Abstract
The treatment of staphylococcal prosthetic joint infection (PJI) with debridement, antibiotics, and retention of the implant (DAIR) often results in failure. An important evidence gap concerns the treatment with rifampicin for PJI. A systematic review and meta-analysis were conducted to assess the outcome of staphylococcal hip and/or knee PJI after DAIR, focused on the role of rifampicin. Studies published until September 2, 2020 were included. Success rates were stratified for type of joint and type of micro-organism. Sixty-four studies were included. The pooled risk ratio for rifampicin effectiveness was 1.10 (95% confidence interval, 1.00–1.22). The pooled success rate was 69% for Staphylococcus aureus hip PJI, 54% for S aureus knee PJI, 83% for coagulase-negative staphylococci (CNS) hip PJI, and 73% for CNS knee PJI. Success rates for MRSA PJI (58%) were similar to MSSA PJI (60%). The meta-analysis indicates that rifampicin may only prevent a small fraction of all treatment failures.
Keywords: DAIR, meta-analysis, rifampicin, staphylococcal PJI, systematic review
In this review, the outcome of staphylococcal hip and/or knee prosthetic joint infection (PJI) after debridement and implant retention was assessed in 64 studies. Success rates were significantly lower for S. aureus and knee PJI compared to CNS and hip PJI. The risk ratio for rifampicin effectiveness was 1.10 (95% CI 1.00-1.22). The data expose a need to address the role of rifampicin for staphylococcal PJI in a large randomized controlled trial.
A prosthetic joint infection (PJI) is a severe complication of orthopedic surgery and it is associated with significant morbidity and mortality. Staphylococcus aureus or coagulase-negative staphylococci (CNS) are the most common causative pathogens of PJI, accounting for approximately two third of all cases [1]. Treatment of acute PJI, aimed at maintaining the implant, consists of thorough surgical debridement of the implant and of the infected tissue around the implant, followed by antibiotic treatment (summarized as debridement, antibiotics, and implant retention [DAIR]). Nevertheless, failure rates with this treatment strategy are high, ranging from 10% to 45% in some of the largest studies [2, 3]. An important evidence gap concerns the causes for these high failure rates. The type of joint, the type of micro-organism, and the antibiotic treatment that was used for PJI are risk factors that have been put forward to explain these high failure rates. Most international guidelines have adopted rifampicin combination therapy as the cornerstone antibiotic treatment for staphylococcal PJI treated with DAIR, based on experimental animal models, 1 randomized trial, and several cohort studies. However, rifampicin combination therapy is associated with significant side effects and drug-drug interactions, making its use less patient-friendly [4, 5]. Moreover, the literature regarding the effect of rifampicin combination therapy against staphylococcal hip and knee PJI after DAIR has not yet been explored systematically. Most observational PJI studies also included patients with PJI caused by other micro-organisms. Furthermore, not all studies specify details regarding the outcome per affected joint (hip or knee) or per causative staphylococcal species (S aureus or CNS), both of which may influence success rate. Therefore, we conducted a literature search to systematize and appraise the available evidence concerning outcome of staphylococcal PJI treated with DAIR, with a specific focus on the outcome with or without rifampicin use. A secondary objective was to relate outcomes to the type of joint (hip or knee), the type of micro-organism (S aureus and CNS), and susceptibility to methicillin (methicillin-resistant S aureus [MRSA] and methicillin-sensitive S aureus [MSSA]).
METHODS
Search Strategy and Selection Criteria
The reporting of this systematic review and meta-analysis is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The population of interest included all patients evaluating the outcome after DAIR for the treatment of staphylococcal hip and/or knee PJI, as defined by Infectious Diseases Society of America (IDSA) or Musculoskeletal Infection Society (MSIS) criteria [6]. Studies that also included other types of surgical strategy, other joints, or other micro-organisms were only included if the outcome was quantified separately for the variables of our interest. The following exclusion criteria were applied: studies that included patients with superficial wound infection, case reports, and studies reporting 20 patients or less with staphylococcal PJI [7]. A meta-analysis was performed for the studies in which patients treated with rifampicin could be compared with patients not treated with rifampicin. The search was limited to articles published until September 2, 2020. Articles were identified searching PubMed, Cochrane Library, and Embase databases (Supplemental Table 1). In addition, bibliographies of relevant articles were cross-checked for references missing in the original search. Two independent reviewers (H.S. and L.M.G.) reviewed all studies. A third reviewer (M.G.J.D.B.) was consulted if disagreements between reviewers could not be solved.
Data Analysis
Texts of selected abstracts were reviewed, as were article texts of abstracts that could not be excluded based on abstract review alone. Data from each study were entered in an SPSS database. Information extracted included study design, number of patients with S aureus and/or CNS PJI, number of hip and/or knee PJI, year of publication, duration of follow-up, rifampicin use (number of patients receiving rifampicin), and treatment outcomes for all these subcategories. Because there is no universally accepted definition for treatment success or failure after PJI, we incorporated the definitions used by the included paper. We contacted study authors and requested individual patient-level data if rifampicin data were not clearly specified.
