Abstract
Background
This study evaluated the survival benefit of asparaginase (ASP)-based versus non-ASP-based chemotherapy combined with radiotherapy in a real-world cohort of patients with early-stage extranodal nasal-type natural killer/T-cell lymphoma (ENKTCL).
Patients and methods
We identified 376 patients who received combined radiotherapy with either ASP-based (ASP, platinum, and gemcitabine; n = 286) or non-ASP-based (platinum and gemcitabine; n = 90) regimens. The patients were stratified into low-, intermediate-, and high-risk groups using the early stage-adjusted nomogram-revised risk index. Overall survival (OS) and distant metastasis (DM)-free survival (DMFS) between the chemotherapy regimens were compared using inverse probability of treatment weighting (IPTW) and multivariable analyses.
Results
ASP-based (versus non-ASP-based) regimens significantly improved 5-year OS (84.5% versus 73.2%, P = 0.021) and DMFS (84.4% versus 74.5%, P = 0.014) for intermediate- and high-risk patients, but not for low-risk patients in the setting of radiotherapy. Moreover, ASP-based regimens decreased DM, with a 5-year cumulative DM rate of 14.9% for ASP-based regimens compared with 25.1% (P = 0.014) for non-ASP-based regimens. The survival benefit of ASP-based chemotherapy and radiotherapy remained consistent after adjusting the confounding variables using IPTW and multivariate analyses; additional sensitivity analyses confirmed these results.
Conclusions
The findings provided support for ASP-based chemotherapy and radiotherapy as a first-line treatment strategy for intermediate- and high-risk early-stage ENKTCL.
Key words: NK/T-cell lymphoma, asparaginase, chemotherapy, radiotherapy, survival outcome
Highlights
-
•
We explored the benefit of ASP-based chemotherapy in first-line combined-modality therapy of early-stage ENKTCL in a large real-world data set.
-
•
Current-era and most commonly used regimens were selected and compared.
-
•
Adding ASP into platinum and gemcitabine-based chemotherapy improved OS and DMFS in intermediate- and high-risk patients.
-
•
This is the first study to demonstrate clear associations of decreased DM and improved OS in early-stage ENKTCL patients.
Introduction
Extranodal nasal-type natural killer/T-cell lymphoma (ENKTCL) is rare worldwide, but is prevalent in East Asia and South America.1, 2, 3 ENKTCL mainly presents as early-stage disease in 65%-90% of patients and arises in the upper aerodigestive tract sites in ~90% of cases.1, 2, 3, 4 ENKTCL treatments have advanced with the induction of non-anthracycline-based chemotherapy and upfront radiotherapy over the past decade.5, 6, 7, 8, 9 The prognosis for patients with ENKTCL has improved, with 5-year overall survival (OS) of ~70% for early-stage disease and 10%-40% for advanced-stage disease.7, 8, 9, 10, 11, 12
Non-anthracycline-based versus anthracycline-based chemotherapy for patients with ENKTCL has improved long-term survival,8, 9, 10, 11 regardless of stage and risk subgroup.8 The most commonly used agents in non-anthracycline-based regimens include asparaginase (ASP; l-ASP or pegaspargase), platinum (PLA; oxaliplatin, cisplatin, or carboplatin), gemcitabine (GEM), methotrexate (MTX), and etoposide. Various non-anthracycline-based regimens have been considered as a first-line option for patients with ENKTCL.9,10,13, 14, 15, 16, 17, 18 For advanced-stage or refractory-relapsed patients, ASP-based regimens, such as SMILE (corticosteroid, MTX, ifosfamide, l-ASP, and etoposide),9,10,13 AspaMetDex (l-ASP, MTX, and dexamethasone),14 DDGP (GEM, pegaspargase, cisplatin, and dexamethasone),15, 16, 17 and LVDP (l-ASP, etoposide, dexamethasone and cisplatin)18 have improved patient survival outcomes.
For early-stage patients, radiotherapy is the mainstay of curative-intent combined-modality therapy (CMT)5, 6, 7,12,19 and is essential even after a complete response (CR) to ASP-based chemotherapy.20,21 Adding chemotherapy to radiotherapy provides survival benefit in intermediate- and high-risk early-stage patients,22 even in the modern chemotherapy era.23 In the setting of CMT, both ASP-based and non-ASP-based (mainly PLA-based) regimens are considered an option for early-stage patients.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Usually, ASP-based regimens, such as modified SMILE,13,24 GELOX (GEM, oxaliplatin, l-ASP),25, 26, 27, 28 P-GEMOX (pegaspargase, GEM, oxaliplatin),27,28 VIDL (etoposide, ifosfamide, cisplatin, l-ASP),29 LVP/D (l-ASP, vincristine, prednisone/dexamethasone),30, 31, 32 MESA (MTX, etoposide, dexamethasone, pegaspargase),33 and GDP-L/P (GEM, dexamethasone, cisplatin, l-ASP, or pegaspargase)16,34 are administrated subsequently with radiotherapy. In addition, less intense PLA-based regimens without inclusion of ASP, such as VIDP (etoposide, ifosfamide, cisplatin, dexamethasone),35,36 DeVIC (dexamethasone, etoposide, ifosfamide, carboplatin),11,37 and GDP (GEM, dexamethasone, cisplatin),38, 39, 40 administrated concurrently or subsequently with radiotherapy, yields favorable outcomes with tolerable toxicities. However, the use of ASP-based or non-ASP-based regimens remains limited to single-arm phase II trials or retrospective studies with small cohorts of patients.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 The optimal chemotherapy regimens remain to be identified, and it is unknown whether ASP is an essential component of non-anthracycline-based regimens in the first-line CMT for early-stage ENKTCL.
Acknowledging discrepancies in treatment between countries and limited high-quality clinical trial data, we designed a study to investigate the role of ASP-based chemotherapy and radiotherapy in early-stage ENKTCL using patient data from the China Lymphoma Collaborative Group (CLCG) database.
Patients and methods
Patient eligibility and study population
Data on patients with newly diagnosed ENKTCL between 2000 and 2016 from the CLCG database were retrospectively reviewed. The institutional review board of our institution approved the study and waived the requirement for informed consent, as patients were deidentified in the database. The eligible criteria included stage I-II patients who received combined radiotherapy and non-anthracycline-based chemotherapy. Patients who received either radiotherapy alone or chemotherapy alone were excluded. Because both ASP- and PLA/GEM-based regimens are most commonly used for early-stage ENKTCL in China,8 PLA/GEM-based regimens with (ASP-based) or without ASP (non-ASP-based) were selected in this study to reduce the heterogeneity of chemotherapy regimens (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100206). A total of 376 patients formed the study population. A CONSORT diagram describing the cohort selection is outlined in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100206.
