BC trends [20,29]. Future studies should consider the role of other BC-related risk factors, including alcohol consumption, sedentary behavior, and obesity (for women over 50 years), [6,[30], [31], [32]] which are prevalent in HIC and have been increasing globally [33,34].
The trend analysis also revealed important findings particularly when examining specific countries. Two countries, Libya and Bhutan, offer an interesting counterfactual consideration (eFig. 1). Both countries had similar starting points –– similarly high fertility rates in 1970 (Libya: 6.7 children per woman; Bhutan: 6.4 children per woman) and low incidence rates in 1990 (Libya: 11.2 cases per 100,000; Bhutan:10.3 cases per 100,000). Additionally, these countries experienced similar declines in fertility to 2007 (Libya, 2007: 2.5 children per woman; Bhutan, 2007: 2.9 children per woman); however, these countries have a 3-fold difference in incidence rate by 2017. Bhutan had a slight increase in incidence rate (12.5 cases per 100,000), whereas Libya experienced a much larger increase (41 cases per 100,000). If fertility trends were a true primary driver of increasing BC incidence, we would expect these countries with nearly identical starting fertility rates, starting incidence rates, and end fertility rates to have similar end incidence rates. These country specific contrasts may prove useful in developing new hypotheses about BC etiology.
Other reports have focused on parity differences to explain BC trends [16,[35], [36], [37]]. For example, using data from the U.S., Pfeiffer et al. examined trends in parity and BC incidence for 1980 – 2008 and found parity to be a driver of increasing rates [35]. When we examined data in the U.S. using a longer time horizon (e.g., 1935 – 2015), we found increases in BC incidence even during high rates of parity [20]. While there is a high correlation between absolute rates of BC incidence and fertility, particularly when evaluating differences between HIC and LIC, we found that fertility trends do not fully explain the increase in BC. We found unexplained significant increases in BC trends even in regions that have been proposed to have lower BC rates because of higher parity rates, such as Sub-Saharan Africa [36].
To examine whether increasing BC rates are driven by longer lifespans, we focused on detailed age-specific analyses. We found significant increases in mortality and incidence rates in women 70 and over, evidence that increased lifespan is contributing to higher rates of BC. However, the increases in incidence for under 50 were just as strong, and even stronger in 5 out of 7 regions and 69% of countries compared to overall (all age groups combined) changes. Additionally, most of our inferences remained the same after further adjusting for the percent of women surviving beyond 65 years in our sensitivity analysis.
This study had many strengths including the inclusion of data from 185 countries in all regions of the world for 27 years. Although the quality of cancer incidence and mortality data has been shown to vary by country, the similarity in inferences across very different settings strengthens the overall conclusion that declining fertility rates cannot be responsible for increasing BC incidence rates. Although increases in screening and BC awareness may contribute to rising incidence rates, they also cannot explain the overall pattern and particularly cannot explain the pattern below age 50, as BC screening generally starts at 50 or over in countries that have routine screening [38]. We also found that our results were consistent when we considered different lag times (5, 10, and 20 years).
The study is not without limitations. First, we did not have individual level fertility and BC data. Thus, it is possible that another factor or factors could explain our findings. For this to be the case, though, the omitted factors would have to be correlated with fertility rates in the same way across countries and regions of the world. Second, all countries have experienced declines in fertility since 1970, with the exception of Chad, Democratic Republic of Congo, and Timor-Leste –– which all had higher fertility rates in 2007 compared to 1970 but had no changes in APC after adjustment (eTable 4). Thus, we are unable to substantially consider the effect of increasing fertility on BC incidence rates. We previously investigated a similar question using 80 years of U.S. data from the State of Connecticut to specifically address whether the increase in BC incidence started before parity rates declined. We do not have the advantage of a longer time horizon in these analyses. However, the sheer number of countries in this study allows us to create contrasts by observing countries with similar fertility and BC rates undergo different changes in BC rates over time. Such country specific contrasts are useful for hypothesis generation on novel risk factors that may be driving the increase. Third, we are unable to separately address delayed timing of pregnancy, which could contribute to higher rates of BC from pregnancy-associated BC risk [39]. Finally, we focused only on fertility rates because parity remains one of the key established risk factors for breast cancer. It is possible that temporal changes in other risk factors such as obesity, alcohol consumption, or hormone replacement therapy [40,41] may partially explain increases in incidence, but these factors still differ substantially by countries and regions which means that they are unlikely to fully explain the changes in incidence.
