Abstract
Background
We updated a 2017 systematic review and compared the effects of HIV self-testing (HIVST) to standard HIV testing services to understand effective service delivery models among the general population.
Methods
We included randomized controlled trials (RCTs) comparing testing outcomes with HIVST to standard testing in the general population and published between January 1, 2006 and June 4, 2019. Random effects meta-analysis was conducted and pooled risk ratios (RRs) were reported. The certainty of evidence was determined using the GRADE methodology.
Findings
We identified 14 eligible RCTs, 13 of which were conducted in sub-Saharan Africa. Support provided to self-testers ranged from no/basic support to one-on-one in-person support. HIVST increased testing uptake overall (RR:2.09; 95% confidence interval: 1.69–2.58; p < 0.0001;13 RCTs; moderate certainty evidence) and by service delivery model including facility-based distribution, HIVST use at facilities, secondary distribution to partners, and community-based distribution. The number of persons diagnosed HIV-positive among those tested (RR:0.81, 0.45–1.47; p = 0.50; 8 RCTs; moderate certainty evidence) and number linked to HIV care/treatment among those diagnosed (RR:0.95, 0.79–1.13; p = 0.52; 6 RCTs; moderate certainty evidence) were similar between HIVST and standard testing. Reported harms/adverse events with HIVST were rare and appeared similar to standard testing (RR:2.52: 0.52–12.13; p = 0.25; 4 RCTs; very low certainty evidence).
Interpretation
HIVST appears to be safe and effective among the general population in sub-Saharan Africa with a range of delivery models. It identified and linked additional people with HIV to care. These findings support the wider availability of HIVST to reach those who may not otherwise access testing.
Keywords: HIV testing services, HIV self-testing, general population, Systematic Review, Meta-analysis
Research in context.
Evidence before this study
A previous 2017 systematic review and meta-analysis identified five randomized controlled trials (RCTs) comparing HIVST to standard HIV testing services. This systematic review informed the 2016 WHO recommendation on HIV self-testing (HIVST). All five included RCTs were published as conference abstracts and only two of them were conducted among the general population in high HIV burden settings in sub-Saharan Africa. At that time, little was known on optimal HIVST implementation and service delivery models, as well as success of linkage to appropriate prevention or treatment services after HIVST.
Added value of this study
We updated the 2017 systematic review to understand which service delivery models are effective with a focus on the general population. Evidence from 14 RCTs showed that HIVST doubled the uptake of HIV testing in the general population compared to standard HIV testing services. Importantly, HIV testing uptake increased with a range of HIVST delivery models and support tools. HIVST can achieve HIV positivity and linkage rates similar to that with standard HIV testing, and identify individuals with HIV who may not otherwise test.
Implications of all the available evidence
Based on the findings of this review, and additional information on user and provider values and preferences, WHO updated the recommendation on HIVST in 2019 and suggested to provide choice in service delivery models and support tools. Review findings can inform country implementation as effective HIVST service delivery models and support tools can be adapted to suit the local context, epidemiology and focus populations. Countries need to consider rapid introduction and scaling up of HIVST to achieve national and global goals. As most of the evidence was from sub-Saharan Africa, findings have greater relevance for this region.
Alt-text: Unlabelled box
1. Introduction
Globally, knowledge of HIV status among people with HIV has increased considerably over the past decade. In 2019, 81% of all people with HIV were estimated to be aware of their HIV status, with 7.9 million people yet to be diagnosed [1]. Efficient and effective approaches to HIV testing services are needed to reach these remaining people with undiagnosed HIV who do not routinely access HIV services. HIV self-testing (HIVST) – whereby a person collects their own specimen, performs a simple rapid diagnostic test and interprets their result – has emerged as a safe, acceptable and effective tool to reach people who do not otherwise access HIV testing services [2,3]. The World Health Organization (WHO) first recommended HIVST as an additional approach to HIV testing services in 2016 [4]. HIVST has since been highlighted as a key intervention to reach the global 95-95-95 goals by 2025, starting with diagnosing 95% of all people with HIV and then linking them to treatment and achieving viral suppression [5,6].
Since 2016, there has been a rapid increase in the number of countries with national policies supportive of HIVST; however, the shift from policy adoption to routine implementation has been slow. As of June 2020, nearly half (n = 41) of all countries with HIVST polices (n = 88) reported routine HIVST implementation, with regional variation in implementation status [7]. Some low- and middle-income countries in east and southern Africa have scaled up HIVST distribution in select public-sector facilities and in the community through catalytic donor investments [8]. Countries introducing HIVST and those transitioning from initial catalytic investment to more sustainable donor or domestic financing need guidance on optimal HIVST delivery and distribution models suited to their context.
WHO's 2016 recommendation on HIVST was based on a systematic review (published 2017) synthesizing evidence from five randomized controlled trials (RCTs); only two RCTs were conducted among the general population in high HIV burden settings [3]. At that time, little was known on HIVST implementation and service delivery models, and linkage to care rates following HIVST, which are important considerations to inform HIVST introduction and scale-up decisions in countries [9]. Since then, additional RCTs have evaluated HIVST service delivery models and related outcomes. We updated the 2017 systematic review [3] to compare the effects of HIVST with standard HIV testing and understand effective service delivery models for the general population. This review is one of a series examining the effects of HIVST in the general population, key populations [10], and user and provider values and preferences on HIVST. Review findings informed an update to the WHO recommendation (2019) on HIVST [2].
2. Methods
This review was conducted according to the WHO Handbook for guideline development [11], the Cochrane handbook for systematic reviews [12], and followed PRISMA guidelines for reporting [13]. See supplementary material S1 for the full systematic review protocol.
2.1. Search strategy and selection criteria
The review focused on the general population, defined for the purpose of this review as populations other than key populations (men who have sex with men, people who inject drugs, people in prisons or other closed settings, sex workers and transgender people) as defined by the WHO [14]. We included RCTs that directly compared HIVST to standard of care (standard facility-based testing by a provider; SoC) in the general population and reported at least one of the outcomes of interest.
The outcomes were ranked and selected by a guidelines development group according to the GRADE framework. The outcomes are defined in Table 1.
Table 1.
Definition of systematic review outcomes.
| Outcome | Definition |
|---|---|
| Uptake of HIV testing | Proportion of participants who tested for HIV among those randomized |
| HIV positivity | Proportion of participants who were diagnosed HIV-positive among those tested |
| Linkage to confirmatory testing | Proportion of participants who were linked to confirmatory testing among those who received reactive HIVST results |
| Linkage to treatment or care | Proportion of participants who were linked to treatment or care (CD4 count or viral load test) among those diagnosed HIV-positive |
| Misuse, social harm or adverse events | Any undesirable effect, or intended or unintended harm associated with HIV testing, for example, coercive testing, partner violence or suicide |
We included articles published in scientific journals and abstracts from major HIV-related conferences with no language or geographic restriction. The search strategy was previously validated for systematic mapping of the HIVST literature [15]. Briefly, we used key terms “HIV” AND “self-test” OR “home test” for electronic databases and only terms for self-testing for conference platforms because of limitations in search functions.
