Abstract
Study Objectives
Whether the cause of daytime sleepiness in narcolepsy type 1 (NT1) is a direct consequence of the loss of orexin (ORX) neurons or whether low orexin reduces the efficacy of the monoaminergic systems to promote wakefulness is unclear. The neurobiology underlying sleepiness in other central hypersomnolence disorders, narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH), is currently unknown.
Methods
Eleven biogenic amines including the monoaminergic neurotransmitters and their metabolites and five trace amines were measured in the cerebrospinal fluid (CSF) of 94 drug-free subjects evaluated at the French National Reference Center for Narcolepsy: 39 NT1(orexin-deficient) patients, 31 patients with objective sleepiness non orexin-deficient (NT2 and IH), and 24 patients without objective sleepiness.
Results
Three trace amines were undetectable in the sample: tryptamine, octopamine, and 3-iodothyronamine. No significant differences were found among the three groups for quantified monoamines and their metabolites in crude and adjusted models; however, CSF 5-hydroxyindoleacetic acid (5-HIAA) levels tended to increase in NT1 compared to other patients after adjustment. Most of the biomarkers were not associated with ORX-A levels, clinical or neurophysiological parameters, but a few biomarkers (e.g. 3-methoxy-4-hydroxyphenylglycol and norepinephrine) correlated with daytime sleepiness and high rapid eye movement (REM) sleep propensity.
Conclusions
We found no striking differences among CSF monoamines, their metabolites and trace amine levels, and few associations between them and key clinical or neurophysiological parameters in NT1, NT2/IH, and patients without objective sleepiness. Although mostly negative, these findings are a significant contribution to our understanding of the neurobiology of hypersomnolence in these disorders that remain mysterious and deserve further exploration.
Keywords: central disorders of hypersomnolence, narcolepsy, hypersomnia, sleepiness, cerebrospinal fluid, monoamine, hypocretin/orexin
Statement of Significance.
To explore the neurobiology of sleepiness in central hypersomnolence disorders, we measured with the latest advanced technique 16 monoamines and metabolites in the cerebrospinal fluid (CSF) of well-characterized patients with narcolepsy type 1 (NT1), type 2, idiopathic hypersomnia, and controls. Patients were evaluated in a National Reference Center for Narcolepsy in France. Three trace amines were undetectable in the sample, and no striking differences were found across the three groups except for a trend toward higher CSF 5-hydroxyindoleacetic acid levels in NT1. Two biomarkers (3-methoxy-4-hydroxyphenylglycol and norepinephrine) correlated with daytime sleepiness and the number of sleep onset REM periods but with uncertain clinical significance. These findings represent a significant contribution to our understanding of the neurobiology of hypersomnolence in these rare diseases, that remain mysterious and deserve first and foremost further exploration.
Introduction
The discovery of orexin (ORX)/hypocretin neuropeptides and their role in sleep and wake regulation was a major advance in the field of sleep research [1, 2]. The importance of the ORX system is most obvious in narcolepsy type 1 (NT1), characterized by excessive daytime sleepiness (EDS), cataplexy, and sleep/wake fragmentation. NT1 is caused by the selective loss of the ORX-producing neurons [3, 4] and, consequently, NT1 patients have reduced cerebrospinal (CSF) ORX levels. Several animal models of narcolepsy have been developed including ORX-null mice [5], mice lacking ORX neurons, and mice in which orexin-containing neurons are conditionally ablated [6, 7], each of which express sleepiness, cataplexy, sleep/wake fragmentation and increased rapid eye movement (REM) sleep propensity, although to varying degrees [8].
The brain circuitry controlling wake and sleep depends on fast neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA) that interact with the classical monoaminergic, cholinergic, and peptidergic modulatory systems [9]. Although all NT1 patients are ORX deficient, the narcolepsy phenotype varies widely regarding the main features of the disease: age of onset, frequency of cataplexy, and severity of EDS or disturbed nocturnal sleep. Whether EDS in NT1 is a direct consequence of this neuronal loss, or whether low ORX reduces the functional activation of other wake-promoting systems remain unclear. The few studies that have quantified levels of neurotransmitters and their metabolites in narcolepsy were conducted before the diagnostic distinction of NT1 versus NT2, but suggested low metabolism of dopamine [10–12]. More recently, CSF levels of histamine and telemethylhistamine have been measured in NT1 patients and found to be either normal or decreased, with higher histidine levels reported with an increased number of histamine neurons [13–17].
Non-ORX deficient central hypersomnolence disorders such as narcolepsy type 2 (NT2) and idiopathic hypersomnia (IH) are less well-defined, without key reliable biomarkers or animal models. Several controversial findings on CSF norepinephrine, histamine-telemethylhistamine and GABA-A activities were reported in NT2 and IH [10, 14–19]. However, whether a primary partial deficit in monoaminergic systems causes the neurobiology of sleepiness in NT2 or IH remains to be elucidated.
Considering the lack of sufficient knowledge regarding monoamine activity in central hypersomnolence disorders as well as the recent progress in monoamine and metabolite measurement technology and in the detection of the trace amines, endogenous amino acid metabolites that regulate monoaminergic neuronal activity [20–22], we assessed 16 analytes in the CSF of patients evaluated for a complaint of hypersomnolence using liquid chromatography–mass spectroscopy (LC–MS/MS). Our objectives were (1) to measure and compare these biomarkers levels in patients with objective hypersomnolence and ORX deficiency (NT1), without ORX deficiency (NT2 and IH), and in a control group with normal ORX levels and without a confirmed hypersomnolence disorder; and (2) to analyze the associations between these biomarkers and clinical and neurophysiological parameters across the whole sample and in patients with NT1.
Methods
Participants
We included 94 consecutive drug-free patients referred for a suspected central hypersomnolence disorder to the French National Reference Center for Narcolepsy, Montpellier, France. Patients with relevant psychiatric and medical comorbidities that could explain sleepiness were not included.
