Abstract
Study Objective
Insomnia has been linked to acute and chronic pain conditions; however, it is unclear whether such relationships are causal. Recently, a large number of genetic variants have been discovered for both insomnia and pain through genome-wide association studies (GWASs) providing a unique opportunity to examine the evidence for causal relationships through the use of the Mendelian randomization paradigm.
Methods
To elucidate the causality between insomnia and pain, we performed bidirectional Mendelian randomization analysis in FinnGen, where clinically diagnosed ICD-10 categories of pain had been evaluated. In addition, we used measures of self-reported insomnia symptoms. We used endpoints for pain in the FinnGen Release 5 (R5) (N = 218,379), and a non-overlapping sample for insomnia (UK Biobank (UKBB) and 23andMe, N = 1,331,010 or UKBB alone N = 453,379). We assessed the robustness of results through conventional Mendelian randomization sensitivity analyses.
Results
Genetic liability to insomnia symptoms increased the odds of reporting pain (odds ratio (OR) [95% confidence interval (CI)] = 1.47 [1.38–1.58], p = 4.12 × 10−28). Manifested pain had a small effect on increased risk for insomnia (OR [95% CI] = 1.04 [1.01–1.07], p < 0.05). Results were consistent in sensitivity analyses.
Conclusions
Our findings support a bidirectional causal relationship between insomnia and pain. These data support a further clinical investigation into the utility of insomnia treatment as a strategy for pain management and vice versa.
Keywords: genetics, GWAS, insomnia, Mendelian randomization, pain, sleep disorders
Statement of Significance.
In order to further elucidate the connection between insomnia and pain, we performed a two-sample Mendelian randomization analysis using summary statistics from genome-wide association studies of sleep and pain. Our results indicate a statistically significant bi-directional causal connection between insomnia and pain. The effect of insomnia exposure on pain sensation as the outcome was overall more robust than vice versa. The data we present here may be used as support for further clinical studies of the use of insomnia treatment for managing pain, and vice versa.
Introduction
Insomnia is a sleep disorder that affects daytime alertness and function due to the difficulty of obtaining sufficient sleep [1, 2]. Clinical insomnia affects approximately 10% of the population, and symptoms of insomnia are substantially more frequent with up to 30% prevalence in the population [1, 3]. Insomnia has been connected both with somatic and psychological traits, and with pain [2]. Sleep issues have been reported in over 50% of chronic pain disorders, while over 40% of individuals reporting insomnia have reported chronic pain [4, 5]. Previous research has suggested that insomnia has a stronger impact on the sensation of pain (chronic and clinical), than the less clear effect of pain on sleep quality [4, 6]. Indeed, while poor sleep in pain-free individuals increases the risk for new-onset cases of chronic pain sufficient sleep has been linked to improved long-term prognosis in those with tension headache, migraine, and chronic musculoskeletal pain [4]. Consequently, previous evidence suggests a relationship between lack of sufficient sleep in general as evidenced in sleep deprivation studies where an increase in pain sensation is seen in model organisms [7, 8]. For example, a consecutive three-night sleep disruption significantly heightened pain perception [9]. In addition, the link between pain and insomnia, has been reported in patients as significant comorbidity with pain interference and severity [5].
Chronic pain itself, similarly to sleep disorders, is a prevalent social and medical health issue spanning several somatic diseases. Furthermore, while pain at different sites of the body and caused by different diseases may have different biological etiologies, the sensitivity to perceived pain may be common across these different conditions [10–12]. Similarly, earlier genome-wide association studies (GWASs) on multisite pain from originating from different parts of the body identified uniform genetic pathways that increase the risk for perceived pain [13]. In addition, chronic pain is oftentimes challenging to medically manage, highlighting the need for novel treatment strategies. The robust prospective relationship between insomnia and different chronic pain conditions described above raise a question if insomnia itself has an impact in chronic or acute pain sensation and if managing sleep should be explored as one option to manage pain.
GWASs have become a standard tool for discovering the genetic association and underlying biology of human diseases [14–16]. In addition, cohorts have emerged where such discoveries are possible. In this study, FinnGen, United Kingdom Biobank (UKBB), and 23andMe were used. These are large-scale biobanks that aim to discover novel disease associations and ultimately improve patient health care.