Assessment of Quality of Evidence
Estimates of associations in observational studies may deviate from true underlying relationships due to confounding or biases. Confounding may occur because patients with comorbidity or use of immunosuppressants, implying a higher a priori risk for a poor outcome, may not be selected for rifampicin treatment. Survival bias occurs when only patients “surviving” the first weeks after debridement are included in the rifampicin group. The Newcastle-Ottawa Quality Scale was used to assess the quality of the studies included in the meta-analysis (Supplemental Table 3). Because this scale only addresses basic methodological factors and not important confounding factors or survival bias, studies will also be reviewed qualitatively in the discussion.
Statistical Methods
For the meta-analysis, we used the Hedges random-effects model to pool the risk ratio (RR) of individual studies to estimate an overall RR along with its associated confidence interval (CI). The choice for a random-effects method was based on the assumption that underlying risk factors for outcome were expected to vary between studies regarding underlying host comorbidities, type of joint, and the severity of PJI. Patients were excluded from the meta-analysis if failure occurred in the first week after debridement and before initiation of rifampicin, to prevent survivor bias. The extent of statistical heterogeneity was assessed by calculating I2 statistics. A funnel plot was constructed for studies reporting the primary outcome to assess the possibility of publication bias. Success rates were compared in predetermined subgroups (hip versus knee, S aureus versus CNS, MRSA versus MSSA) using t test. A linear regression model, including success rate, proportion of rifampicin use, and type of joint, was used to further explore the relationship between rifampicin use and success rates. Descriptive statistics were performed using SPSS 23.0 (IBM Corp., Armonk, NY). Stata was used for the meta-analysis (version 16; StataCorp, College Station, TX). The study-protocol was registered a priori with PROSPERO (registration number CRD42020155132).
RESULTS
Study Selection and Study Characteristics
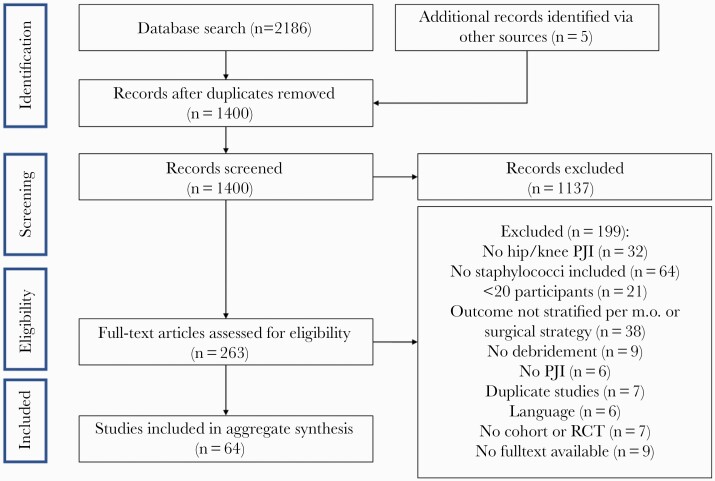
The review process identified 2186 articles, 263 full-text articles of which were assessed for eligibility (Figure 1). In total, 64 studies (4380 patients) were included, published between 1990 and September 2, 2020 (Supplemental Table 2). Only 2 studies were published before 2005. All studies were observational cohort studies (3 prospective, 59 retrospective), except for 2 randomized controlled trials (RCTs). The median study size was 50 patients; 10 studies included more than 100 patients. Staphylococcus aureus was the causative micro-organism in 3142 patients, CNS in 915 patients, and the staphylococcal species was not specified in 323 patients. Of 1797 patients with S aureus PJI in which the methicillin susceptibility of the isolates was reported, 416 (21%) were MRSA. Use of rifampicin for staphylococcal PJI was mentioned in 49 studies. Of those studies, outcome of treatment with or without rifampicin was reported in 30 studies (Table 1 and Supplemental Table 4). Except for 1 RCT, no studies compared baseline characteristics between patients treated and not treated with rifampicin. The study by Karlsen et al [8] was the only RCT that could be included in the meta-analysis. In this study, 48 patients with staphylococcal PJI were randomized between rifampicin combination therapy (23 patients) and beta-lactam monotherapy (25 patients).
Figure 1.
Flow chart of study selection. m.o., micro-organism; PJI, prosthetic joint infection; RCT, randomized controlled trial.
Table 1.