Risk stratification and treatment
The patients were staged with the Ann Arbor system and stratified according to the nomogram-revised risk index (NRI).41,42 The early-stage-adjusted NRI (ES-NRI) includes five risk factors as follows: age >60 years, stage II, elevated lactate dehydrogenase, poor performance status [Eastern Cooperative Oncology Group (ECOG) score ≥2], and primary tumor invasion. Early-stage patients in the study were stratified into the low- (0), intermediate- (1-2), and high- (≥3) risk groups.
All patients received CMT. Bone marrow function, normal liver and kidney function, underlying cardiovascular diseases, and contraindications to chemotherapy drugs were evaluated according to each institutional guideline before treatment. Chemotherapy was administrated with ASP-based (ASP/PLA/GEM; n = 286) or non-ASP-based (PLA/GEM; n = 90) regimens (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100206). ASP-based regimens included GELOX [GEM (1000 mg/m2) on days 1 and 8, oxaliplatin (130 mg/m2) on day 1, l-ASP (6000 IU/m2) on days 1-7, every 3 weeks], P-GEMOX [GEM (800-1000 mg/m2) on days 1 and 8, oxaliplatin (100-130 mg/m2) on day 1, pegaspargase 2500 IU/m2 on day 1, every 3 weeks], GDP-L [GEM (1000 mg/m2) on days 1 and 8, cisplatin (25 mg/m2) on days 1-3, dexamethasone (20 mg) on days 1-3, l-ASP 6000 IU/m2 on days 1-7 or 200 IU/kg on days 1-10, every 3 weeks], and DDGP [GEM (800 mg/m2) on days 1 and 8, cisplatin (20 mg/m2) on days 1-4, pegaspargase (2500 IU/m2) on day 1, dexamethasone (15 mg/m2) on days 1-5, every 3 weeks]. Non-ASP-based regimens were defined as PLA/GEM-based regimens such as GDP [GEM (1000 mg/m2) on days 1 and 8, cisplatin (25 mg/m2) on days 1-3, dexamethasone (20 mg) on days 1-4 and 11-14, every 3 weeks], GEMOX [GEM (800-1000 mg/m2) on days 1 and 8, oxaliplatin (100-130 mg/m2) on day 1, every 3 weeks], and GP [GEM (1000 mg/m2) on days 1 and 8 and cisplatin (25 mg/m2) on days 1-3, every 3 weeks]. The median chemotherapy cycles were four. Involved-site radiation therapy was given with a median dose of 55 Gy (interquartile range 50-56 Gy).43
Endpoints
Primary endpoints included OS and distant metastasis (DM) free survival (DMFS). OS was defined as the period from the date of initial treatment to the date of any death. Progression-free survival (PFS) was defined as the period from the date of initial treatment to the date of locoregional and/or distant disease failure. DMFS was defined as the period from the date of initial treatment to the date of extranodal and/or distant lymphatic dissemination, and locoregional recurrence (LRR)-free survival (LRRFS) was defined as the period from the date of initial treatment to the date of disease failure in the primary site and/or regional lymph node.
Statistical analyses
The differences in clinical characteristics between ASP-based and non-ASP-based regimens were adjusted by inverse probability of treatment weighting (IPTW) method.44 IPTW aims to simulate a cohort in which treatments are randomly assigned to patients. The probability of being in the two groups (ASP-based and non-ASP-based regimens) was estimated from a logistic regression model that incorporated prognostic factors associated with the receipt of ASP-based regimen. Standardized mean difference was used to assess the balance of covariate distribution between treatment groups before and after weighting.
The categorical data and response rates between groups were compared with the chi-square test. Survival data before or after weighting were analyzed with the Kaplan–Meier method and compared with the log-rank test. Weighted Cox proportional hazards model was applied to calculate the IPTW-adjusted hazard ratio (HR).45 Additional sensitivity analyses without assumptions were conducted to assess the potential influence of unmeasured confounders on OS. The cumulative incidences of DM and LRR were investigated via competing risk analysis using the Gray’s test. Statistical analyses were performed using SPSS 26.0 (IBM, New York, NY) and the simPH, survival, cmprsk, and RISCA packages in R, version 4.0.2 (http://www.r-project.org/).
Results
Baseline characteristics and outcomes
The baseline clinical features for the entire cohort are summarized in Table 1. The majority of patients (89.6%) were ≤60 years old (median age 43 years; range 13-78 years). The male-to-female ratio was 2.42:1. Most patients showed good performance status (ECOG score 0-1, 97.3%) and upper aerodigestive tract site (96.5%). Elevated lactate dehydrogenase, primary tumor invasion, and stage II disease were present in 27.1%, 65.4%, and 44.1% of patients, respectively. According to the ES-NRI, the majority of patients (84.3%) were classified into intermediate- and high-risk groups, whereas 15.7% of patients were classified into the low-risk group.
Table 1.
Clinical characteristics of early-stage ENKTCL patients treated with CMT in unweighted and weighed population.
| Characteristic | All patients, n (%) | Unweighted population |
Weighted population (IPTW) |
||||
|---|---|---|---|---|---|---|---|
| ASP-based, n (%) | Non-ASP-based, n (%) | SMDa | ASP-based, % | Non-ASP-based, % | SMDa | ||
| All early-stage patients | 376 | 286 | 90 | ||||
| Sex, male | 266 (70.7) | 205 (71.7) | 61 (67.8) | 0.085 | 71.1 | 71.9 | 0.018 |
| Age, >60 years | 39 (10.4) | 34 (11.9) | 5 (5.6) | 0.226 | 10.5 | 10.2 | 0.008 |
| B symptoms | 172 (45.7) | 126 (44.1) | 46 (51.1) | 0.142 | 45.7 | 46.4 | 0.014 |
| ECOG score, ≥2 | 10 (2.7) | 7 (2.4) | 3 (3.3) | 0.053 | 2.6 | 2.2 | 0.026 |
| Stage II | 166 (44.1) | 132 (46.2) | 34 (37.8) | 0.170 | 44.5 | 48.0 | 0.072 |
| PTI | 246 (65.4) | 177 (61.9) | 69 (76.7) | 0.325 | 65.1 | 62.5 | 0.055 |
| Elevated LDH | 102 (27.1) | 69 (24.1) | 33 (36.7) | 0.275 | 26.6 | 23.7 | 0.066 |
| Primary UADT | 363 (96.5) | 275 (96.2) | 88 (97.8) | 0.095 | 96.5 | 95.1 | 0.067 |
| ES-NRI | |||||||
| Low-risk (0) | 59 (15.7) | 50 (17.5) | 9 (10.0) | 0.219 | 16.2 | 13.9 | 0.063 |
| Intermediate-risk (1-2) | 262 (69.7) | 196 (68.5) | 66 (73.3) | 0.106 | 69.2 | 70.6 | 0.029 |
| High-risk (≥3) | 55 (14.6) | 40 (14.0) | 15 (16.7) | 0.074 | 14.6 | 15.5 | 0.026 |
| Intermediate- and high-risk early-stage | 317 | 236 | 81 | ||||
| Sex, male | 222 (70.0) | 168 (71.2) | 54 (66.7) | 0.098 | 70.4 | 70.7 | 0.005 |
| Age, >60 years | 39 (12.3) | 34 (14.4) | 5 (6.2) | 0.274 | 12.4 | 12.5 | 0.003 |
| B symptoms | 146 (46.1) | 104 (44.1) | 42 (51.9) | 0.156 | 46.2 | 48.1 | 0.038 |
| ECOG score, ≥2 | 10 (3.2) | 7 (3.0) | 3 (3.7) | 0.041 | 3.0 | 2.5 | 0.035 |
| Stage II | 166 (52.4) | 132 (55.9) | 34 (42.0) | 0.282 | 52.7 | 56.6 | 0.078 |
| PTI | 246 (77.6) | 177 (75.0) | 69 (85.2) | 0.257 | 77.3 | 74.0 | 0.077 |
| Elevated LDH | 102 (32.2) | 69 (29.2) | 33 (40.7) | 0.243 | 31.5 | 28.1 | 0.074 |
| Primary UADT | 308 (97.2) | 228 (96.6) | 80 (98.8) | 0.144 | 97.1 | 95.4 | 0.089 |
| ES-NRI | |||||||
| Intermediate-risk (1-2) | 262 (82.6) | 196 (83.1) | 66 (81.5) | 0.041 | 83.0 | 80.7 | 0.059 |
| High-risk (≥3) | 55 (17.4) | 40 (16.9) | 15 (18.5) | 0.041 | 17.0 | 19.3 | 0.059 |
ASP, asparaginase; CMT, combined-modality therapy; ECOG, Eastern Cooperative Oncology Group; ENKTCL, extranodal nasal-type natural killer/T-cell lymphoma; ES-NRI, early stage-adjusted nomogram-revised risk index; IPTW, inverse probability of treatment weighting; LDH, lactate dehydrogenase; PTI, primary tumor invasion; SMD, standardized mean difference; UADT, upper aerodigestive tract.