The gold standard to test for a causal effect of an exposure on an outcome, such as disease or pain, on medical research is randomized controlled trials. An example of such a trial in the context of the current study is treatment or improving insomnia on having an impact on disease, pain. However, large blinded randomized controlled clinical trials may not be either feasible, practical or as in the case of insomnia and pain; still in the stages of being performed [17, 18]. One option to determine whether or not a causal association between sleep and pain is the Mendelian randomization (MR) analysis, which use single nucleotide polymorphisms (SNPs) in individuals as instrumental variables to examine the evidence for causal relationships between a trait and a disease [17, 19]. MR was originally developed as a “one-sample” based analysis, relying on individual-level data, which are generally more difficult to utilize for researchers and are not used for multiple GWASs [20]. Thus, as the SNPs associated with exposure risk traits and outcome diseases or conditions are often found in different GWASs, the two-sample MR method has been developed. The two-sample MR allows for more power by taking advantage of large independent cohorts, and summary statistics instead of individual-level data [17, 20]. On an epidemiological note, this approach is less susceptible to confounders and reverse causality, because it imposes strict limits on usable instruments; an SNP must be associated with the exposure trait, have no direct association with the outcome and does not have an association with potential confounders [21]. However, as many genetic variants have associations with multiple traits (pleiotropy) this may impact their validity as instruments for MR [22]. These effects can be tested for using pleiotropy tests (such as the Egger intercept method) [21–23].
Using these methods and the rationale above, we aimed to examine the causal relationships between insomnia and pain. To do this, we used bidirectional two-sample MR analysis of biobank GWAS summary statistics from GWASs of both insomnia and specific different pain diagnoses. The MR analysis was performed using summary statistics from FinnGen (ICD-10 coded pain cohorts) and UKBB and earlier 23andMe data (insomnia cohorts). Through this MR analysis we saw a significant bi-directional causality between insomnia and different types of pain, but a stronger and more complex impact of insomnia on pain than the reverse. The results provided in this investigation highlight the significant genetic bidirectional causality between insomnia symptoms and pain, which may be investigated further for clinical management of the two conditions.
Methods
Genetic associations with insomnia
We obtained GWAS summary statistics for insomnia from two cohorts; the United Kingdom Biobank (UKBB) and 23andMe based significant (p < 5 × 10−8) SNPs presented by Jansen et al. (2019) Supplementary Table S6 [24] and the summary statistics based on the UKBB presented by Lane et al. [1]. The Jansen et al. SNPs were used for exposure instruments as it contains more independent lead SNPs, whereas the Lane et al. summary statistics were used for outcome data. This approach avoids overestimating or skewing the estimates of GWAS significant variants where data had been included by 23andMe in Jansen et al. Classification of individuals into insomnia cases or controls in the 23andMe cohort was assessed using scoring of answers to a 7-concept sleep-related questionnaire. The UKBB used the question “Do you have trouble falling asleep at night, or do you wake up in the middle of the night?” [1, 24].
Genetic associations with pain
We used an independent cohort to estimate the genetic associations with pain in two-sample MR so as to avoid overlap of individuals with insomnia and patients with pain [23]. Therefore, pain summary statistics were collected from the FinnGen Release 5 (R5) release (described in detail below), and were comprised of cases defined as carrying ICD10 diagnosis codes for disorders with a clear pain component including general pain (occurring in joints, limbs, neck, head, abdomen, back, similar as the categorization in other pain studies as part of overall pain [11]. The data comprises a total of 87,242 cases and 131,127 controls, and in the sub-categories; joint pain, limb pain, ocular pain, throat and chest pain, pain in the abdomen and pelvic area and low back pain (Supplementary Table S1). We selected significant lead SNPs (p < 5 × 10−8) by removing highly correlated SNPs by linkage disequilibrium (LD) pruning (primary r2 threshold of 0.6 and a secondary r2 of 0.1) in the FinnGen R5 general Pain GWAS summary statistics using the FUMA platform [25]. Additionally, we performed proxy SNP detection for the Jansen et al. lead SNPs and the FinnGen general Pain SNPs using the LDproxy function of the LDlink platform (an r2-value of >0.9 was used as a cut-off for using a potential proxy SNP) [26]. In total there were only seven significant FinnGen general pain lead SNPs used for MR with insomnia. Thus, as a secondary one-sample analysis further was performed to support the causality from pain to insomnia, we used the lead SNPs in the multisite chronic pain (MCP) GWAS data produced by Johnston et al. [13]. This provided 64 SNPs used in MR with insomnia, increasing the power of the analysis. The study focused on chronic pain (pain experienced over 3 months) that also occurred in a number of sites where this pain was experienced. The MCP cohort is larger in a number of individuals (approximately 380,000 UKBB participants) and has larger power to examine shared (pleiotropic) effects, and provided a larger amount of significant lead SNPs for analysis.
