Abstract
Study Objectives
To determine whether actigraphy-measured sleep was independently associated with risk of frailty and mortality over a 5-year period among older adults.
Methods
We used data from Waves 2 (W2) and 3 (W3) (2010–2015) of the National Social Life, Health and Aging Project, a prospective cohort of community-dwelling older adults born between 1920 and 1947. One-third of W2 respondents were randomly selected to participate in a sleep study, of whom N = 727 consented and N = 615 were included in the analytic sample. Participants were instructed to wear a wrist actigraph for 72 h (2.93 ± 0.01 nights). Actigraphic sleep parameters were averaged across nights and included total sleep time, percent sleep, sleep fragmentation index, and wake after sleep onset. Subjective sleep was collected via questionnaire. Frailty was assessed using modified Fried Frailty Index. Vital status was ascertained at the time of the W3 interview. W3 frailty/mortality status was analyzed jointly with a four-level variable: robust, pre-frail, frail, and deceased. Associations were modeled per 10-unit increase.
Results
After controlling for baseline frailty (robust and pre-frail categories), age, sex, education, body mass index, and sleep time preference, a higher sleep fragmentation index was associated with frailty (OR = 1.70, 95% CI: 1.02–2.84) and mortality (OR = 2.12, 95% CI: 1.09–4.09). Greater wake after sleep onset (OR = 1.24, 95% CI: 1.02–1.50) and lower percent sleep (OR = 0.41, 95% CI: 0.17–0.97) were associated with mortality.
Conclusions
Among community-dwelling older adults, actigraphic sleep is associated with frailty and all-cause mortality over a 5-year period. Further investigation is warranted to elucidate the physiological mechanisms underlying these associations.
Keywords: sleep, actigraphy, aging, frailty, mortality
Statement of Significance.
Few cohort studies have evaluated the associations of sleep with frailty and mortality using objective sleep measures among older adults. We found that older adults with actigraphic indices of disrupted sleep had an increased risk of becoming frail and dying over a 5-year period.
Introduction
Decreases in child mortality and increases in average life expectancy have contributed to the growing number of older adults worldwide. By 2050, over a fifth of the world’s population (>2 billion people) will be 60 years of age or older, representing a 9.2% increase in this age group since 1990 [1]. Frailty is a common age-related, multidimensional condition that often precipitates negative outcomes, including mortality [2–4]. There are two distinct, but widely recognized, frailty paradigms [5]: the Deficit Accumulation Model [6] and the Frailty Phenotype [7]. The Deficit Accumulation Model views frailty in terms of health deficits, or the number of conditions/diseases present, and is often used to explain the variability in health status as individuals age [6]. The Frailty Phenotype considers frailty as a syndrome and is characterized by decreased reserve across multiple physiologic systems. The Frailty Phenotype is often measured by the Fried Frailty Index, which uses pre-determined symptoms to assess the presence of frailty (e.g. three or more of the following symptoms indicates frailty: low lean muscle mass, self-reported exhaustion, muscle weakness, slow walking speed, and low energy expenditure) [8]. The Frailty Phenotype may be more useful to interrogate mechanisms of sub-clinical/clinical frailty because individuals are stratified into distinct risk categories and shared pathways can be identified for prevention and remediation [6].
Older adults who are frail are more vulnerable to poor health outcomes (e.g. disability, hospitalization, and mortality) when faced with stressors due to a decline in function and physiologic reserve [8–10]. Frailty may be reversible when treated with appropriate interventions [9]. Therefore, identifying individuals at risk for frailty and developing upstream interventions that promote physiologic recovery may prevent progression to frailty and lengthen healthspan for older adults. Physiologic restoration and repair for multiple organ systems occur during sleep [11], and sleep is a modifiable target for behavioral interventions. Well-described age-related changes in sleep, including decrements in non-rapid eye movement (NREM) sleep and reductions in sleep quality and quantity [12], may limit sleep-facilitated recovery and further erode physiologic reserve [11–14]. Half of older adults report difficulties sleeping and many experience short sleep duration (<7 h/night) [15], more frequent awakenings, and more fragmented sleep [11, 14, 16–18].
Sleep may impact frailty in several ways, including reduced energy expenditure, tissue growth and repair, a heightened inflammatory response, and disturbed hormonal pathways (e.g. plasma growth hormones, insulin-like growth factor I, prolactin, and leptin) [19, 20]. These pathways and others may explain the associations observed among sleep problems, frail states, and mortality at advanced ages. In particular, sleep fragmentation has been associated with decreased energy and increased role limitations [21]—two symptoms of frailty [8].
Previous studies suggest that poor sleep is a risk factor for frailty [22–26] and mortality [18, 23, 27–35]. However, findings are inconsistent and many of these studies are based on self-reported sleep assessments and cross-sectional designs. Additionally, poor sleep may partially explain the higher prevalence of frailty observed among women [36], since women report more sleep problems, including trouble initiating and maintaining sleep [37, 38], and sex differences through shared pathways (e.g. inflammatory cytokines and muscle quality) have been reported [39]. Of the few published prospective studies using objectively measured sleep, most use sex-specific cohorts, which limit our understanding of sleep characteristics in the general population and formal examination of sex differences. For example, in the Osteoporotic Fractures in Men Study (MrOS), Ensrud et al. found that greater nighttime wakefulness, as measured by wake after sleep onset (WASO), was associated with frailty and mortality over an average follow-up period of 3.4 years [23]. In the Women’s Health Initiative Study, Kripke et al. found associations of lower baseline actigraphic sleep efficiency and abnormal sleep duration (i.e. <300 and >390 min) with reduced survival among postmenopausal women [29, 40]. To the best of our knowledge, there are no published longitudinal studies of community-dwelling older adults that have used an actigraphic sleep fragmentation index that integrates the total sleep time (TST), and the frequency and duration of awakenings during the night [41] to assess the potential link between sleep fragmentation and frailty/mortality. This is an important research gap because older adults often experience recurrent awakenings at night, which is not necessarily captured with other measures of sleep fragmentation, such as WASO.