Imbalance between treatment groups was defined as an SMD ≥0.1; balance between treatment groups was defined as an SMD <0.1.
With a median follow-up of 50 months, the 5-year OS and PFS were 83.4% and 68.7% for all patients, respectively. The ES-NRI is a powerful predictor for survival and showed an excellent 5-year OS of 93% for the low-risk group and worse outcomes (81.5%) for intermediate- and high-risk groups.
Survival benefit of ASP-based chemotherapy
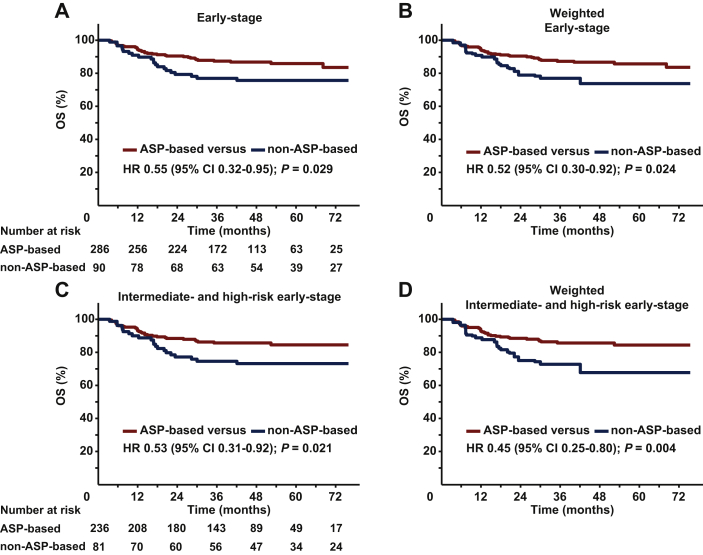
We evaluated the effect of ASP-based chemotherapy on OS. The 5-year OS was 85.9% for ASP-based regimens compared with 75.7% for non-ASP-based regimens [HR 0.55; 95% confidence interval (CI), 0.32-0.95; P = 0.029; Figure 1A]. Before weighting, clinical features differed between ASP-based and non-ASP-based groups (Table 1), and ASP-based regimens tended to have more low-risk patients. After IPTW adjustment, the prognostic factors were well balanced between the two groups. The adjusted 5-year OS was 85.7% for ASP-based regimens compared with 73.7% for non-ASP-based regimens (HR 0.52; 95% CI, 0.30-0.92; P = 0.024; Figure 1B). Furthermore, multivariable analysis demonstrated that ASP-based chemotherapy was an independent prognostic factor for OS (HR 0.52; 95% CI, 0.30-0.91; P = 0.022; Table 2).
Figure 1.
OS stratified by chemotherapy regimens in early-stage patients.
OS of ASP-based regimens versus non-ASP-based regimens in the entire cohort (A) before and (B) after IPTW. OS of ASP-based regimens versus non-ASP-based regimens in intermediate- and high-risk early-stage patients (C) before and (D) after IPTW.
ASP, asparaginase; CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; OS, overall survival.
Table 2.
Multivariable analysis of OS, DMFS, and LRRFS for early-stage ENKTCL patients treated with CMT.