FinnGen
Patients and control subjects in FinnGen provided informed consent for biobank research, based on the Finnish Biobank Act. Alternatively, older research cohorts, collected prior the start of FinnGen (in August 2017), were collected based on study-specific consents and later transferred to the Finnish biobanks after approval by Fimea, the National Supervisory Authority for Welfare and Health. Recruitment protocols followed the biobank protocols approved by Fimea. The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS) approved the FinnGen study protocol no. HUS/990/2017.
The FinnGen study is approved by Finnish Institute for Health and Welfare (THL), approval number THL/2031/6.02.00/2017, amendments THL/1101/5.05.00/2017, THL/341/6.02.00/2018, THL/ 2222/6.02.00/2018, THL/283/6.02.00/2019, THL/1721/5.05.00/2019, digital and population data service agency VRK43431/2017-3, VRK/6909/2018-3, VRK/4415/2019-3 the Social Insurance Institution (KELA) KELA 58/522/2017, KELA 131/522/2018, KELA 70/522/2019, KELA 98/522/2019, and Statistics Finland TK-53-1041-17.
The Biobank Access Decisions for FinnGen samples and data utilized in FinnGen Data Freeze 5 include: THL Biobank BB2017_55, BB2017_111, BB2018_19, BB_2018_34, BB_2018_67, BB2018_71, BB2019_7, BB2019_8, BB2019_26, Finnish Red Cross Blood Service Biobank 7.12.2017, Helsinki Biobank HUS/359/2017, Auria Biobank AB17-5154, Biobank Borealis of Northern Finland_2017_1013, Biobank of Eastern Finland 1186/2018, Finnish Clinical Biobank Tampere MH0004, Central Finland Biobank 1-2017, and Terveystalo Biobank STB 2018001.
Mendelian randomization of causality between insomnia and general pain
The lead SNPs demonstrated by Jansen et al. were used as genetic proxies for insomnia in a two-sample MR analysis against the FinnGen R5 Pain summary statistics. The Jansen et al.’s study included 23andMe data only for calculating the statistically most significant variants, which we use here as insomnia exposure instruments. We therefore used data from Lane et al. [1] when examining pain to insomnia effects. This approach avoids overestimating or skewing the estimates of those variants where data had been included by 23andMe. Furthermore, we double-checked for potential weak instrument bias of the exposure instruments by calculating their F-statistic [27, 28]. All exposure instruments used here had an F-statistic >10. The instruments provided by Jansen et al. exhibited an average F of 42, Johnston et al. an average of 35 and FinnGen general pain instruments exhibited an average F of 32. The MR was performed using the TwoSampleMR R package [29, 30]. There are multiple methods for MR analysis. The ones we focused on in this study were inverse-variance weighting (IVW) [31] method, MR Egger [22] and weighted median [32]. The IVW method is a weighted regression that averages the association ratio estimates (weights) of multiple uncorrelated exposure instruments to the outcome to calculate an overall causal estimate, with a pleiotropic intercept term set to zero [22, 32]. The MR Egger method is similar to IVW, but it does not set the intercept term to zero, but rather estimates it during the calculation [22]. The weighted median method provides more weight to instruments with more precise causal estimates, more robust to potential outliers than IVW and MR Egger but less efficient overall [21, 22, 32]. All three methods use the inverse-variance of instruments weights. Statistical significance was determined using the IVW method (p < 0.05).
Sensitivity analysis
We tested for pleiotropic effects using the Egger intercept methods as part of the TwoSampleMR package and the MR-PRESSO package [33]. The MR PRESSO method is similar to IVW and MR Egger, and uses three steps: (1) the calculation of pleiotropy using a global test, (2) identifying outliers and removing these to correct the pleiotropy, and (3) a distortion test estimating the significance of causal estimates differences before and after correcting for outliers [33]. We performed a leave-one-out analysis to assess whether individual variants had strong effects on the MR estimate. We used the FinnGen R5 Pain sub-categories (Supplementary Table S1) as outcome instruments against the Jansen et al. lead SNPs in order to further validate the analysis results, and to ensure that no independent (1) location of pain or (2) ICD10 code included, would bias the estimates of our analysis. Additionally, we also performed pleiotropic effect and leave-one-out analysis of Jansen et al. lead SNPs excluding those also found to be significantly (p < 5 × 10−8) associated with other sleep traits (sleep duration, short sleep, long sleep, daytime naps, chronotype and excessive daytime sleepiness) in the UKBB.