The goal of this study was to investigate the association between objectively measured sleep, quantified using wrist actigraphy, and 5-year risk of frailty and all-cause mortality among community-dwelling older adults from the National Health, Social Life, and Aging Project (NSHAP) ancillary sleep study [42]. We hypothesized that greater periods of wakefulness after sleep onset (i.e. WASO), more fragmented sleep, lower percent sleep, and short (<7 h) and long (≥8 h) TST (vs. intermediate-duration sleep) [15], would be associated with an increased risk of frailty and all-cause mortality over the 5-year follow-up period. Based on evidence that women experience more sleep problems [37, 38] and frailty, [39] we hypothesized that the sleep-frailty/mortality association would differ by sex [43].
Methods
Data source
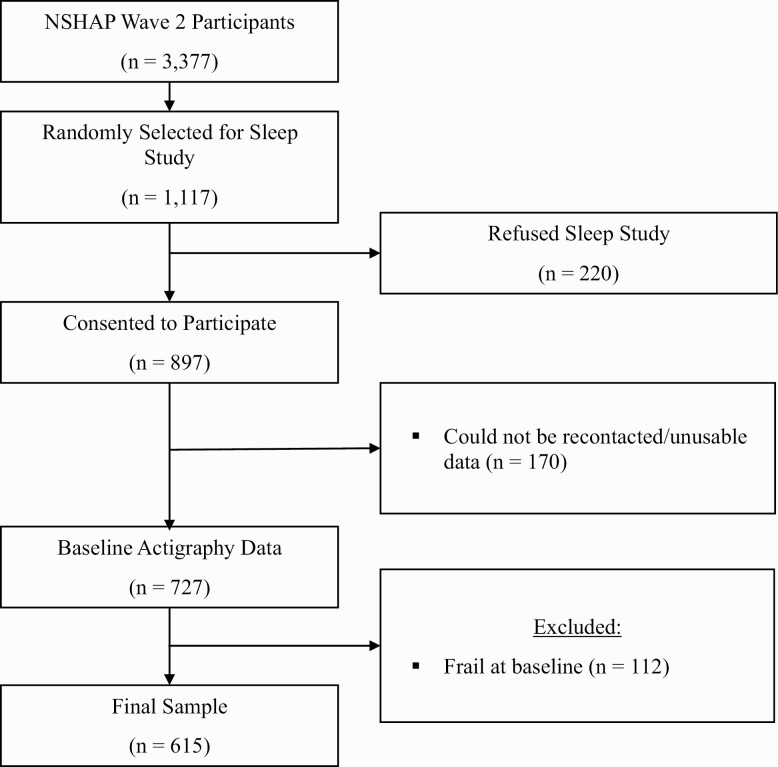
NSHAP is a prospective cohort of community-dwelling older adults born between 1920 and 1947 with three waves of data collection (2005–2015) [44, 45]. At each wave, home interviews were conducted by trained field interviewers to collect survey, biometric, and functional data. In wave 2, one-third of respondents were randomly selected to participate in an ancillary sleep study (n = 1,117) [42]. Of these, 220 refused to participate and 170 could not be re-contacted or did not have usable data. To assess frailty risk, we excluded individuals who were frail at baseline (n = 112), resulting in a total sample of 615 participants (Figure 1). The distribution of the analytic sample was comparable to the distributions of the sociodemographic and sleep variables in the original sleep study (Supplementary Table 1). Herein, we refer to the wave 2 sleep study as “baseline” and wave 3 as “follow-up.” NSHAP data are publicly available and considered exempt from IRB review.
Figure 1.
Derivation of the analytic sample.
Outcome: frailty status and mortality
Frailty status was assessed using a modified version of the Fried Frailty Index, a commonly used measure to discern the frailty phenotype with high reliability and validity [8, 46, 47]. A description of the frailty components and their measures are described in Table 1. One point was assigned for each frailty component present and scores were summed to create a composite variable using the established cut points: 0 = robust, 1–2 = pre-frail, ≥3 = frail (range = 0–5). Mean replacement was used for participants with missing data on the individual frailty components (n = 50) prior to creating a composite score, whereby missing values were replaced by the mean of each particular component across the analytic sample [48]. NSHAP investigators confirmed mortality status either by speaking with the respondent (if living), through a proxy interview, or examination of public records (if deceased). Deceased individuals were defined as those that died between the baseline and follow-up exams. Since frailty and mortality are competing risks, we jointly analyzed frailty and mortality status at follow-up using an ordinal outcome variable with four levels: robust, pre-frail, frail, and deceased [23].
Table 1.
Frailty Measurement in the National Social Life, Health and Aging Project (NSHAP) Cohort
| Allocation of points | ||
|---|---|---|
| Fried frailty index | NSHAP measure | One point was assigned if the following criteria were met: |
| 1.Slowness | Timed 3-m walk tests were conducted by a trained field interviewer during the home visit. | The test was completed in ≥5.7 s, or if the participant was wheelchair bound or could not complete it independently. |
| 2.Weakness | Timed chair stands were conducted by a trained field interviewer during the home visit. | The test was completed in ≥16.7 s, or if the participant was wheelchair bound or could not complete it independently. |
| 3.Exhaustion | Self-reported on the questionnaire Centers for Epidemiologic Studies and Depression Scale items “everything was an effort” or “they could not get going.” | Participants reported that “everything was an effort” or “they could not get going” occasionally or most of the time. |
| 4.Shrinking | Weight assessments taken by the field interviewer were used to derive weight loss between waves. | Calculated weight loss of 10% or more. |
| 5.Low physical activity | Self-reported exercise on the questionnaire | Participant reported exercising <3 times per month. |
Scores were summed to create a composite variable based on established cut points (range 0–5): 0 = robust, 1–2 = pre-frail, ≥ 3 = frail.
