Abstract
Primordial germ cells (PGCs) are the precursors of germline cells that generate sperm and ova in adults. Thus, they are promising tools for gene editing and genetic preservation, especially in avian species. In this study, we established stable male and female PGC lines from 6Hungarian indigenous chicken breeds with derivation rates ranging from 37.5 to 50 percent. We characterized the PGCs for expression of the germ cell-specific markers during prolonged culture in vitro. An in vivo colonization test was performed on PGCs from four Hungarian chicken breeds and the colonization rates were between 76 and 100%. Cryopreserved PGCs of the donor breed (Partridge color Hungarian) were injected into Black Transylvanian Naked Neck host embryos to form chimeric progeny that, after backcrossing, would permit reconstitution of the donor breed. For 24 presumptive chimeras 13 were male and 11 were female. In the course of backcrossing, 340 chicks were hatched and 17 of them (5%) were pure Partridge colored. Based on the backcrossing 1 hen and 3 roosters of the 24 presumptive chimeras (16.6%) have proven to be germline chimeras. Therefore, it was proven that the original breed can be recovered from primordial germ cells which are stored in the gene bank. To our knowledge, our study is a first that applied feeder free culturing conditions for both male and female cell lines successfully and used multiple indigenous chicken breeds to create a gene bank representing a region (Carpathian Basin).
Key words: PGC line, indigenous chicken breed, germline transmission, cryopreservation, gene bank
INTRODUCTION
Over the last decade, the European Union has strongly supported the establishment of gene banks and the storage and use of gene bank samples. Since the 1950s, native Hungarian poultry and waterfowl species and breeds have been kept (Biszkup and Beke, 1951; Báldy, 1954) as living gene bank populations (ex situ in vivo). To coordinate the use of genetic material in gene banks within the European Union and to improve techniques (ex situ in vitro methods), we set out to create primordial germ cell (PGC) gene bank samples besides the already stored Hungarian indigenous poultry semen samples. In avian species PGC storage in gene banks has a great importance, because recently this has become the most practical way to preserve the genetic material present in the female W chromosome and mitochondrial DNA. In birds, unlike mammals, the male has the homogametic ZZ chromosomes, while the female has the heterogamous ZW chromosome pair. Since only Z haploid genetic material can be conserved in sperm, 6 to 8 steps of backcrossing is required to reconstruct the original genome (Blesbois, 2007). The use of stem cells in birds for breed constitution began in the 1990s (Tajima et al., 1993; Naito et al., 1994). However, to take advantage of their potential, a method was needed to propagate PGCs in vitro to increase their number. Van De Lavoir et al., (2006) first developed a long-term culturing method, and later on further improvements had been made by other researchers (Macdonald et al., 2010; Whyte et al., 2015; Miyahara et al., 2016; Tonus et al., 2016). Nowadays, PG cells can be readily obtained from the blood of avian embryos (48–65 h after laying in chicken), they can be cultivated, deep-frozen and then reinjected into the circulation system of a recipient embryo. Thus, they can be used for gene conservation, research, and genetic modification purposes. As there are many research papers on this topic (Pain et al., 1996; Nakamura et al., 2010; 2013a; Macdonald et al., 2010; Kim et al., 2013; Rikimaru et al., 2014; Whyte et al., 2015; Tonus et al., 2016), nonetheless there is no established practice and widely accepted standard protocol on the production and validation of PGC lines to be stored in gene banks, in this study a methodology is proposed for this purpose.
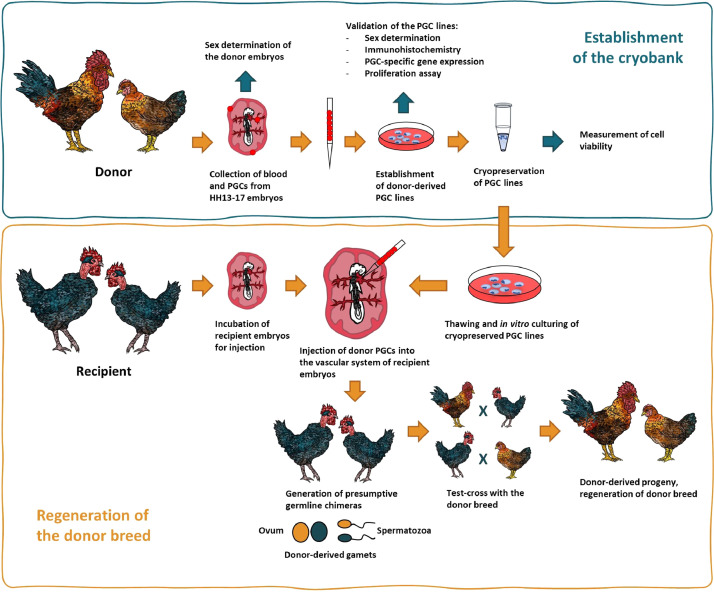
A gene bank had been built with PGC samples from 6 Hungarian native breeds which represent great national value and genetic diversity. During the experiments we could recover the donor breed from the stored PGCs with high efficiency. Another important result was that both male and female PGCs were maintained in feeder free cell culture conditions and both sexes were retrieved after reinjection. Therefore, the results presented in this article are recommended to other in vitro gene preservation institutions/gene banks. A general summary of producing donor-derived hatchlings with cryopreserved indigenous PGC lines is presented on Figure 1.