We searched nine electronic databases (Pubmed, Embase, Global Index Medicus, Social Policy and Practice, PsycINFO, Health Management Information Consortium, EBSCO CINAHL Plus, Cochrane Library and Web of Science) for articles published between January 1, 2006 and June 4, 2019, as HIVST articles are unlikely to have been published before this period. Conferences searched included the African Society for Laboratory Medicine, AIDS Impact, Conference on Retroviruses and Opportunistic Infections (2014–2019), International AIDS Conference, and International AIDS Society Conference. Secondary reference searching was conducted on all studies included in the review. We also contacted field experts to identify additional studies.
Titles and abstracts were reviewed in duplicate and full texts of shortlisted studies were reviewed independently by two authors (MSJ and TCW) to assess eligibility. Disagreements were resolved by discussion and consensus among authors (CJ, IEW, MSJ, TCW).
2.2. Data analysis
Data were extracted and entered into a relational database tool (airtable.com) using standardized extraction forms by one author (MSJ or IEW), and independently reviewed and checked by a second author (MSJ or IEW). Any disagreements were resolved by consensus. Where required information was not available, study authors were contacted for additional information.
Meta-analyses were conducted using random effects models (generic inverse-variance method) in R statistical software [16]. Where cluster RCTs were included, cluster-adjusted effect estimates from manuscripts were preferentially used; where cluster-adjusted effect estimates were not available, we adjusted the effective sample size using guidance from the Cochrane handbook [12]. We calculated pooled risk ratios (RRs) and 95% confidence intervals (CIs). Statistical heterogeneity was evaluated using the DerSimonian–Laird estimator for Tau2 and the associated I2 statistic. We generated forest plots overall and by subgroups (sex, young people aged 16–24 years, HIVST distribution model, and support tools) when relevant data were available from at least two RCTs. WHO defines young people as those between the ages of 10 and 24 [17], however for this review we focused on ages 16–24 years because the lowest age range for eligibility in included studies was 16 years.
For outcomes reported at multiple time-points, we used the time-point for primary endpoint as reported in the article. For RCTs with multiple intervention arms: (i) where differences in interventions were determined to be not likely to influence the outcome, data from intervention arms was combined and observations from the SoC arm were adjusted (divided by the number of intervention arms included in the meta-analysis); (ii) where differences in interventions were determined as likely to influence the outcome, the intervention arms were not combined; (iii) where one of the intervention arms was an enhanced/optimized version of SoC, we did not include it in the meta-analysis.
Studies with relevant outcomes that were not suitable for inclusion in the meta-analysis were described narratively.
2.3. Quality assessment
We used the Cochrane risk of bias tool I to assess the risk of bias in included studies [18]. Funnel plots were used to assess reporting bias when at least 10 studies were included in the meta-analysis. Studies were determined to be at high risk of bias overall if at least one domain was rated at high risk of bias. We followed the GRADE methodology to assess the overall certainty of evidence for each outcome across all included RCTs [11,12].
2.4. Role of the funding source
The funder of the study had no role in study design, data analysis, interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
The search strategy yielded 14,333 citations. After removing duplicate citations, 5908 unique titles and abstracts were screened, and 628 full-text articles were assessed for eligibility. Fourteen RCTs were eligible for inclusion [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], including seven cluster RCTs [19,20,22,25,29,32,33] (Fig. 1).
Fig. 1.
Study selection.
All RCTs were conducted in sub-Saharan Africa except one in the United States [30]. Across all RCTs, populations studied included male partners of antenatal women (4 RCTs) [19,20,23,28], partners of people with HIV or ART clients (2 RCTs) [20,21], male truck drivers (2 RCTs) [26,27], clients in the outpatient or emergency department (3 RCTs) [22,30,31], and individuals approached in the community or households (4 RCTs) [24,25,29,32] (Table 2). Two RCTs focused exclusively on young people (age range: 16–24 years) [29,31] and six RCTs focused on reaching men through direct or secondary HIVST distribution [19,20,23,[26], [27], [28]]. Included RCTs provided between one and five oral fluid-based HIVST kits free of cost to participants.
Table 2.
Characteristics of included studies.
| Study | Country | Total sample (randomized) | Study population | Control arm | Intervention(s) / support tools | HIVST distribution method |
|---|---|---|---|---|---|---|
| Choko 2019a [19]* | Malawi | 2349 (2349 women) | Male partners of antenatal women | An invitation letter given to women addressing their male partner and inviting them to seek HIV testing at facility | • Five intervention arms: HIVST only; HIVST + US$ 3 incentive; HIVST + US$ 10 incentive; HIVST + US$ 30 lottery; HIVST + phone reminder (incentive/reminder related to linkage after HIVST) • a clinic invitation letter given to women addressing their male partner in all arms |
Secondary distribution by antenatal women to their male partner |
| Choko 2019b [20]* | Malawi | 5136 (4428 ANC women, 708 index clients) | Partner(s) of antenatal women and HIV-positive (index) clients | Invitation letter given to antenatal women or HIV-positive index clients inviting their partner(s) to test at a facility | • HIVST kit and invitation letter given to antenatal women and HIV-positive clients inviting their partner(s) to attend the facility if reactive HIVST result in one arm • HIVST kit and invitation letter given to antenatal women and HIV-positive clients inviting their partner(s) to attend the facility irrespective of HIVST results in the other arm |
Secondary distribution by antenatal women to their male partner Secondary distribution by HIV-positive clients to their partner(s) |
| Dovel 2018 [22]* | Malawi | 5885 (2252 men, 3633 women) | Adult clients in 15 high-burden outpatient facilities | Standard facility-based HIV testing | • HIVST distributed for use in outpatient waiting spaces with private spaces for results interpretation • Pre-test information and HIVST demonstration in group setting; optional post-test counselling |