| Variables | OS |
DMFS |
LRRFS |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| All early-stage patients | ||||||
| Sex (female versus male) | 0.96 (0.55-1.69) | 0.891 | 0.93 (0.52-1.67) | 0.801 | 0.77 (0.43-1.37) | 0.371 |
| Age (>60 versus ≤60) | 1.53 (0.68-3.47) | 0.305 | 2.50 (1.17-5.34) | 0.018∗ | 1.42 (0.63-3.19) | 0.396 |
| B symptoms (yes versus no) | 0.78 (0.45-1.35) | 0.380 | 0.44 (0.24-0.81) | 0.008∗ | 1.52 (0.89-2.59) | 0.123 |
| ECOG score (≥2 versus 0-1) | 2.05 (0.48-8.78) | 0.333 | 2.79 (0.63-12.32) | 0.177 | 2.02 (0.47-8.67) | 0.342 |
| Ann Arbor stage (II versus I) | 1.37 (0.80-2.34) | 0.255 | 1.87 (1.05-3.31) | 0.033∗ | 2.23 (1.28-3.87) | 0.004∗ |
| Elevated LDH (yes versus no) | 1.22 (0.69-2.16) | 0.485 | 1.03 (0.56-1.88) | 0.934 | 0.67 (0.36-1.27) | 0.224 |
| PTI (yes versus no) | 1.21 (0.66-2.21) | 0.531 | 2.00 (1.00-4.01) | 0.050∗ | 0.78 (0.45-1.38) | 0.396 |
| Primary UADT (yes versus no) | 0.37 (0.13-1.05) | 0.062 | 0.15 (0.06-0.38) | <0.001∗ | 0.26 (0.10-0.66) | 0.005∗ |
| Regimen (ASP-based versus non-ASP-based) | 0.52 (0.30-0.91) | 0.022∗ | 0.37 (0.21-0.66) | 0.001∗ | 1.02 (0.56-1.87) | 0.955 |
| Intermediate- and high-risk early-stage patients | ||||||
| Sex (female versus male) | 0.98 (0.55-1.74) | 0.932 | 1.00 (0.55-1.84) | 0.989 | 0.69 (0.36-1.30) | 0.249 |
| Age (>60 versus ≤60 years) | 1.30 (0.56-3.03) | 0.538 | 2.36 (1.08-5.15) | 0.031∗ | 1.34 (0.58-3.10) | 0.499 |
| B symptoms (yes versus no) | 0.78 (0.44-1.38) | 0.391 | 0.39 (0.20-0.74) | 0.004∗ | 1.52 (0.85-2.70) | 0.158 |
| ECOG score (≥2 versus 0-1) | 1.75 (0.40-7.57) | 0.455 | 2.82 (0.63-12.69) | 0.176 | 1.90 (0.44-8.28) | 0.394 |
| Ann Arbor stage (II versus I) | 1.22 (0.70-2.11) | 0.492 | 1.82 (1.00-3.29) | 0.049 | 2.15 (1.16-3.98) | 0.015∗ |
| Elevated LDH (yes versus no) | 1.13 (0.64-1.99) | 0.683 | 1.02 (0.56-1.87) | 0.948 | 0.68 (0.36-1.29) | 0.234 |
| PTI (yes versus no) | 0.92 (0.48-1.77) | 0.804 | 1.81 (0.84-3.92) | 0.131 | 0.74 (0.39-1.41) | 0.366 |
| Primary UADT (yes versus no) | 0.56 (0.13-2.38) | 0.435 | 0.15 (0.05-0.45) | 0.001∗ | 0.36 (0.11-1.19) | 0.095 |
| Regimen (ASP-based versus non-ASP-based) | 0.49 (0.27-0.86) | 0.014∗ | 0.34 (0.19-0.63) | 0.001∗ | 0.97 (0.51-1.86) | 0.933 |
ASP, asparaginase; CI, confidence interval; CMT, combined-modality therapy; DMFS, distant metastasis-free survival; ECOG, Eastern Cooperative Oncology Group; ENKTCL, extranodal nasal-type natural killer/T-cell lymphoma; HR, hazard ratio; LDH, lactate dehydrogenase; LRRFS, locoregional recurrence-free survival; OS, overall survival; PTI, primary tumor invasion; UADT, upper aerodigestive tract.
Significant P value.
With a small number of low-risk patients in this cohort, OS was comparably favorable between the ASP-based and non-ASP-based groups (P = 0.333; Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2021.100206). For intermediate- and high-risk patients, the 5-year OS was 84.5% for ASP-based regimens compared with 73.2% for non-ASP-based regimens (HR 0.53; 95% CI 0.31-0.92; P = 0.021; Figure 1C). After IPTW adjustment, the 5-year OS was 84.4% for ASP-based regimens versus 67.7% for non-ASP-based regimens (HR 0.45; 95% CI 0.25-0.80; P = 0.004; Figure 1D). These results suggested that ASP-based chemotherapy and radiotherapy significantly improved OS in intermediate- and high-risk early-stage patients.
Decreased DM and improved DMFS with ASP-based chemotherapy
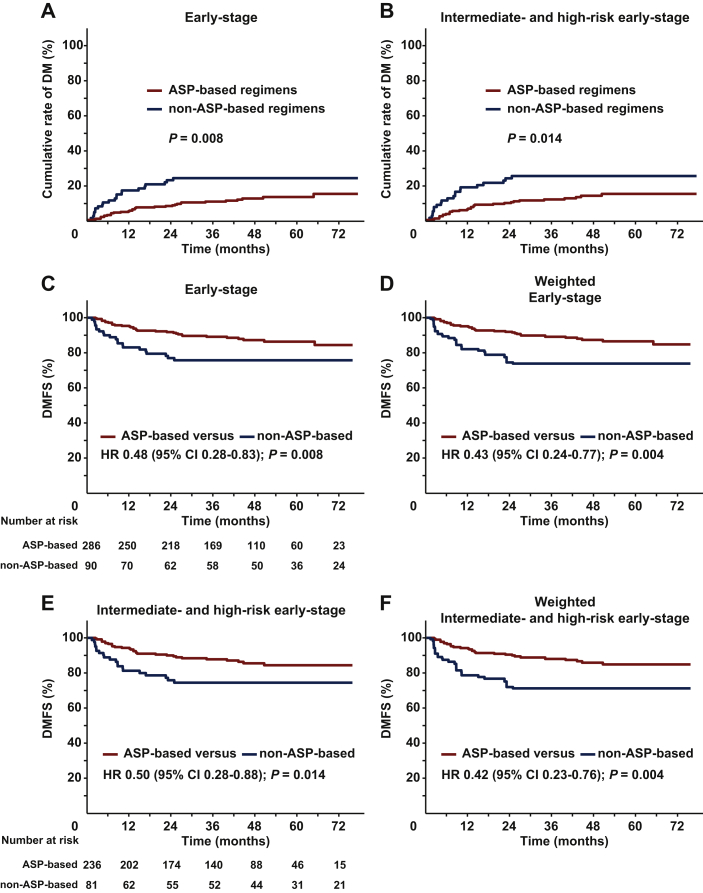
We further investigated the effect of ASP-based chemotherapy on DM and DMFS. Among the entire cohort, 54 (14.4%) of the 376 patients developed DM after CMT. The crude DM rate was significantly higher in non-ASP-based regimens (21/90, 23.3%) compared with ASP-based regimens (33/286, 11.5%; P = 0.005). ASP-based regimens showed a significantly lower 5-year cumulative DM rate (13.1% versus 23.9%; P = 0.008; Figure 2A) than non-ASP-based regimens. Similarly, in intermediate- and high-risk patients, the 5-year cumulative DM rate was 14.9% for ASP-based regimens compared with 25.1% for non-ASP-based regimens (P = 0.014; Figure 2B). These results indicated that ASP-based chemotherapy and radiotherapy significantly decreased distant and extranodal failure in intermediate- and high-risk early-stage ENKTCL.
Figure 2.
Cumulative incidence of DM and DMFS stratified by chemotherapy regimens in early-stage patients.
Cumulative incidence of DM (A) in the entire cohort and (B) in the intermediate- and high-risk early-stage patients. DMFS of ASP-based regimens versus non-ASP-based regimens in the entire cohort (C) before and (D) after IPTW, and in the intermediate- and high-risk early-stage patients (E) before and (F) after IPTW.
ASP, asparaginase; CI, confidence interval; DM, distant metastasis; DMFS, distant metastasis-free survival; HR, hazard ratio; IPTW, inverse probability of treatment weighting.