Outcome of 30 Studies That Reported Individual Patient Data Regarding the Use of Rifampicin or Not
| Category | N Studies | N Patients | Cure With Rifampicinb | Cure Without Rifampicinb | RR (95% CI) |
|---|---|---|---|---|---|
| Hip PJIa | |||||
| Staphylococcus aureus | 0 | ||||
| CNS | 0 | ||||
| Combined | 4 | 157 | 102/123 (83%) | 28/34 (82%) | 1.01 (0.85–1.20) |
| Knee PJIa | |||||
| S aureus | 1 | 22 | 9/22 (41%) | ||
| CNS | 0 | ||||
| Combined | 2 | 108 | 56/69 (81%) | 17/34 (50%) | 1.62 (1.14–2.31) |
| Hip and knee PJIc | |||||
| S aureus | 3 | 135 | 100/125(80%) | 4/10 (40%) | 2.00 (0.93–4.29) |
| CNS | 0 | ||||
| Combined | 24 | 1652 | 903/1298 (70%) | 186/354 (53%) | 1.32 (1.19–1.47) |
Abbreviations: CI, confidence interval; CNS, coagulase-negative staphylococci; PJI, prosthetic joint infection; RR, risk ratio.
aPooled individual patient data in N studies.
bPer-category studies are included if outcome is reported apart for S aureus and/or CNS apart or combined if outcome for all staphylococci is summarized.
cStudies are included in this category if outcome was reported only for hip and knee PJI together.
Outcome After Debridement, Antibiotics, and Retention of the Implant Related to Micro-Organism
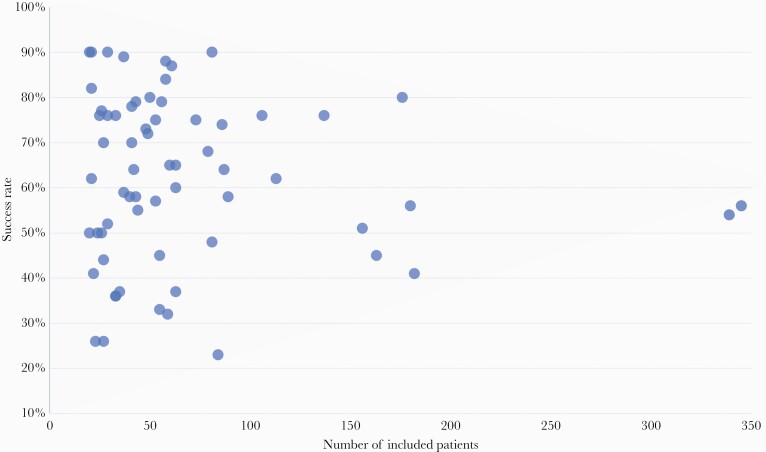
Outcome of treatment for staphylococcal PJI is presented in Table 2. The pooled success rate in all included studies was 60%. In smaller cohorts (<100 patients), the reported success rates varied from 23% to 90% (Figure 2). Cure rates in the 2 largest cohort studies (both containing more than 300 patients and likely more closely reflecting a real-life clinical situation) were 54% and 56% [2, 9]. Pooled success rate for S aureus PJI after DAIR was 62% (2922 analyzed patients in 54 studies) and for CNS PJI 73% (36 studies, 760 patients) (Table 2). Outcome for MRSA and MSSA PJI was reported in 25 and 28 studies, respectively (Table 3); success rate after DAIR was not different between both groups (MRSA 58%, MSSA 60%, P = .459). Outcomes between MRSA and MSSA PJI were not different when stratified for type of joint (data not shown). Pooled success rate of S aureus PJI after DAIR was 67% if PJI occurred within 3 months after arthroplasty and 49% in patients with later onset of S aureus PJI (990 analyzed patients in 9 studies) [2, 10–17].
Table 2.
Reported Outcome After DAIR, Stratified for Micro-Organism and/or Type of Joint Using Individual Patient Data From 64 Included Studies
| Micro-Organism and/or Type of Joint | n Studiesa |
n Patientsa | Pooled Success Rate of All Individual Patient Data |
RR (95% CI)b |
|---|---|---|---|---|
| All | 64 | 4380 | 60% | - |
| Per micro-organism | ||||
| Staphylococcus aureus | 54 | 2922 | 61% | ref. |
| CNS | 36 | 761 | 74% | 1.50 (1.32–1.70) |
| Per Affected Joint | ||||
| Knee | 27 | 1106 | 55% | ref. |
| Hip | 24 | 904 | 69% | 1.45 (1.29–1.63) |
| Per Affected Joint and Micro-Organism | ||||
| S aureus knee PJI | 19 | 692 | 54% | ref. |
| CNS knee PJI | 12 | 187 | 73% | 1.72 (1.33–2.21) |
| S aureus hip PJI | 19 | 547 | 69% | 1.48 (1.27–1.72) |
| CNS hip PJI | 13 | 145 | 83% | 2.66 (1.85–3.84) |
Abbreviations: CI, confidence interval; CNS, coagulase-negative staphylococci; DAIR, debridement, antibiotics, and retention of the implant; PJI, prosthetic joint infection; ref., reference category; RR, risk ratio.
aThe columns ‘n studies’ and ‘n patients’ displays the number of studies and patients for which the specific outcome regarding affected joint and/or micro-organism was reported. For example: one study could report outcome for both S aureus and CNS but not stratifying outcome for type of joint, whereas other studies only reported outcome for the total population without stratification for either type of joint or micro-organism. Therefore, numbers in this table cannot be summed.
bRelative risks for success were calculated for micro-organisms (with S aureus PJI as reference), for type of joint (with knee PJI as a reference), and for the 4 groups (with S aureus knee PJI as a reference).