All patients underwent a standardized clinical evaluation with a medical interview by a sleep expert. Age, gender, body mass index (BMI, categorized as normal/overweight/obese), drug status (naïve, i.e. never treated for sleepiness), or withdrawal condition (i.e. they stopped all medication that could influence sleep at least 2 weeks before evaluation), age at first symptoms onset, Epworth Severity Scale (ESS) scores [23] and, when present, cataplexy frequency were collected. All participants had a video-polysomnography (PSG) recording followed by a multiple sleep latency test (MSLT) and a lumbar puncture after the last nap, which was performed between 6:00 pm and 7:00 pm.
Sleep was scored manually based on the standard method [24], and the following data were collected: total sleep time (TST), sleep efficiency, and wake after sleep onset (WASO). On the MSLT, mean sleep latency and the number of sleep onset REM periods (SOREMPs) were recorded.
This study was approved by the local ethics committees (Comité de Protection des Personnes, France: Constitution of a cohort and of a clinical, neurophysiological and biological bank of rare hypersomnolence disorders-NARCOBANK PHRC AOM07-138). Assent was provided by all participants prior to participation, and written consent by adults, and by both parents for minors.
Diagnosis and classification of the subjects
According to ICSD-3 criteria [25], 70 subjects had a diagnosis of central disorder hypersomnolence: 39 with NT1, 24 NT2, and 7 IH. All patients with NT2 or IH had a short MSLT mean sleep latency (<8 min). NT2 subjects had two or more SOREMPs and IH subjects had zero or one SOREMP. The seven IH patients also had a prolonged bed-rest PSG recording which showed TST >19 h over a 32-h recording period [26].
The other 24 other subjects did not meet the objective criteria for central disorder hypersomnolence: they were without objective sleepiness on MSLT (mean sleep latency >8 min for all, nine subjects between 9.5 and 12 min and the other 15 subjects >12 min), without SOREMP or abnormalities on nocturnal PSG, no drug intake, and no other significant psychiatric, neurological, or medical disorders. Two patients also underwent a 32-h bed-rest PSG recording, which excluded a diagnosis of idiopathic hypersomnia with long sleep duration [26]. The remaining subjects did not complain of prolonged duration of nighttime sleep. This group of patients was called nonspecified hypersomnolence (NSH).
Thus, subjects were classified in three groups: (1) NT1 patients (all with CSF ORX-A levels < 110 pg/mL), (2) NT2 and IH patients (all with normal CSF ORX-A levels > 200 pg/mL and MSLT mean sleep latency < 8 min), and (3) NSH subjects (all with CSF ORX-A levels > 200 pg/mL).
Measurements of analytes in CSF
CSF samples (2–3 mL) were collected and divided into 0.5 mL aliquots, which were frozen and stored immediately at –80°C. Aliquots were maintained at –80°C for several months to years with a median at 37.9 months [range 1.4–234] prior to monoamine measurements. None of the aliquots were thawed prior to the measurement and were sent frozen from the sleep center directly to Charles River Laboratories for LC–MS/MS analysis.
CSF ORX-A level was determined in duplicate using the [125I]-radio-immuno-assay (RIA) kit from Phoenix Pharmaceuticals, Inc. (Belmont, CA), according to the manufacturer’s recommendations. All values were back-referenced to the Stanford reference samples (Stanford University Center for Narcolepsy, Palo Alto, CA). CSF ORX-A levels below 110 pg/mL were considered low, and below 10 pg/mL undetectable.
Sixteen monoamine and their metabolites were measured in the CSF by HPLC with tandem mass spectrometry (MS/MS): eleven biogenic amines [serotonin, 5-hydroxyindoleacetic acid (5-HIAA), dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3-MT), homovanillic acid (HVA), 3-O-methyldopa (3-OMD), epinephrine, norepinephrine, 3-methoxy-4-hydroxyphenylglycol (MHPG), vanillylmandelic acid (VMA), and five trace amines (β-phenylethylamine, tyramine, tryptamine, octopamine, and 3-iodothyronamine]. All CSF samples were thawed on the day of analysis and diluted twofold with a solution of artificial CSF (aCSF) containing 0.01% ascorbic acid and 0.1% formic acid prior to analysis. All three LC–MS assays (Supplementary Material) were performed with the same sample aliquot as they were analyzed on the same day. Samples were prepared shortly before analysis in order to minimize exposure to room temperature. CSF concentrations of these analytes were measured blind to diagnosis by Charles River Laboratories (San Francisco, USA, www.criver.com). The procedures are detailed in Supplementary Material.
The limit of detection for each of the analytes was defined as the lowest concentration that could be detected in a sample and was derived by determining the average background signal and then adding three standard deviations to that mean value. The lower limit of quantitation (LLOQ) was defined as the minimum value calculated on any sample or standard by the analysis program, including values reported by the software as extrapolated. For data mining, individual values below the LLOQ were replaced with 50% of the LLOQ value measured in the data set.
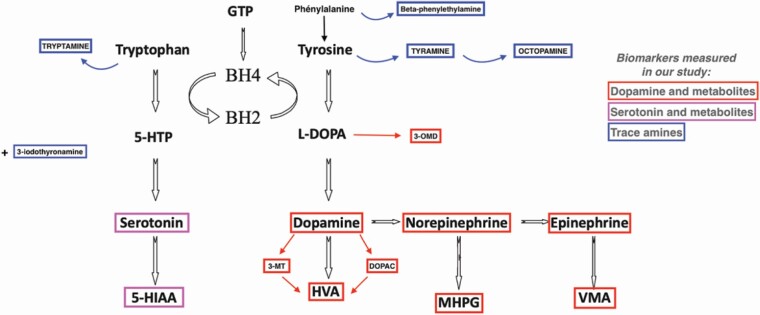
CSF biomarker levels were determined in duplicate for 31 subjects (10 NT1, 11 NT2/IH, 10 NSH) without between measurement differences, except for 5-HIAA and epinephrine being higher in the second measurements [median 65.4 nM (range 19.8–214.0) vs 73.6 nM (range 18.30–244), p = 0.01)] and [0.11 nM (0.02–0.46) vs 0.15 (0.02–0.56), p = 0.03], respectively. The monoamines and their metabolites tested in this study, as well as their synthesis pathways, are presented in Figure 1.