Results
Mendelian randomization exhibits bidirectional causality between insomnia and pain
Genetically proxied insomnia increased odds of reporting pain (OR [95% confidence interval (CI)] = 1.47 [1.38–1.58], P = 4.12 × 10−28, Table 1, Figures 1, A and 2). When stratified by pain subtype, there were consistently significant effects of genetic liability to insomnia on all pain categories (Table 2).
Table 1.
MR results assessing the causal effect of insomnia on FinnGen R5 pain (general)
| MR method | SNPs | OR [95% CI] | P |
|---|---|---|---|
| Inverse-variance weighted | 231 | 1.47 [1.38–1.58] | 4.12 × 10−28 |
| MR Egger | 231 | 1.31 [0.99–1.73] | 5.57 × 10−2 |
| Weighted median | 231 | 1.33 [1.22–1.46] | 3.50 × 10−10 |
Exposure instruments for insomnia from Jansen et al.
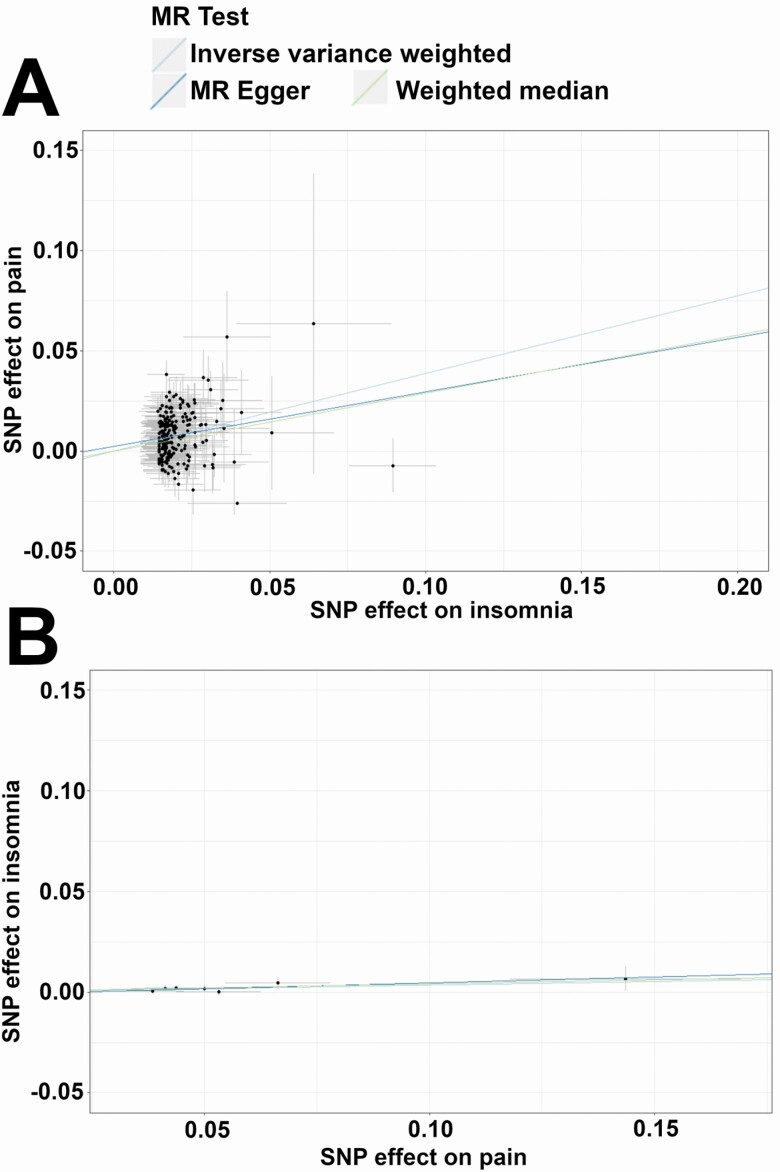
Figure 1.
Mendelian randomization analyses demonstrate stronger associations of insomnia with pain than vice versa. Scatterplots showing association of single variants and slopes from IVW, MR Egger, and weighted median analyses. Jansen et al. insomnia exposure and FinnGen general pain outcome (A), and FinnGen pain exposure with Lane et al. insomnia symptoms outcome (B).