Actigraphic parameters of sleep–wake patterns
Data collection for the NSHAP Sleep Study has been described elsewhere [42, 49]. Briefly, participants were asked to wear an actigraph (Actiwatch Spectrum; Philips Respironics, Murrysville, Pennsylvania) for 72 h (mean = 2.93 ± 0.01 nights). Data were analyzed using the validated settings in the Philips Respironics software (version 5.59). Actigraphic data were scored by NSHAP investigators blinded to the other data collected for the participant. Rest intervals were set according to data from the light sensor on the actigraph and the time stamp on the participant-initiated event marker at each bed and waking time and were cross-checked with the participants’ sleep diary. The software scores each 15-s epoch as either sleep or non-sleep based on activity counts within each epoch and adjacent counterparts. The sleep interval is defined as the period within each rest interval beginning with the first epoch scored as sleep and ending with the last epoch scored as sleep [42].
Sleep measures derived from the software included nighttime TST, WASO, percent sleep, and sleep fragmentation. TST and WASO are the summed duration of all epochs scored as sleep and wake after sleep inception, respectively. Percent sleep is the percentage of the sleep interval spent asleep and is measured as the TST divided by the length of the sleep interval. The sleep fragmentation index measures restless sleep and is defined by the manufacturer’s software as the sum of two percentages: (1) the percentage of epochs in the sleep period spent moving; and (2) the percentage of immobile periods that last for only one minute in duration. Fragmentation is a useful metric because it is an indicator of sleep disruption or disturbance [50, 51]. Additionally, sleep fragmentation attempts to estimate microarousals [52], which have been associated with poor health outcomes [53]. Sleep parameters were averaged over the total number of nights the actigraph was worn and were analyzed as continuous variables per 10-unit increase. TST was categorized as <7 h, ≥7 to <8 h [reference], and ≥8 h based on the recommendations of the National Sleep Foundation for older adults [15] and analyzed as a categorical variable to account for the non-linear relationship with frailty/mortality status.
Self-reported sleep characteristics
Participants were asked to report the time they tried to start falling asleep and the time they awoke on weekdays and weekends during the in-home interview. The duration of sleep was calculated as the average between wake and sleep times for weekdays and weekends separately. The average number of hours slept per week was calculated as: [((weekday sleep hours × 5) + (weekend sleep hours × 2))/7] and was categorized similar to the TST (<7 h, ≥7 to <8 h, [reference], and ≥8 h). Participants were asked to report their sleep quality with the following questions: “how often do you feel really rested when you wake up in the morning?” and “during the past week, how often was your sleep restless?” Response options for both questions were rarely, never, sometimes, and most of the time, and were dichotomized for analyses (rarely/never vs. sometimes/most of the time). Napping was assessed by the question “during the past week, on how many days did you nap for an hour or two?” Based on the distribution, responses were collapsed into none vs. any napping. Participants were also surveyed on the use of sleep medications or other sleep aides (yes/no).
Covariates
A number of covariates were selected as potential confounders based on their known associations with sleep and frailty/mortality in the literature, including age (62–65, 66–75, 76+ years), race (white/non-white), sex, marital status (married or cohabitating partner/single), education (high school or less/some college or more), self-reported health (excellent/good vs. fair/poor), smoking (current vs. former/never), alcohol use (≥4 days per week vs. <4 days per week), current psychotropic medication use (yes/no), NSAID use (yes/no), body mass index (BMI), social isolation, a modified version of the Charlson Comorbidity Index, depressive symptoms, the Montreal Cognitive Assessment adapted for Survey Administration (MoCA-SA), sleep time preference, season of data collection, and baseline frailty (robust and pre-frail categories). Height and weight were collected by field interviewers during the home interview; BMI was calculated as [(weight (lbs)/height (in)2)*703] [54]. The social isolation index is a validated scale that includes emotional and tangible support from spouses, family and friends, perceived feelings of isolation, a lack of companionship, and feeling left out (range 0–12) [55]. A modified Charlson Comorbidity Index was constructed based on self-reported survey responses and included heart conditions, stroke, cancer, diabetes, hypertension, COPD/asthma, arthritis, urinary incontinence, and Alzheimer’s disease/dementia (range 0–12) [56]. Depressive symptomatology was assessed using the 11-item Iowa short version of the Centers for Epidemiologic Studies-Depression (CES-D) scale, excluding the items “restless sleep,” “everything was an effort,” and “could not get going” [57]. The MoCA-SA is a scale that assesses eight domains of cognitive function, including orientation, naming, visuoconstruction, executive function, attention, abstraction, memory, and language (range 0–20) [58]. Scores for the social isolation, comorbidity, CES-D, and MoCA-SA scales were summed separately with higher scores indicating higher levels of social isolation, number of comorbidities, depressive symptomatology, and cognitive function, respectively. Sleep time preference (i.e. sleep midpoint) relates to the timing of biological and behavioral activities, including when individuals go to bed and wake up in the morning. In accordance with previously published methods, sleep time preference was derived from actigraphy data and calculated as the average midpoint of the actigraphic sleep interval over the 72-h period and was categorized as 8:00 pm to 1:59 am, 2:00–2:59 am, 3:00–8:59 am [59, 60]. The season in which actigraphy data were collected was derived by extracting the month from the date of data collection. The months October–March were coded as seasons “fall/winter” and April–September as “spring/summer,” in accordance with other NSHAP studies” [60].
Statistical analysis
Differences in participant characteristics at baseline by frailty/mortality status at follow-up were compared using chi-squared tests for categorical variables and simple linear regression for continuous variables (Tables 2 and 3). Pearson correlations between sleep and frailty/mortality parameters with Bonferroni Adjustment are presented in Supplementary Table 2. The associations between sleep parameters and the ordinal outcome, frailty/mortality status, were first assessed using ordinal logistic regression models. However, the proportionality assumption was not satisfied, and therefore we used multinomial logistic regression, comparing each level of the outcome to the robust category. Sex was identified a priori as a potential effect modifier and was tested by adding an interaction term to the models. The interaction was not statistically significant, and sex was treated as a potential confounder instead.
Table 2.
