Abstract
Background
More than 80% of anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) patients harbor the (nucleophosmin) NPM1-ALK fusion gene t(2;5) chromosomal translocation. We evaluated the preclinical and clinical efficacy of ceritinib treatment of this aggressive lymphoma.
Materials and methods
We studied the effects of ceritinib treatment in NPM1-ALK+ T-cell lymphoma cell lines in vitro and on tumor size and survival advantage in vivo utilizing tumor xenografts. We treated an NPM1-ALK+ ALCL patient with ceritinib. We reviewed all hematologic malignancies profiled by a large hybrid-capture next-generation sequencing (NGS)-based comprehensive genomic profiling assay for ALK alterations.
Results
In our in vitro experiments, ceritinib inhibited constitutive activation of the fusion kinase NPM1-ALK and downstream effector molecules STAT3, AKT, and ERK1/2, and induced apoptosis of these lymphoma cell lines. Cell cycle analysis following ceritinib treatment showed G0/G1 arrest with a concomitant decrease in the percentage of cells in S and G2/M phases. Further, treatment with ceritinib in the NPM1-ALK+ ALCL xenograft model resulted in tumor regression and improved survival. Of 19 272 patients with hematopoietic diseases sequenced, 58 patients (0.30%) harbored ALK fusions that include histiocytic disorders, multiple myeloma, B-cell neoplasms, Castleman's disease, and juvenile xanthogranuloma. A multiple relapsed NPM1-ALK+ ALCL patient treated with ceritinib achieved complete remission with ongoing clinical benefit to date, 5 years after initiation of therapy.
Conclusions
This ceritinib translational study in NPM1-ALK+ ALCL provides a strong rationale for a prospective study of ceritinib in ALK+ T-cell lymphomas and other ALK+ hematologic malignancies.
Key words: precision medicine, NPM1-ALK+ ALCL, ALK inhibitor, apoptosis, clinical trial, complete response
Graphical abstract

Highlights
-
•
The fusion kinase NPM1-ALK is constitutively activated in a ligand-independent manner.
-
•
Preclinically, ceritinib inhibits fusion kinase activity, along with inducing apoptosis in NPM1-ALK+ ALCL cells.
-
•
Tumors beyond ALK+ lymphomas such as histiocytic disorders, multiple myeloma, B-cell neoplasms, harbor ALK fusions.
-
•
Ceritinib therapy documented durable complete response in an NPM1-ALK+ ALCL patient corroborating our preclinical results.
Introduction
Anaplastic lymphoma kinase (ALK) rearranged anaplastic large cell lymphoma (ALCL) is a subtype of T-cell non-Hodgkin's lymphoma and is clinicopathologically known as ALK+ ALCL.1 It comprises 10%-30% of pediatric and 5%-10% of adult non-Hodgkin's lymphoma.2,3 ALK, a receptor tyrosine kinase, belongs to the insulin receptor superfamily and plays an important role in the pathogenesis of several cancers by either chromosomal translocations or point mutations.4,5 In >80% of the ALK+ ALCL patients, ALK is present in the form of the nucleophosmin (NPM1)-ALK fusion gene, generated by the t(2;5) chromosomal translocation.6 Nucleophosmin is a nucleolar phosphoprotein that serves as a molecular chaperone involved in the shuttling of proteins and nucleic acids between the nucleus and cytoplasm.7 The fusion protein, NPM1-ALK (p80), consists of the amino-terminal oligomerization domain of NPM1 and the entire carboxy-terminal domain of ALK, including the tyrosine kinase domain.8 As a result of its fusion with NPM1, the extracellular domain of ALK is lost.9 NPM1-ALK is localized in the cytoplasm but it is also capable of translocating to the nucleus as a result of heterodimerization with wild-type NPM1.10 Homodimerization of the fusion protein through the NPM1-oligomerization domain triggers constitutive ALK kinase activity in a ligand-independent manner.11 Similar to other fusion oncogenes, activated NPM1-ALK mediates interaction and activation of downstream signaling molecules responsible for cell proliferation and survival.5 STAT3, AKT, and ERK1/2 are some of the major downstream effectors that are activated and subsequently mediate oncogenic signaling conferring a survival advantage to NPM1-ALK+ ALCL cells.12, 13, 14, 15
NPM1-ALK+ ALCL is an aggressive neoplasm. The current first-line therapy is limited to multi-agent chemotherapy using CHOP (cyclophosphamide, hydroxy doxorubicin, vincristine, prednisone) or chemoimmunotherapy comprising the CD30 antibody-drug conjugate, brentuximab vedotin with CHP (cyclophosphamide, hydroxy doxorubicin, prednisone).16,17 Although most ALK+ T-cell lymphoma patients initially respond to CHOP therapy, >50% of patients relapse within 5 years, leading to increased disease-related morbidity and mortality.18,19 Second-line therapy for relapsed or refractory ALK+ T-cell lymphoma is not well established.20,21 Recent advances in cancer therapeutics have led to the development of many novel targeted therapies for cancers with actionable molecular targets.22 The discovery of small molecule inhibitors specific for ALK revolutionized precision medicine in ALK+ malignancies.23 Over the past decade, several selective inhibitors have been developed to target ALK dysregulated neoplasms.4 For instance, crizotinib, a first-generation ALK inhibitor, has been used as a standard of care for ALK-rearranged cancers. Unfortunately, ∼30% acquired resistance to crizotinib due to acquired mutation in the ALK kinase domain or amplification of the ALK fusion gene.24, 25, 26, 27 Ceritinib is a second-generation oral ATP-competitive ALK inhibitor approved by the US Food and Drug Administration) for the treatment of ALK-driven metastatic non-small-cell lung cancer (NSCLC).28 Due to structural differences, ceritinib binds to ALK with a higher affinity than crizotinib and shows efficacy in crizotinib-resistant NSCLC.29,30 Based on ceritinib anti-ALK efficacy data in naive and resistant ALK-driven solid tumors, we evaluated this inhibitor in the treatment of ALK-rearranged ALCL.
Herein, we present the preclinical data evaluating the efficacy of ceritinib on in vitro NPM1-ALK+ rearranged ALCL cell lines and in vivo xenograft mouse models. In conjunction with our preclinical data, we report a durable complete response (CR) in an NPM1-ALK+ ALCL patient treated with ceritinib in a phase II clinical trial.
Materials and methods
A detailed description of the methodology for analysis of western blot, flow cytometry, cell cycle, clonogenic assay, CD30 expression, pathological/immunohistochemical/immunophenotypic analysis of the clinical sample, and statistical analyses used in the study is available in the Supplementary Methods section (S1), available at https://doi.org/10.1016/j.esmoop.2021.100172.