Figure 1.
Monoamines synthesis pathway. 3-MT, 3-Methoxytyramine; 5-HIAA, 5-hydroxyindoleacetic acid; BH2, quinonoid dihydrobiopterin; BH4, tetrahydrobiopterin; DOPAC, 3,4-dihydroxyphenylacetic acid; GTP, guanosine triphosphate; HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol, OMD, 3-O-methyldopa; VMA, vanillylmandelic acid.
Statistical analysis
Categorical variables were presented as percentages and quantitative variables as medians with ranges. Univariate multinomial regression models were used to determine differences in demographic and clinical characteristics among the three groups of patients (NSH, IH/NT2, NT1). Variables associated at p < 0.10 were included as potential confounders in a multivariate model to examine the relationships between CSF monoamine and their metabolite levels across the three groups. When comparisons were statistically significant across the three groups, pairwise comparisons were subsequently conducted with Bonferroni correction for multiple comparisons.
When the percentage of undetectable measures was high for a particular CSF analyte (i.e. biomarker), it was not possible to execute the analysis using that analyte as a continuous variable. Consequently, the following rules were implemented to report the levels of each biomarker: (1) if the undetectable biomarker rate was less than 10%, the biomarker was considered to be a continuous variable but, if the model did not satisfy the linear assumptions of logistic regression, the biomarker was divided into tertiles; (2) if the biomarker was undetectable in 10%–33% of the samples, the biomarker levels were divided into tertiles of the whole sample; (3) between 33% and 50% undetectability, the first class of the categorized biomarker was the detection level and the second and third classes were defined by the median of the detectable values; (4) at an undetectable rate of 50% or greater, the biomarker was categorized into detectable and undetectable classes.
To analyze the associations between the CSF analytes and clinical (i.e. age, gender, EDS, duration of the evolution of EDS, and frequency of cataplexy) and neurophysiological characteristics (i.e. nocturnal sleep efficiency, WASO, mean sleep latency on the MSLT, and the number of SOREMPs on the MSLT and PSG). Chi-square or Fisher’s exact tests were used to compare categorical variables; Mann–Whitney or Student’s t-test to compare continuous variables of two groups; and the Kruskal–Wallis test or analysis of variance (ANOVA) to compare continuous variables for more than two groups. Spearman’s rank order correlations were used to determine associations between continuous variables. Given the exploratory nature of this part of the analysis, multiple test adjustments were not made [27]. Significance level was set at p < 0.05. Analyses were performed with the SAS statistical software (version 9.4; SAS, Cary, NC).
Results
Characteristics of the population
Among the 94 participants, 52 (55.32%) were men with a median age of 25.5 years old (12; 63) including 23 (24.47%) children, and 48 (68.57%) drug-naïve patients. The median CSF ORX-A level of the whole sample was 247 (0; 615) pg/mL. Among ORX-deficient patients, 14 (35.9%) had undetectable CSF ORX-A levels. No patient had intermediate (110–200 pg/ml) ORX-A levels. Patients with either a diagnosis of NT2 (n = 27) or IH (n = 7) had a short MSLT mean sleep latency (<8 min) and normal CSF ORX levels (>200 pg/mL), without significant between-group differences except gender (83.3% of women in IH and 28.6% in NT2) and, by definition, the number of SOREMPs. We pooled those patients into one group of NT2/IH as a non-ORX deficient, objectively sleepy group. Clinical and neurophysiological characteristics were compared between the three groups with differences for gender, BMI, ESS score, MSLT sleep latency, and number of SOREMPs (Table 1).
Table 1.
Characteristics of the study population according to diagnosis groups
| Variable | NSH N = 24 | IH/NT2 N = 31 | NT1 N = 39 | p | Post-hoc comparisons |
|---|---|---|---|---|---|
| n(%) | n(%) | n(%) | |||
| Gender, male | 7(29.17) | 22(70.97) | 23(58.97) | 0.01 | NSH < IH/NT2 |
| Age, years* | 29.0(12.0; 55.0) | 24.6(15.5; 63.0) | 30.0(12.2; 55.0) | 0.94 | – |
| Age, <18 years | 7(29.17) | 4(12.90) | 12(30.77) | 0.20 | – |
| BMI, kg/m2 * | 20.65(13.67; 33.20) | 22.90(16.53; 31.44) | 24.22(17.21; 43.42) | <0.01 | NSH < NT1 |
| Overweight/obese, yes | 2(8.33) | 3(9.68) | 8(20.51) | 0.30 | – |
| Duration of evolution of sleepiness, years* | 4.03(0.20; 40.02) | 5.50(0.27; 45.73) | 3.41(0.02; 37.84) | 0.33 | – |
| Age of onset of sleepiness, years* | 16.00(3.00; 46.00) | 17.00(9.00; 48.00) | 20.00(10.00; 48.00) | 0.18 | – |
| Drug-naïve, yes | 14 (58.33) | 25(80.65) | 23(58.97) | 0.06 | – |
| ESS score* | 15.00(0.00; 19.00) | 16.00(10.00; 23.00) | 19.00(12.00; 24.00) | 0.0005 | NSH, IH/NT2 < NT1 |
| MSLT, mean latency, min* | 13.70(9.40; 19.80) | 5.40(0.80; 8.00) | 2.70(0.80; 14.67) | 0.0002 | NSH > IH/NT2 > NT1 |
| MSLT, number of SOREMP* | 0.00(0.00; 3.00) | 2.00(0.00; 5.00) | 4.00(2.00; 5.00) | <0.0001 | NSH < IH/NT2 < NT1 |
| CSF ORX-A levels, pg/mL* | 306.5(210; 615) | 318.0(228; 467) | 15.0(0; 97) | NA |
*Continuous variables are expressed as median with minimum value and maximum value.