HIVST distribution for use at facilities |
| Dovel 2019 [21] | Malawi | 484 (113 men, 371 women) | Partners of adult ART clients attending facilities | Referral slips given to ART clients for their partners | • HIVST kits to given to HIV-positive clients for their partners along with referral slips for confirmatory testing at a facility • In-person demonstration to ART (index) client, locally tailored instructions, and information about testing facilities |
Secondary distribution by ART clients to their partner(s) |
| Gichangi 2018 [23] | Kenya | 1410 (1410 women) | Male partners of antenatal women | Women given a standard card inviting male partners to attend a facility for general health check of the family (not HIV-specific) | • Two HIVST kits given to women for distribution to their male partner along with standard invitation card • Counselling on strategies to introduce HIVST kit to their male partner • Brief orientation on HIVST kit use |
Secondary distribution by antenatal women to their male partner |
| Indravudh 2018 [24]* | Malawi | 3457 | Men and women (>15 years) in rural and peri-urban areas | Standard facility-based HIV testing | • Door-to-door distribution of HIVST kits by resident community-based distributors with continuous access to HIVST as needed • In-person demonstration • Post-test support and provider-assisted referral for confirmatory testing or ART services |
Home-based (door-to-door) HIVST distribution |
| Indravudh 2019 [25]* | Malawi | 7880 | Men and women (>15 years) in rural communities | Standard facility-based HIV testing | • HIVST kits distributed during a 7-day community-led campaign • Community volunteers distributed kits, provided in-person demonstration and supported linkage |
Community-based distribution (campaign-based) |
| Kelvin 2018 [27] | Kenya | 305 (305 men) | Male truck drivers | Standard facility-based HIV testing |
|
HIVST distribution for use at facilities |
| Kelvin 2019 [26] | Kenya | 2262 (2262 men) | Male truck drivers | One SMS inviting participants to attend a facility for HIV testing |
|
HIVST distribution for use at facilities |
| Masters 2016 [28] | Kenya | 600 (600 women) | Male partners of antenatal women | Women given an invitation card for male partner inviting them to attend a facility for HIV testing | • Two HIVST kits for distribution to male partner • HIVST demonstration to antenatal women |
Secondary distribution by antenatal women to male partner |
| Nichols 2019 [29]* | Zambia | Not reported | Men and women aged 16–24 years | Standard facility-based HIV testing | • HIVST distribution during community campaigns • Pre-test information and post-test counselling • Optional HIVST demonstration and assistance with results interpretation • Linkage to care for those tested positive through either self-referral card only (first half of trial) or self-referral card with optional patient escort to a health facility (second half of trial) |
Community-based distribution (campaign-based) |
| Patel 2018 [30] | USA | 100 (34 men, 66 women) | Adult patients (≥18 years) who declined HIV testing during emergency department visit | Information pamphlet on importance of HIV testing and list of local facilities for HIV testing | • HIVST kits given to participants in emergency department with instructions on performing the test and interpreting results • Optional results reporting via website • Five referral cards for peers to request HIVST kits |
Facility-based distribution |
| Pettifor 2018 [31] | South Africa | 284 (284 women) | Young women (18–24 years) | Five invitations to attend a facility for standard HIV testing | • Choice of free facility-based HIV testing or HIVST kits • Five HIVST kits (one for personal use and four for distribution to peers or partners |
|
| Tsamwa 2018 [32]* | Zambia | 5005 | Men and women aged ≥16 years living in eligible households | Standard facility-based HIV testing | • Door-to-door HIVST distribution + distribution from community distributors’ home + facility-based distribution • In-person demonstration, home visits and referral advice • Financial incentive for distributors for distributing kits (US$ 0.6/kit) and returning used kits (0.3/kit) |
• Community-based distribution • Home-based HIVST • Facility-based distribution |
HIVST: HIV self-testing; USA: United States of America.
Cluster RCTs
All studies were determined to be at high risk of bias overall. The certainty of evidence for outcomes overall ranged from very low to moderate. See supplementary material S2 for full risk of bias assessment and GRADE tables.
Several HIVST service delivery models and support tools were used in included RCTs. Delivery models included community-based or home-based (door-to-door) distribution and facility-based distribution for HIVST use within those facilities, for later use, or for secondary distribution to partners (Table 3). Most studies provided some support to participants for self-testing in addition to the manufacturer instructions for use or hotline [[19], [20], [21], [22], [23],[25], [26], [27], [28], [29],32,33]. The type of support provided varied and included enhancement of kit instructions for use (translations or adaptations), in-person, one-on-one or group demonstration, and in-person observation or supervision (Table 4).
Table 3.
Classification of HIVST service delivery and distribution models.
| Distribution method | Description | Studies |
|---|---|---|
| Community-based HIVST distribution | HIVST kits distributed by community distributors door-to-door or during community campaigns or events | Indravudh 2018, Indravudh 2019, Nichols 2019, Tsamwa 2018 * [24,25,29,32] |
| Facility-based HIVST distribution | HIVST kits distributed to clients at a facility or study clinic | Patel 2018, Pettifor 2018⁎⁎ [30,31] |
| HIVST use at facilities | HIVST kits distributed in facilities for use at those facilities (optional give-away for home use in two studies) | Dovel 2018, Kelvin 2018, Kelvin 2019 [22,26,27] |
| Secondary distribution by women to male partners | HIVST kits distributed to antenatal women to give to their male partners | Choko 2019a, Choko 2019b, Gichangi 2018, Masters 2016 [19,20,23,28] |
| Secondary distribution by HIV-positive clients to partners | HIVST kits distributed to HIV-positive or ART clients to give to their partners | Choko 2019b, Dovel 2019 [20,21] |
ART: antiretroviral therapy; HIVST: HIV self-testing.
facility and community-based HIV distribution but most kits distributed in the community
also included kits for peer distribution, only relevant outcomes for trial participants reported
Table 4.
Classification of HIVST support tools.