Human cell lines and culture conditions
SU-DHL-1, SUP-M2 (NPM1-ALK-expressing ALCL), and OCI-AML3 (acute myeloid leukemia; FAB-M4) cell lines were purchased from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany). HL-60 (acute myeloid leukemia; FAB-M2) and MV-411 (biphenotypic acute leukemia) cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA). The cell lines SU-DHL-1 and SUP-M2 were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), OCI-AML3 in minimal essential medium (MEM)/Alpha with 10% FBS, HL-60 and MV4-11 in Iscove's Modified Dulbecco's Medium (IMDM) with 10% FBS and 1% penicillin/streptomycin at 37°C in 5% carbon dioxide. All cells were grown logarithmically.
Antibodies
Antibodies were purchased from the following: NPM1-ALK (#3333), phospho-NPM1-ALK (Y138, which corresponds to Y1078 in ALK) (#12127), AKT (#2920), phospho-AKT (S473) (#4060), ERK1/2 (#4695), phospho-STAT3 (Y705) (#9145), c-Myc (#5605), Survivin (#2802), phospho-FLT3 (Y591) (#3466S), (Cell Signaling Technologies, Danvers, MA), STAT3 (#610189), phospho-ERK1/2 (T202/Y204) (#612358), phospho-AKT (S473) (#560397), PARP (#556494), FITC-CD30 (#555829), FITC-IgGκ-Isotype control (#555748), FITC-Annexin V (#556419) (BD Biosciences, San Jose, CA), FLT3 (#SC480) (Santa Cruz Biotechnology, Santa Cruz, CA), FLAG (#F1804), MCL-1 (#M8434 ) and β-actin (#A5316) (Sigma-Aldrich, St. Louis, MO).
Chemicals and reagents
All reagents were purchased from Selleck Chemicals, Houston, TX (Ceritinib-S7083), Sigma-Aldrich, St. Louis, MO (Propidium iodide-P4170); Molecular Probes, Eugene, OR (TO-PRO-3); Thermo Scientific, Waltham, MA (RPMI-1640-SH30027; IMDM-SH30228FS; MEM/Alpha- SH30265FS); Corning Life Sciences, Tewksbury, MA (FBS-35010CV Penicillin/Streptomycin-30-002-CI); STEMCELL Technologies, Vancouver, BC (MethoCult-H4100-04100).
In vivo NPM1-ALK+ ALCL xenograft model
Five to 7-week-old female Hsd:Athymic Nude-Foxn1nu mice (Envigo, Indianapolis, IN) were subcutaneously injected with 5 million SU-DHL-1 cells in the flank. When tumors were palpable, the mice were randomized into two groups (each group had eight mice): a control group treated with vehicle alone (0.5% w/methylcellulose and 0.5% w/w Tween 80, Fisher Scientifics, Pittsburgh, PA) and an experimental group treated with 50 mg/kg ceritinib by oral gavage per day for 21 days. Tumors were measured by Vernier caliper and tumor volumes were calculated using the modified ellipsoid formula: ½ (length × width2). Mice were euthanized when tumor size reached 2000 mm3 or the tumors became necrotic. Further treated mice were followed for survival analysis. All in vivo studies were carried out in accordance with an institutional animal care and use committee-approved protocol.
Clinical trial using ceritinib in an NPM1-ALK+ ALCL patient
Treatment, data collection, and consent for investigational trials were carried out in accordance with the guidelines of the University of Texas MD Anderson Cancer Center Institutional Review Board (IRB) and Quorum Central IRB. This was a phase II (NCT02186821), open-label study to determine the efficacy and safety of treatment with ceritinib (Zykadia®, Novartis Pharmaceuticals, Cambridge, MA) in patients with a diagnosis of solid tumors or hematological malignancies that harbor ALK or ROS1 positive mutations, translocations, rearrangements, or amplifications and whose disease has progressed on or after standard treatment. The brief eligibility criteria included that patient has a confirmed diagnosis of a solid tumor (except ALK+ NSCLC) or hematological malignancy and is in need of treatment because of radiologic progression or relapse. Lymphoma patients should have at least one measurable nodal lesion (≥2 cm in the long axis at screening) according to Cheson criteria.31 In a case where the patient has no measurable nodal lesions ≥2 cm, then the patient must have at least one measurable extra-nodal lesion. The patient had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1 and adequate bone marrow as described below: (i) absolute neutrophil count (ANC) ≥1.5 × 109/l. (ii) Platelets (PLT) ≥75 × 109/l. (iii) Hemoglobin (Hgb) ≥8 g/dl. Tumor NPM1-ALK fusion transcript were analyzed by NGS (Foundation Medicine, Cambridge, MA). Tumor ALK expression levels and distribution were analyzed by immunohistochemistry (IHC) staining. One patient was treated in the study and the presented data represent a data cutoff of 56+ months.
NGS-based comprehensive genomic profiling of ALK mutations in hematologic malignancies
Tissues from 370 096 unique advanced cancers were sequenced during the course of routine clinical care by hybrid-capture, NGS-based comprehensive genomic profiling of 406 genes plus introns from 31 genes commonly rearranged in cancer, as well as RNA for 265 genes for a portion of these cases. We reviewed all hematologic malignancies profiled by a large comprehensive genomic profiling database – acute leukemias, bone marrow failure, histiocytosis, Hodgkin's lymphoma, lymphoproliferative disease, myeloid neoplasm, non-Hodgkin's lymphoma, plasma cell neoplasm, and thymic thymoma.