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; ESS, Epworth sleepiness scale; IH, idiopathic hypersomnia; MSLT, mean sleep latency test; NA, test not applicable; NSH, nonspecified hypersomnolence, NT1, narcolepsy type 1; NT2, narcolepsy type 2; ORX-A, orexin-A; SOREMP, sleep onset rapid eye movement period.
Measurement of CSF analytes
Among the 16 biomarkers tested, three trace amines were undetectable in the sample: octopamine, tryptamine, and 3-iodothyronamine. Five biomarkers (serotonin, 3-MT, VMA, β-phenylethylamine, tyramine) were undetectable in at least 50% of the subjects, two biomarkers (epinephrine and DOPAC) were undetectable in 33%–50% of the subjects, dopamine was undetectable in 10%–33% of the subjects, and the undetectability rate was less than 10% for five biomarkers (norepinephrine, HVA, 5-HIAA, MHPG, and 3-OMD).
A cluster of correlations was found in patients with NT1 between DOPAC and HVA, 5-HIAA, MHPG, norepinephrine, and 3-OMD (0.39 < r < 0.71), 5-HIAA and MHPG, norepinephrine, and 3-OMD (0.37 < r < 0.63), MHPG and norepinephrine and 3-OMD (0.59 < r < 0.74), HVA, and 5-HIAA and dopamine (r = 0.51 and r = –0.42). The same profiles of results were found in the whole sample.
CSF ORX levels were not associated with any other analytes except with β-phenylethylamine. For detectable β-phenylethylamine versus not, CSF ORX levels were 10 pg/mL (0–14) versus 18.6 (0–97.2), p = 0.03) in patients with NT1 only.
Between-group comparisons of CSF analytes
No significant differences were found between the three groups (NT1, NT2/IH, and NSH) for any of the biomarkers tested, nor for the three often reported ratios (i.e. 5-HIAA/serotonin, HVA/dopamine, and MHPG/norepinephrine) in crude and adjusted statistical models (Models 1 and 2, respectively, in Table 2). However, comparing patients with NT1 versus the two other groups (non-ORX deficient patients with NT2/IH or NSH), we found higher CSF 5-HIAA levels in patients with NT1 in the unadjusted model, with a similar trend after adjustment for BMI (Tables 3 and 4).
Table 2.
Between-group comparisons of cerebrospinal analyte levels (when detectable)
| CSF monoamine and metabolites levels | NSH N = 24 | IH/NT2 N = 31 | NT1 N = 39 | Model 1 | Model 2 |
|---|---|---|---|---|---|
| n(%) | n(%) | n(%) | p | p | |
| Serotonergic system | |||||
| Serotonin (nM) | |||||
| ≤0.02 | 18(75.00) | 26(83.87) | 27(69.23) | 0.38 | 0.51 |
| >0.02 | 6(25.00) | 5(16.13) | 12(30.77) | ||
| 5-HIAA (nM)* | 58.60 (20.60;155.00) | 55.60 (19.80; 113.00) | 65.40 (27.70; 214.00) | 0.09 | 0.20 |
| Dopaminergic system | |||||
| Dopamine (nM) | |||||
| ≤0.0868 | 10(41.67) | 10(32.26) | 12(30.77) | 0.83 | 0.93 |
| ]0.0868–1.32] | 8(33.33) | 11(35.48) | 12(30.77) | ||
| >1.32 | 6(25.00) | 10(32.26) | 15(38.46) | ||
| HVA (nM)* | 35.20 (14.90;155.00) | 43.30 (10.30; 294.00) | 46.90 (17.00; 633.00) | 0.46 | 0.60 |
| DOPAC (nM) | |||||
| ≤0.5 | 8(33.33) | 11(35.48) | 13(33.33) | 0.19 | 0.14 |
| ]0.5–2.795[ | 12(50.00) | 10(32.26) | 9(23.08) | ||
| ≥2.795 | 4(16.67) | 10(32.26) | 17(43.59) | ||
| 3-MT (nM) | |||||
| ≤0.125 | 21(87.50) | 30(96.77) | 37(94.87) | 0.38 | 0.72 |
| >0.125 | 3(12.50) | 1(3.23) | 2(5.13) | ||
| OMD (nM) * | 15.75 (9.92; 25.70) | 13.20 (9.27; 22.40) | 13.20 (5.76; 45.10) | 0.29 | 0.19 |
| Noradrenergic system | |||||
| Norepinephrine (nM)* | 0.29 (0.02; 0.86) | 0.36 (0.15; 1.01) | 0.38 (0.08; 2.05) | 0.24 | 0.46 |
| MHPG (nM) * | 52.60 (28.20; 86.00) | 51.90 (36.20; 69.40) | 52.00 (21.30; 138.00) | 0.39 | 0.54 |
| MHPG/norepinephrine* | 177.71 (57.38; 4193.33) | 127.15 (60.59; 457.33) | 140.16 (40.54; 423.22) | 0.31 | 0.57 |
| Epinephrine (nM) | |||||
| ≤0.02 | 10(41.67) | 10(32.26) | 13(33.33) | 0.45 | 0.55 |
| ]0.05–0.1190[ | 10(41.67) | 9(29.03) | 11(28.21) | ||
| ≥0.1190 | 4(16.67) | 12(38.71) | 15(38.46) | ||
| VMA (nM) | |||||
| ≤0.125 | 23(95.83) | 29(93.55) | 33(84.62) | 0.30 | 0.49 |
| >0.125 | 1(4.17) | 2(6.45) | 6(15.38) | ||
| Trace amines | |||||
| β-Phenylethylamine (nM) | |||||
| ≤0.125 | 19(79.17) | 31(100.00) | 34(87.18) | NA | NA |
| >0.125 | 5(20.83) | 0(0.00) | 5(12.82) | ||
| Tyramine (nM) | |||||
| ≤0.025 | 21(87.50) | 28(90.32) | 32(82.05) | 0.60 | 0.62 |
| >0.025 | 3(12.50) | 3(9.68) | 7(17.95) |
* Continuous variables are expressed as median with minimum value and maximum value.
Model 1: crude association.
Model 2: adjustment for gender and BMI.