Figure 1.
General outline of producing donor-derived hatchlings with cryopreserved indigenous PGC lines. The migration of PGCs reaches its peak in the bloodstream between HH stages 13-17 (48–65 h after laying in chicken); thus this is the optimal stage for collecting donor PGCs, and also this is the suitable stage for injecting them back to the recipient embryo. After the isolation, using a selective media, in vitro cultures of PGCs can be established. The stable cell lines need to be validated for sex, PGC specific gene expression by qPCR, PGC specific proteins by immunohistochemistry and proliferation rate by proliferation assay. For long-term storage, the cells are cryopreserved and kept in liquid nitrogen. As a next step, cryopreserved PGCs are injected into the recipient embryo. After the hatching, the presumptive germline chimaeras are backcrossed with the original breed to regenerate the donor genotype.
MATERIALS AND METHODS
Maintenance of Domestic Fowl Experimental Stocks
The fertile eggs which were used for establishing primordial germ cell lines, came from the gene bank stock of the National Centre for Biodiversity and Gene Conservation, Institute for Farm Animal Gene Conservation. The old Hungarian chicken breeds were kept in barns with large outdoor areas in the institute. The stocking density 5 to 6 birds/m2, the sex ratio: 7 hens, 1 cockerel. There are nest boxes (5 hens/nest) for collection of eggs. Breeding flocks are fed with laying mash in addition to limestone grit. The eggs are collected twice a day, and then stored in a refrigerated room. The hatched Black Transylvanian Naked Neck presumptive germline chimera chickens were grown in a special chick rearing box until 4 wks of age (0.5 m2/10 individuals). The box is equipped with automatic heating and lighting. The temperature was gradually reduced weekly from 30°C to the final 22 to 24°C. After 4 wks of age they were placed on deep litter. The young individuals were fed ad libitum with granulated starter mash (Szinbád Ltd., Gödöllő, Hungary) and a water supply (Molnár et al., 2019).
Artificial Insemination
Semen from cockerels was collected by abdominal massage according to Burrows and Quinn, (1935). The fresh, pooled and diluted semen was inseminated in a dose of 100 ± 20 million spermatozoa per female in all cases. For the calculation of sperm concentration Lake's diluent was used. Artificial insemination of hens was performed as described by Bakst and Dymond, (2013).
Isolation, Establishment and Cryopreservation of Stable PGC Lines
Isolation of PGCs From Embryonic Blood
Blood was collected individually from every embryo between HH stages 13 to 17 (2.5–3 days old). About 1 to 3 µL blood was isolated from the dorsal aorta under stereomicroscope, using sterile pulled glass microcapillary (~ 20 µm in diameter at the end) and a mouth pipette, then immediately placed into 48-well plates, in selective media for chicken PGCs (Whyte et al., 2015). Tissue samples for sex-determination were collected from every isolated embryo and stored at −20°C until further use. The time of isolation, the exact age of the embryos (HH stages) and the presence / absence of developmental abnormalities were recorded.
Establishment and Maintenance of PG Cell Lines
The isolated blood from single embryos, which contains the cellular components of blood including the PGCs, was cultured in vitro in selective media for PGCs (Whyte et al., 2015), in thermostat at 38°C with 5% of CO2 concentration for 3 wk. This period of culturing ensures enough time for elimination of all cell types except the PGCs. In the selective medium, PGCs start to divide, and a homogeneous cell population can be established. If the cell number of PGCs from one embryo reached 1.0 × 105 cells in 3 wks, the establishment of the line was considered successful.