Figure 2.
Relation between study size and outcome of staphylococcal prosthetic joint infection) treated with debridement, antibiotics, and retention of the implant (DAIR) (n = 64 studies).
Table 3.
Outcome of MSSA Versus MRSA PJI Treated With DAIR
| Study | N Studiesa | N Patients | Pooled Success Rateb |
|---|---|---|---|
| MSSA PJI | 28 | 1381 | 60% |
| MRSA PJI | 26 | 416 | 58% |
| Hip MSSA PJI | 2 | 32 | 81% |
| Hip MRSA PJI | 1 | 12 | 92% |
| Knee MSSA PJI | 3 | 56 | 66% |
| Knee MRSA PJI | 3 | 78 | 64% |
Abbreviations: DAIR, debridement, antibiotics, and retention of the implant; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; PJI, prosthetic joint infection.
aPer category, studies were included if they reported specific or combined outcome for hip and/or knee MSSA and MRSA.
bBased on individual patient data.
Outcome After Debridement, Antibiotics, and Retention of the Implant Related to Type of Joint
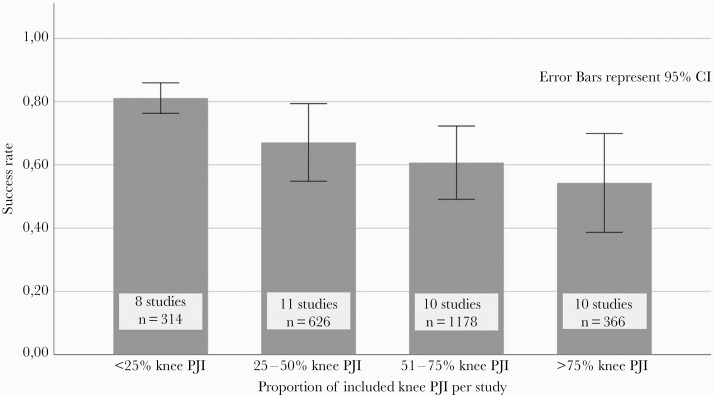
Outcome per affected joint was specified in 33 studies. Pooled success rate after DAIR for S aureus hip PJI was 69%, whereas pooled success rate after S aureus knee PJI was 54% (Table 2). Pooled success rates after DAIR for CNS hip PJI was 83% and 73% for CNS knee PJI. Using linear regression analysis, reported success rates positively correlated with the proportion of included hip PJI per study: success rates increased from 54% in studies with <25% of patients with hip PJI to 82% in studies with >75% of patients with hip PJI (P = .002), indicating that reported outcome of PJI is strongly affected by the type of joint included in studies (Figure 3). The high success rates for hip PJI could not be attributed to rifampicin use: success rates were 83% for patients on rifampicin and 82% for patients who were not treated with rifampicin (RR, 1.01; 95% CI, 0.85–1.20; evaluable in 4 studies with 157 patients) (Table 1).
Figure 3.
Success rates in 39 studies that could be categorized by knee-to-hip-ratio. CI, confidence interval; RR, risk ratio.