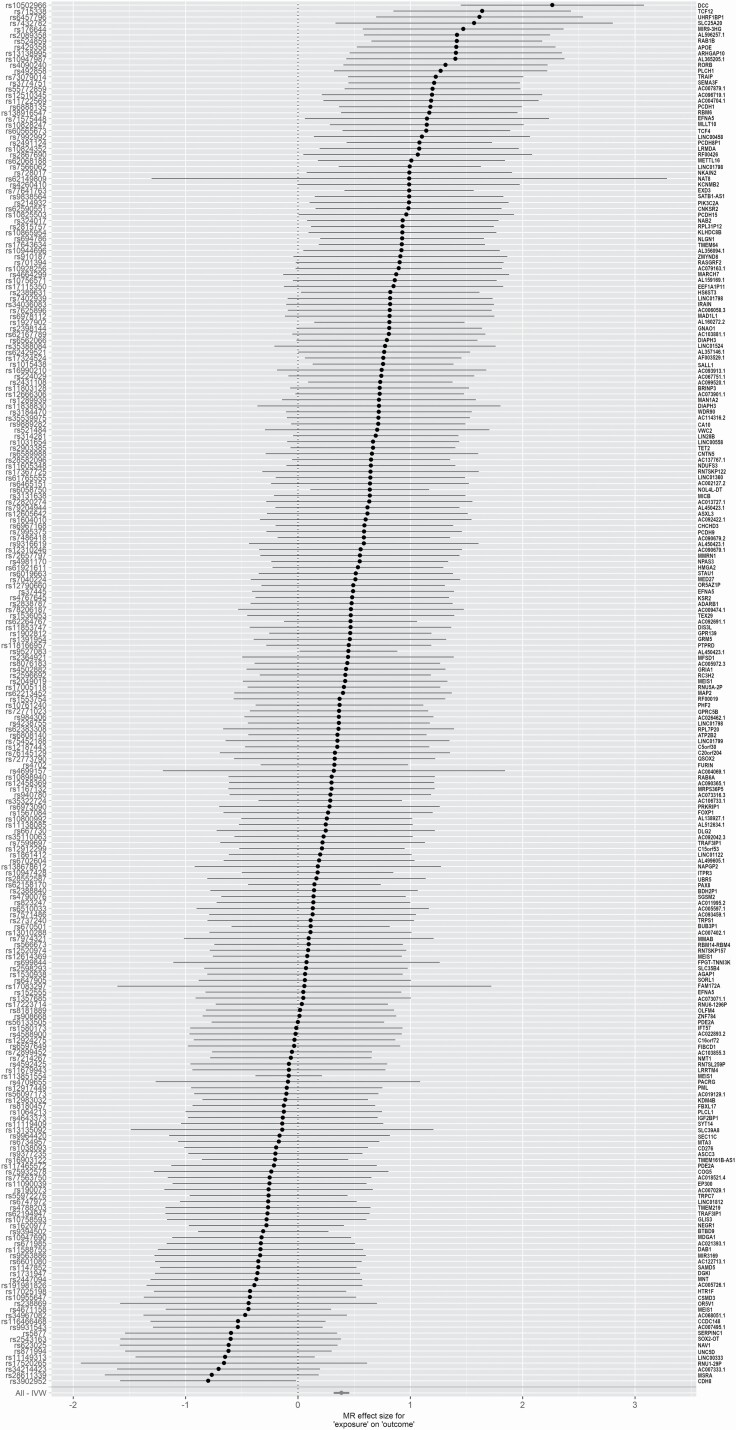
Figure 2.
Jansen et al. insomnia demonstrates a significant positive effect size on pain outcome. Forest plot of the MR-based effect sizes of Jansen et al. insomnia exposure instruments on FinnGen general pain outcome. The names of nearest genes are displayed on the right hand side of the plot.
Table 2.
MR results assessing causal effects of insomnia on specific pain category cohorts within FinnGen R5
| Pain type | Cases | Controls | OR [95% CI] | P | Bonferroni P |
|---|---|---|---|---|---|
| Abdominal and pelvic area pain | 49,416 | 161,968 | 1.40 [1.30–1.52] | 8.30 × 10−18 | 4.98 × 10−17 |
| Joint pain | 13,419 | 131,550 | 1.62 [1.44–1.82] | 1.65 × 10−15 | 9.9 × 10−14 |
| Limb pain | 12,606 | 167,641 | 1.48 [1.31–1.66] | 6 × 10−11 | 3.6 × 10−10 |
| Low back pain | 13,178 | 164,682 | 1.70 [1.50–1.93] | 1.46 × 10−16 | 8.76 × 10−16 |
| Ocular pain | 893 | 216,919 | 1.77 [1.22–2.57] | 0.003 | 0.018 |
| Throat and chest pain | 24,609 | 163,123 | 1.37 [1.24–1.51] | 5.58 × 10−10 | 3.35 × 10−9 |
Exposure instruments for insomnia from Jansen et. al, Bonferroni corrected p (accounting for six pain types) threshold = 0.05 (corresponding to 0.0083 nominal p).