The unadjusted 5-year DMFS was 86.3% for ASP-based regimens compared with 75.7% for non-ASP-based regimens (HR 0.48; 95% CI, 0.28-0.83; P = 0.008; Figure 2C). After IPTW, the adjusted 5-year DMFS was 86.5% for ASP-based regimens compared with 73.9% for non-ASP-based regimens (HR 0.43; 95% CI 0.24-0.77; P = 0.004; Figure 2D). In the multivariable analysis, ASP-based regimens resulted in better DMFS than PLA/GEM regimens (HR 0.37, 95% CI 0.21-0.66; P = 0.001; Table 2).
For intermediate- and high-risk patients, the 5-year DMFS was 84.4% for ASP-based regimens compared with 74.5% for non-ASP-based regimens (HR 0.50; 95% CI 0.28-0.88; P = 0.014; Figure 2E). After IPTW adjustment, the 5-year DMFS was 84.9% for ASP-based regimens compared with 71.2% for non-ASP-based regimens (HR 0.42; 95% CI 0.23-0.76; P = 0.004; Figure 2F). For low-risk patients, there was no difference in DMFS between the two groups (P = 0.623; Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2021.100206). These results indicated that ASP-based chemotherapy and radiotherapy significantly improved DMFS in intermediate- and high-risk patients.
Effect of ASP-based chemotherapy on LRRFS
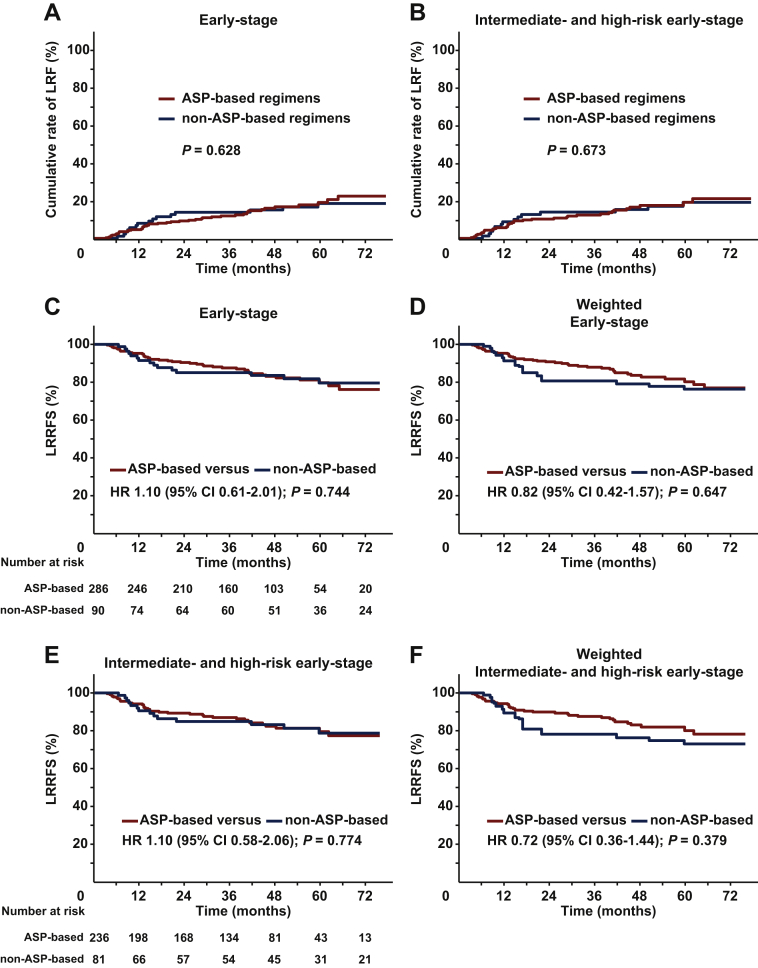
We evaluated the effect of ASP-based chemotherapy on LRR and LRRFS. As many as 44 of the 286 patients (15.4%) in ASP-based regimens and 15 of the 90 patients (16.7%) in non-ASP-based regimens developed LRR. ASP-based and non-ASP-based regimens resulted in a comparable 5-year cumulative LRR rate for the entire cohort (19.0% versus 18.4%, P = 0.628; Figure 3A), as well as for intermediate- and high-risk patients (19.1% versus 19.0%, P = 0.673; Figure 3B).
Figure 3.
Cumulative incidence of LRR and LRRFS stratified by chemotherapy regimens in early-stage patients.
Cumulative incidence of LRR (A) in the entire cohort and (B) in the intermediate- and high-risk early-stage patients. LRRFS of ASP-based regimens versus non-ASP-based regimens in the entire cohort (C) before and (D) after IPTW, and in the intermediate- and high-risk early-stage patients (E) before and (F) after IPTW.
ASP, asparaginase; CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LRR, locoregional recurrence; LRRFS, locoregional recurrence-free survival.
The 5-year LRRFS was 79.7% for ASP-based regimens compared with 79.6% for non-ASP-based regimens (HR 1.10; 95% CI 0.61-2.01; P = 0.744; Figure 3C). After IPTW adjustment, the 5-year LRRFS was 80.2% for ASP-based regimens compared with 76.3% for non-ASP-based regimens (HR 0.82; 95% CI 0.42-1.57; P = 0.647; Figure 3D). In the multivariable analysis, ASP-based chemotherapy showed no effect on LRRFS (HR 1.02; 95% CI 0.56-1.87; P = 0.955; Table 2).
For intermediate- and high-risk patients, the LRRFS remained comparable between the two groups, with a 5-year LRRFS of 79.5% for ASP-based regimens versus 78.7% for non-ASP-based regimens (HR 1.10; 95% CI 0.58-2.06; P = 0.774; Figure 3E). After IPTW adjustment, the 5-year LRRFS rate was 80.1% for ASP-based regimens versus 73.0% for non-ASP-based regimens (HR 0.72; 95% CI 0.36-1.44; P = 0.379; Figure 3F). Similarly, no difference in LRRFS was observed in low-risk patients (P = 0.688; Supplementary Figure S2C, available at https://doi.org/10.1016/j.esmoop.2021.100206). These results indicated that there was no additive effect of ASP-based chemotherapy on LRR or LRRFS in intermediate- and high-risk early-stage patients in the setting of radiotherapy.
Sensitivity analysis
A sensitivity analysis without assumptions showed that the results remained consistent under the influence of unmeasured confounders (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100206). In addition, we selected GDP with or without ASP to test the stability of our findings. GDP-ASP compared with GDP resulted in better OS (HR 0.49; 95% CI 0.21-1.15; P = 0.095) and DMFS (HR 0.28; 95% CI 0.10-0.84; P = 0.015), but similar LRRFS (HR 1.00; 95% CI 0.41-2.47; P = 0.996; Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100206).