Outcome After Debridement, Antibiotics, and Retention of the Implant Related to Treatment With Rifampicin
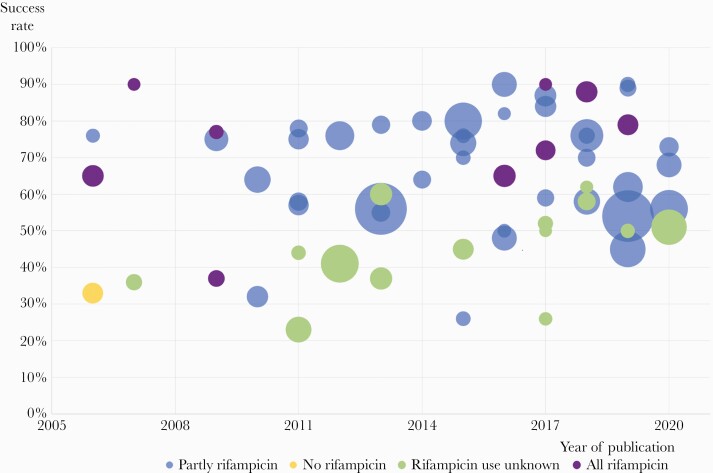
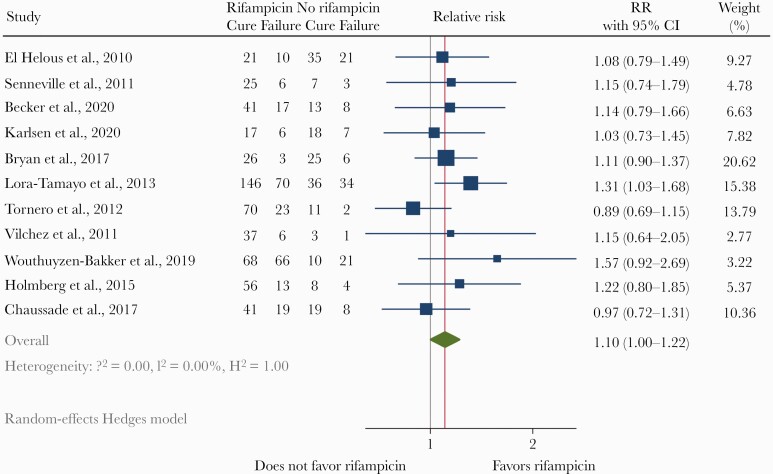
The reported success rates over the years, stratified by treatment with rifampicin, are shown in Figure 4. Success rates were higher in studies in which rifampicin was prescribed (64% in 34 studies with 2884 patients) compared to studies in which rifampicin was not prescribed or not mentioned by the authors (44% in 18 studies with 976 patients). In 12 studies, all included patients were treated with rifampicin resulting in a pooled success rate of 71% (Table 1). These studies were likely hampered by selection bias because outcome of patients who did not use rifampicin were not evaluated herein. Twelve observational studies and 1 RCT reported outcome for both patients treated and not treated with rifampicin. In 2 of these studies, the group of patients without rifampicin was too small for comparative evaluation [18, 19]. Outcome of the remaining 11 studies was evaluated with a random-effects meta-analysis (Figure 5). Survivor bias could be corrected in 2 of those studies, in which 5 of 17 and 6 of 13 patients failed before initiation of rifampicin [20, 21]. From 1 study, comparing 2 historical groups and 1 prospective group, only the historical groups were included in the meta-analysis because these groups could be compared with each other while a control group for the prospective cohort was absent. The only included RCT in the meta-analysis (by Karlsen et al [8]) reported similar cure rates between the rifampicin group (74%) and the beta-lactam group (72%). The pooled risk ratio for rifampicin effectivity from 11 studies in the meta-analysis was 1.10 (95% CI, 1.00–1.22). The funnel plot was asymmetric (Supplemental Figure 1). A trim-and-fill analysis to explore this possible publication bias suggested 4 missing studies, which after correction would result in an adjusted relative risk for success of 1.04 (95% CI, 0.94–1.14).
Figure 4.
Success rates over the years for staphylococcal prosthetic joint infection treated with debridement, antibiotics, and retention of the implant (DAIR) and related to use of rifampicin. Different bubble sizes represent differences in study size.
Figure 5.
Meta-analysis of 11 studies in which outcome for staphylococcal prosthetic joint infection (PJI) after debridement, antibiotics, and retention of the implant (DAIR) could be compared between patients treated and not treated with rifampicin. The point estimate (relative risk) for each study is represented by a square. The 95% confidence interval (CI) for each study is represented by a horizontal line intersecting the square. The size of the square represents the relative precision of the study estimates: the bigger the square, the more precise the study.
DISCUSSION
Despite gradually improving success rates over the years, the reported outcome of staphylococcal PJI is still heterogeneous, ranging from 23% to 90%. Overall, the pooled risk ratio for success was slightly higher in patients treated with rifampicin. Success rates were considerably better for hip and CNS PJI than for knee and S aureus PJI. Success rates of MRSA and MSSA PJI after DAIR were similar. Of note, the ratio of S aureus to CNS PJI remained stable over the years (between 72% and 76%), indicating that success rates are probably not influenced by changing epidemiology of causative staphylococci.
The pooled estimated effect of rifampicin on treatment outcome in our meta-analysis differs from a recently published meta-analysis that did not find a positive association between treatment with rifampicin and success rates [22]. Several studies in that meta-analysis included other micro-organisms than staphylococci or patients with other surgical strategies whom we excluded [23–26]. Moreover, with a broader search strategy, we were able to include 7 other studies in our meta-analysis.