Abbreviations: 3-MT, 3-methoxytyramine; 5-HIAA, 5-hydroxyindoleacetic acid; BMI, body mass index; CSF, cerebrospinal fluid; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; IH, idiopathic hypersomnia; MHPG, 3-methoxy-4-hydroxyphenylglycol; NA, test not applicable; NSH, nonspecified hypersomnolence; NT1, narcolepsy type 1; NT2, narcolepsy type 2; OMD, 3-O-methyldopa; VMA, vanillylmandelic acid.
Table 3.
Characteristics of the study population according to their CSF orexin levels (normal levels: NSH/ IH/ NT2 vs low levels in NT1)
| Variable | NSH/ IH/NT2 N = 55 | NT1 N = 39 | |
|---|---|---|---|
| n(%) | n(%) | p | |
| Gender, male | 29 (52.73) | 23 (58.97) | 0.55 |
| Age, years* | 25.00 (12.00; 63.00) | 30.0 (12.2; 55.0) | 0.80 |
| Age, <18 years | 11 (20.00) | 12 (30.77) | 0.23 |
| BMI, kg/m2 * | 22.20 (13.67; 33.20) | 24.22 (17.21; 43.42) | 0.01 |
| Overweight/obese, yes | 5 (9.09) | 8 (20.51) | 0.12 |
| Duration of evolution of sleepiness, years* | 5.22 (0.20; 45.73) | 3.41 (0.02; 37.84) | 0.15 |
| Age of onset of sleepiness, years* | 17.00 (3.00; 48.00) | 20.00 (10.00; 48.00) | 0.07 |
| Drug-naïve, yes | 25 (80.65) | 23 (58.97) | 0.06 |
| ESS score* | 15.50 (0.00; 23.00) | 19.00 (12.00; 24.00) | 0.0002 |
| MSLT, mean latency (min)* | 7.20 (0.80; 19.80) | 2.70 (0.80; 14.67) | <0.0001 |
| MSLT, number of SOREMP* | 1.00 (0.00; 5.00) | 4.00 (2.00; 5.00) | <0.0001 |
| CSF ORX-A levels (pg/mL)* | 308.00 (210.00; 615.00) | 15.0 (0; 97) | NA |
* Continuous variables are expressed as median with minimum value and maximum value.
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; ESS, Epworth sleepiness scale; IH, idiopathic hypersomnia; MSLT, mean sleep latency test; NA, test not applicable; NSH, nonspecified hypersomnolence; NT1, narcolepsy type 1; NT2, narcolepsy type 2; ORX-A, orexin-A; SOREMP, sleep onset rapid eye movement period.
Table 4.
Between-group comparisons of cerebrospinal analyte levels (when detectable) according to their CSF orexin-A levels (normal levels in NSH/IH/NT2 vs low levels in NT1)
| CSF monoamine and metabolites levels | NSH/ IH/NT2 N = 55 | NT1 N = 39 | Model 1 | Model 2 |
|---|---|---|---|---|
| n(%) | n(%) | p | p | |
| Serotonergic system | ||||
| Serotonin (nM) | ||||
| ≤0.02 | 44 (80.00) | 27 (69.23) | 0.23 | 0.58 |
| >0.02 | 11 (20.00) | 12 (30.77) | ||
| 5-HIAA (nM)* | 58.30 (19.80; 155.00) | 65.40 (27.70; 214.00) | 0.03 | 0.09 |
| Dopaminergic system | ||||
| Dopamine (nM) | ||||
| ≤0.0868 | 20 (36.36) | 12 (30.77) | 0.63 | 0.82 |
| ]0.0868–1.32] | 19 (34.55) | 12 (30.77) | ||
| >1.32 | 16 (29.09) | 15 (38.46) | ||
| HVA (nM)* | 38.90 (10.30; 294.00) | 46.90 (17.00; 633.00) | 0.32 | 0.38 |
| DOPAC (nM) | ||||
| ≤0.5 | 19 (34.55) | 13 (33.33) | 0.13 | 0.09 |
| ]0.5–2.795[ | 22 (40.00) | 9 (23.08) | ||
| ≥2.795 | 14 (25.45) | 17 (43.59) | ||
| 3-MT (nM) | ||||
| ≤0.125 | 51 (92.73) | 37 (94.87) | 0.68 | 0.70 |
| >0.125 | 4 (7.27) | 2 (5.13) | ||
| OMD (nM) * | 14.00 (9.27; 25.70) | 13.20 (5.76; 45.10) | 0.75 | 0.63 |
| Noradrenergic system | ||||
| Norepinephrine (nM)* | 0.33 (0.02; 1.01) | 0.38 (0.08; 2.05) | 0.19 | 0.34 |
| MHPG (nM)* | 52.10 (28.20; 86.00) | 52.00 (21.30; 138.00) | 0.20 | 0.36 |
| MHPG/norepinephrine* | 147.06 (57.38; 4193.33) | 140.16 (40.54; 423.22) | 0.44 | 0.53 |
| Epinephrine (nM) | ||||
| ≤0.02 | 20 (36.36) | 13 (33.33) | 0.62 | 0.78 |
| ]0.05–0.1190[ | 19 (34.55) | 11 (28.21) | ||
| ≥0.1190 | 16 (29.09) | 15 (38.46) | ||
| VMA (nM) | ||||
| ≤0.125 | 52 (94.55) | 33 (84.62) | 0.12 | 0.23 |
| >0.125 | 3 (5.45) | 6 (15.38) | ||
| Trace amines | ||||
| β-Phenylethylamine (nM) | ||||
| ≤0.125 | 50 (90.91) | 34 (87.18) | 0.57 | 0.56 |
| >0.125 | 5 (9.09) | 5 (12.82) | ||
| Tyramine (nM) | ||||
| ≤0.025 | 49 (89.09) | 32 (82.05) | 0.33 | 0.39 |
| >0.025 | 6 (10.91) | 7 (17.95) |
*Continuous variables are expressed as median with minimum value and maximum value.
Model 1: crude association.
Model 2: adjustment for gender and BMI.