Baseline Characteristics of Participants According to Frailty* and Mortality Status at Follow-Up (n = 615)
| Overall | Robust | Pre-Frail | Frail | Deceased | ||
|---|---|---|---|---|---|---|
| n = 615 | n = 151 | n = 285 | n = 90 | n = 89 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | P # | |
| Age, years | ||||||
| 62–65 | 133 (24.5) | 44 (31.5) | 68 (27.0) | 13 (15.6) | 8 (11.5) | <0.01 |
| 66–75 | 284 (46.2) | 84 (53.6) | 136 (48.0) | 41 (46.8) | 23 (24.3) | |
| 76+ | 198 (29.2) | 23 (14.9) | 81 (25.0) | 36 (37.6) | 58 (64.2) | |
| Race | ||||||
| White | 465 (84.5) | 123 (87.8) | 209 (85.2) | 61 (75.1) | 72 (86.2) | 0.06 |
| Nonwhite | 148(15.5) | 28 (12.2) | 75 (14.8) | 28 (24.8) | 17 (13.8) | |
| Gender | ||||||
| Male | 304 (48.5) | 73 (48.2) | 136 (45.9) | 39 (43.2) | 56 (64.0) | 0.09 |
| Female | 311 (51.5) | 78 (51.8) | 149 (54.1) | 51 (56.8) | 33 (36.0) | |
| Marital status | ||||||
| Married/cohabitating partner | 450 (69.5) | 117 (73.5) | 217 (72.7) | 61 (59.3) | 55 (61.6) | 0.09 |
| Not married | 165 (30.5) | 34 (26.5) | 68 (27.3) | 29 (40.7) | 34 (38.4) | |
| Education | ||||||
| High school or less | 371 (63.3) | 96 (63.9) | 177 (65.4) | 42 (51.1) | 56 (68.1) | 0.17 |
| Some college or more | 244 (36.7) | 55 (36.1) | 108 (34.6) | 48 (48.9) | 33 (31.9) | |
| Self-reported health | ||||||
| Excellent or good | 310 (52.3) | 109 (73.0) | 150 (53.6) | 27 (29.9) | 24 (30.9) | <0.01 |
| Fair, poor, or very poor | 305 (47.7) | 42 (27.0) | 135 (46.4) | 63 (70.1) | 65 (69.1) | |
| Modified Charlson Comorbidity Index (range 0–11),† mean, SE | 2.2 (0.1) | 1.6 (0.1) | 2.1 (0.1) | 2.7 (0.2) | 3.1 (0.2) | <0.01 |
| Depressive symptoms (range 0–15), mean, SE | 2.3 (0.1) | 1.5 (0.3) | 2.6 (0.2) | 3.0 (0.5) | 2.5 (0.2) | 0.02 |
| MoCA-SA score (range 0–20),‡ mean, SE | 14.1 (0.1) | 14.3 (0.2) | 14.1 (0.2) | 13.7 (0.3) | 13.9 (0.3) | 0.15 |
| BMI§ | 28.9 (0.3) | 27.5 (0.5) | 29.2 (0.4) | 31.2 (0.8) | 28.0 (0.7) | 0.04 |
| Current Smoker | 68 (13.0) | 13 (11.9) | 31 (13.3) | 11 (10.2) | 13 (17.2) | 0.80 |
| Alcohol Use >4 days per week | 102 (28.9) | 30 (31.5) | 46 (29.0) | 13 (22.6) | 13 (29.6) | 0.78 |
| Social Isolation Index (range 0–12)||, mean, SE | 6.2 (0.1) | 6.3 (0.2) | 6.3 (0.1) | 6.3 (0.2) | 5.8 (0.4) | 0.28 |
| Current psychotropic medication use¶ | 132 (22.4) | 17 (12.3) | 60 (23.7) | 27 (30.4) | 28 (29.3) | 0.01 |
| Current NSAID Use | 74 (12.2) | 21 (12.4) | 33 (12.7) | 13 (15.7) | 7 (5.8) | 0.35 |
| Baseline frailty | ||||||
| Robust | 222 (37.6) | 89 (57.7) | 97 (34.1) | 18 (29.0) | 18 (19.8) | <0.01 |
| Pre-frail | 393 (62.4) | 62 (42.3) | 188 (65.9) | 72 (71.0) | 71 (80.2) |
Bold font indicates p < 0.05.
BMI = body mass index, MoCA-SA = Montreal Cognitive Assessment adapted for Survey Administration, SE = standard error of the mean.
*Frailty scale: slow chair stand, slow gait speed, exhaustion, weight loss, and low physical activity.
†Modified Charlson Comorbidity Index included heart conditions, stroke, cancer, diabetes, hypertension, chronic obstructive pulmonary disease/asthma, arthritis, depression, urinary incontinence, and Alzheimer’s disease/dementia.
‡MoCA-SA: orientation, naming, visuoconstruction, executive function, attention, abstraction, memory, and language.
§BMI was calculated as weight (kg)/height (m) [2].
||Social Isolation Index included emotional and tangible support from spouses, family, and friends, perceived feelings of isolation, a lack of companionship, and feeling left out.
¶Psychotropic medication use included antidepressants, antipsychotics, anxiolytics/sedatives, hypnotics, and CNS stimulants.
# p-Value is derived from χ 2 tests or simple linear regression for continuous variables using the survey procedures in SAS and weighted for non-response in Wave 2.
Table 3.