Results
Ceritinib inhibits NPM1-ALK-mediated signaling in NPM1-ALK+ ALCL cells
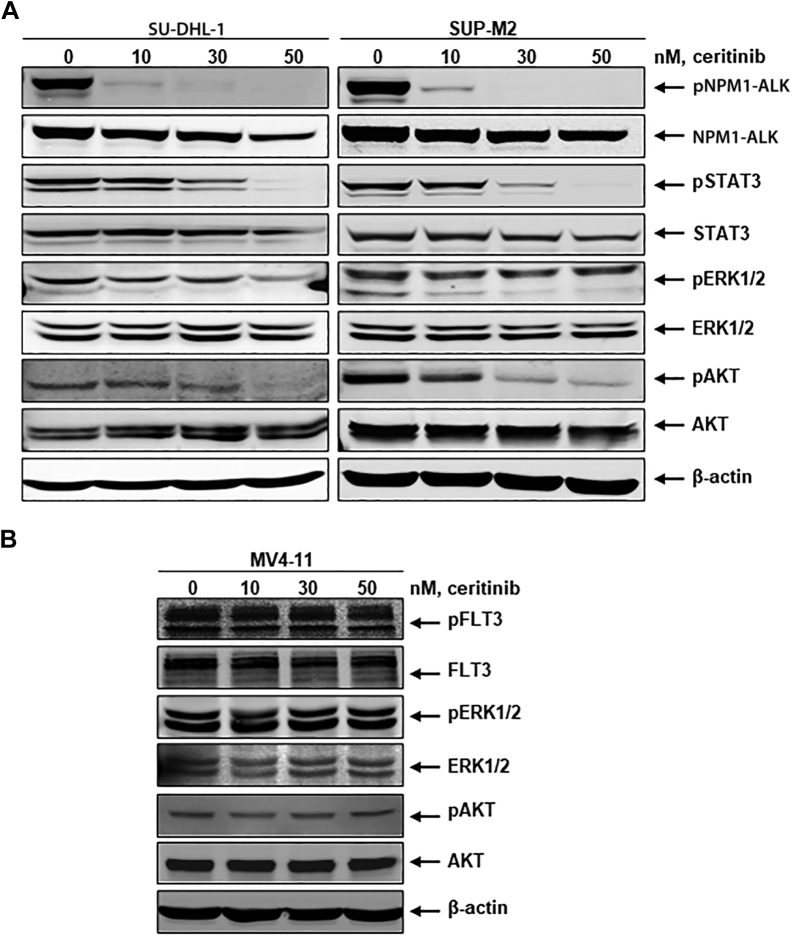
Constitutive activation of the NPM1-ALK fusion tyrosine kinase signaling axis includes the upregulation of downstream effector molecules STAT3, ERK1/2, and AKT, which are known to induce proliferation and survival of ALK+ ALCL cells. Since fusion kinase activation is the first step of initiating the survival signaling cascade, we examined the effects of ceritinib on NPM1-ALK activation and downstream targets STAT3, ERK1/2, and AKT. In this study, we utilized two NPM1-ALK+ cell lines (SU-DHL-1 and SUP-M2) and one NPM1-ALK-negative cell line (MV4-11). MV4-11 is an acute myeloid leukemia cell line positive for FLT3 (FMS-like tyrosine kinase 3) mutation (FLT3-ITD), which is constitutively activated, resulting in downstream ERK1/2, AKT, and STAT5 signaling cascade. We used the MV4-11 cell line to demonstrate that ceritinib induces selective inhibition of ALK. Both NPM1-ALK+ and NPM1-ALK-negative cell lines were treated with ceritinib at concentrations ranging from 10-50 nM for 24 h. Both control and treated cell lysates were prepared and subjected to western blot analysis using pSTAT3/STAT3, pERK1/2/ERK1/2, and pAKT/AKT antibodies. As shown in Figure 1A, ceritinib inhibits NPM1-ALK activation in a dose-dependent manner. Ceritinib-mediated inhibition of NPM1-ALK phosphorylation led to decreased phosphorylated STAT3, ERK1/2, and AKT. There was no inhibition of FLT3 kinase activation and corresponding downstream signaling pathways ERK1/2 and AKT in MV4-11 cells (Figure 1B). The data supported the high selectivity of ceritinib on fusion kinase NPM1-ALK kinase. Overall, these findings indicate that ceritinib selectively and effectively inhibits the oncogenic fusion kinase NPM1-ALK and its downstream survival signaling partners.
Figure 1.
Ceritinib inhibits NPM1-ALK mediated signaling in NPM1-ALK+ ALCL cells.
(A) Ceritinib inhibits NPM1-ALK mediated signaling. SU-DHL-1 and SUP-M2 cells were treated with the indicated concentrations of ceritinib for 24 h. At the end of treatment, total cell lysates were prepared and analyzed by immunoblotting for phospho-NPM1-ALK, total NPM1-ALK, and downstream signaling molecules phospho-STAT3, phospho-ERK1/2, phospho-AKT, and the corresponding total protein content. The expression levels of β-actin in the lysates served as the loading control. (B) Ceritinib selectively inhibits NPM1-ALK signaling. FLT3-ITD expressing MV4-11 cells were treated with the indicated concentrations of ceritinib for 24 h. At the end of treatment, total cell lysates were prepared and analyzed by immunoblotting for phospho-FLT3, total FLT3, and downstream signaling molecules, including phospho-ERK1/2, phospho-AKT, and the corresponding total protein content. The expression levels of β-actin in the lysates served as the loading control.
Ceritinib-mediated inhibition of NPM1-ALK signaling induces apoptosis in NPM1-ALK+ ALCL cells
Next, we examined the effects of ceritinib-mediated inhibition of NPM1-ALK signaling on apoptosis. We used the NPM1-ALK+ cell lines, SU-DHL-1 and SUP-M2, and NPM1-ALK- cell lines, HL-60, OCI-AML3, and MV-411, to demonstrate ceritinib selectivity for NPM1-ALK. Both cell lines (NPM1-ALK+ and NPM1-ALK−) were treated with gradually increasing concentrations of ceritinib (10-50 nM) and incubated for 48 h. The cells were then analyzed for apoptosis using annexin V and TO-PRO-3 staining by flow cytometry. As shown in Figure 2A, ceritinib-induced apoptosis in the NPM1-ALK+ cell lines SU-DHL-1 and SUP-M2 in a dose-dependent manner, whereas no significant changes were observed in the NPM1-ALK− cell lines (Figure 2A). Interestingly, SUP-M2 cells were more sensitive to ceritinib even at the lowest concentration of 10 nM. In both SUP-M2 and SU-DHL-1 cell lines, ceritinib induced significant apoptosis from 20 to 50 nM. To analyze the biochemical changes associated with ceritinib-mediated apoptosis, we carried out western blotting for anti-apoptotic proteins MCL-1 and survivin. We also assessed the cleavage of PARP, which is considered a hallmark of early apoptosis. The data (Figure 2B) indicates that the treatment with ceritinib was associated with dose-dependent decreased MCL-1 and survivin levels along with increased PARP cleavage. Our findings further revealed that ceritinib treatment reduced the levels of the c-Myc protein, which is one of the downstream targets of ALK.32 Collectively, these results indicate that ceritinib induces apoptosis exclusively in NPM1-ALK+ cells, but not in NPM1-ALK− cells.
Figure 2.
Inhibition of NPM1-ALK signaling induces apoptosis in NPM1-ALK+ ALCL cells.
(A) Ceritinib induces apoptosis. SU-DHL-1, SUP-M2, HL-60, OCI-AML3, and MV4-11 cells were treated with the indicated concentrations of ceritinib for 48 h. Then cells were stained with annexin V and TO-PRO-3, the percentage of apoptotic cells was determined by flow cytometry. Columns represent the mean of three independent experiments; bars represent the SEM. P values <0.05 were considered as statistically significant (∗P < 0.05, significant; ∗∗P < 0.005, very significant; ∗∗∗P < 0.0005, extremely significant). (B) Ceritinib downregulates anti-apoptotic proteins. SU-DHL-1 and SUP-M2 cells were treated with indicated concentrations of ceritinib for 24 h. Subsequently, total cell lysates were prepared and subjected to immunoblotting for survivin, MCL-1, c-Myc, and PARP cleavage. The expression levels of β-actin in the lysates served as the loading control.