| Category | Description | Studies |
|---|---|---|
| No or basic support | Standard or manufacturer provided instructions for use Manufacturer hotline or customer support No additional support |
Patel 2018, Pettifor 2018 [30,31] |
| Instructions for use enhancement |
Tailored, translated or pictorial instructions designed specifically for the study Hotline or phone support provided by the study |
Choko 2019a, Choko 2019b, Dovel 2019, Gichangi 2018, Masters 2016 [[19], [20], [21],23,28] |
| In-person demonstration | One-on-one self-test use demonstration or training provided to participants by the study staff Does not include observing or supervising participants during self-testing |
Indravudh 2018, Indravudh 2019, Nichols 2019, Tsamwa 2018 [24,25,29,32] |
| Group demonstration | Demonstration of self-test use by the study staff in a group setting Does not include observing or supervising participants during self-testing |
Dovel 2018 [22] |
| In-person observation or supervision | Observation or supervision by study staff during self-testing Staff can intervene if requested or in case of errors |
Kelvin 2018, Kelvin 2019 [26,27] |
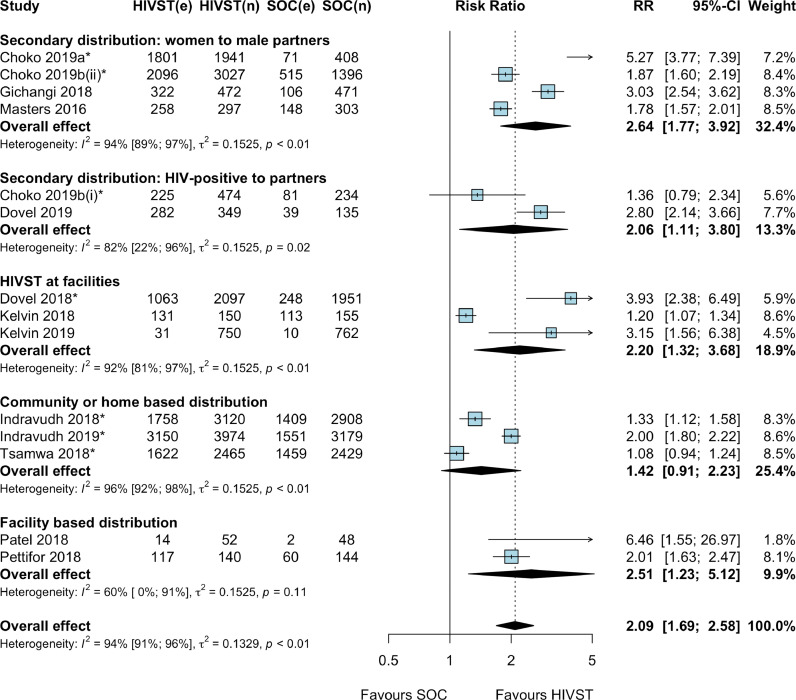
Thirteen RCTs (14 comparisons due to one multi-arm RCT) reported uptake of HIV testing with HIVST compared to SoC [[19], [20], [21], [22], [23],[25], [26], [27], [28],[30], [31], [32], [33]]. Meta-analysis showed that HIVST doubled HIV testing uptake compared to SoC (RR: 2.09, 95%CI: 1.69–2.58; p < 0.0001; I2: 94%; 13 RCTs; low certainty evidence; Fig. 2). A subgroup meta-analysis showed that HIVST more than doubled HIV testing uptake among men (RR: 2.40, 95%CI: 1.84–3.13; p < 0.0001; I2: 93%; 10 RCTs; low certainty evidence) [[19], [20], [21], [22], [23], [25], [26], [27], [28],33], women (RR: 2.05, 95%CI: 1.21–3.48; p < 0.0001; I2: 93%; 3 RCTs; low certainty evidence) [22,31,33] and young people aged 16–24 years (RR: 2.43, 95%CI: 1.65–3.57; p < 0.0001; I2: 92%; 4 RCTs; low certainty evidence) [22,25,31,33] compared to SoC (see supplementary material S3).
Fig. 2.
Uptake of HIV testing by service delivery model, HIV self-testing compared to standard facility-based HIV testing * Cluster RCTs. Note: Choko 2019b (i): HIVST distribution by HIV-positive (index) clients to their partners; Choko 2019b (ii): HIVST distribution by antenatal women to their male partners. Abbreviations: CI: confidence interval; e: number of events; HIVST: HIV self-testing; n: denominator; RR: risk ratio; SOC: standard of care.
Subgroup meta-analysis by HIVST service delivery model showed that HIVST increased HIV testing uptake compared to SoC with: (i) secondary HIVST distribution by antenatal women to male partner (RR: 2.64, 95% CI: 1.77–3.92; p < 0.0001; I2: 94%; 4 RCTs; moderate-certainty evidence) [19,20,23,28]; (ii) secondary HIVST distribution by HIV-positive or ART clients to partners (RR: 2.06, 95% CI: 1.11–3.80; p = 0.02; I2: 82%; 2 RCTs; low certainty evidence) [20,21]; (iii) HIVST distribution for use within facilities (RR: 2.20, 95% CI: 1.32–3.68; p < 0.01; I2: 92%; 3 RCTs; moderate certainty evidence) [22,26,27]; and (iv) facility-based HIVST distribution for later use (RR: 2.51, 95% CI: 1.23–5.12; p = 0.01; I2: 60%; 2 RCTs; low certainty evidence); [30,31]. HIV testing uptake also increased with (v) community- or home-based HIVST distribution (RR: 1.42, 95% CI: 0.91–2.23; p = 0.17; I2: 96%; 3 RCTs; low certainty evidence), the 95% CIs cross 1 so it might have little or no effect (see supplementary material S3) [25,32,33].
Subgroup meta-analyses by type of support offered to participants for self-testing showed that HIVST increased HIV testing uptake compared to SoC with: (i) no or basic support (RR: 2.45, 95% CI: 1.27–4.75; p = 0.01; I2: 60%; 2 RCTs; low certainty evidence); [30,31] and (ii) instructions for use enhancement (RR: 2.45, 95% CI: 1.81–3.33; p < 0.0001; I2: 92%; 5 RCTs; moderate certainty evidence) [[19], [20], [21],23,28]. HIV testing uptake also increased with (iii) in-person demonstration or training (RR: 1.43, 95% CI: 0.95–2.15; p < 0.15; I2: 96%; 3 RCTs; low certainty evidence) [25,32,33] and (iv) in-person observation or supervision (RR: 1.66, 95% CI: 0.93–2.95; p = 0.11; I2: 86%; 2 RCTs; moderate certainty evidence) [26,27], the 95% CIs cross 1 so it might have little or no effect (see supplementary material S3). One study that offered group pre-test information and HIVST demonstration in outpatient waiting rooms showed an increase in HIV testing uptake (RR: 3.93, 95% CI: 1.66–9.27; p < 0.0001; very low certainty evidence) [22].
Eight RCTs (9 comparisons due to one multi-arm RCT) reported the outcome of HIV positivity with HIVST compared to SoC among those tested [[19], [20], [21], [22],[26], [27], [28], [29]]. Meta-analysis showed that there was no difference in HIV positivity with HIVST compared to SoC (RR: 0.81, 95% CI: 0.45–1.47; p = 0.50; I2: 38%; 8 RCTs; moderate certainty evidence; Fig. 3).
Fig. 3.
HIV positivity among those tested, HIV self-testing compared to standard facility-based HIV testing *cluster RCTs. Note: Choko 2019b (i): HIVST distribution by HIV-positive (index) clients to their partners; Choko 2019b (ii): HIVST distribution by antenatal women to their male partners. Abbreviations: CI: confidence interval; e: number of events; HIVST: HIV self-testing; n: denominator; RR: risk ratio; SOC: standard of care.