This comprehensive analysis of global BC rates addresses whether rising rates can be explained by declining fertility rates or longer life expectancy. We found that while both may partially explain the trends, they cannot fully explain the trends, particularly in women under 50 years. These data support the need for research focused on the role of environmental exposures in the development of BC, given that genetic factors cannot explain change over such short time horizon. The urgency and potential public health impact of identifying environmental drivers of cancer risk is underscored by the fact that BC is only one of several types of cancer currently increasing in younger adults.
Funding
This study was conducted using the resources of the Breast Cancer Research Foundation (Dr Terry) and National Cancer Institute (T32CA094061). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
Sarah M Lima, MPH: conceptualization, data curation, analysis, visualization, writing – original draft. SML has verified the underlying data.
Rebecca D Kehm, PhD: methodology, analysis and validation, visualization, writing – review and editing.
Mary Beth Terry, PhD: conceptualization, methodology, supervision, writing – original draft, review and editing.
Data sharing statement
BC country-level data were pulled from Global Health Data Exchange (GHDx) at Institute for Health Metrics and Evaluation (IHME) and are publicly available. Data are available at http://ghdx.healthdata.org/gbd-results-tool
Fertility country-level data were pulled from World Bank Data Catalog and are publicly available. Data are available at https://data.worldbank.org/indicator/SP.DYN.TFRT.IN
Declaration of Competing Interest
Dr. Terry has nothing to disclose.
Acknowledgments
Dr. Kehm was supported by grant T32CA094061 from the National Cancer Institute.
MB Terry gratefully acknowledges the Breast Cancer Research Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100985.
Contributor Information
Sarah M. Lima, Email: sl4280@cumc.columbia.edu.
Rebecca D. Kehm, Email: rk2967@cumc.columbia.edu.
Mary Beth Terry, Email: mt146@cumc.columbia.edu, mt146@columbia.edu.
Appendix. Supplementary materials
References
- 1.Fitzmaurice C., Dicker D. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Bray F., Ferlay J., Lortet-Tieulent J., Anderson B.O., Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomark Prev. 2015;24(10):1495–1506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 3.Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global Cancer Incidence and Mortality Rates and Trends–An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 4.Hashim D., Boffetta P., La Vecchia C. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27(5):926–933. doi: 10.1093/annonc/mdw027. [DOI] [PubMed] [Google Scholar]
- 5.Althuis M.D., Dozier J.M., Anderson W.F., Devesa S.S., Brinton L.A. Global trends in breast cancer incidence and mortality 1973-1997. Int J Epidemiol. 2005;34(2):405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 6.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 7.Azamjah N., Soltan-Zadeh Y., Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20(7):2015–2020. doi: 10.31557/APJCP.2019.20.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J., Héry C., Autier P., Sankaranarayanan R. Global burden of breast cancer. Breast cancer epidemiology. 2010:1–19. In: Li C. [Google Scholar]
- 10.Rivera-Franco M.M., Leon-Rodriguez E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer: Basic Clin Res. 2018;12 doi: 10.1177/1178223417752677. 1178223417752677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantelhardt E.J., Cubasch H., Hanson C. Taking on breast cancer in East Africa: global challenges in breast cancer. Curr Opin Obstetr Gynecol. 2015;27(1) doi: 10.1097/GCO.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 12.Torre L.A., Islami F., Siegel R.L., Ward E.M., Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomark Prev. 2017;26(4):444. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- 13.Gu X., Zheng R., Xia C. Interactions between life expectancy and the incidence and mortality rates of cancer in China: a population-based cluster analysis. Cancer Commun. 2018;38(1):44. doi: 10.1186/s40880-018-0308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancik R., Ries L.A. Cancer in older persons: an international issue in an aging world. Semin Oncol. 2004;31(2):128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Youlden D.R., Cramb S.M., Dunn N.A., Muller J.M., Pyke C.M., Baade P.D. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Colditz G.A., Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am J Epidemiol. 2000;152(10):950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 18.Rosner B., Colditz G.A., Willett W.C. Reproductive risk factors in a prospective study of breast cancer: the Nurses' Health Study. Am J Epidemiol. 1994;139(8):819–835. doi: 10.1093/oxfordjournals.aje.a117079. [DOI] [PubMed] [Google Scholar]
- 19.United Nations Department of Economic and Social Affairs, Population Division (2015). World Fertility Patterns 2015 – Data Booklet (ST/ESA/SER.A/370). Available at: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Feb/un_2015_worldfertilitypatterns_databooklet.pdf.