Discussion
In this CLCG multicenter study, we explored the benefit of ASP-based chemotherapy for the first-line CMT of early-stage ENKTCL. In the context of radiotherapy, adding ASP into PLA/GEM-based chemotherapy significantly improved OS in intermediate- and high-risk early-stage patients. Improved OS after ASP-based chemotherapy was associated with decreased DM and increased DMFS, but not with LRRFS. The survival benefit of ASP-based chemotherapy remained constant after adjustment of the confounding variables by IPTW and multivariate analyses. These results provided additional evidence for the clinical use of ASP-based CMT for early-stage ENKTCL.
Risk-adapted therapy with introduction of non-anthracycline-based regimens and early implementation of radiotherapy have been the most important advances for the first-line treatment of early-stage ENKTCL.5, 6, 7, 8, 9, 10, 11 Despite the absence of high-level evidence, ASP-based regimens have been widely accepted as the standard of care in advanced-stage disease.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Given favorable outcomes with upfront radiotherapy for early-stage ENKTCL, adding chemotherapy to radiotherapy conferred survival benefit for intermediate- and high-risk patients but not for low-risk patients.22,23 A variety of ASP-based or PLA-based regimens have been used as the appropriate CMT approach.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 There are no comparative data describing the benefit of ASP-based regimens over non-ASP-based regimens for early-stage ENKTCL. The goal of this CLCG study was to identify active agents and optimal combinations to improve survival in early-stage ENKTCL. We confirmed that some portion of intermediate- and high-risk early-stage patients could benefit from the addition of ASP to PLA/GEM-based CMT. In this study, the 5-year OS rate of 286 early-stage patients that received ASP-based CMT was ~85%, higher than previous large multi-institutional reports from Japan (72%),11 China (73.3%),8 Asian joint (72-74%),46 and the International T-cell Project registry data (median, 59 months),47 probably because of the use of heterogeneous non-anthracycline-based regimens in the latter studies. We could not differentiate the efficacy of ASP-based versus non-ASP-based regimens in a small cohort of low-risk early-stage patients with favorable prognoses. These findings suggest that ASP may be a fundamental agent of non-anthracycline-based regimens in the first-line CMT for patients with intermediate- and high-risk early-stage ENKTCL. However, different types of treatment selection bias may have existed in these patients. Patients with favorable prognostic factors tended to receive non-ASP-based chemotherapy, while patients who are younger or in good performance status were more likely to receive ASP-based chemotherapy.
In the present CLCG study of early-stage patients treated with radiotherapy, ASP-based chemotherapy significantly decreased DM and improved DMFS and OS. Multivariate analyses revealed that ASP-based chemotherapy was an independent prognostic factor for DMFS and OS. In addition, ASP-based chemotherapy showed limited effect on locoregional control and LRRFS in the CMT setting. The high DMFS rate with ASP-based chemotherapy could translate into a significant improvement in OS. In our previous CLCG studies,19,48 improved locoregional control using optimal radiotherapy was associated with prolonged PFS and OS in early-stage patients,19 and the survival probability increased and failure hazard decreased in a risk-dependent manner.48 Moreover, in a subgroup analysis of 240 early-stage patients who achieved CR after ASP-based chemotherapy, additional radiotherapy significantly decreased LRR and improved survivals.21 The 5-year OS, disease-free survival, and locoregional control rates were 84.9%, 76.2%, and 84.9% for CMT compared with 58.9% (P = 0.006), 43.6% (P = 0.001), and 62.1% (P = 0.026) for chemotherapy alone. Based on competing risk analysis, the 5-year cumulative rates of LRR and systemic recurrence were 14.2% and 10.1% for CMT compared with 30.6% (P = 0.044) and 18.4% (P = 0.225) for ASP-based chemotherapy alone. These data suggest that in contrast to radiotherapy, ASP-based chemotherapy improves OS by acting on distant micrometastatic disease more than via locoregional effects. Early-stage ENKTCL is not only a localized disease, but also a systemic disease. The use of upfront locoregional radiotherapy and systematic ASP-based chemotherapy provide the ideal spatial combination for first-line therapy in intermediate- and high-risk early-stage ENKTCL patients.
The sequencing strategy for chemotherapy and radiotherapy in early-stage ENKTCL varies across countries and between collaborative study groups.11,23,46,47 In a recent CLCG study of 1360 NRI-stratified early-stage patients, the sequence and optimal cycles of non-anthracycline-based chemotherapy have been especially addressed in the setting of radiotherapy.23 For intermediate- and high-risk patients treated with sequential chemoradiotherapy, radiotherapy-first CMT showed similar 5-year OS (77.7% versus 72.4%; P = 0.290) and PFS (67.1% versus 63.1%; P = 0.592) to chemotherapy-first CMT.23 Moreover, for patients who achieved CR after induction chemotherapy, initiation of radiotherapy within or beyond three cycles of non-anthracycline-based chemotherapy resulted in comparable 5-year OS (78.2% versus 81.7%; P = 0.915) and PFS (68.2% versus 69.9%; P = 0.519). However, for patients who did not achieve CR, early radiotherapy within three cycles of chemotherapy yielded better PFS compared with delayed radiotherapy beyond three cycles of chemotherapy (63.4% versus 47.6%; P = 0.019).23 In another study of 244 early-stage patients,46 concurrent (early radiotherapy) versus sequential non-anthracycline-based chemotherapy and radiotherapy (delayed radiotherapy) yielded equivalent OS (P = 0.582) but better PFS (P = 0.035). These findings suggest that sequential combination of forefront radiotherapy and short-course ASP-based chemotherapy may constitute a reasonable treatment option for selected early-stage patients.
The strengths of this study included the heterogeneous early-stage population, use of current-era chemotherapy regimens, and unique effect of ASP-based regimens on DMFS. First, because of the heterogeneous population in the cohort of early-stage patients, we examined the variability of outcomes with risk-adapted first-line CMT and improved the generalizability of our study. Second, the most commonly used chemotherapy for early-stage ENKTCL was ASP-based or PLA-based regimens in China.8,23 Although the comparison of different ASP-based regimens has not been extensively investigated, the emerging results suggested that incorporation of ASP into PLA-based regimens is effective and safe for early-stage ENKTCL.16,25, 26, 27, 28, 29,34 Third, this study was the first to demonstrate clear associations of decreased DM and improved DMFS with OS benefit in early-stage ENKTCL patients; this unique finding is critical to understand that the therapeutic efficacy of ASP-based chemotherapy may mostly rely on DMFS. The analysis of DMFS as an endpoint, which is not commonly reported in lymphomas, was not confounded by locoregional factors related to radiotherapy. DMFS may provide an additional clinically relevant endpoint to evaluate the effect of novel systemic therapy in ENKTCL patients.