Three RCTs reported on linkage to additional or confirmatory HIV testing after receiving reactive HIVST results in the HIVST arm. Two RCTs reported that 20–25% of those with reactive HIVST results sought confirmatory testing within four weeks to three months [21,28]. Another RCT reported that of 27 participants who had reactive HIVST results when they used self-tests within facilities and did not already know their HIV-positive status, the “majority” received confirmatory testing on the same day [22].
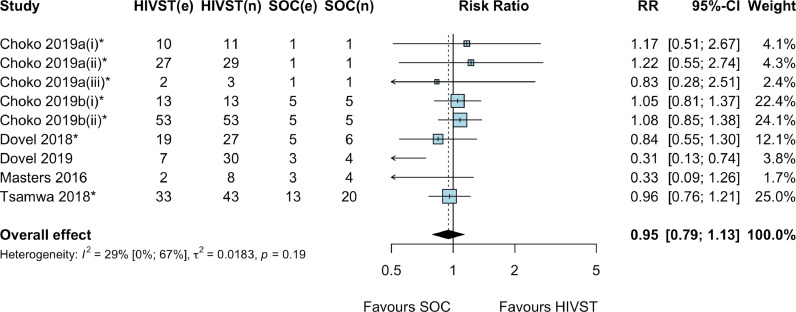
Six RCTs (9 comparisons due to two multi-arm RCTs) reported on linkage to HIV care or treatment among those diagnosed in the HIVST arm compared to SoC [[19], [20], [21], [22],28,32]. Meta-analysis showed that there was no difference in linkage to care or treatment with HIVST among those diagnosed compared to SoC (RR: 0.95, 95% CI: 0.79–1.13; p = 0.52; I2: 29%; 6 RCTs; moderate certainty evidence; Fig. 4). Sub-group meta-analysis by type of linkage support showed similar results whether no linkage support with HIVST was offered (RR: 0.79, 95% CI: 0.54–1.15; p = 0.19; I2: 59%; 5 RCTs; moderate certainty evidence) [[19], [20], [21], [22],28] or financial incentives were offered (RR: 1.12, 95% CI: 0.67–1.88; p = 0.68; I2: 0%; 2 RCTs; moderate certainty evidence) (see supplementary material S3) [19,20]. A study that offered a phone call reminder for linkage in one of the HIVST arms reported little or no effect on linkage to care or treatment compared to SoC (RR: 0.83, 95% CI: 0.28–2.51; p = 0.21; very low certainty evidence) [19]. Another study that offered home visits or in-person referral by community HIVST distributors to support linkage also showed little or no effect on linkage to care or treatment compared to SoC (RR: 0.96, 95% CI: 0.52–1.76; p = 0.90; low certainty evidence) [32].
Fig. 4.
Linkage to HIV care or treatment among those diagnosed, HIV self-testing compared to standard facility-based HIV testing, by type of linkage support *cluster RCTs. Note: Choko 2019a(i): HIVST alone; Choko 2019a(ii): HIVST + linkage provider financial incentive arms; Choko 2019a(iii): HIVST + phone reminder arm. Choko 2019b (i): HIVST distribution by HIV-positive (index) clients to their partners; Choko 2019b(ii): HIVST distribution by ANC women to their male partners. Abbreviations: CI: confidence interval; e: number of events; HIVST: HIV self-testing; n: denominator; RR: risk ratio; SOC: standard of care.
Meta-analysis of four RCTs showed no difference in occurrence of social harms or adverse events with HIVST compared to SoC, however certainty of evidence for this outcome was very low (RR: 2.52: 95%CI: 0.52–12.13; p = 0.25; I2: 0%; very low-certainty evidence; Fig. 5). All four RCTs involved secondary distribution of HIVST kits and reported adverse events included two instances of verbal abuse [21], three temporary self-resolving separations (resolved within 3 days) in one study [19], and four temporary self-resolving separations in another study [20]. An additional RCT not included in the meta-analysis reported three adverse events (one mild, two severe, details not provided) among 2581 participants in the HIVST arm [24]. Another RCT reported no instances of coercion to test or to disclose results among 1063 participants who performed HIVST at facilities. In the same study, 2.6% (29/1124) of participants reported being coerced to test in the SoC and optimized SoC arms [22]. No suicide was reported across any included RCT.
Fig. 5.
Social harms or adverse events among randomized participants, HIVST compared to SoC Abbreviations: CI: confidence interval; e: number of events; HIVST: HIV self-testing; n: denominator; RR: risk ratio; SOC: Standard of care.
4. Discussion
This systematic review showed that HIVST doubled the uptake of HIV testing in the general population compared to SoC. Nearly all the studies (12 out of 13) were conducted in sub-Saharan Africa. Testing uptake increased with a range of HIVST delivery models and support tools. HIV positivity and linkage to care rates in the HIVST arms were similar to those of SoC. HIVST appears to be safe and social harms or adverse events were rare. In some instances, harm, such as relationship breakdown between serodiscordant couples, was temporary.
Our finding of increased testing uptake with HIVST in the general population strengthens evidence synthesized in a previous systematic review that informed the 2016 WHO HIVST recommendation [3]. In our review, testing uptake doubled with HIVST overall and results were consistent across included RCTs. Only one of 13 RCTs showed an increase in testing uptake that was non-significant [32]. This RCT involved community-based HIVST distribution in Zambia, a setting with generally high HIV testing and treatment coverage and large “test and treat” trials [34,35]. Qualitative research has explored the drivers of high acceptability of HIVST and shows that users value the convenient, private and confidential nature of HIVST and potential for addressing stigma and reducing opportunity costs related to visiting health-care facilities [36], [37], [38]. Many users prefer the ease of use and painless nature of oral fluid-based HIVST kits, while others perceive blood-based kits to be more accurate. All studies included in this review used oral fluid-based HIVST kits provided free of cost to participants. To date, WHO has prequalified four HIVST kits, including three blood-based kits [39,40]. Programmatically, countries are increasingly procuring a mix of oral fluid- and blood-based HIVST kits to offer choice to users and ensure supplier diversity. It is also important that HIVST kits are affordable for users, whether offered through country programmes or in the private sector, to achieve and maintain its benefits.
A range of HIVST service delivery models were used across included studies. Our review showed that these models can be effective in increasing testing uptake, such as community-based distribution, facility-based distribution and secondary distribution models. Other promising HIVST delivery models for reaching priority populations in high HIV burden settings in east and southern Africa, such as men, have been assessed in observational and pilot studies. These include offering HIVST through workplaces [41], [42], [43] and faith-based organizations [44,45]. HIVST can also expand options for partner services for individuals diagnosed with HIV, particularly in instances where provider-assisted referral (assisted partner notification or index testing) is not feasible or acceptable and in the context of multiple partners [46]. In ANC settings, HIVST can be used to support retesting among pregnant women in high HIV burden settings or women at ongoing risk who need to test more frequently [46]. Overall, these results should encourage countries and programmes to introduce or scale up HIVST, with regular review of programme outcomes to inform adjustment and optimization of service delivery models.