We performed sensitivity analyses to assess for biases in our MR estimates. These analyses revealed no significant pleiotropy (MR Egger intercept test for insomnia exposure with pain outcome p = 0.40, and pain exposure with insomnia outcome p = 0.68, Supplementary Table S2).
In addition to effect from insomnia to pain we observed a smaller effect from pain to insomnia (OR [95% CI] = 1.04 [1.01–1.07], p < 0.05, Table 3, Figure 1, B, Supplementary Figure 1), with an MR-PRESSO global test p = 0.95 (Supplementary Table S2).
Table 3.
MR results assessing the causal effects of general pain on frequent insomnia symptoms (UKBB)
| MR method | SNPs | OR [95% CI] | P |
|---|---|---|---|
| Inverse-variance weighted | 7 | 1.04 [1.01–1.07] | 0.009 |
| MR Egger | 7 | 1.06 [0.96–1.17] | 0.31 |
| Weighted median | 7 | 1.04 [1.01–1.08] | 0.016 |
Exposure instruments from FinnGen R5 Pain (general) GWAS.
Leave-one-out analysis removing individual Jansen et al. insomnia SNPs (exposure instruments) on the general pain outcome demonstrated robust effects from insomnia to pain regardless of which SNPs were removed (Supplementary Figure 2). Similar to pleiotropy analysis above, a funnel plot of the IVW analysis on the Jansen et al. insomnia SNPs suggested no significant pleiotropy (Figure 3). Vice versa, a leave-one-out analysis and funnel plot of pain exposure on insomnia outcome suggested no significant pleiotropy (Supplementary Figure 3).
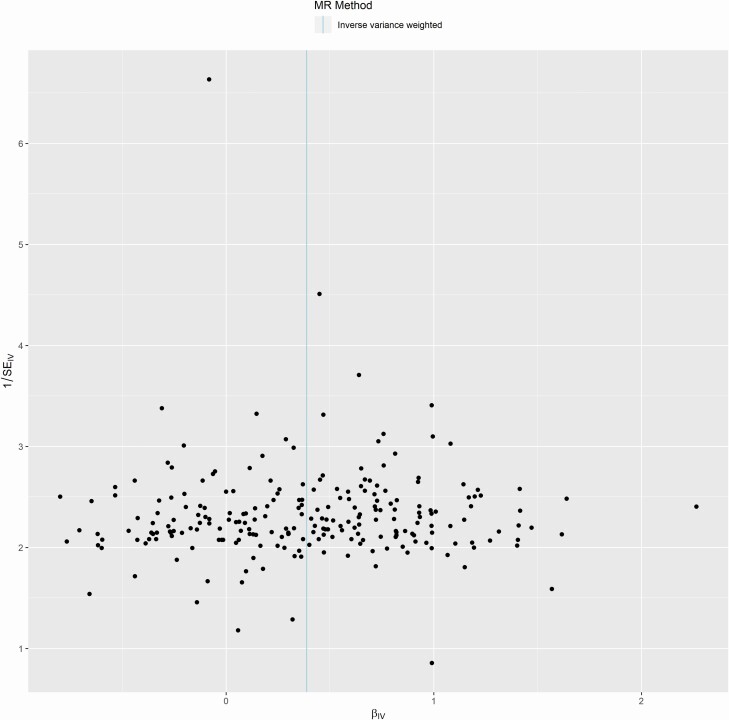
Figure 3.
Jansen et al. insomnia instruments show no significant pleiotropy or experimental bias. A funnel plot of the IVW analysis for the 231 insomnia SNP instruments as exposure on general pain outcome.
A secondary bi-directional MR analysis using the MCP lead SNPs produced by Johnston et al. also demonstrated significant bidirectional causality with insomnia without significant pleiotropic effects. The MR analysis of MCP to insomnia (Lane et al.) produced an IVW OR [95% CI] = 1.32 [1.26–1.38], P = 1.58 × 10−36, while the vice versa using Jansen et al. lead SNPs resulted in an IVW OR [95% CI] = 1.36 [1.32–1.41], p = 2.04 × 10−81 (Supplementary Figure 4, Supplementary Table S3). Comparing Jansen et al. lead SNPs with other UKBB sleep trait analyses we found 26 SNPs significantly associated with other sleep traits. We removed those and re-calculated the potential pleiotropic effect, and obtained an Egger intercept p = 0.55 (no significant pleiotropy). We also performed a leave-one-out analysis suggesting still a consistent robust effect by the SNPs (Supplementary Figure 5).