Interpreting the association between rifampicin and success rates after DAIR in the meta-analysis is complicated by survival bias and selection bias, because 10 studies in the meta-analysis were observational. In 3 studies, survival bias could be ruled out by excluding patients who failed early after debridement and before start of rifampicin [2, 20, 21], but survival bias was likely present in more studies. The positive association between duration of rifampicin and success rates after DAIR in the study of Becker et al [27] could be explained by survival bias and selectively excluding patients from the analysis who developed a failure while on rifampicin treatment [28]. Lora-Tamayo et al [2] described the strongest association between rifampicin use and outcome. This study addressed survivor bias and performed multivariate regression analysis to correct for confounding factors, which did not change the outcome of the study [2]. The trim-and-fill analysis suggested that publication biases have influenced the outcome of the meta-analysis. However, this analysis is a statistical measure that presumes that negative studies were not published, which, in our opinion, is not very likely given the many studies presented in this review with negative results.
Because most studies in this review were observational, confounding factors that influence both the choice for antibiotic strategy and outcome after DAIR were present in these studies. It is unfortunate that a comparison of baseline characteristics between rifampicin and nonrifampicin users is almost absent in the literature summarized in this review. Survival bias may explain the increased effectiveness of long-term rifampicin compared to short-term rifampicin in the study by Lesens et al [12], because patients could only be analyzed in the group with long-term rifampicin if they survived the first weeks of treatment. Confounding by indication was described in the studies of Morata et al [23] and Ascione et al [23] in which patients who were not treated with rifampicin had diabetes, rheumatoid arthritis, and liver disease more often [23, 26].
The well known RCT of Zimmerli et al [29] was excluded from this review due to the low number of patients (18 patients with PJI, 8 of whom received rifampicin) and because outcome was not stratified per micro-organism (both S aureus and CNS included) and type of infection (both osteosynthesis-associated infection and PJI were included). Patients were randomized in this trial between rifampicin combination therapy or ciprofloxacin monotherapy. Intention-to-treat analysis showed a nonsignificant 89% versus 60% cure rate in favor of rifampicin; significance was reached in the per-protocol analysis. However, the choice for ciprofloxacin monotherapy in the control arm, nowadays regarded as inferior therapy for staphylococcal PJI, played a major role in the outcome because 4 of 5 failures this group were due to ciprofloxacin resistance. The RCT of Karlsen et al [8] contained 3 times as many patients as the trial of Zimmerli et al [29] and had a different comparator arm (beta-lactams instead of ciprofloxacin).
The timing of rifampicin initiation and the duration of treatment with rifampicin may also affect outcome. In the 2 RCTs discussed above and 1 observational study, rifampicin was started immediately or from day 1 postoperatively [21, 29, 30]. In these studies, rifampicin resistance had not developed in patients with positive cultures after failure. Rifampicin resistance in patients with failure after DAIR has been reported, but this was in patients who were not treated with adequate debridement or with combination therapy [31, 32]. Whether the duration of rifampicin combination therapy affects outcome is unknown. Treatment duration was 3 months in most studies included in this review. In some observational studies, shorter rifampicin treatment was associated with more treatment failure, but these results should be interpreted cautiously because studying treatment duration in observational studies is inherently affected by selection bias and survival bias [12, 27, 28]. More research is needed to gain more evidence regarding the timing and duration of rifampicin.
This review reveals that success rates are strongly influenced by the ratio of knee-to-hip PJI per study. Are higher success rates, usually attributed to rifampicin use, in fact explained by a decreased knee-to-hip ratio in studies? To explore this further, we related the knee-to-hip ratio to rifampicin use. We unexpectedly found that the knee-to-hip ratio per study was inversely related to rifampicin use. The knee-to-hip PJI ratio in studies was 0.90 (meaning more knees than hips) if rifampicin was not used, 0.77 if rifampicin use was not mentioned, 0.40 if a certain proportion of patients used rifampicin, and 0.35 in studies in which all patients were treated with rifampicin. As a derived measure, we performed linear regression analysis with proportion of rifampicin use per study as predictor variable for success weighted by proportion of included knee PJIs. Results revealed that the significant correlation between rifampicin use and successful outcome (P = .01) disappeared after correction for type of joint (P = .17), indicating that both rifampicin and type of joint influence the outcome of PJI. We hypothesize that adjunctive rifampicin use will not yield a further increase in success rate in patients with hip PJI with a priori higher chances for cure. The poor outcomes of knee PJI may relate to the surgical debridement, which is more complicated for infected knee prostheses than for hip prostheses due the anatomical barriers that hinder a proper debridement of a knee prosthesis. Of note, outcome for knee PJI was better in patients treated with rifampicin (81%) compared to patients not treated without rifampicin (50%) (RR, 1.62; 95% CI, 1.14–2.31), but this risk ratio could be obtained from only 2 studies.