Testing uptake increased when either basic support (manufacturer-provided instructions) or a range of additional support options were used, ranging from tailored instructions for use to in-person demonstration, training or supervision. It is important to consider the feasibility and sustainability of support options when introducing or scaling up HIVST. For example, in-person support options are likely to be costly, human resource-intensive and less scalable, thus may be considered as a time-limited option or for specific population groups [47]. New innovative support tools, including digital platforms, video instructions and mobile-based applications, may be more sustainable and acceptable to some populations, such as young people, and can be considered [46].
An important contribution of this review is the finding that the proportion linked to treatment or care among those diagnosed HIV-positive in the HIVST arms was similar to SoC, irrespective of whether any support for linkage was provided or not. Overall, a larger number of individuals were diagnosed and linked to care with HIVST compared to SoC. There is some evidence from RCTs that HIVST and a linkage intervention, such as in-person referral or navigation [29], home antiretroviral therapy (ART) initiation [48] or fixed and conditional provider financial incentive (compared to only conditional financial incentive) [49], may improve linkage compared to HIVST alone. Programmes can adapt these or other interventions to support linkage; however, it is important to consider resource needs and sustainability as some of these interventions may be costly. To date, RCTs have not focused on continuity of care or viral suppression outcomes after HIVST, which require a larger sample size and longer follow up, as well as linkage to prevention services among those who are HIV-negative and at ongoing risk. These outcomes should be addressed through programmatic implementation and data.
Misuse of HIVST kits, social harm or adverse events with HIVST were rare in the included RCTs. Adverse events, when reported, were often temporary such as relationship breakdown, and resolved within days [20]. No self-harm or suicide was reported in any of the RCTs. Data from observational studies and implementation research support these findings. For example, in the HIV Self-Testing Africa (STAR) Initiative, 25 serious harms were reported from 10 self-testers following distribution of 175,683 HIVST kits over six years in Malawi [50]. A cluster RCT of community-based HIVST distribution in Zambia reported nine adverse events among 13,267 participants and that social harms were exacerbated by pre-existing issues within couples, such as alcohol abuse and history of gender-based violence [51]. The initial reactions of fear and anxiety after a reactive result are not unique to HIVST, but also relate to HIV testing through other standard approaches. The immediate negative reactions to an HIV-positive diagnosis may change over time as individuals start to realize the benefits of treatment for themselves and their partners. Despite serious harms being rarely reported in studies, it is important to disseminate appropriate information and messages in the communities to mitigate them, and include appropriate monitoring systems in HIVST programmes.
Providing choice and options for service delivery models and support tools in HIVST programmes can address the needs of diverse populations groups and individual preferences [9,46]. Communities, including community-based organizations, civil society and networks of people with HIV, need to be effectively engaged when selecting and adapting HIVST models and support tools for implementation and scale up. Community-led HIV testing and HIVST models have been shown to be feasible in Viet Nam [52], Uganda [53] and Malawi [25], and effective in reaching first-time testers and identifying undiagnosed HIV infections. Such models can be adapted in other settings. Trained peers can also distribute HIVST kits and support linkage [46]. Community members and peers can also play an important role in raising awareness in the communities on appropriate use of HIVST kits, steps to take after using HIVST and prevent misuse and harm.
Globally, HIVST country policy adoption has been rapid; however, implementation and large-scale procurement has lagged behind [7]. Many countries and programmes have hesitated in introducing HIVST due to concerns around linkage and social harm [54]. This review shows that HIVST appears to be a safe intervention and consistently effective in identifying additional people with HIV with a range of service delivery and support models and linking them to care with similar success as SoC. Further, successful implementation experiences suggest that countries should not delay introducing or scaling up HIVST programmes. In-country policy and regulatory barriers hindering the availability of HIVST kits and their scale up need to be addressed, such as by adopting accelerated product registration processes (e.g. WHO collaborative registration procedure [55]). In the current context of public health responses to the COVID-19 pandemic [56], where physical distancing measures may limit access to standard testing, HIVST can be an important tool to ensure continued access to HIV testing [57], [58], [59]. Countries without HIVST are likely to miss out on these benefits, potentially slowing progress towards achieving national and global HIV goals. Communities and stakeholders should advocate for faster and wider availability of HIVST.
This review updated a previous systematic review and synthesized a large body of evidence on HIVST in the general population using a comprehensive validated search strategy. This review addresses key gaps in the literature on implementation models and linkage rates with HIVST. There are limitations that need to be considered when interpreting the findings. First, using the GRADE framework, the certainty of evidence for review outcomes was determined to be very low to moderate. A key reason was downgrading of the certainty of evidence for risk of bias, often due to lack of blinding of participants and personnel (performance bias) and/or lack of blinding of outcome assessment (detection bias). Due to the nature of the intervention, that is, distribution of HIVST kits, blinding of participants or distributors allocated to the study arm is not possible and may introduce performance bias. The outcomes assessment often relied on self-reporting, thus it was subject to detection bias. For subgroup analyses, certainty of evidence was often downgraded due to imprecision and wide confidence intervals. Second, reporting bias could be assessed only for the uptake of HIV testing meta-analysis, which had more than 10 studies. A funnel plot for uptake showed a benefit from all studies, including smaller studies. In our view, this likely represents the true effect of the intervention and the fact that meta-analysis included only RCTs, which were sufficiently powered for this outcome. These findings of increased uptake with HIVST is similar for key populations [10]. Third, several RCTs were available only as conference abstracts; thus the complete information needed for all risk of bias assessment domains was not available. Fourth, a small number of studies contributed to subgroup analyses, suggesting caution in interpreting the results and limiting generalizability. Fifth, RCTs included in this review were not powered for assessing HIV positivity or linkage outcomes; however, findings for these outcomes were consistent across included studies. We also noted some variation in definition and measurement (self-reported vs. validated) of positivity and linkage outcomes across studies. Sixth, we used an operational definition for the general population as any population other than key populations. In fact, programmes have often focused on certain priority populations depending on epidemic, testing gaps or limited available commodities (for example, truck drivers, fisher-folk, migrants and partners of people with HIV). HIVST may not be a suitable approach for everyone and should be used strategically to complement existing services. Thus, our definition does not necessarily reflect how HIVST will be used in programmes. Last, nearly all studies were conducted in sub-Saharan Africa, limiting generalisability to other settings. However, most high HIV burden countries with general population-focused HIV programmes are in this region.