SEM, standard error of the mean.
Ceritinib induces G0/G1 phase cell cycle arrest and inhibits clonogenic potential and CD30 expression in NPM1-ALK+ ALCL cells
In order to explore the effect of ceritinib on cell cycle progression, we treated SU-DHL-1 and SUP-M2 cell lines with ceritinib concentrations ranging from 10 to 50 nM for 24 h. After treatment, cells were washed, fixed, and stained with propidium iodide, and cell cycle status was analyzed by flow cytometry. As shown in Figure 3A and B, ceritinib significantly increased the percentage of cells in the G0/G1 [SU-DHL-1: Control (56.9%) versus 50 nM ceritinib (87.5%); SUP-M2: Control (50.8%) versus 50 nM ceritinib (72.1%)] with a concomitant decrease in S [(SU-DHL-1: Control (20.9%) versus 50 nM ceritinib (6.9%); SUP-M2: Control (17.7%) versus 50 nM ceritinib (11.6%)], and G2/M [(SU-DHL-1: Control (23.3%) versus 50 nM ceritinib (5.1%); SUP-M2: Control (31.75%) versus 50 nM ceritinib (16%)] phases (Figure 3A and B).
Figure 3.
Ceritinib induces G0/G1 cell cycle arrest and inhibits clonogenic growth and CD30 expression of NPM1-ALK+ ALCL cells.
(A and B) Ceritinib induces G0/G1 cell cycle arrest. SU-DHL-1 and SUP-M2 cells were treated with the indicated concentrations of ceritinib for 24 h. Then, cells were fixed and stained with propidium iodide, and cell cycle status was determined by flow cytometry. Values represent the mean of three independent experiments. ∗P values <0.05 were considered as statistically significant. (C) Ceritinib inhibits colony growth: SU-DHL-1 and SUP-M2 cells were treated with the indicated concentrations of ceritinib for 24 h. After treatment, cells were resuspended in MethoCult semisolid media, plated, and incubated for 7 days. Colony count was made under a brightfield microscope and expressed as a percentage compared with the untreated. ∗P values <0.05 were considered as statistically significant. (D) Ceritinib decreases CD30 expression: SU-DHL-1 cells were treated with designated concentrations of ceritinib for 24 h. After end of the treatment, cells were washed and stained with FITC-IgG1κ isotype control or FITC-conjugated CD30 antibody. Representative flow histograms of CD30 levels with isotype control are presented.
Next, we carried out a clonogenic assay to determine ceritinib-mediated growth inhibitory effects on proliferation and survival of NPM1-ALK+ ALCL cells. Both SU-DHL-1 and SUP-M2 cells were treated with ceritinib (25-50 nM) for 24 h; then cells were washed, resuspended in MethoCult semisolid medium, and incubated for 7 days. Colonies were counted in untreated and treated wells and expressed as a percentage compared with control cells. As shown in Figure 3C, ceritinib treatment reduced anchorage-independent colony formation potential of lymphoma cells compared with control untreated cells, i.e., colony growth was reduced at 25 nM ceritinib (26% and 40%) and 50 nM (56% and 77%) in SU-DHL-1 and SUP-M2, respectively.
NPM1-ALK+ ALCL is characterized by a strong, universal expression of CD30, a member of the tumor necrosis factor receptor superfamily. Constitutive activation of NPM1-ALK signaling and downstream effector molecules STAT3 and ERK1/2 transcriptionally increase the expression of CD30.33 CD30 signaling activates NF-κB, which is a major downstream mediator contributing to lymphoma cell proliferation and survival.34,35 Previous studies showed that downregulation of NPM1-ALK by small interfering RNA (siRNA) was associated with decreased expression of CD30 in NPM1-ALK+ T-cell lymphoma cells.33 Therefore, we studied the effects of ceritinib-mediated inhibition of NPM1-ALK signaling on CD30 expression in SU-DHL-1 cells. Cells were treated with ceritinib at concentrations ranging from 10 to 50 nM for 24 h. Cells were then harvested, washed, incubated with CD30-FITC and IgG-kappa-isotype control antibodies and CD30-positive cells were determined by flow cytometry. As shown in Figure 3D, treatment with ceritinib decreases CD30 expression in a dose-dependent manner [right to left peaks; red (10 nM), light blue (20 nM), light green (30 nM), violet (40 nM), and dark blue (50 nM)] compared with untreated control cells (maroon peak). Overall, the data infers that ceritinib-mediated inhibition of NPM1-ALK signaling decreases CD30 surface expression in these cells.
Ceritinib inhibits tumor growth and potentiates survival advantage in NPM1-ALK+ ALCL xenograft model
Recent studies demonstrated that the ALK inhibitor ceritinib overcomes crizotinib resistance in ALK+ NSCLC in vitro and in vivo.36 To assess in vivo antitumor activity of ceritinib in NPM1-ALK+ ALCL, 5 million SU-DHL-1 cells were subcutaneously injected in the flank of Hsd: Athymic Nude-Foxn1nu mice. When tumors were palpable, mice were randomized into two groups (eight mice each), control (vehicle alone), and ceritinib (50 mg/kg administered via oral gavage for 21 days). As shown in Figure 4A, B-I (tumor) and C, there was a significant difference in tumor size between control and ceritinib-treated groups (P values remain significant from day 7 (P = 0.009) to day 15 (P = 0.0007). Additionally, tumor progression was associated with splenomegaly in control group (Figure 4B-II spleen). To determine overall survival benefit, we treated mice with the vehicle or ceritinib until they required euthanasia due to tumor burden following the institutional protocol. The Kaplan–Meier survival analysis demonstrates that treatment with ceritinib conferred a significant survival advantage (P ≤ 0.001) in the ceritinib-treated group compared with the vehicle-treated group (Figure 4D). While the vehicle-treated mice group had a median survival of 20 days, six out of eight mice in the ceritinib-treated group were alive at day 60, after which the experiment was terminated.
Figure 4.
Ceritinib inhibits tumor progression and potentiates survival advantage in the NPM1-ALK+ ALCL xenograft model.
(A and B) Hsd athymic nude mice were injected with SU-DHL-1 cells in the flank via a subcutaneous route (n = 8 in each control and treated groups). Treatment with ceritinib inhibited tumor growth (A, B-I) and associated splenomegaly (B-II); representation of resected tumor and spleen from treated and control groups. (C) Tumor volume decreased significantly in the ceritinib-treated mice group compared with control. (D) Kaplan–Meier survival plots for control and ceritinib-treated mice following indicated days of treatment.
Collectively, these preclinical in vivo findings suggest that treatment with ceritinib inhibited tumor growth and improved survival of mice harboring the NPM1-ALK+ ALCL xenografts.