The definition of treatment failure varied across included studies. In most studies, a second debridement within the first 3 weeks of antibiotic treatment was not regarded as failure, whereas other studies defined all subsequent debridements as failure. Furthermore, the use of chronic suppressive antibiotic therapy with a well functioning prothesis is defined as a failure in some but not all studies, also affecting cure rates in studies [33]. Of all included studies, 30 studies did not report whether chronic antibiotic suppression was part of the definition of failure or was regarded as success in patients with a functioning prosthesis. Of note, success rates ware comparable between 30 studies that defined chronic suppressive antibiotics as failure (61%) and 34 studies that did not mention suppressive therapy or regarded suppressive therapy as success (60%), but interpretation is difficult because most studies did not specify the number of patients on suppressive antibiotic treatment. Uniform definitions of treatment failure are needed to make comparison between studies more accurate.
In this review, higher success rates were reached in early postoperative PJI (within 3 months after arthroplasty) compared to later onset of PJI. Wouthuyzen-Bakker et al [10] reported lower treatment success of late acute (hematogenous) PJI compared to early postoperative PJI for both S aureus (34% versus 75%) and CNS (46% versus 88%). Poor outcome of late acute PJI may relate to the hematogenous origin with seeding of inaccessible parts of the prosthesis such as the stem, which cannot be surgically debrided, possibly resulting in more treatment failure.
CONCLUSIONS
Taken together, this review and meta-analysis found that the outcome of staphylococcal PJI after DAIR is largely determined by the type of joint and the type of causative micro-organism. Outcome for MRSA PJI seems to equal outcome for MSSA PJI. Use of rifampicin was associated with a 10% increase in success rate, but studies were hampered by confounding, publication bias, and selection bias. The supporting evidence for rifampicin combination treatment is weak and possibly restricted to knee PJI, but good-quality data from randomized studies are scarce. Given this paucity of evidence, the accumulated data expose an urgent need to address the role and duration of rifampicin for staphylococcal PJI in a large randomized controlled trial.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are indebted to Jaime Lora-Tamayo and Marjan Wouthuyzen-Bakker who kindly provided us with additional detailed information regarding rifampicin use in 2 cohort studies that were included in this review.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Tande AJ, Osmon DR, Greenwood-Quaintance KE, et al. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio 2014; 5:e01910–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lora-Tamayo J, Murillo O, Iribarren JA, et al. ; REIPI Group for the Study of Prosthetic Infection . A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56:182–94. [DOI] [PubMed] [Google Scholar]
- 3. Lora-Tamayo J, Senneville É, Ribera A, et al. ; Group of Investigators for Streptococcal Prosthetic Joint Infection . The not-so-good prognosis of streptococcal periprosthetic joint infection managed by implant retention: the results of a large multicenter study. Clin Infect Dis 2017; 64:1742–52. [DOI] [PubMed] [Google Scholar]
- 4. Nguyen S, Robineau O, Titecat M, et al. Influence of daily dosage and frequency of administration of rifampicin-levofloxacin therapy on tolerance and effectiveness in 154 patients treated for prosthetic joint infections. Eur J Clin Microbiol Infect Dis 2015; 34:1675–82. [DOI] [PubMed] [Google Scholar]
- 5. Vollmer NJ, Rivera CG, Stevens RW, et al. Safety and tolerability of fluoroquinolones in patients with staphylococcal periprosthetic joint infections. Clin Infect Dis 2021. doi: 10.1093/cid/ciab145 [DOI] [PubMed] [Google Scholar]
- 6. Osmon DR, Berbari EF, Berendt AR, et al. ; Infectious Diseases Society of America . Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 7. Pijls BG, Dekkers OM, Middeldorp S, et al. AQUILA: assessment of quality in lower limb arthroplasty. An expert Delphi consensus for total knee and total hip arthroplasty. BMC Musculoskelet Disord 2011; 12:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karlsen ØE, Borgen P, Bragnes B, et al. Rifampin combination therapy in staphylococcal prosthetic joint infections: a randomized controlled trial. J Orthop Surg Res 2020; 15:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowik CAM, Parvizi J, Jutte PC, et al. Debridement, antibiotics and implant retention is a viable treatment option for early periprosthetic joint infection presenting more than four weeks after index arthroplasty. Clin Infect Dis 2019. doi: 10.1093/cid/ciz867 [DOI] [PubMed] [Google Scholar]
- 10. Wouthuyzen-Bakker M, Sebillotte M, Huotari K, et al. ; ESCMID Study Group for Implant-Associated Infections (ESGIAI) . Lower success rate of débridement and implant retention in late acute versus early acute periprosthetic joint infection caused by Staphylococcus spp. Results from a matched cohort study. Clin Orthop Relat Res 2020; 478:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aboltins CA, Page MA, Buising KL, et al. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect 2007; 13:586–91. [DOI] [PubMed] [Google Scholar]