Our retrospective study had several limitations. First, ASP-based and non-ASP-based chemotherapy regimens were not randomly assigned. We attempted to reduce the selection bias using IPTW and multivariable analyses; however, there remains a chance that the underlying confounders may have influenced the treatment outcomes. Before generalizing the hypothetical survival benefit of additional ASP proposed in this retrospective study, it needs further evidence from prospective studies controlling confounding factors. Second, the toxicity profiles were not available in this retrospective, observational study. The toxicities of the chemotherapy regimens affected clinical decision making and could be considered in different risk groups of early-stage patients. However, the toxicity profiles of two categories of regimens in early-stage ENKTCL have been reported in other studies.25,27,38,40,49,50 The addition of ASP into PLA and GEM would inevitably result in increased toxicities. Previous studies showed that non-ASP-based (PLA/GEM) CMT in early-stage ENKTCL resulted in less grade 3/4 toxicities (18%-30% versus 12.5%-65.8%) than ASP-based (ASP/PLA/GEM) CMT.25,27,38,40,49,50 The thrombosis, hypersensitivity, and pancreatitis induced by ASP in early-stage ENKTCL were rare, but should be taken into consideration. However, both ASP-based (ASP/PLA/GEM) regimens and non-ASP-based (PLA/GEM) regimens were well tolerated in early-stage ENKTCL, when combined with radiotherapy.25,27,38,40,49,50 In addition, our study focused on the efficacy comparison of ASP/PLA/GEM versus PLA/GEM regimens and did not include other ASP-based regimens with inclusion of MTX and/or etoposide because of a small number of patients.8 Thus, our results may not be applicable to patients receiving ASP/MTX-based regimens. However, given the severe toxicities9,10,14 and low risk of central nervous system involvement,51 the role of incorporation of MTX into ASP-based regimens remains uncertain for the first-line CMT of early-stage ENKTCL. Using PFS at 24 months as an efficacy endpoint,52 further work is needed to optimize systemic therapies or novel regimens with inclusion of ASP and radiotherapy in intermediate- and high-risk early-stage patients.
In summary, ASP-based chemotherapy was associated with improved OS and DMFS. With the limited data available, patients with intermediate- and high-risk early-stage ENKTCL could be offered subsequent ASP-based chemotherapy and radiotherapy. This approach needs to be validated in a prospective trial.
Acknowledgments
Funding
This work was supported by grants from the Chinese Academy of Medical Science (CAMS) Innovation Fund for Medical Sciences (CIFMS), [grant number 2016-I2M-1-001], the National Key Projects of Research and Development of China [grant number 2016YFC0904600], and National Natural Science Foundation of China [grant number 81670185]. The funding sources had no influence on the design, performance, or reporting of the study.
Disclosure
The authors have declared no conflicts of interest.
Contributor Information
S.N. Qi, Email: medata@163.com.
Y.X. Li, Email: yexiong12@163.com, yexiong@yahoo.com.
Supplementary data
References
- 1.Vose J., Armitage J., Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Liu Q.F., Wang W.H., Wang S.L. Immunophenotypic and clinical differences between the nasal and extranasal subtypes of upper aerodigestive tract natural killer/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2014;88:806–813. doi: 10.1016/j.ijrobp.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Kim T.M., Lee S.Y., Jeon Y.K. Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 2008;19:1477–1484. doi: 10.1093/annonc/mdn147. [DOI] [PubMed] [Google Scholar]
- 4.Qi S.N., Xu L.M., Yuan Z.Y. Effect of primary tumor invasion on treatment and survival in extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: a multicenter study from the China Lymphoma Collaborative Group (CLCG) Leuk Lymphoma. 2019;60:2669–2678. doi: 10.1080/10428194.2019.1602265. [DOI] [PubMed] [Google Scholar]
- 5.Li Y.X., Yao B., Jin J. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. [DOI] [PubMed] [Google Scholar]
- 6.Li Y.X., Wang H., Jin J. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1809–1815. doi: 10.1016/j.ijrobp.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Wu T., Yang Y., Zhu S.Y. Risk-adapted survival benefit of IMRT in early-stage NKTCL: a multicenter study from the China Lymphoma Collaborative Group. Blood Adv. 2018;2(18):2369–2377. doi: 10.1182/bloodadvances.2018021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi S.N., Yang Y., Song Y.Q. First-line non-anthracycline-based chemotherapy for extranodal nasal-type NK/T-cell lymphoma: a retrospective analysis from the CLCG. Blood Adv. 2020;4:3141–3153. doi: 10.1182/bloodadvances.2020001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong Y.L., Kim W.S., Lim S.T. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi M., Kwong Y.L., Kim W.S. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi M., Suzuki R., Oguchi M. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. 2017;35(1):32–39. doi: 10.1200/JCO.2016.68.1619. [DOI] [PubMed] [Google Scholar]
- 12.Vargo J.A., Patel A., Glaser S.M. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer. 2017;123:3176–3185. doi: 10.1002/cncr.30697. [DOI] [PubMed] [Google Scholar]
- 13.Qi S.N., Yahalom J., Hsu M. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016;57(11):2575–2583. doi: 10.1080/10428194.2016.1180689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaccard A., Gachard N., Marin B. Efficacy of l-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z., Li X., Chen C. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. 2014;93(11):1889–1894. doi: 10.1007/s00277-014-2136-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., Jia S., Ma Y. Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced-stage extranodal natural killer/T-cell lymphoma: interim analysis of a phase 4 study NCT01501149. Oncotarget. 2016;7(34):55721–55731. doi: 10.18632/oncotarget.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Cui Y., Sun Z. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res. 2016;22(21):5223–5228. doi: 10.1158/1078-0432.CCR-16-0153. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y.Q., Yang Y., Zhuo H.Y. Trial of LVDP regimen (l-asparaginase, etoposide, dexamethasone, and cisplatin) followed by radiotherapy as first-line treatment for newly diagnosed, stage III/IV extranodal natural killer/T cell lymphoma. Med Oncol. 2015;32(2):435. doi: 10.1007/s12032-014-0435-4. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Cao J.Z., Lan S.M. Association of improved locoregional control with prolonged survival in early-stage extranodal nasal-type natural killer/T-cell lymphoma. JAMA Oncol. 2017;3:83–91. doi: 10.1001/jamaoncol.2016.5094. [DOI] [PubMed] [Google Scholar]
- 20.Li Y.Y., Feng L.L., Niu S.Q. Radiotherapy improves survival in early stage extranodal natural killer/T cell lymphoma patients receiving asparaginase-based chemotherapy. Oncotarget. 2017;8:11480–11488. doi: 10.18632/oncotarget.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng X.W., Wu J.X., Wu T. Radiotherapy is essential after complete response to asparaginase-containing chemotherapy in early-stage extranodal nasal-type NK/T-cell lymphoma: a multicenter study from the China Lymphoma Collaborative Group (CLCG) Radiother Oncol. 2018;129:3–9. doi: 10.1016/j.radonc.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Zhu Y., Cao J.Z. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126:1424–1432. doi: 10.1182/blood-2015-04-639336. [DOI] [PubMed] [Google Scholar]