Baseline Sleep Characteristics by Frailty and Mortality Status at Follow-Up
| Robust | Pre-frail | Frail | Deceased | ||
|---|---|---|---|---|---|
| n = 151 | n = 285 | n = 90 | n = 89 | ||
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | P* | |
| Actigraphic sleep characteristics | |||||
| WASO, minutes | 32.9 (1.7) | 36.9 (1.6) | 39.2 (2.4) | 44.3 (3.2) | <0.01 |
| Fragmentation index | 12.8 (0.5) | 13.7 (0.4) | 15.2 (0.7) | 16.4 (0.9) | <0.01 |
| Percent sleep | 92.8 (0.4) | 92.2 (0.3) | 91.7 (0.5) | 91.0 (0.8) | 0.01 |
| Total sleep time, minutes | 424.9 (5.9) | 437.5 (5.7) | 427.9 (12.6) | 432.2 (12.7) | 0.77 |
| Total sleep time, hours | |||||
| <7 h | 68 (46.4) | 113 (36.1) | 38 (46.1) | 39 (44.9) | 0.27 |
| 7–8 h | 54 (34.9) | 107 (39.4) | 25 (28.9) | 21 (26.5) | |
| 8+ h | 29 (18.7) | 65 (24.5) | 27 (26.5) | 29 (28.6) | |
| Sleep time preference† | |||||
| 8:00 pm to 1:59 am | 33 (21.9) | 51 (16.1) | 27 (38.5) | 22 (26.1) | <0.01 |
| 2:00 am to 2:59 am | 54 (41.5) | 90 (36.7) | 15 (19.1) | 30 (36.6) | |
| 3:00 am to 9:00 am | 58 (36.6) | 128 (47.2) | 39 (42.4) | 34 (37.3) | |
| Season‡ | |||||
| Spring/summer | 34 (22.8) | 59 (17.1) | 8 (9.8) | 15 (18.3) | 0.11 |
| Fall/winter | 117 (77.2) | 226 (82.9) | 82 (90.2) | 74 (81.7) | |
| Self-reported sleep characteristics | |||||
| Sleep duration, hours | |||||
| <7 h | 12 (9.1) | 20 (7.7) | 6 (9.1) | 8 (10.8) | 0.16 |
| 7–8 h | 42 (34.3) | 47 (22.5) | 13 (17.7) | 12 (19.6) | |
| 8+ h | 81 (56.7) | 164 (69.9) | 57 (73.2) | 54 (69.5) | |
| Wake feeling rested (rarely/never) | 10 (7.2) | 34 (14.1) | 12 (11.3) | 9 (10.7) | 0.19 |
| Restless sleep (most or some of the time) | 25 (19.1) | 52 (19.0) | 28 (27.8) | 16 (19.4) | 0.52 |
| Napped for 1–2 h (past week) | 42 (33.3) | 98 (37.2) | 42 (54.2) | 35 (49.7) | 0.04 |
| Sleep medication/treatment (past 2 weeks) | 25 (15.0) | 53 (21.1) | 21 (27.0) | 14 (14.3) | 0.20 |
Bold font indicates p < 0.05.
SE = standard error of the mean, WASO = wake after sleep onset.
*p-Value is derived from χ 2 tests or simple linear regression using the survey procedures in SAS and weighted for non-response in Wave 2.
†Sleep time preference is defined as the average midpoint of the sleep interval over the 3 days of actigraphy.
‡The seasons “fall/winter” were coded as months October–March and “spring/summer” as months “April–September.”
Covariates were included in the final multivariable model if they were statistically significantly associated with frailty/mortality status at follow-up. Sleep parameters were tested in separate models to avoid multicollinearity. We conducted three sets of nested models for each sleep parameter. The first model was adjusted for baseline frailty (robust and pre-frail categories) and sociodemographic factors (age, sex, and education). The second model controlled for model 1 covariates plus BMI and sleep time preference. The third model controlled for model 1 and 2 covariates and additionally adjusted for the modified Charlson Comorbidity Index, depressive symptoms, self-rated health, psychotropic medication use, and napping. The results were similar across models 2 and 3 (Supplementary Table 3), so we present the two most parsimonious models (models 1 and 2).
To determine if including the pre-frail category at baseline influenced the associations between sleep disturbances and frailty/mortality status at follow-up, we performed a sensitivity analysis limiting the sample to robust participants at baseline. Additionally, we compared participants with complete frailty data to those with mean replaced missing data. The conclusions of the sensitivity analyses were the same (Supplementary Tables 4 and 5) and therefore, we retained pre-frail individuals in the analytic sample and present the analysis with mean replacement. Additionally, to assess the impact of potential actigraphic measurement error, we conducted a sensitivity analysis excluding participants with percent sleep <50% and/or a TST of ≤180 or ≥720 min (n = 22). Results were similar, so we retained these individuals in the final models (data not shown). Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). All p-values were from 2-sided tests and results were considered statistically significant at p < 0.05. Analyses were performed with SAS version 9.4 using the survey procedures to account for the complex survey design.
Results
Participant characteristics
Among the 615 older adults (mean age = 71.5 years) included in the sample, 222 (37.6%) were robust and 393 (62.4%) were pre-frail at baseline. Most of the participants were aged 66–75 years old (46.2%), white (84.5%), married (69.5%), had a high school education or less (63.3%), and self-reported excellent or very good health (52.3%) at baseline (Table 2). The mean MoCA-SA score, number of comorbidities, and depressive symptoms were 14.1 (SE = 0.1), 2.2 (SE = 0.1), and 2.3 (SE = 0.1), respectively. At the 5-year follow-up (mean 4.8 years), 151 (24.7%) individuals were classified as robust, 285 (46.2%) were pre-frail, 90 were frail (14.8%), and 89 (14.3%) were deceased. The average WASO was 37.2 min (SE = 1.4 min) and the mean TST was 432.2 min (SE = 2.7 min) (Table 3). The mean sleep fragmentation index was 14.0 (0.4) and percent sleep was 92.1 (SE = 0.3). Older age, poorer self-rated health, higher BMI, having more comorbidities and depressive symptoms, taking psychotropic medications, napping, and sleep time preference at baseline were significantly associated with frailty/mortality status at follow-up (Tables 2 and 3).
Correlations of sleep parameters at baseline
WASO, sleep fragmentation, and percent sleep were strongly correlated with each other (Bonferroni adjusted p < 0.003, Supplementary Table 2). TST was moderately correlated with sleep fragmentation (r = –0.25, p < 0.003) and percent sleep (r = 0.23, p < 0.003). TST was not correlated with self-reported sleep duration (r = –0.08, p > 0.003).