Abbreviations: 3-MT, 3-methoxytyramine; 5-HIAA, 5-hydroxyindoleacetic acid; BMI, body mass index; CSF, cerebrospinal fluid; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; IH, idiopathic hypersomnia; MHPG, 3-methoxy-4-hydroxyphenylglycol; NA, test not applicable; NSH, nonspecified hypersomnolence; NT1, narcolepsy type 1; NT2, narcolepsy type 2; OMD, 3-O-methyldopa; VMA, vanillylmandelic acid.
Association between CSF analytes, and clinical and neurophysiological characteristics
We found almost no significant association between any of the 13 detectable analytes and most of clinical (drug naïve vs withdrawal condition, ESS, duration of hypersomnolence) or neurophysiological characteristics (sleep efficiency, WASO, mean sleep latency on the MSLT, and the number of SOREMPS on the MSLT-PSG) in the whole sample, except for the nominal associations between tyramine and disease duration (detectable vs not, 5.6 years (3.7–37.8) vs 3.4 (0.02–45.7), p = 0.02), VMA and WASO (detectable vs not, 80.5 min (17–142) vs 35 (5–175), p = 0.03). We also found positive correlations between the number of SOREMPs and CSF 5-HIAA (r = 0.26, p = 0.03), MHPG (r = 0.32, p = 0.006), and norepinephrine levels (r = 0.38, p = 0.00002), and a negative correlation between 3-OMD and ESS (r = –0.26, p = 0.02).
In NT1 patients, we found almost no significant association between these analytes and clinical/neurophysiological characteristics (i.e. the same data tested above and frequency of cataplexy), except for the nominal associations between tyramine and disease duration (detectable vs not, 5.6 years (3.7–37.8) vs 2.6 (0.02–19.3), p = 0.01) and with WASO (detectable vs not, 91.0 min (59.0–120.0) vs 53 (17.0–175.0), p = 0.03). We found positive correlations between the number of SOREMPs and CSF MHPG (r = 0.46, p = 0.003) and OMD levels (r = 0.42, p = 0.009), and negative correlations between 3-OMD and ESS (r = –0.42, p = 0.01), and MHPG and ESS (r = –0.40, p = 0.02).
Discussion
In this study, we used the latest analytical technique, LC–MS/MS, to measure eleven biogenic amines and five trace amines in the CSF of 94 subjects referred for hypersomnolence: patients with NT1 (ORX deficient), with NT2 or IH (objective hypersomnolence without ORX deficiency), and patients without any confirmed central hypersomnolence disorder and with normal ORX levels (NSH). The following main results were obtained: (1) three trace amines were completely undetectable and five analytes were detected in less than 50% of samples; (2) no significant differences were found between the three groups for quantified biomarkers in adjusted statistical models, but CSF 5-HIAA levels tended to increase in NT1 compared to all other patients in the adjusted model; (3) in the whole sample and patients with NT1, most of the biomarkers were not significantly associated with ORX-A levels, clinical characteristics nor with neurophysiological parameters, but a few biomarkers (e.g. MHPG and norepinephrine) correlated with some parameters, ESS and the number of SOREMPs. However, given the number of comparisons made, the clinical significance of these nominal associations remains uncertain.
The brain circuitry controlling wake and sleep depends on several neurotransmitters, including glutamate and GABA interconnected with cholinergic and monoaminergic arousal systems [9]. Monoaminergic neurons that drive arousal produce norepinephrine, serotonin, dopamine, or histamine and, with the exception of the dopamine cells, share similar firing patterns with high firing rates during the wake, slow firing during NREM sleep, and almost no firing during REM sleep [28]. HVA, 5-HIAA and MHPG are the major degradation products of the monoamines dopamine, serotonin and noradrenaline, respectively, and their concentrations in the CSF reflect the turnover rates of the monoamines in the brain [29]. Studies published prior to the discovery of ORX found a decrease in CSF dopamine and 5-HIAA in both patients with IH and with narcolepsy [10]. Another group also reported dysregulation of the dopamine system in narcolepsy and of the norepinephrine system in IH [11, 12]. Together, these data suggested dysfunction of aminergic arousal systems in IH and narcolepsy. Since these publications, however, narcolepsy has been recognized as two subtypes, NT1 and NT2, which raises the possibility that some patients identified as IH in the previous studies would now be diagnosed as NT2. The neurobiology and neural basis of hypersomnolence in these rare sleep diseases are still unknown, and it is also unclear whether or not EDS is a direct consequence of ORX deficiency in NT1 [30].
Research on biogenic amines and their measurement in the CSF has benefited from research on the inherited monoamine neurotransmitter disorders [31]. In contrast to the earlier studies on narcolepsy, neurotransmitter profiles are now analyzed at specialized centers using advanced technologies such as HPLC with tandem mass spectrometry (MS/MS), providing reliable results to measure monoamines dopamine, serotonin and noradrenaline and several of their main metabolites. All analytes measured in the current study met the following bioanalytical criteria: percentage of accuracy within ±25% of theoretical (spiked) concentration, percentage of CV within 15%, and percentage of quality control (QCs) >67% at all QC levels. We confirmed here the feasibility, the sensitivity and selective quantification of these biomarkers in CSF; however, some analytes were at very low levels in human CSF samples (i.e. DOPAC, 3-MT, VMA). We also quantified five trace amines but only two, β-phenylethylamine and tyramine, were detectable. Trace amines are endogenous amino acid metabolites, colocalized and coreleased with biogenic amine transmitters, and are metabolized by monoamine oxidase [32]. To our knowledge, trace amines have never been measured in the CSF of patients with hypersomnolence, but their interest was due to recent studies showing that trace amine-associated receptor 1 (TAAR1) partial agonism increases wakefulness and decreases NREM and REM sleep in rats [21, 22], mice [33] and nonhuman primates [34]. Furthermore, TAAR1 partial agonists have beneficial effects in two mouse models of narcolepsy [35].