In this systematic review, HIVST was found to be safe and effective in increasing HIV testing uptake among the general population in sub-Saharan Africa with a range of service delivery models and support tools. HIV positivity and linkage rates with HIVST were comparable to that with SoC, and HIVST can identify additional people with HIV and link them to care. These findings support wider availability of HIVST to reach those who may not otherwise access HIV testing. Communities need to be meaningfully engaged when selecting and adapting HIVST models that should be regularly reviewed and adapted to optimize impact. Based on this review, and additional information reviewed at an expert meeting, WHO guidance on HIVST was updated to recommend that HIVST be offered as an HIV testing services approach [46].
Declaration of Competing Interest
Dr. Corbett reports grants from London School of Hygiene & Tropical Medicine, outside the submitted work. Dr. Geng reports a Viiv Healthcare research grant. Ms. Johnson reports grants from the Bill and Melinda Gates Foundation, Unitaid, and the United States Agency for International Development, during the conduct of the study; grants from the Bill and Melinda Gates Foundation, Unitaid, and the United States Agency for International Development outside the submitted work. Dr. Witzel, Dr. Rodger and Dr. Weatherburn report grants from the National Instituet of Health Research during the conduct of the study. All other authors have nothing to declare.
Acknowledgments
Contributors
CJ, MSJ and TCW designed the study. CF, IEW, MSJ and TCW screened abstracts and manuscripts and extracted data. IEW and EG analysed the data. All authors contributed to interpretation of results. MSJ wrote the first draft of the manuscript. All authors reviewed and commented on manuscript drafts and approved the final manuscript.
Funding
This review was funded by the Bill and Melinda Gates Foundation (OPP1177903), Unitaid (PO#10140–0-600 and PO#8477–0-600) and the United States Agency for International Development (US-2015-0839 and US-2016-940). TCW, AJR, PW received grants during the conduct of the study (National Institute for Health Research Programme Grants for Applied Research Programme [PG-482 1212-20006]). EC (London School of Hygiene & Tropical Medicine), EG (ViiV Healthcare research grant) and CJ (the Bill and Melinda Gates Foundation, Unitaid, and the United States Agency for International Development) received grants outside of submitted work. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Data sharing statement
See supplementary material S4 for systematic review data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100991.
Appendix. Supplementary materials
References
- 1.Joint United Nations Programme on HIV/AIDS; Geneva: 2019. Global AIDS update 2019 — communities at the centre. [Google Scholar]
- 2.World Health Organization; Geneva: 2019. Consolidated guidelines on HIV testing services 2019. [PubMed] [Google Scholar]
- 3.Johnson C.C., Kennedy C., Fonner V. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. 2017;20(1):21594. doi: 10.7448/IAS.20.1.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization; Geneva: 2016. Guidelines on HIV self-testing and partner notification: a supplement to the consolidated guidelines on HIV testing services. [PubMed] [Google Scholar]
- 5.Joint United Nations Programme for HIV/AIDS; Geneva: 2017. Ending AIDS: progress towards the 90–90–90 targets. [Google Scholar]
- 6.2025 AIDS targets. 2020. https://aidstargets2025.unaids.org/#:~:text=The%202025%20targets%20prioritize%20sexual,to%20life%2Dsaving%20treatment%20services. (accessed December 31, 2020.
- 7.Global AIDS monitoring 2019. Geneva: Joint United Nations Progarmme on HIV/AIDS, 2019.
- 8.World Health Organization, UNITAID; Geneva: 2018. Market and technology landscape: HIV rapid diagnostic tests for self-testing: fourth edition. [Google Scholar]
- 9.Kelvin E.A., Akasreku B. The evidence for HIV self-testing to increase HIV testing rates and the implementation challenges that remain. Curr HIV/AIDS Rep. 2020;17(4):281–289. doi: 10.1007/s11904-020-00504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witzel T.C., Eshun-Wilson I., Jamil M.S. Comparing the effects of HIV self-testing to standard HIV testing for key populations: a systematic review and meta-analysis. BMC Med. 2020;18(1):381. doi: 10.1186/s12916-020-01835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization; Geneva: 2014. Handbook for guideline development-2nd edition. [Google Scholar]
- 12.Higgins J., Green S., editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane; 2011. [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization; 2016. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva. [PubMed] [Google Scholar]
- 15.Witzel T.C., Weatherburn P., Burns F.M., Johnson C.C., Figueroa C., Rodger AJ. Consolidating emerging evidence surrounding HIVST and HIVSS: a rapid systematic mapping protocol. Syst Rev. 2017;6(1):72. doi: 10.1186/s13643-017-0452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. A language and environment for statistical computing. [Google Scholar]
- 17.World Health Organization; Geneva: 2016. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations: 2016 update. [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Altman D.G., Gøtzsche P.C. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choko A.T., Corbett E.L., Stallard N. HIV self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: an adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med. 2019;16(1) doi: 10.1371/journal.pmed.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choko A.T., Neuman M., Fielding K. Reaching partners of antenatal and index HIV-positive patients in Malawi: a pragmatic cluster randomized trial evaluating uptake, yield, and accuracy of secondary distribution of HIV self-test kits. Proceedings of the AIDS impact 14th international conference; London; 2019. [Google Scholar]
- 21.Dovel K., Balakasi K., Shaba F. CROI. Seattle; Washington: 2019. A randomized trial on index HIV self-testing for partners of art clients in Malawi. [Google Scholar]
- 22.Dovel K., Nyirenda M., Shaba F. Facility-based HIV self-testing for outpatients dramatically increases HIV testing in Malawi: a cluster randomized trial. Proceedings of the 22nd international AIDS conference; 2018 23-27 July; Amsterdam, the Netherlands; 2018. [Google Scholar]
- 23.Gichangi A., Wambua J., Mutwiwa S. Impact of HIV self-test distribution to male partners of ANC clients: results of a randomized controlled trial in Kenya. J Acquir Immune Defic Syndr. 2018;79(4):467–473. doi: 10.1097/QAI.0000000000001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Indravudh P., Fielding K., Neuman M. Increasing knowledge of HIV status and demand for ART using community-based HIV self-testing in rural communities: a cluster randomised trial in Malawi (THPDC0103). Proceedings of the 22nd international AIDS conference; Amsterdam, the Netherlands; 2018. [Google Scholar]