Associations of sleep at baseline with frailty and mortality at follow-up
On average, individuals who were frail or deceased at follow-up had greater baseline WASO and sleep fragmentation, and lower percent sleep (p ≤ 0.01, Table 3). Adjusting for baseline frailty and sociodemographic factors, a higher sleep fragmentation index was associated with a greater odds of frailty (OR = 1.95, 95% CI: 1.12–3.40, per 10-unit increase) and mortality (OR = 2.19, 95% CI: 1.16–4.12, per 10-unit increase) (Table 4). Greater WASO (OR = 1.24, 95% CI: 1.04–1.49, per 10-min increase) and lower percent sleep (OR = 0.40, 95% CI: 0.17–0.92, per 10-percent increase) were also associated with mortality.
Table 4.
Associations of Baseline Sleep Characteristics with Frailty and Mortality Status at Follow-Up*
| Model 1† | Model 2‡ | |||||
|---|---|---|---|---|---|---|
| Pre-frail | Frail | Deceased | Pre-frail | Frail | Deceased | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| WASO, minutes§ | 1.10 (0.99–1.23) | 1.14 (0.98–1.33) | 1.24 (1.04–1.49) | 1.10 (1.00–1.21) | 1.12 (0.97–1.31) | 1.24 (1.02–1.50) |
| Fragmentation index§ | 1.24 (0.79–1.95) | 1.95 (1.12–3.40) | 2.19 (1.16–4.12) | 1.19 (0.75–1.89) | 1.70 (1.02–2.84) | 2.12 (1.09–4.09) |
| Percent sleep§ | 0.65 (0.38–1.10) | 0.54 (0.26–1.13) | 0.40 (0.17–0.92) | 0.67 (0.41–1.09) | 0.62 (0.30–1.29) | 0.41 (0.17–0.97) |
| Total sleep time, hours | ||||||
| <7 h | 0.66 (0.37–1.17) | 1.17 (0.44–3.12) | 1.08 (0.47–2.48) | 0.57 (0.33–1.01) | 0.86 (0.34–2.16) | 1.06 (0.48–2.34) |
| 7–8 h | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 8+ h | 1.07 (0.58–1.97) | 1.30 (0.58–2.88) | 1.58 (0.71–3.51) | 1.01 (0.57–1.80) | 1.12 (0.51–2.45) | 1.48 (0.64–3.43) |
Bold indicates p < 0.05.
95% CI = 95% confidence interval, OR = odds ratio, SE = standard error of the mean, WASO = wake after sleep onset.
*Models estimated using multinomial logistic regression using the robust category as the reference group.
†Model 1 controls for baseline frailty (robust and pre-frail categories), age, sex, and education.
‡Model 2 controls for baseline frailty (robust and pre-frail categories), age, sex, education, BMI, and sleep time preference.
§WASO, fragmentation index, and percent sleep are modeled per 10-unit increase.
After further adjustment for BMI and sleep time preference (model 2), a higher sleep fragmentation index was associated with a greater odds of frailty (OR = 1.70, 95% CI: 1.02–2.84, per 10-unit increase) and mortality (OR = 2.12, 95% CI: 1.09–4.09, per 10-unit increase). Greater WASO (OR = 1.24, 95% CI: 1.02–1.50, per 10-min increase) and lower percent sleep were associated with a greater odds of mortality (OR = 0.41, 95% CI: 0.17–0.97, per 10-percent increase).
Discussion
The present study highlights the importance of disrupted sleep with respect to critical late-life outcomes of frailty and mortality. This study is the first, to the best of our knowledge, to show that greater sleep fragmentation (i.e. higher frequency and duration of awakenings throughout the night), as measured using an actigraphic index of sleep fragmentation, is associated with an increased risk of frailty and mortality over a 5-year period in a sample of community-dwelling older adults. Our results are consistent with findings from other cohort studies using actigraphy-derived sleep measures, demonstrating statistically significant associations of greater nighttime wakefulness (measured by WASO) with mortality [23, 32]. Sleep interventions that improve symptoms of insomnia (i.e. nighttime awakenings) may reduce sleep fragmentation and help consolidate sleep [61].
In our study, actigraphically measured sleep duration was not associated with frailty or mortality. Prior cohort studies assessing the link between sleep duration, frailty, and mortality have reported inconsistent associations, with some investigations showing that longer [22, 24, 25, 27, 28, 30, 35, 62] and shorter sleep duration [25, 28, 29] are associated with mortality, while others report null findings [23, 31–33]. Such divergent conclusions may be due to differences in measurement and/or measurement error, as subjective sleep duration is systematically overreported [63] and poor agreement between self-reported and actigraphic measures of sleep duration have been described [63–66]. For example, most studies reporting an association of long sleep duration with frailty and mortality used self-report sleep measures [22, 24, 25, 27, 30, 35, 62], and several studies that used actigraphy reported null findings [23, 32, 33], in line with our results.
Sleep has been shown to play an important role in maintaining cognitive [67, 68] and physical health [68]. However, disease processes and normative aging affect sleep architecture, such that older adults spend less time in non-REM slow-wave sleep (stage N3) or “deep sleep” and more time in the lighter stages of sleep (stages N1 and N2) [11, 12, 14]. Stage N3 is the deepest and most rejuvenative of the sleep stages, impacting multiple organ systems. For example, stage N3 sleep is where energy is restored, hormones essential for muscle growth and development are released, and tissue growth and repair occurs [69]. Sleep fragmentation erodes deep sleep and may interrupt or perturb these restorative processes, contributing to multisystem functional decline (i.e. frailty). For older adults with diminishing physiologic reserve, impaired restoration resulting from fragmented sleep may further decrease resilience to physical and mental stressors and accelerate progression to a frail state (or death) because compensatory mechanisms and repair processes have become exhausted.