The ALK inhibitor ceritinib causes a rapid and sustained CR in a patient with NPM1-ALK+ ALCL
A 21-year-old male presented with erythematous, non-painful, raised 1.5 and 0.5 cm lesions on his back and right inner thigh, respectively. Fludeoxyglucose (FDG) positron emission tomography computed tomography scan (PET CT) showed an FDG avid left supraclavicular lymph node, several left axillary lymph nodes, increased cutaneous radiotracer uptake overlying the right thoracic paraspinal muscles, a right iliac wing lesion, a right internal iliac lymph node, and a right inguinal lymph node. A punch biopsy of the two lesions revealed a dense lymphoid infiltrate that in some areas involved the epidermis. The infiltrate was comprised of large, atypical cells with moderately large nuclei, some of which were pleomorphic. Flow cytometry immunophenotypic studies were carried out using a lymph node biopsy from the left upper arm. A neoplastic T-cell population was identified that comprised ∼70% of the entirely analyzed cellular events, and expressed CD7 (subpopulation), CD25, CD26, CD30, and CD45. These cells were negative for CD2, CD3, CD4, CD5, CD8, CD10, TCRα/β, and TCRγ/δ. These findings were consistent with a diagnosis of T-cell ALCL (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100172). Histopathological features of the patient's tumor in a lymph node biopsy hematoxylin-eosin (H&E) staining show total effacement of the normal nodal architecture by neoplastic lymphoid cells that are small to intermediate in size with abundant eosinophilic cytoplasm, round to oval nuclei, and occasional prominent nucleoli (Figure 5A and B). Immunohistochemical (IHC) staining of a section from the lymph node further shows that the neoplastic lymphoid cells are strongly positive for ALK1 (Figure 5C) and that CD30 is universally expressed in the lymphoma cells (Figure 5D). The expression of CD30 is seen in the membrane, cytoplasm, and the paranuclear Golgi body. Overall, IHC staining demonstrates cytoplasmic and nuclear localization of ALK, consistent with the pattern of expression of NPM1-ALK in the lymphoma cells. In addition, the NGS panel showed an NPM1-ALK fusion gene. Altogether, these immunophenotypic and histopathologic results were consistent with NPM1-ALK+ ALCL diagnosis.
Figure 5.
Histopathological features of the patient's NPM1-ALK+ T-cell tumor in a lymph node biopsy.
(A) Touch imprint preparation (Wright–Giemsa stain) demonstrates an example of the neoplastic lymphoid cells (yellow arrowhead). The cell is relatively large in size with abundant cytoplasm and folded nucleus (original magnification: ×1000). (B) Histologic section from the lymph node (hematoxylin and eosin stain) shows total effacement of the normal nodal architecture by neoplastic lymphoid cells (yellow arrowheads) that are small to intermediate in size with abundant eosinophilic cytoplasm and round to oval nuclei with occasional prominent nucleoli. Scattered, atypical mitotic figures are present (green arrowhead). The background of the neoplastic lymphoid cells includes a mix of inflammatory cells that are composed of histiocytes, small reactive lymphocytes, and plasma cells (original magnification: ×400). (C) Immunohistochemical staining of a section from the lymph node shows that the neoplastic lymphoid cells are strongly positive for ALK1. The staining demonstrates cytoplasmic and nuclear localization, consistent with the pattern of expression of NPM1-ALK in the lymphoma cells. Numerous inflammatory and stromal cells are present in the background and lack the expression of ALK (original magnification: ×400). (D) Immunohistochemical staining of a section from the lymph node shows that CD30 is universally expressed in the lymphoma cells. The expression of CD30 is seen in the membrane, cytoplasm, and the paranuclear Golgi body. Background inflammatory and stromal cells are negative for CD30 (original magnification: ×1000).
Depicted in Figure 6A is the treatment timeline. The patient was started on CHOP [cyclophosphamide (750 mg/m2 i.v. on day 1), doxorubicin (50 mg/m2 i.v. on day 1), vincristine (2 mg i.v. on day 1), and prednisone (100 mg orally once daily on days 1-5)] every 3 weeks with granulocyte colony-stimulating factor (G-CSF) support. A PET CT following four cycles and again after six cycles of CHOP showed CR. However, 2 months later, an enlarging left axillary lymph node was noticed. Fine needle aspirate of the left axillary lymph node revealed recurrent NPM1-ALK+ T-cell lymphoma. PET CT showed a new FDG avid left axillary mass and numerous hypermetabolic scattered subcutaneous and intramuscular soft tissue nodules. Bone marrow aspiration and biopsy showed no evidence of ALCL. He was started on an antibody conjugate targeting CD30 brentuximab vedotin (1.8 mg/kg i.v.) every 3 weeks. PET CT following two cycles of brentuximab vedotin showed partial response (PR) with significant improvement in the left axillary mass and near resolution of all other subcutaneous nodules. Upon completion of six cycles of brentuximab vedotin, PET CT showed CR.
Figure 6.
Ceritinib induces a deeper molecular response in NPM1-ALK+ ALCL patient.
(A) Timeline and clinical history of a patient. (B) PET CT images describing therapeutic response of NPM1-ALK+ ALCL patient to ceritinib. The patient had multiple lung and several soft tissue lesions. Representative lesions in the right upper lobe (black arrow) and right gluteal subcutaneous fat (white arrow) are shown (December 2015 scan). There was a response to ceritinib therapy on the first follow-up scan in February 2016, with residual scarring at the site of the right upper lobe metastasis and residual right gluteal skin thickening. There is a persistent positive response as of the most recent scan in October 2018.
BEAM, carmustine (BCNU), etoposide, aracytin, and melphalan; CHOP, cyclophosphamide, hydroxy doxorubicin, vincristine, prednisone; CR, complete response; CT, computed tomography; HSCT, hematopoietic stem cell transplantation; PD, progressive disease; PET, positron emission tomography; PR, partial response.