- 25.Indravudh P.P., Fielding K., Kumwenda M.K. Community-led delivery of HIV self-testing to improve HIV testing, ART initiation and broader social outcomes in rural Malawi: study protocol for a cluster-randomised trial. BMC Infect Dis. 2019;19(1):814. doi: 10.1186/s12879-019-4430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelvin E.A., George G., Kinyanjui S. Announcing the availability of oral HIV self-test kits via text message to increase HIV testing among hard-to-reach truckers in Kenya: a randomized controlled trial. BMC Public Health. 2019;19(1) doi: 10.1186/s12889-018-6345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelvin E.A., George G., Mwai E. Offering self-administered oral HIV testing to truck drivers in Kenya to increase testing: a randomized controlled trial. AIDS Care. 2018;30(1):47–55. doi: 10.1080/09540121.2017.1360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masters S.H., Agot K., Obonyo B., Napierala M.S, Maman S., Thirumurthy H. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med. 2016;13(11) doi: 10.1371/journal.pmed.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols B., Cele R., Chasela C. Cost and impact of community-based, assisted HIV self-testing amongst youth in Zambia. Proceedings of the 26th conference on retroviruses and opportunistic infections (CROI); Seattle, Washington; 2019. [Google Scholar]
- 30.Patel A.V., Abrams S.M., Gaydos C.A. Increasing HIV testing engagement through provision of home HIV self-testing kits for patients who decline testing in the emergency department: a pilot randomisation study. Sex Transm Infect. 2018;95(5):358–360. doi: 10.1136/sextrans-2018-053592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettifor A., Kahn K., Kimaru L. Proceedings of the 25th conference on retroviruses and opportunistic infections (CROI) 2018. HIV self-testing increases testing in young South African women: results of an RCT. [Google Scholar]
- 32.Tsamwa D., Handima N., Sigande L. AIDS Res Human Retroviruses; Madrid: 2018. Does community distribution of HIV self-test kits increase uptake of HIV testing at population level? results of a cluster-randomised trial in Zambia. [Google Scholar]
- 33.Indavudh P., Choko A., Corbett E. Scaling up HIV self-testing in sub-Saharan Africa: a review of technology, policy and evidence. Curr Opin HIV Res. 2018;31(1) doi: 10.1097/QCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes R.J., Donnell D., Floyd S. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART) N Engl J Med. 2019;381(3):207–218. doi: 10.1056/NEJMoa1814556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joint United Nations Progarmme on HIV/AIDS; Geneva: 2020. UNAIDS Data 2020. [Google Scholar]
- 36.Figueroa C., Johnson C., Verster A., Baggaley R. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS Behav. 2015;19(11):1949–1965. doi: 10.1007/s10461-015-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Njau B., Covin C., Lisasi E. A systematic review of qualitative evidence on factors enabling and deterring uptake of HIV self-testing in Africa. BMC Public Health. 2019;19(1):1289. doi: 10.1186/s12889-019-7685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens D.R., Vrana C.J., Dlin R.E., Korte JE. A global review of HIV self-testing: themes and implications. AIDS Behav. 2018;22(2):497–512. doi: 10.1007/s10461-017-1707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization; Geneva: 2018. Overview of the prequalification of in vitro diagnostics assessment. [Google Scholar]
- 40.WHO list of prequalified in vitro diagnostic products. https://www.who.int/diagnostics_laboratory/evaluations/PQ_list/en/. 2021
- 41.World Health Organization and International Labour Organization; Geneva: 2018. HIV self-testing at the workplace: policy brief. [Google Scholar]
- 42.Hatzold K., Gudukeya S., Mutseta M. HIV self-testing: breaking the barriers to uptake of testing among men and adolescents in sub-Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. 2019;22(Suppl 1):e25244. doi: 10.1002/jia2.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebina L., Seatlholo N., Taruberekera N. Feasibility of community-based HIV self-screening in South Africa: a demonstration project. BMC Public Health. 2019;19(1):898. doi: 10.1186/s12889-019-7122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer R. Proceedings of the 20th international conference on AIDS and STIs in Africa. Kigali. 2019. Partnering with faith communities to deliver HIV targeted testing services to vulnerable populations: the Eastern Deanery AIDS Relief Program (EDARP) [Google Scholar]
- 45.Hills S. Proceedings of the 20th international conference on AIDS and STIs in Africa. Kigali. 2019. Faith and community Initiative: partnering with communities of faith to reach men and children living with HIV in 10 countries. [Google Scholar]
- 46.World Health Organization; Geneva: 2019. Consolidated guidelines on HIV testing services for a changing epidemic. [Google Scholar]
- 47.World Health Organization; Geneva: 2018. HIV self-testing strategic framework: a guide for planning, introducing and scaling up. [Google Scholar]
- 48.MacPherson P., Lalloo D.G., Webb E.L. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. JAMA. 2014;312(4):372–379. doi: 10.1001/jama.2014.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sibanda E., Neuman M., Tumushime M. Linkage to care after HIV self-testing in Zimbabwe: a cluster-randomised trial. Proceedings of the conference on opportunistic infections and retroviruses; Boston, USA; 2018. [Google Scholar]
- 50.Kumwenda M.K., Johnson C.C., Choko A.T. Exploring social harms during distribution of HIV self-testing kits using mixed-methods approaches in Malawi. J Int AIDS Soc. 2019;22(Suppl 1):e25251. doi: 10.1002/jia2.25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulubwa C., Hensen B., Phiri M.M. Community based distribution of oral HIV self-testing kits in Zambia: a cluster-randomised trial nested in four HPTN 071 (PopART) intervention communities. Lancet. 2019;6(2):e81–e92. doi: 10.1016/S2352-3018(18)30258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen V.T.T., Phan H.T., Kato M. Community-led HIV testing services including HIV self-testing and assisted partner notification services in Vietnam: lessons from a pilot study in a concentrated epidemic setting. J Int AIDS Soc. 2019;22(Suppl 3):e25301. doi: 10.1002/jia2.25301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collaboration S. Evaluating the feasibility and uptake of a community-led HIV testing and multi-disease health campaign in rural Uganda. J Int AIDS Soc. 2017;20(1):21514. doi: 10.7448/IAS.20.1.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choko A.T., Jamil M.S., MacPherson P. Measuring linkage to HIV treatment services following HIV self-testing in low-income settings. J Int AIDS Soc. 2020;23(6):e25548. doi: 10.1002/jia2.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collaborative procedure for accelerated registration. https://extranet.who.int/prequal/content/collaborative-procedure-accelerated-registration. 2021
- 56.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- 57.HIV/AIDS Q&A. https://www.who.int/news-room/q-a-detail/hiv-aids. 2021
- 58.World Health Organization and the United Nations Children's Fund; Geneva: 2020. Community-based health care, including outreach and campaigns, in the context of the COVID-19 pandemic. [Google Scholar]
- 59.World Health Organization; Geneva: 2020. COVID-19: Operational guidance for maintaining essential health services during an outbreak: interim guidance. 25 March 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.