- 20.Lima S.M., Kehm R.D., Swett K., Gonsalves L., Terry M.B. Trends in parity and breast cancer incidence in US women younger than 40 years from 1935 to 2015. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute for Health Metrics and Evaluation Cancer Registry Database. Date accessed: 7/22/2020. In:http://ghdx.healthdata.org/about-ghdx/our-information-sources.
- 22.World Bank. Fertility rate, total (births per woman). Indicator ID: SP.DYN.TFRT.IN. World Development Indicators. Available at: https://data.worldbank.org /indicator/SP.DYN.TFRT.IN
- 23.Institute for Health Metrics and Evlaution; March 2020. Protocol for the global burden of diseases, injuries, and risk factors study (GBD)http://www.healthdata.org/sites/default/files/files/Projects/GBD/March2020_GBD%20Protocol_v4.pdf [Google Scholar]
- 24.World Bank. Survival to age 65, female (% of cohort). Indicator ID: SP.DYN.TO65.FE.ZS. Accessed April 2021. https://data.worldbank.org/indicator/SP.DYN.TO65.FE.ZS.
- 25.Hu K., Ding P., Wu Y., Tian W., Pan T., Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2018-028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puliti D., Zappa M. Breast cancer screening: are we seeing the benefit? BMC Med. 2012;10(1):106. doi: 10.1186/1741-7015-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yip C.H., Anderson B.O. The Breast Health Global Initiative: clinical practice guidelines for management of breast cancer in low- and middle-income countries. Expert Rev Anticancer Ther. 2007;7(8):1095–1104. doi: 10.1586/14737140.7.8.1095. [DOI] [PubMed] [Google Scholar]
- 28.DMeae P. IARC Scientific Publications; 2003. Cancer in Africa: epidemiology and Prevention. No. 153. [PubMed] [Google Scholar]
- 29.Bellanger M., Lima S.M., Cowppli-Bony A., Molinié F., Terry M.B. Effects of fertility on breast cancer incidence trends: comparing France and US. Cancer Causes Control. 2021 doi: 10.1007/s10552-021-01440-2. In press. [DOI] [PubMed] [Google Scholar]
- 30.Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shield K.D., Soerjomataram I., Rehm J. Alcohol use and breast cancer: a critical review. Alcohol Clin Exp Res. 2016;40(6):1166–1181. doi: 10.1111/acer.13071. [DOI] [PubMed] [Google Scholar]
- 32.Jones M.E., Schoemaker M.J., Wright L.B., Ashworth A., Swerdlow A.J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 2017;19(1):118. doi: 10.1186/s13058-017-0908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manthey J., Shield K.D., Rylett M., Hasan O.S.M., Probst C., Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. The Lancet. 2019;393(10190):2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 34.Porter P. Westernizing" women's risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358(3):213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer R.M., Webb-Vargas Y., Wheeler W., Gail M.H. Proportion of U.S. trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol BiomarkPrev. 2018;27(10):1214–1222. doi: 10.1158/1055-9965.EPI-18-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hortobagyi G.N., de la Garza Salazar J., Pritchard K. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 37.Parkin D.M., Fernández L.M. Use of statistics to assess the global burden of breast cancer. Breast J. 2006;12(Suppl 1):S70–S80. doi: 10.1111/j.1075-122X.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 38.Organization WH . World Health Organization; 2014. WHO position paper on mammography screening. [PubMed] [Google Scholar]
- 39.Wohlfahrt J., Andersen P.K., Mouridsen H.T., Melbye M. Risk of Late-stage Breast Cancer after a Childbirth. Am J Epidemiol. 2001;153(11):1079–1084. doi: 10.1093/aje/153.11.1079. [DOI] [PubMed] [Google Scholar]
- 40.McDonald J.A., Goyal A., Terry M.B. Alcohol intake and breast cancer risk: weighing the overall evidence. Curr Breast Cancer Rep. 2013;5(3) doi: 10.1007/s12609-013-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picon-Ruiz M., Morata-Tarifa C., Valle-Goffin J.J., Friedman E.R., Slingerland J.M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.