Following this, the patient underwent high-dose chemotherapy with BEAM (carmustine [bis-chloroethylnitrosourea (BCNU)]-etoposide, aracytin, and melphalan) standard conditioning and autologous peripheral blood stem cell transplant. Approximately 6 weeks later, he noticed enlargement of the left axillary lymph node and cutaneous lesions. Excisional biopsy of the lymph node showed recurrent NPM1-ALK+ T-cell lymphoma. PET CT showed new hypermetabolic enlarged left axillary lymph node and right paratracheal lymph nodes. He restarted brentuximab vedotin with a quick response in cutaneous lesions. Following three cycles of brentuximab vedotin, he presented to MD Anderson Cancer Center for clinical trial consideration. A repeat PET CT showed FDG avid 7 mm left axillary lymph node and a new soft tissue nodule on the medial aspect of the left proximal arm. Excisional biopsy of the left forearm skin lesion showed NPM1-ALK+ ALCL. He had no measurable disease following resection of the left forearm cutaneous lesion and felt like his left axillary lymph node was shrinking. Therefore, he was not eligible for any clinical trials and opted for observation. Two months later, PET CT showed diffuse disease progression involving lymph nodes, bilateral lungs, and subcutaneous tissue. He was briefly hospitalized with left axillary surgical wound infection, which resolved with a 2-week course of levofloxacin and metronidazole. One month later, he was enrolled in a phase II basket clinical trial of ceritinib (750 mg orally once a day) (NCT02186821). Ceritinib was overall well tolerated and resulted in significant regression in all lesions (measurable and non-measurable). Per Cheson's criteria, the maximal initial response was a 96% reduction of the target lesions, eventually resulting in a CR (Figure 6B). Adverse effects, including nausea, diarrhea, elevated liver enzymes, leukopenia, and elevated serum creatinine, were all grade 1 per Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. Two years later, upon the end of the trial, he continued ceritinib off-protocol. He remains on ceritinib to date (>56 months) with an ongoing CR and clinical benefit.
NGS-based comprehensive genomic profiling of ALK mutations in hematologic malignancies
Here we used NGS-based comprehensive genomic profiling data to analyze the number of ALK-rearranged cases in hematologic malignancies from Foundation Medicine data. Of 19 272 patients sequenced with hematopoietic diseases, 58 patients (0.30%) harbored ALK fusions. ALCL accounted for the majority of these cases (32), which was 35.2% (32/91) of all cases of ALCL. Of the ALK-positive ALCL cases, 25/32 (78.1%) were NPM1-ALK fusions. In addition to ALCL, 10 diffuse large B-cell lymphomas (DLBCL) harbored ALK fusions (0.95% of total DLBCL), with three cases harboring NPM1-ALK fusions. The other hematopoietic diseases harboring ALK fusion events were histiocytosis (9/120; 7.5%), multiple myeloma (2/2662; 0.07%), and acute leukemia (2/3211; 0.06%). Diseases infrequent in this series with one instance of an ALK fusion included Castleman's disease (1/11), juvenile xanthogranuloma (JXG) (1/10), and an unspecified B-cell neoplasm (1/265). One case of histiocytic disorder in this series was previously reported as responding to an ALK inhibitor.37 Recurrent 5′ partners other than NPM1 (n ≥ 2) included clathrin heavy chain (4 DLBCL, 1 histiocytosis, 1 B-cell neoplasm NOS), KIF5B (6 histiocytosis, ), TPM3 (2 ALCL, 1 histiocytosis), SEC31A (1 DLBCL and multiple myeloma), TRK-fused gene (1 ALCL and JXG), 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase (2 ALCL), and RANBP2 (1 DLCBL and AML). All breakpoints in ALK were within intron 19, and all the NPM1 breakpoints were in intron 4. The majority of fusions were detected in RNA (49/58). Of the nine cases where the fusions was only seen in DNA, four did not have RNA sequencing results and four had suboptimal coverage for the RNA portion of the assay.
Discussion
The current advancement in omics profiling techniques has rapidly changed oncology, enabling the identification of novel molecular targets and the development of targeted therapeutics to treat clinically actionable mutations for many cancers.38,39 The discovery of ALK tyrosine kinase specific small molecule inhibitors forever transformed precision medicine in ALK+ malignancies.40 Recently several ALK gene mutations have been found in various solid and hematologic malignancies.41 ALK is a receptor tyrosine kinase rendered as an oncogene through mutations or chromosomal translocations in various solid and hematologic malignancies. The most common oncogenic ALK fusions partners are the NPM1 gene in ALCL, TPM3/TPM4 genes in inflammatory myofibroblastic tumors (IMT), and EML4 (Echinoderm microtubule-associated protein-like 4) gene in NSCLC. In this study, we present evidence for the efficacy of ceritinib, a potent second-generation ALK inhibitor, against NPM1-ALK fusion-driven ALCL.9,29,30
In clinical practice, many refractory NPM1-ALK+ ALCL patients are treated with crizotinib as an alternative to standard chemotherapy.42 Recently, the FDA also approved crizotinib in children and young adults with relapsed or refractory ALK+ ALCL. NPM1-ALK fusion-driven pediatric lymphomas showed an objective response rate to crizotinib therapy while a subset of patients progressed in the first 120 days of treatment. Increased expression of IL10RA was indicated to be responsible for crizotinib resistance in NPM1-ALK-driven ALCL cells.42 A phase I clinical trial result showed that ceritinib had marked antitumor activity on crizotinib (a first-generation ALK inhibitor)-resistant EML4-ALK+ NSCLC tumors, including those with ALK mutations at amino acid residues at position 1196, 1269, 1171, or 1206. The preclinical data from crizotinib-resistant NPM1-ALK+ ALCL clones shared some common ALK mutations that are found in NSCLC (amino acid residues at 1196 and 1171).43 Data from the crizotinib-resistant ALK mutational analysis suggest that ceritinib also has future therapeutic potential in crizotinib-resistant ALK gene rearranged lymphomas.
A previous report44 showed efficacy of ceritinib in ALK-rearranged lymphomas, but the relatively short follow-up (20 months) and limited scope of the preclinical data are now addressed in this report. Here we show durable 56-month NPM1-ALK+ T-cell ALCL patient CR in addition to more comprehensive molecular analyses in preclinical in vivo and in vitro assays. As demonstrated in previous studies on the inhibition of NPM1-ALK signaling by ALK inhibitors, ceritinib-mediated blockade of NPM1-ALK oncogenic signaling results in significant downregulation of STAT3, AKT, and ERK1/2 phosphorylation levels.12 Our results supported the high selectivity of ceritinib in targeting ALK kinase. Since NPM1-ALK-mediated signaling triggers the survival of ALCL cells, we investigated the effect of ceritinib effect on apoptosis. Our findings reveal that inhibition of NPM1-ALK oncogenic signaling by ceritinib arrested ALCL cells in G0/G1 phase, decreased the clonogenic potential, and significantly induced apoptosis in ALCL cells.