- 23.Qi S.N., Yang Y., Zhang Y.J. Risk-based, response-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: a China Lymphoma Collaborative Group study. Am J Hematol. 2020;95:1047–1056. doi: 10.1002/ajh.25878. [DOI] [PubMed] [Google Scholar]
- 24.Ghione P., Qi S., Imber B.S. Modified SMILE (mSMILE) and intensity-modulated radiotherapy (IMRT) for extranodal NK-T lymphoma nasal type in a single-center population. Leuk Lymphoma. 2020;14:3331–3341. doi: 10.1080/10428194.2020.1811864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Wang Z.H., Chen X.Q. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119:348–355. doi: 10.1002/cncr.27752. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Wang Z.H., Chen X.Q. First-line combination of GELOX followed by radiation therapy for patients with stage IE/IIE ENKTL: an updated analysis with long-term follow-up. Oncol Letters. 2015;10:1036–1040. doi: 10.3892/ol.2015.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi X.W., Xia Y., Zhang W.W. Radiotherapy and PGEMOX/GELOX regimen improved prognosis in elderly patients with early-stage extranodal NK/T-cell lymphoma. Ann Hematol. 2015;94:1525–1533. doi: 10.1007/s00277-015-2395-y. [DOI] [PubMed] [Google Scholar]
- 28.Li J.W., Li Y.J., Zhong M.Z. Efficacy and tolerance of GELOXD/P-GEMOXD in newly diagnosed nasal-type extranodal NK/T-cell lymphoma: a multicenter retrospective study. Eur J Haematol. 2018;100:247–256. doi: 10.1111/ejh.13004. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.J., Yang D.H., Kim J.S. Concurrent chemoradiotherapy followed by l-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol. 2014;93(11):1895–1901. doi: 10.1007/s00277-014-2137-6. [DOI] [PubMed] [Google Scholar]
- 30.Jiang M., Zhang H., Jiang Y. Phase 2 trial of “sandwich” l-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118:3294–3301. doi: 10.1002/cncr.26629. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Ming Jiang M., Xie L. Five-year analysis from phase 2 trial of “sandwich” chemoradiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer Med. 2016;5(1):33–40. doi: 10.1002/cam4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y., Chen M., Song Y. Study of l-asparaginase, vincristine, and dexamethasone combined with intensity-modulated radiation therapy in early-stage nasal NK/T-cell lymphoma. Am J Clin Oncol. 2020;43(4):257–262. doi: 10.1097/COC.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 33.Xu P.P., Xiong J., Cheng S. A phase II study of methotrexate, etoposide, dexamethasone and pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage IE to IIE extranodal natural-killer/T-cell lymphoma, nasal-type. EBioMedicine. 2017;25:41–49. doi: 10.1016/j.ebiom.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T., Zhu F., Xiao Y. Pegaspargase, gemcitabine, dexamethasone, and cisplatin (P-GDP) combined chemotherapy is effective for newly diagnosed extranodal NK/T-cell lymphoma: a retrospective study. Cancer Manag Res. 2018;10:5061–5069. doi: 10.2147/CMAR.S179567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S.J., Kim K., Kim B.S. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 36.Tsai H.J., Lin S.F., Chen C.C. Long-term results of a phase II trial with frontline concurrent chemoradiotherapy followed by consolidation chemotherapy for localized nasal natural killer/T-cell lymphoma. Eur J Haematol. 2015;94(2):130–137. doi: 10.1111/ejh.12405. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi M., Tobinai K., Oguchi M. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27:5594–5600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- 38.Ke Q.H., Zhou S.Q., Du W. Concurrent IMRT and weekly cisplatin followed by GDP chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell lymphoma. Blood Cancer. 2014;4:e267. doi: 10.1038/bcj.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian S., Li R., Wang T. Gemcitabine, dexamethasone, and cisplatin (GDP) chemotherapy with sandwiched radiotherapy in the treatment of newly diagnosed stage IE/IIE extranodal natural killer/T-cell lymphoma, nasal type. Cancer Med. 2019;8:3349–3358. doi: 10.1002/cam4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi F., Wang W.H., He X.H. Phase 2 study of first-line intensity modulated radiation therapy followed by gemcitabine, dexamethasone, and cisplatin for high-risk, early stage extranodal nasal-type NK/T-cell lymphoma: The GREEN Study. Int J Radiat Oncol Biol Phys. 2018;102:61–70. doi: 10.1016/j.ijrobp.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Zhang Y.J., Zhu Y. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015;29:1571–1577. doi: 10.1038/leu.2015.44. [DOI] [PubMed] [Google Scholar]
- 42.Chen S.Y., Yang Y., Qi S.N. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making. Leukemia. 2021;35:130–142. doi: 10.1038/s41375-020-0791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi S.N., Li Y.X., Specht L. Modern radiation therapy for extranodal nasal-type NK/T-cell lymphoma: risk-adapted therapy, target volume and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2021;110:1064–1081. doi: 10.1016/j.ijrobp.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin P.C. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–5655. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwong Y.L., Kim S.J., Tse E. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol. 2018;29:256–263. doi: 10.1093/annonc/mdx684. [DOI] [PubMed] [Google Scholar]
- 47.Fox C.P., Civallero M., Ko Y.H. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol. 2020;7:e284–e294. doi: 10.1016/S2352-3026(19)30283-2. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Wu T., Zhu S.Y. Risk-dependent conditional survival and failure hazard after radiotherapy for early-stage extranodal natural killer/T-cell lymphoma. JAMA Netw Open. 2019;2(3):e190194. doi: 10.1001/jamanetworkopen.2019.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Wuxiao Z.J., Zhu J.Y. Comparison of gemcitabine, oxaliplatin and l-asparaginase and etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone as first-line chemotherapy in patients with stage IE to IIE extranodal natural killer/T-cell lymphoma: a multicenter retrospective study. Leuk lymphoma. 2015;56:971–977. doi: 10.3109/10428194.2014.939964. [DOI] [PubMed] [Google Scholar]
- 50.Jing X.M., Zhang Z.H., Wu P. Efficacy and tolerance of pegaspargase, gemcitabine and oxaliplatin with sandwiched radiotherapy in the treatment of newly-diagnosed extranodal nature killer (NK)/T cell lymphoma. Leuk Res. 2016;47:26–31. doi: 10.1016/j.leukres.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Kim H., Jeong H., Yamaguchi M. Prediction and prevention of central nervous system relapse in patients with extranodal natural killer/T-cell lymphoma. Blood. 2020;136(22):2548–2556. doi: 10.1182/blood.2020005026. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y., Wang Y., Liu X. Progression-free survival at 24 months and subsequent survival of patients with extranodal NK/T-Cell lymphoma: a China Lymphoma Collaborative Group (CLCG) study. Leukemia. 2021;35(6):1671–1682. doi: 10.1038/s41375-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.