Circadian disruption is another potential mechanism that may underlie the manifestation of sleep fragmentation in older adults and contribute to poor health outcomes. For example, older adults experience an age-related decrease in sensitivity to light from either inadequate light during the day or exposure to light at night, resulting in sleep difficulties [70, 71]. Finally, other factors such as nocturia may disrupt sleep. While we did not measure nocturia, we controlled for urinary incontinence within the comorbidity index and robust associations remained. Further research is needed to better understand the mechanisms that may link sleep disruption and fragmentation to frailty and mortality risk.
Interventions aimed at improving sleep may benefit individuals at risk for frailty. For example, behavioral therapies for insomnia, such as sleep restriction and stimulus control may reduce sleep fragmentation and help consolidate sleep [61]. Additionally, fragmented daytime physical activity is associated with mortality [72, 73], and evidence suggests that exercise and increased physical activity improve many aspects of sleep [7, 74–76] and directly targets several frailty symptoms (e.g. weakness, slowness, and low physical activity) [77]. Future studies should consider the synergistic effects of sleep and exercise interventions to prevent/reverse frailty.
The strengths of this study include the large sample size, access to a random subset of a population-based cohort of community-dwelling older adult men and women with 5-year follow-up data and both objective and self-reported measures of sleep. Additionally, our analyses excluded individuals who were considered frail at baseline, permitting evaluation of incident frailty. Despite these strengths, this study has several limitations. First, NSHAP sleep study participants were a relatively healthy sample of older adults with good sleep hygiene. Attrition due to frailty or poor sleep over the 5-year period may have underestimated the study findings. Second, our study used measures similar to the original Fried Frailty Index, but was limited to those available in the dataset. Third, including pre-frail individuals at baseline and using mean replacement for individuals with missing frailty data at follow-up may have introduced biases. However, sensitivity analyses led to similar conclusions, suggesting that any potential bias introduced was likely minimal. Fourth, actigraphy may over-estimate sleep periods for frail older adults with low physical activity. Fifth, inclusion of more sleep-related data would have strengthened the findings. For example, a limited number of subjective sleep measures were collected. Furthermore, we controlled for self-reported napping since napping data from actigraphy were unavailable. Additionally, some sleep disorders were not assessed in our study (e.g. sleep-disordered breathing, restless legs syndrome) and unmeasured confounding may have influenced the results. Finally, we could not assess changes in sleep over time, nor discern habitual sleep patterns from these data. Longer, serial actigraphic sleep assessments are needed to better understand sleep’s role in both health and longevity.
Conclusion
Among non-frail older adults, greater sleep fragmentation was associated with a higher risk of frailty at the 5-year follow-up. Nighttime wakefulness, sleep fragmentation, and percent sleep were associated with mortality risk. Together, these findings indicate that sleep disruption is an important risk factor for poor outcomes. Future research is warranted to replicate these findings, assess frailty as an intermediary outcome, and determine if interventions aimed at improving sleep produce changes in frailty and mortality.
Supplementary Material
Financial Disclosure
This work was supported by the Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD. Adam Spira is supported by grants from the National Institute on Aging. Adam Spira received honoraria from Springer Nature Switzerland AG for Guest Editing a Special Issue of Current Sleep Medicine Reports.
Non-Financial Disclosure
No conflicts of interest to report.
References
- 1. Sander M, et al. The challenges of human population ageing. Age Ageing. 2015;44(2):185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crow RS, et al. Mortality risk along the frailty spectrum: data from the National Health and Nutrition Examination Survey 1999 to 2004. J Am Geriatr Soc. 2018;66(3):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garre-Olmo J, et al. Prevalence of frailty phenotypes and risk of mortality in a community-dwelling elderly cohort. Age Ageing. 2013;42(1):46–51. [DOI] [PubMed] [Google Scholar]
- 4. Rodríguez-Mañas L, et al. ; FOD-CC group (Appendix 1). Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cesari M, et al. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10–12. [DOI] [PubMed] [Google Scholar]
- 6. Rockwood K, et al. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 7. Passos GS, et al. Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med. 2010;6(3):270–275. [PMC free article] [PubMed] [Google Scholar]
- 8. Fried LP, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 9. Clegg A, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ofori-Asenso R, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mander BA, et al. Sleep and human aging. Neuron. 2017;94(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, et al. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 14. Neikrug AB, et al. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirshkowitz M, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 16. Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. [DOI] [PubMed] [Google Scholar]
- 17. Gulia KK, et al. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. 2018;18(3):155–165. [DOI] [PubMed] [Google Scholar]
- 18. Young T, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 19. Piovezan R, et al. Frailty and sleep disturbances in the elderly: possible connections and clinical implications. Sleep Sci. 2013;6(2013):175–179. [Google Scholar]
- 20. Everson CA, et al. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286(6):E1060–E1070. [DOI] [PubMed] [Google Scholar]
- 21. Bennett LS, et al. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytime sleepiness, and effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 1999;159(6):1884–1890. [DOI] [PubMed] [Google Scholar]
- 22. Baniak LM, et al. Long sleep duration is associated with increased frailty risk in older community-dwelling adults. J Aging Health. 2020;32(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ensrud KE, et al. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang I, et al. Sleep latency in men and sleep duration in women can be frailty markers in community-dwelling older adults: The Korean Frailty and Aging Cohort Study (KFACS). J Nutr Health Aging. 2019;23(1):63–67. [DOI] [PubMed] [Google Scholar]
- 25. Nakakubo S, et al. Long and short sleep duration and physical frailty in community-dwelling older adults. J Nutr Health Aging. 2018;22(9):1066–1071. [DOI] [PubMed] [Google Scholar]
- 26. Sun XH, et al. Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. 2020;20(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen-Mansfield J, et al. Sleep duration, nap habits, and mortality in older persons. Sleep. 2012;35(7):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gangwisch JE, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31(8):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 29. Kripke DF, et al. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwok CS, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J Am Heart Assoc. 2018;7(15):e008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morgan K, et al. Sleep duration and all-cause mortality: links to physical activity and prefrailty in a 27-year follow up of older adults in the UK. Sleep Med. 2019;54:231–237. [DOI] [PubMed] [Google Scholar]