Discussion
In this Mendelian randomization study using both two-sample and one-sample analyses using data from 10 different GWAS datasets (Figure 4), we found evidence to support potentially casual bidirectional relationships between insomnia and reported pain. The overlap of lead SNPs between Jansen et al., Johnston et al., Lane et al., and FinnGen was relatively small (Supplementary Table S4). All utilized GWAS datasets here have corrected for confounding factors such as sex and age. Primarily, insomnia symptoms are a risk factor for generalized pain and pain in different parts of the body. Conversely, the pain has a small but significant effect on insomnia. These results support earlier observations from model organisms, from experimental sleep deprivation (a primary manifestation of insomnia) studies in humans, from prospective epidemiologic studies and clinical findings [7–9].
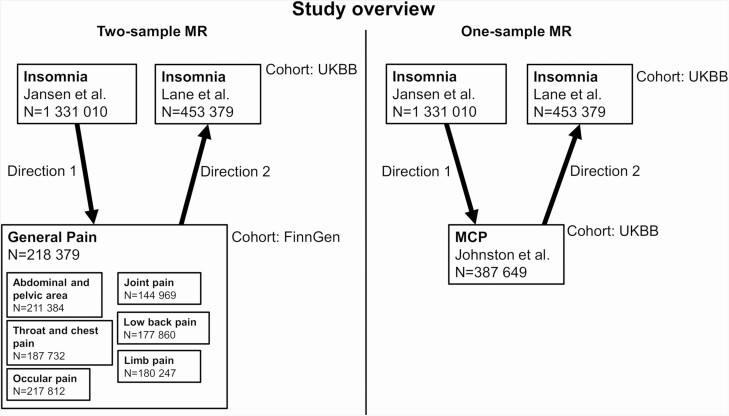
Figure 4.
Overview of MR analyses and datasets used in this study. Sample sizes (N) for all datasets and cohorts and the different directions of MR exposure-outcome analyses (arrows).
Our results demonstrate that insomnia is significantly causal to general pain (Egger intercept p = 0.4, indicating no significant pleiotropy). The results are concordant with previous findings linking insomnia to pain [2, 5, 7, 8]. Some findings have suggested a state of over-activation of pain-inhibitory circuits in patients with insomnia, causing pain sensation independent from medication, which may relate to the importance of sleep to modulate neural development and maintenance [34]. Other studies have linked the effects of insomnia on pain to neuronal modifications in the caudate nucleus, which is involved in pain suppression [6, 35]. This demonstrates the importance of treating insomnia in order to alleviate pain.
In the other direction, we report a significant but smaller effect of pain on insomnia (Egger intercept p = 0.68, indicating no significant pleiotropy). In part, this may be due to only obtaining seven lead SNPs from the FinnGen general pain data used for MR (64 SNPs from Johnston et al.), whereas the Jansen et al. data contained 248 lead SNPs associated with insomnia. Thus, there is a significant difference in power between the two different MR directions in this bi-directional study, which may be the reason for the difference in significances. The Johnston et al. dataset was used to perform a one-sample MR analysis (both datasets were obtained using the UKBB cohort) in order to support our two-sample MR of FinnGen general pain exposure on insomnia. There has been research indicating a significant effect of chronic pain on the onset of insomnia symptoms [12, 36]. However, this effect seems to be long-term (years) instead of more immediate effects of insomnia on pain sensation (days), and seems to be a complex condition with some confounding effects by limited social participation on the insomnia outcome as highlighted by Tang et al. [9, 12, 36]. The social aspect was linked to pain leading to reduced participation in social and physical activities that generate sleep pressure and sleep promotion [12, 36].
There are limitations to consider in interpreting the results of this study. The pain diagnoses in FinnGen are currently not divided into chronic or acute pain, and this remains an important avenue to further exploration. The difference between pain types is essential to take into account as acute and chronic pain are likely mediated by distinct mechanisms. There is also a need to untangle the differences between genetic effects and short-term clinical intervention for treating these conditions, and account for possible pleiotropy in MR. Conclusively, we have strong indications that sleep management in terms of co-occurring insomnia may be a practical avenue for future research in clinical managing of pain sensation, while pain management may also reduce insomnia. The bidirectional causal link between pain and insomnia highlighted this study is supported by more observational studies of pain and insomnia, where both traits were found to significantly affect each other [6, 37, 38]. The maintenance of good sleep hygiene could thus prove a cost-effective, widely available, and safe way to aid in the management of pain. However, as discussed by Wei et al., the primary effect of treating insomnia may be to avoid enhanced pain sensation after a bad night’s sleep, rather than aiming to alleviate the immediate pain sensation by a good night’s sleep, and vice-versa from pain to sleep [6].