In the current study, all subjects underwent a standardized evaluation with systematic clinical, biological and neurophysiological assessments. We included only drug-free, well-characterized patients with NT1, NT2, and IH, all of whom had CSF ORX measurements and objective EDS on the MSLT, excluding IH patients who were characterized by an isolated increase in TST [36]. Overall, we found no between-group differences for the main stable monoamine metabolites (i.e. HVA, 5-HIAA, and MHPG) but also for the other neurotransmitters, metabolites, and trace amines tested. However, we found higher CSF 5-HIAA levels in patients with NT1 compared to all non-ORX deficient patients pooled together (NT2/IH and NSH) in the unadjusted model, with a similar trend after adjusting for BMI. This result is of interest given the well-known permissive role of serotonin neurotransmission for REM sleep expression. The serotoninergic neurons of the dorsal raphe nucleus are REM-sleep inhibiting neurons since they are silent during REM sleep [37, 38].
CSF ORX levels were not associated with analyte levels except β-phenylethylamine in patients with NT1; however, that biomarker was detectable in only one-third of patients. We found few associations between tyramine and the duration of EDS in the whole population or NT1, and negative correlations between 3-OMD, MHPG, and ESS scores in NT1. We also found positive correlations between the number of SOREMPs, 5-HIAA, MHPG, and norepinephrine levels in the whole population, and with MHPG and OMD levels in NT1. We suggest that these associations may relate to a decrease in locus coeruleus norepinephrine neuron activity that may contribute to EDS symptoms and high REM sleep propensity in NT1 [39]. If confirmed, the question of whether this association reveals a direct consequence of ORX deficiency or a compensatory mechanism in response to years of sleepiness remains open. A longer WASO was associated with detectable VMA in the whole sample and with detectable tyramine in NT1, highlighting a potential role for these biomarkers in increasing wakefulness during the nocturnal phase since fragmented nighttime sleep is common in NT1 [40]. However, these two biomarkers were detectable in less than 50% of samples with very low CSF concentrations. Moreover, given the number of statistical comparisons made and the absence of corrections for multiple testing, the nominal associations reported here are uncertain and need to be further confirmed. Finally, we did not find any association between CSF norepinephrine and serotoninergic metabolite levels and cataplexy frequency in NT1, yet both systems are apparently involved in the neurobiology of cataplexy in both animal and human narcolepsy [39, 41].
There are several limitations to the present study. Since lumbar puncture is an invasive procedure, we did not include healthy controls; rather, as a control group, we used subjects with a complaint of sleepiness who underwent the same standardized evaluation as other patients and who were without neurological or psychiatric comorbidities and without objective criteria of central hypersomnolence disorders. Due to limited power to individualize the groups and our strict inclusion criteria, we pooled patients with IH and NT2 into a single group with normal ORX levels but abnormal MSLT latency. Differential diagnosis of these disorders may be due to a difference of only one SOREMP on the MSLT, an often unstable marker in these conditions [42, 43]. Only very few patients with IH were included in our study, so it remains to be determined whether patients with IH, especially those with prolonged sleep time, have abnormal CSF monoamine levels. Our global population sample is small, especially when subdivided by patient groups, endophenotypes, and considering the numerous statistical tests performed. We have computed post hoc power calculations for the main three stable metabolites (i.e. HVA, 5-HIAA, and MHPG) for the three groups in our population. With the means of 69.1, 63.7, and 84.4 nM of CSF 5-HIAA levels in the groups of NSH, IH/NT2, and NT1, a common standard deviation of 39.8 and alpha risk of 0.016, 1,168 subjects per group (3,504 in total) would have been necessary to show significant between-group differences with a power of 0.80. Similar calculations indicate the necessity to include 4,446 patients for CSF HVA, and 4,011 patients to detect CSF MHPG level differences. Such sample sizes are almost impossible to obtain for orphan diseases. Even though the lumbar puncture procedure was standardized and performed between 6:00 pm and 7:00 pm after the last MSLT nap, ventricular CSF reaches the lumbar sac after several hours. Sleepiness, although objectively measured with MSLT, may be a fluctuating condition throughout the day. Although we used the latest advanced techniques to quantify these biomarkers, the deep-freezing and thawing of the samples may have affected the levels for some samples, as several metabolites and trace amines were at very low levels or even undetectable in the CSF. Also, we did not measure CSF histamine and telemethylhistamine levels and GABA-A receptor potentiation in this population, since such measurements have been previously reported [14, 17–19] and because some of the participants of the present study had been included in our previous studies [14, 17–19]. However, due to conflicting results to date [12–16], further studies should measure the levels of histamine, telemethylhistamine and histidine in the CSF of patients with hypersomnolence disorders. Another recent study on the CSF including a few patients with NT1 showed an increase in histidine (a precursor of histamine) and a decrease in histamine compared to a control population, suggesting decreased histamine synthesis in NT1, but without comparison with patients with NT2 and IH [15]. Finally, due to the lack of nonsleepy controls, it remains questionable to conclude whether the levels of CSF monoamine biomarkers are unrelated to the sleepiness condition or simply that they are universally abnormal among sleepy participants. Further studies are required to compare the results of CSF monoamine levels between sleepy and nonsleepy participants.
To conclude, we found no striking differences in CSF biogenic amines, their metabolites or trace amine levels, and few associations between analytes and key clinical and neurophysiological parameters in patients with NT1 (i.e. ORX deficiency), patients with objective EDS who were not ORX deficient, and patients without objective EDS. The levels of these biomarkers of the central monoaminergic systems did not vary between patients with IH and NT2, NT1, and NSH, except for a trend of higher CSF 5-HIAA levels in NT1 compared to non-orexin deficient patients. The clinical significance of these nominal associations remains uncertain. Nonetheless, these findings are a meaningful contribution to the field since there is a pressing need to identify new biomarkers, as well as to increase our understanding of the neurobiology of sleepiness in central hypersomnolence disorders, in order to better diagnose and treat the patients for personalized and precision medicine.
Supplementary Material
Acknowledgments
We thank all collaborators at the National Reference Center for Narcolepsy, Montpellier, France, especially for this study Sabine Scholz and Marie-Lou Rollin. We are indebted to all study participants.