To further explore the molecular mechanisms involved in ceritinib-induced apoptosis, we evaluated the anti-apoptotic proteins survivin and MCL-1. STAT3 and AKT signaling pathways have been shown to regulate apoptosis by increased expression of anti-apoptotic molecules, such as MCL1 and survivin, providing strong anti-apoptotic signals.45 Our results demonstrate that ceritinib significantly decreased the levels of survivin and MCL-1. In NPM1-ALK-driven ALCL cells, the fusion kinase also induces c-Myc expression, which is known to regulate cellular growth and apoptosis.32 Our data indicate that ceritinib effectively decreased anti-apoptotic proteins, increased PARP cleavage, and downregulated c-Myc protein levels. All of these findings correlate with increased apoptosis of the ALCL cells in a dose-dependent manner. Treatment with ceritinib also decreased the expression of the cytokine receptor CD30 in T-cell lymphoma cells. CD30 is transcriptionally increased through NPM1-ALK-dependent ERK1/2 and STAT3 pathways.46 CD30 expression induces NF-κB activation and serves as a survival factor of lymphoma cells.47 Treatment with ceritinib downregulated NPM1-ALK signaling on CD30 expression in ALCL cells, correlating with the inhibition of NPM1-ALK signaling data. Finally, we assessed the in vitro growth inhibitory effects of ceritinib in vivo using the NPM1-ALK+ ALCL xenograft tumor model. The in vivo results demonstrated that while control mice had robust tumor progression in association with splenomegaly, the ceritinib-treated group showed a significant reduction in tumor volumes and decreased splenomegaly. Importantly, we observed that ceritinib-mediated tumor regression potentiated survival advantage in the ceritinib-treated group.
Our case study further confirms the benefit of ceritinib treatment in patients with recurrent NPM1-ALK+ ALCL. Overall, ceritinib treatment in a heavily pretreated lymphoma patient resulted in a significant reduction in lesions and was well tolerated. Adverse effects, including nausea, diarrhea, elevated liver enzymes, leukopenia, and elevated serum creatinine, were limited to grade 1 per NCI CTCAE v 4.03. Two years later, upon the end of the trial, the patient continued ceritinib off-protocol. He remains on ceritinib to date (>56 months) after initiation with a complete ongoing response and significant clinical benefit. Ceritinib has led to salutary effects in a relapsed/refractory patient with NPM1-ALK+ ALCL. In addition to ALCL, our NGS-based comprehensive genomic profiling data showed that ALK fusions exist in multiple hematologic malignancies, including multiple myeloma, DLBCL, histiocytosis, B-cell neoplasms, acute leukemias, and other rare disorders like Castleman's disease and juvenile xanthogranuloma. One case of histiocytosis was previously reported as responding to an ALK inhibitor.37 These data call for systematic identification of ALK fusions in the clinical evaluation of hematologic malignancy patients with refractory disease and suggest opening more ALK inhibitor basket trials for refractory ALK+ malignancies.
In conclusion, our study provides an understanding of ceritinib-mediated molecular events and potential molecular markers of ceritinib action along with clinical trial data achieving a deep and durable molecular response in NPM1-ALK+ ALCL patient.
Acknowledgments
Funding
This work was supported in part by The Cancer Prevention and Research Institute of Texas [grant number RP1100584]; the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy [grant number 1U01 CA180964]; NCATS [grant number UL1 TR000371] (Center for Clinical and Translational Sciences); and The MD Anderson Cancer Center support grant [grant number P30 CA016672]. VS is supported by NIH/NCI [grant number 1R01CA242845-01A1]. DRW thanks support from the Hall Family Foundation endowed Chair in Molecular Medicine and the National Foundation for Cancer Research. RAJ is a recipient of [grant number P30-CA168524] from NCI. Parts of this work have been funded by grant to HMA [grant number R01 CA151533]. RB acknowledges the Sosland Family Foundation Research Award; Hale Family Foundation Research Award; Frontiers Clinical and Translational Pilot Award UL1 TR000001; and American Cancer Society-Institutional Research Grant [grant number ACS-IRG-16-194-07]. We would like to thank Luke Juckett for the initial analysis of the Foundation Medicine Database.
Disclosure
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. Research funding/grant support for clinical trials: FUJIFILM Pharmaceuticals USA, Inc., Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, PharmaMar, D3, Pfizer, MultiVir, Amgen, AbbVie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Loxo Oncology, Medimmune, Altum, Dragonfly Therapeutics, Takeda and Roche/Genentech, National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center. VS was part of a phase II basket trial sponsored by Novartis. Travel: Novartis, PharmaMar, ASCO, ESMO, Helsinn, Incyte, US-FDA. Consultancy/advisory board: Helsinn, LOXO Oncology/Eli Lilly, R-Pharma US, INCYTE, Medimmune, Novartis. SG reports personal fees from Astellas, Seattle Genetics, Sanofi, Daiichi Sankyo, Kite Pharma, Kadmon, and BMS outside the submitted work. JPM reports advisory board, honoraria, and research funding from AlloVir HCP, Juno Therapeutics, Inc., and Gilead-Kite Pharmaceuticals; advisory board for Magenta Therapeutics; and research funding from Novartis, Fresenius Biotech, Astellas, Bellicum Pharmaceuticals, Gamida Cell, and Pluristem Ltd. outside the submitted work. SMA was a former employee at Foundation Medicine. RWM, JMV are employees of Foundation Medicine. All other authors declare no conflicts of interest.
Contributor Information
V. Subbiah, Email: vsubbiah@mdanderson.org.
R. Balusu, Email: rbalusu@kumc.edu.
Supplementary data
Supplementary Figure 1.
Supplementary Figure S1. Flow cytometric immunophenotypic analysis of lymph node biopsy of NPM-ALK+ ALCL specimen.
Flow cytometry immunophenotypic analysis panels carried out using a left upper arm lymph node specimen show that the neoplastic T lymphocytes express CD25 and CD30. These cells are negative for CD3, CD4, CD8, and CD10.