- 32. Smagula SF, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. Actigraphy- and polysomnography-measured sleep disturbances, inflammation, and mortality among older men. Psychosom Med. 2016;78(6):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wallace ML, et al. Which sleep health characteristics predict all-cause mortality in older men? An application of flexible multivariable approaches. Sleep. 2018;41(1). doi: 10.1093/sleep/zsx189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rich J, et al. All-cause mortality and obstructive sleep apnea severity revisited. Otolaryngol Head Neck Surg. 2012;147(3):583–587. [DOI] [PubMed] [Google Scholar]
- 35. Lee WJ, et al. Long sleep duration, independent of frailty and chronic Inflammation, was associated with higher mortality: a national population-based study. Geriatr Gerontol Int. 2017;17(10):1481–1487. [DOI] [PubMed] [Google Scholar]
- 36. Gordon EH, et al. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. [DOI] [PubMed] [Google Scholar]
- 37. Green MJ, et al. The longitudinal course of insomnia symptoms: inequalities by sex and occupational class among two different age cohorts followed for 20 years in the west of Scotland. Sleep. 2012;35(6):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jung KI, et al. Gender differences in nighttime sleep and daytime napping as predictors of mortality in older adults: the Rancho Bernardo study. Sleep Med. 2013;14(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Canon ME, et al. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J Nutr Health Aging. 2011;15(8):695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beverly Hery CM, et al. Contributions of the Women’s Health Initiative to understanding associations between sleep duration, insomnia symptoms, and sleep-disordered breathing across a range of health outcomes in postmenopausal women. Sleep Health. 2020;6(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. da Silva AA, et al. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open. 2016;6(2):e008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lauderdale DS, et al. Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 2):S125–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas. 2011;68(3):224–232. [DOI] [PubMed] [Google Scholar]
- 44. Waite LJ, et al. Assessment National Social Life, Health, and Aging Project (NSHAP): Wave 1, [United States], 2005–2006. In: Inter-university Consortium for Political and Social Research [distributor]; 2019.
- 45. Waite LJ, et al. National Social Life, Health, and Aging Project (NSHAP): wave 2 and Partner Data Collection, [United States], 2010–2011. In: Inter-university Consortium for Political and Social Research [distributor]; 2019.
- 46. Huisingh-Scheetz M, et al. Geriatric syndromes and functional status in NSHAP: rationale, measurement, and preliminary findings. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 2):S177–S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dent E, et al. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. [DOI] [PubMed] [Google Scholar]
- 48. Shrive FM, et al. Dealing with missing data in a multi-question depression scale: a comparison of imputation methods. BMC Med Res Methodol. 2006;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kurina LM, et al. Actigraphic sleep characteristics among older Americans. Sleep Health. 2015;1(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rimmer J, et al. Sleep disturbance in persistent allergic rhinitis measured using actigraphy. Ann Allergy Asthma Immunol. 2009;103(3):190–194. [DOI] [PubMed] [Google Scholar]
- 51. Loewen A, et al. Sleep disruption in patients with sleep apnea and end-stage renal disease. J Clin Sleep Med. 2009;5(4):324–329. [PMC free article] [PubMed] [Google Scholar]
- 52. Kushida CA, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. [DOI] [PubMed] [Google Scholar]
- 53. Thomas RJ. Sleep fragmentation and arousals from sleep-time scales, associations, and implications. Clin Neurophysiol. 2006;117(4):707–711. [DOI] [PubMed] [Google Scholar]
- 54. Obesity: preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 55. Cornwell EY, et al. Measuring social isolation among older adults using multiple indicators from the NSHAP study. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i38–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams SR, et al. Measures of chronic conditions and diseases associated with aging in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i67–i75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shiovitz-Ezra S, et al. Quality of life and psychological health indicators in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i30–i37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kotwal AA, et al. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 2015;29(4):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roenneberg T, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. [DOI] [PubMed] [Google Scholar]
- 60. Morgan E, et al. Sleep characteristics and daytime cortisol levels in older adults. Sleep. 2017;40(5). doi: 10.1093/sleep/zsx043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sharma MP, et al. Behavioral interventions for insomnia: theory and practice. Indian J Psychiatry. 2012;54(4):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee JS, et al. Long sleep duration is associated with higher mortality in older people independent of frailty: a 5-year cohort study. J Am Med Dir Assoc. 2014;15(9):649–654. [DOI] [PubMed] [Google Scholar]
- 63. Lauderdale DS, et al. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Van Den Berg JF, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17(3):295–302. [DOI] [PubMed] [Google Scholar]
- 65. Hughes JM, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Girschik J, et al. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cricco M, et al. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49(9):1185–1189. [DOI] [PubMed] [Google Scholar]
- 68. Song Y, et al. Association between sleep and physical function in older veterans in an Adult Day Healthcare Program. J Am Geriatr Soc. 2015;63(8):1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Van Cauter E, et al. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Duffy JF, et al. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28(5):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scheuermaier KD, et al. Phase Shifts to a Moderate Intensity Light Exposure in Older Adults: a Preliminary Report. J Biol Rhythms. 2019;34(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wanigatunga AA, et al. Association of total daily physical activity and fragmented physical activity with mortality in older adults. JAMA Netw Open. 2019;2(10):e1912352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smirnova E, et al. The predictive performance of objective measures of physical activity derived from accelerometry data for 5-year all-cause mortality in older adults: National Health and Nutritional Examination Survey 2003-2006. J Gerontol A Biol Sci Med Sci. 2020;75(9):1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–65, xi. [DOI] [PubMed] [Google Scholar]
- 75. Driver HS, et al. Exercise and sleep. Sleep Med Rev. 2000;4(4):387–402. [DOI] [PubMed] [Google Scholar]
- 76. Santos RV, et al. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. 2012;60(3):731–735. [DOI] [PubMed] [Google Scholar]
- 77. Angulo J, et al. Physical activity and exercise: strategies to manage frailty. Redox Biol. 2020;35:101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.