Funding
Agence Nationale Recherche (ANR)-R14066FF, National Institutes of Health (NIH) R01 NS103529, and R01 NS098813.
Conflict of interest statement. Y. Dauvilliers received funds for seminars, board engagements. and travel to conferences by UCB Pharma, Jazz, Theranexus, Flamel. and Bioprojet. L. Barateau received funds for travelling to conferences by UCB Pharma. Claudio Ciardiello and Julien Roeser are full-time employees of Charles River Laboratories, CA, USA. T.S. Kilduff is a consultant for Alkermes plc, Idorsia Pharmaceuticals, Ltd, and Vida Ventures and has received research funding from Alkermes plc and Supernus Pharmaceuticals.
Author Contributions
Y.D.: data analysis and interpretation; study concept; study supervision; drafting/revising the manuscript; LB: data analysis and interpretation; drafting/revising the manuscript; I.J.: statistical analysis; revising the manuscript; C.C. and J.R.: data analysis and interpretation; revising the manuscript; T.K.: study concept; revising the manuscript.
References
- 1. de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 3. Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. [DOI] [PubMed] [Google Scholar]
- 4. Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. [DOI] [PubMed] [Google Scholar]
- 6. Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. [DOI] [PubMed] [Google Scholar]
- 7. Tabuchi S, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19): 6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tisdale RK, et al. Animal models of narcolepsy and the hypocretin/orexin system: past, present, and future. Sleep. Published online December 12, 2020. doi: 10.1093/sleep/zsaa278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saper CB, et al. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montplaisir J, et al. Narcolepsy and idiopthic hypersomnia: biogenic amines and related compounds in CSF. Neurology. 1982;32(11):1299–1302. [DOI] [PubMed] [Google Scholar]
- 11. Faull KF, et al. Cerebrospinal fluid monoamine metabolites in narcolepsy and hypersomnia. Ann Neurol. 1983;13(3):258–263. [DOI] [PubMed] [Google Scholar]
- 12. Faull KF, et al. Monoamine interactions in narcolepsy and hypersomnia: a preliminary report. Sleep. 1986;9(1 Pt 2):246–249. doi: 10.1093/sleep/9.1.246 [DOI] [PubMed] [Google Scholar]
- 13. Bassetti CL, et al. Cerebrospinal fluid histamine levels are decreased in patients with narcolepsy and excessive daytime sleepiness of other origin. J Sleep Res. 2010;19(4):620–623. [DOI] [PubMed] [Google Scholar]
- 14. Dauvilliers Y, et al. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep. 2012;35(10):1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimada M, et al. Metabolome analysis using cerebrospinal fluid from narcolepsy type 1 patients. Sleep. 2020;43(11). doi: 10.1093/sleep/zsaa095 [DOI] [PubMed] [Google Scholar]
- 16. Kanbayashi T, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;32(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishino S, et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009;32(2):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dauvilliers Y, et al. Absence of GABA-A receptor potentiation in central hypersomnolence disorders. Ann Neurol. 2016;80(2):259–268. [DOI] [PubMed] [Google Scholar]
- 19. Rye DB, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med. 2012;4(161):161ra151. [DOI] [PubMed] [Google Scholar]
- 20. Lindemann L, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324(3):948–956. [DOI] [PubMed] [Google Scholar]
- 21. Revel FG, et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry. 2012;72(11):934–942. [DOI] [PubMed] [Google Scholar]
- 22. Revel FG, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18(5):543–556. [DOI] [PubMed] [Google Scholar]
- 23. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 24. Iber C, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, NY: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 25. AASM: American Academy of Sleep Medicine. ICSD-3: International Classification of Sleep Disorders. 3rd ed.Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evangelista E, et al. Alternative diagnostic criteria for idiopathic hypersomnia: a 32-hour protocol. Ann Neurol. 2018;83(2):235–247. [DOI] [PubMed] [Google Scholar]
- 27. Bender R, et al. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54(4):343–349. [DOI] [PubMed] [Google Scholar]
- 28. Scammell TE, et al. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanley M, et al. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci. 1985;37(14):1279–1286. [DOI] [PubMed] [Google Scholar]
- 30. Mahoney CE, et al. The neurobiological basis of narcolepsy. Nat Rev Neurosci. 2019;20(2):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng J, et al. Monoamine neurotransmitter disorders—clinical advances and future perspectives. Nat Rev Neurol. 2015;11(10):567–584. [DOI] [PubMed] [Google Scholar]
- 32. Gainetdinov RR, et al. Trace amines and their receptors. Pharmacol Rev. 2018;70(3):549–620. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz MD, et al. Trace amine-associated receptor 1 regulates wakefulness and EEG spectral composition. Neuropsychopharmacology. 2017;42(6):1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goonawardena AV, et al. Trace amine-associated receptor 1 agonism promotes wakefulness without impairment of cognition in Cynomolgus macaques. Neuropsychopharmacology. 2019;44(8):1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Black SW, et al. Trace amine-associated receptor 1 agonists as narcolepsy therapeutics. Biol Psychiatry. 2017;82(9):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lammers GJ, et al. Reply to Maski K et al. commentary on diagnosis of central disorders of hypersomnolence: challenges in defining central disorders of hypersomnolence. Sleep Med Rev. 2020;52:101326. [DOI] [PubMed] [Google Scholar]
- 37. Monti JM. Serotonin control of sleep–wake behavior. Sleep Med Rev. 2011;15(4):269–281. [DOI] [PubMed] [Google Scholar]
- 38. Wisor JP, et al. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14(2):233–238. [DOI] [PubMed] [Google Scholar]
- 39. Szabo ST, et al. Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med Rev. 2019;43:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roth T, et al.. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2013;9(9):955–965. doi: 10.5664/jcsm.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hasegawa E, et al. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124(2):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trotti LM, et al. Test–retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9(8):789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lopez R, et al.. Test–retest reliability of the multiple sleep latency test in central disorders of hypersomnolence. Sleep. 2017;40(12):zsx164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.