References
- 1.Ferreri A.J., Govi S., Pileri S.A., Savage K.J. Anaplastic large cell lymphoma, ALK-positive. Crit Rev Oncol Hematol. 2012;83:293–302. doi: 10.1016/j.critrevonc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Vu K., Ai W. Update on the treatment of anaplastic large cell lymphoma. Curr Hematol Malig Rep. 2018;13:135–141. doi: 10.1007/s11899-018-0436-z. [DOI] [PubMed] [Google Scholar]
- 3.Amin H.M., Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110:2259–2267. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holla V.R., Elamin Y.Y., Bailey A.M. ALK: a tyrosine kinase target for cancer therapy. Cold Spring Harb Mol Case Stud. 2017;3:a001115. doi: 10.1101/mcs.a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiarle R., Voena C., Ambrogio C., Piva R., Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 6.Morris S.W., Kirstein M.N., Valentine M.B. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 7.Falini B., Brunetti L., Sportoletti P., Martelli M.P. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood. 2020;136:1707–1721. doi: 10.1182/blood.2019004226. [DOI] [PubMed] [Google Scholar]
- 8.Kunchala P., Kuravi S., Jensen R., McGuirk J., Balusu R. When the good go bad: mutant NPM1 in acute myeloid leukemia. Blood Rev. 2018;32:167–183. doi: 10.1016/j.blre.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 9.George S.K., Vishwamitra D., Manshouri R., Shi P., Amin H.M. The ALK inhibitor ASP3026 eradicates NPM-ALK(+) T-cell anaplastic large-cell lymphoma in vitro and in a systemic xenograft lymphoma model. Oncotarget. 2014;5:5750–5763. doi: 10.18632/oncotarget.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischof D., Pulford K., Mason D.Y., Morris S.W. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997;17:2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2:495–502. doi: 10.1158/2159-8290.CD-12-0009. [DOI] [PubMed] [Google Scholar]
- 12.Mosse Y.P., Wood A., Maris J.M. Inhibition of ALK signaling for cancer therapy. Clin Cancer Res. 2009;15:5609–5614. doi: 10.1158/1078-0432.CCR-08-2762. [DOI] [PubMed] [Google Scholar]
- 13.Khoury J.D., Medeiros L.J., Rassidakis G.Z. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:3692–3699. [PubMed] [Google Scholar]
- 14.Bai R.Y., Ouyang T., Miething C., Morris S.W., Peschel C., Duyster J. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96:4319–4327. [PubMed] [Google Scholar]
- 15.Amin H.M., McDonnell T.J., Ma Y. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene. 2004;23:5426–5434. doi: 10.1038/sj.onc.1207703. [DOI] [PubMed] [Google Scholar]
- 16.Cederleuf H., Bjerregard Pedersen M., Jerkeman M., Relander T., d'Amore F., Ellin F. The addition of etoposide to CHOP is associated with improved outcome in ALK+ adult anaplastic large cell lymphoma: a Nordic Lymphoma Group study. Br J Haematol. 2017;178:739–746. doi: 10.1111/bjh.14740. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz S., O'Connor O.A., Pro B. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393:229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limat S., Demesmay K., Voillat L. Early cardiotoxicity of the CHOP regimen in aggressive non-Hodgkin's lymphoma. Ann Oncol. 2003;14:277–281. doi: 10.1093/annonc/mdg070. [DOI] [PubMed] [Google Scholar]
- 19.Vose J., Armitage J., Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Soma L.A., Fromm J.R. Targeted therapy for Hodgkin lymphoma and systemic anaplastic large cell lymphoma: focus on brentuximab vedotin. Onco Targets Ther. 2013;7:45–56. doi: 10.2147/OTT.S39107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Xu W., Liu H., Li J. Therapeutic options in peripheral T cell lymphoma. J Hematol Oncol. 2016;9:37. doi: 10.1186/s13045-016-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bode A.M., Dong Z. Recent advances in precision oncology research. NPJ Precis Oncol. 2018;2:11. doi: 10.1038/s41698-018-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piva R., Chiarle R., Manazza A.D. Ablation of oncogenic ALK is a viable therapeutic approach for anaplastic large-cell lymphomas. Blood. 2006;107:689–697. doi: 10.1182/blood-2005-05-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katayama R., Shaw A.T., Khan T.M. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doebele R.C., Pilling A.B., Aisner D.L. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw A.T., Kim D.W., Mehra R. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller I.B., De Langen A.J., Honeywell R.J., Giovannetti E., Peters G.J. Overcoming crizotinib resistance in ALK-rearranged NSCLC with the second-generation ALK-inhibitor ceritinib. Expert Rev Anticancer Ther. 2016;16:147–157. doi: 10.1586/14737140.2016.1131612. [DOI] [PubMed] [Google Scholar]
- 28.Chuang J.C., Neal J.W. Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Transl Lung Cancer Res. 2015;4:639–641. doi: 10.3978/j.issn.2218-6751.2015.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santarpia M., Daffina M.G., D'Aveni A. Spotlight on ceritinib in the treatment of ALK+ NSCLC: design, development and place in therapy. Drug Des Devel Ther. 2017;11:2047–2063. doi: 10.2147/DDDT.S113500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsilje T.H., Pei W., Chen B. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl) phenyl) pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56:5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 31.Cheson B.D., Pfistner B., Juweid M.E. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 32.Raetz E.A., Perkins S.L., Carlson M.A., Schooler K.P., Carroll W.L., Virshup D.M. The nucleophosmin-anaplastic lymphoma kinase fusion protein induces c-Myc expression in pediatric anaplastic large cell lymphomas. Am J Pathol. 2002;161:875–883. doi: 10.1016/S0002-9440(10)64248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu F.Y., Johnston P.B., Burke K.A., Zhao Y. The expression of CD30 in anaplastic large cell lymphoma is regulated by nucleophosmin-anaplastic lymphoma kinase-mediated JunB level in a cell type-specific manner. Cancer Res. 2006;66:9002–9008. doi: 10.1158/0008-5472.CAN-05-4101. [DOI] [PubMed] [Google Scholar]
- 34.Hauer J., Puschner S., Ramakrishnan P. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jost P.J., Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 36.Friboulet L., Li N., Katayama R. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross J.S., Ali S.M., Fasan O. ALK fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncologist. 2017;22:1444–1450. doi: 10.1634/theoncologist.2016-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu K.H., Snyder M. Omics profiling in precision oncology. Mol Cell Proteomics. 2016;15:2525–2536. doi: 10.1074/mcp.O116.059253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awad M.M., Shaw A.T. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12:429–439. [PMC free article] [PubMed] [Google Scholar]
- 41.Grande E., Bolos M.V., Arriola E. Targeting oncogenic ALK: a promising strategy for cancer treatment. Mol Cancer Ther. 2011;10:569–579. doi: 10.1158/1535-7163.MCT-10-0615. [DOI] [PubMed] [Google Scholar]
- 42.Prokoph N., Probst N.A., Lee L.C. IL10RA modulates crizotinib sensitivity in NPM1-ALK+ anaplastic large cell lymphoma. Blood. 2020;136:1657–1669. doi: 10.1182/blood.2019003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ceccon M., Mologni L., Bisson W., Scapozza L., Gambacorti-Passerini C. Crizotinib-resistant NPM-ALK mutants confer differential sensitivity to unrelated Alk inhibitors. Mol Cancer Res. 2013;11:122–132. doi: 10.1158/1541-7786.MCR-12-0569. [DOI] [PubMed] [Google Scholar]
- 44.Richly H., Kim T.M., Schuler M. Ceritinib in patients with advanced anaplastic lymphoma kinase-rearranged anaplastic large-cell lymphoma. Blood. 2015;126:1257–1258. doi: 10.1182/blood-2014-12-617779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabbo F., Barreca A., Piva R., Inghirami G. ALK signaling and target therapy in anaplastic large cell lymphoma. Front Oncol. 2012;2:41. doi: 10.3389/fonc.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S.Y., Lee S.Y., Kandala G., Liou M.L., Liou H.C., Choi Y. CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity. Proc Natl Acad Sci U S A. 1996;93:9699–9703. doi: 10.1073/pnas.93.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright C.W., Rumble J.M., Duckett C.S. CD30 activates both the canonical and alternative NF-kappaB pathways in anaplastic large cell lymphoma cells. J Biol Chem. 2007;282:10252–10262. doi: 10.1074/jbc.M608817200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.