Supplementary Material
Acknowledgments
We thank Iyas Daghlas and Richa Saxena for commenting the analysis plan and the manuscript. The authors would like to thank the FinnGen consortium for GWAS summary statistics. This work was funded by grants from Finnish Cultural Foundation (M.B.), Lastentautien Tutkimussäätiö (M.B.), and the Instrumentarium Science Foundation (H.M.O.).
Author contributions
M.B. and H.M.O. conceived and developed the original study design. M.B. collected the data and performed the computational analyses. M.B. and H.M.O. interpreted the results and wrote the manuscript.
Conflict of interest statement. The authors have no potential conflict of interest.
References
- 1. Lane JM, et al. ; HUNT All In Sleep. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51(3):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morin CM, et al. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. [DOI] [PubMed] [Google Scholar]
- 3. Morin C, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 4. Finan PH, et al. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alföldi P, et al. Comorbid insomnia in patients with chronic pain: a study based on the Swedish quality registry for pain rehabilitation (SQRP). Disabil Rehabil. 2014;36(20):1661–1669. [DOI] [PubMed] [Google Scholar]
- 6. Wei Y, et al. Insomnia really hurts: effect of a bad night’s sleep on pain increases with insomnia severity. Front Psychiatry. 2018;9:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexandre C, et al. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat Med. 2017;23(6):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewin DS, et al. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr. 1999;20(4):244–252. [DOI] [PubMed] [Google Scholar]
- 9. Tang NK, et al. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16(1):85–95. [DOI] [PubMed] [Google Scholar]
- 10. Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13(6):513–520. [DOI] [PubMed] [Google Scholar]
- 11. Kaur S, et al. Gender differences in health care utilization among veterans with chronic pain. J Gen Intern Med. 2007;22(2):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang NK, et al. Impact of musculoskeletal pain on insomnia onset: a prospective cohort study. Rheumatology (Oxford). 2015;54(2):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston KJA, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019;15(6):e1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bush WS, et al. Chapter 11: genome-wide association studies. PLoS Comput Biol. 2012;8(12):e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marchini J, et al. Genotype imputation for genome-wide association studies. Nat Rev Genet. 2010;11(7):499–511. [DOI] [PubMed] [Google Scholar]
- 16. Hirschhorn JN, et al. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6(2):95–108. [DOI] [PubMed] [Google Scholar]
- 17. Porcu E, et al. ; eQTLGen Consortium; BIOS Consortium. Mendelian randomization integrating GWAS and eQTL data reveals genetic determinants of complex and clinical traits. Nat Commun. 2019;10(1):3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gill D, et al. The effect of iron status on risk of coronary artery disease: a Mendelian randomization study-brief report. Arterioscler Thromb Vasc Biol. 2017;37(9):1788–1792. [DOI] [PubMed] [Google Scholar]
- 19. Mokry LE, et al. Obesity and multiple sclerosis: a Mendelian randomization study. PLoS Med. 2016;13(6):e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng J, et al. Use of Mendelian randomization to examine causal inference in osteoporosis. Front Endocrinol (Lausanne). 2019;10:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slob EAW, et al. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44(4):313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burgess S, et al. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess S, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jansen PR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394–403. doi: 10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 25. Watanabe K, et al. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machiela MJ, et al. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31(21):3555–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daghlas I, et al. Habitual sleep disturbances and migraine: a Mendelian randomization study. Ann Clin Transl Neurol. 2020:7(12): 2370–2380. doi: 10.1002/acn3.51228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burgess S, et al. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hemani G, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hemani G, et al. Correction: orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(12):e1007149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burgess S, et al. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowden J, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol. 2016;40(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verbanck M, et al. Publisher correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(8):1196. [DOI] [PubMed] [Google Scholar]
- 34. Haack M, et al. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16(4):522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stoffers D, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137(Pt 2):610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang NKY, et al. The role of pain, physical disability, and reduced social participation in insomnia onset in community dwelling older adults: a prospective cohort study. The Lancet. 2013;382:S95. doi: 10.1016/S0140-6736(13)62520-9 [DOI] [Google Scholar]
- 37. Skarpsno ES, et al. Do physical activity and body mass index modify the association between chronic musculoskeletal pain and insomnia? Longitudinal data from the HUNT study, Norway. J Sleep Res. 2018;27(1):32–39. [DOI] [PubMed] [Google Scholar]
- 38. Uhlig BL, et al. Insomnia and risk of chronic musculoskeletal complaints: longitudinal data from the HUNT study, Norway. BMC Musculoskelet Disord. 2018;19(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.