- 12. Lesens O, Ferry T, Forestier E, et al. ; Auvergne-Rhône-Alpes Bone and Joint Infections Study Group . Should we expand the indications for the DAIR (debridement, antibiotic therapy, and implant retention) procedure for Staphylococcus aureus prosthetic joint infections? A multicenter retrospective study. Eur J Clin Microbiol Infect Dis 2018; 37:1949–56. [DOI] [PubMed] [Google Scholar]
- 13. Joulie D, Girard J, Mares O, et al. Factors governing the healing of Staphylococcus aureus infections following hip and knee prosthesis implantation: a retrospective study of 95 patients. Orthop Traumatol Surg Res 2011; 97:685–92. [DOI] [PubMed] [Google Scholar]
- 14. Dx Duffy S, Ahearn N, Darley ES, et al. Analysis of the KLIC-score; an outcome predictor tool for prosthetic joint infections treated with debridement, antibiotics and implant retention. J Bone Jt Infect 2018; 3:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sendi P, Banderet F, Graber P, Zimmerli W. Clinical comparison between exogenous and haematogenous periprosthetic joint infections caused by Staphylococcus aureus. Clin Microbiol Infect 2011; 17:1098–100. [DOI] [PubMed] [Google Scholar]
- 16. Bouaziz A, Uçkay I, Lustig S, et al. Non-compliance with IDSA guidelines for patients presenting with methicillin-susceptible Staphylococcus aureus prosthetic joint infection is a risk factor for treatment failure. Med Mal Infect 2018; 48:207–11. [DOI] [PubMed] [Google Scholar]
- 17. Shohat N, Goswami K, Tan TL, et al. Increased failure after irrigation and debridement for acute hematogenous periprosthetic joint infection. J Bone Joint Surg Am 2019; 101:696–703. [DOI] [PubMed] [Google Scholar]
- 18. Peel TN, Buising KL, Dowsey MM, et al. Outcome of debridement and retention in prosthetic joint infections by methicillin-resistant staphylococci, with special reference to rifampin and fusidic acid combination therapy. Antimicrob Agents Chemother 2013; 57:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moojen DJ, Zwiers JH, Scholtes VA, et al. Similar success rates for single and multiple debridement surgery for acute hip arthroplasty infection. Acta Orthop 2014; 85:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmberg A, Thorhallsdottir VG, Robertsson O, Dahl A, Stefansdottir A. 75% success rate after open debridement, exchange of tibial insert, and antibiotics in knee prosthetic joint infections. Acta Orthop 2015:86:457–62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect 2011; 17:439–44. [DOI] [PubMed] [Google Scholar]
- 22. Aydın O, Ergen P, Ozturan B, et al. Rifampin-accompanied antibiotic regimens in the treatment of prosthetic joint infections: a frequentist and Bayesian meta-analysis of current evidence. Eur J Clin Microbiol Infect Dis 2021; 40:665–71. [DOI] [PubMed] [Google Scholar]
- 23. Morata L, Senneville E, Bernard L, et al. A retrospective review of the clinical experience of linezolid with or without rifampicin in prosthetic joint infections treated with debridement and implant retention. Infect Dis Ther 2014; 3:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puhto AP, Puhto T, Niinimäki T, et al. Predictors of treatment outcome in prosthetic joint infections treated with prosthesis retention. Int Orthop 2015; 39:1785–91. [DOI] [PubMed] [Google Scholar]
- 25. Chaussade H, Uçkay I, Vuagnat A, et al. Antibiotic therapy duration for prosthetic joint infections treated by debridement and implant retention (DAIR): similar long-term remission for 6 weeks as compared to 12 weeks. Int J Infect Dis 2017; 63:37–42. [DOI] [PubMed] [Google Scholar]
- 26. Ascione T, Pagliano P, Mariconda M, et al. Factors related to outcome of early and delayed prosthetic joint infections. J Infect 2015; 70:30–6. [DOI] [PubMed] [Google Scholar]
- 27. Becker A, Kreitmann L, Triffaut-Fillit C, et al. Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. J Bone Jt Infect 2020; 5:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scheper H, de Boer MGJ. Comment on “Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France” by Becker et al. (2020). J Bone Jt Infect 2020; 6:17–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) study group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 30. Scheper H, van Hooven D, van de Sande M, et al. Outcome of acute staphylococcal prosthetic joint infection treated with debridement, implant retention and antimicrobial treatment with short duration of rifampicin. J Infect 2018; 76:498–500. [DOI] [PubMed] [Google Scholar]
- 31. Achermann Y, Eigenmann K, Ledergerber B, et al. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): a matched case-control study. Infection 2013; 41:431–7. [DOI] [PubMed] [Google Scholar]
- 32. Hellebrekers P, Verhofstad MHJ, Leenen LPH, et al. The effect of early broad-spectrum versus delayed narrow-spectrum antibiotic therapy on the primary cure rate of acute infection after osteosynthesis. Eur J Trauma Emerg Surg 2020; 46:1341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Helou OC, Berbari EF, Lahr BD, et al. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis 2010; 29:961–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.