Freezing and Thawing of PGC Lines
Freshly prepared freezing media for PGCs was used for freezing of the established PGC lines, which contained the following components: DMEM (Thermo Fisher Scientific, Waltham, MA, USA, 21068-028) and sterile water (Thermo Fisher Scientific, Waltham, MA, USA, 15230-089) in 2:1 ratio, 8 % of DMSO (Sigma-Aldrich, St. Louis, MO, USA, 276855), 10% of chicken serum (Sigma-Aldrich, St. Louis, MO, USA, C5405) and 0.75 % of 20 mM CaCl2 (Sigma-Aldrich, St. Louis, MO, USA, C4901). PGCs were suspended carefully from the bottom of the culturing well, and then pipetted into 1.5 mL Eppendorf tubes. After centrifugation (1,000 g, 3 min) the supernatant was removed, then the cells were suspended in 250µL of DMSO free freezing media for PGCs and pipetted into labelled cryotube. 250µL of freezing media for PGCs was added slowly dropwise, and then the tube was placed into the freezer at −70°C. In the case of long-term storage, after one night, the samples were moved into the freezer at −150°C or into liquid nitrogen. For thawing of PGCs, water bath at 37°C was used, and then the total content of the tube was pipetted into 2 mL of culturing media for PGCs. After centrifugation (1,000 g, 3 minutes) the supernatant was removed, then the cells were resuspended in fresh culture media for PGCs and placed into cell culture wells for further growth.
In Vitro Characterization of the PGC Lines
The established PGC lines were submitted to in vitro validation in order to define the essential characteristics and ensure that the cell populations are actually homogenous and have the specific characteristics of this cell type.
DNA Isolation and Sex Determination.
For isolating the DNA, High Pure PCR Template Preparation Kit (Roche Diagnostics, Rotkreuz, Switzerland) was used according to the manufacturer's instruction. The sex of the donor embryos and the established PGC lines were determined with the P2–P8 primer set (Table 1.) as described before by Griffiths and colleagues (Griffiths et al., 1998). The isolated DNA was diluted to 25 ng/μL concentration for PCR reaction and gel electrophoresis. MyTaq Red Mix was used for the reaction (Bioline Reagents Ltd., London, UK). The PCR products were then separated by electrophoresis, using 3% agarose gel stained with ethidium bromide at 100V for 1.5 to 2.0 h. The DNA bands were then visualized under UV illumination and photographed (Lázár et al., 2018).
Table 1.
Primer sequences used in the gene expression and sex PCR experiments.
| Gene Symbol | Gene Full Name (organism) | NCBI number | Primers | Product length (bp) | |
|---|---|---|---|---|---|
| cGAPDH | Glyceraldehyde-3-phosphate dehydrogenase (Gallus gallus) | NM_204305.1 | FW | GACGTGCAGCAGGAACACTA | 112 |
| RV | CTTGGACTTTGCCAGAGAGG | ||||
| cPOUV | POU domain class 5 transcription factor 3 (Pou5f3) (Gallus gallus) | NM_001110178.1 | FW | GAGGCAGAGAACACGGACAA | 109 |
| RV | TTCCCTTCACGTTGGTCTCG | ||||
| CVH | DEAD-box helicase 4 (DDX4)(Gallus gallus) | NM_204708.1 | FW | GAACCTACCATCCACCAGCA | 113 |
| RV | ATGCTACCGAAGTTGCCACA | ||||
| P2/P8-Z | (Gallus gallus) | NC_006127.4 | FW | TCTGCATCGCTAAATCCTTT | 345 |
| RV | CTCCCAAGGATGAGAAATTG | ||||
| P2/P8-W | (Gallus gallus) | NC_006126.4 | FW | TCTGCATCGCTAAATCCTTT | 362 |
| RV | CTCCCAAGGATGAGAAATTG |
Immunostaining of PGCs.
Isolated PGCs were fixed with 4% PFA for 10 min. After washing with PBS (3 times, 5 min each), cells were permeabilized with 0.5% Triton X-100 (Merck Millipore, Burlington, MA) for 5 min. After washing with PBS, to minimize nonspecific binding of antibodies, the fixed cells were blocked for 45 min with a blocking buffer containing PBS with 1% (v/v) BSA. Cells were then washed 3 times with PBS and were incubated with each of the primary antibodies including mouse anti-SSEA-1 (1:100, Developmental Studies Hybridoma Bank, Iowa City, IA) and rabbit anti-VASA (CVH) (1 : 1,000; kindly provided by Bertrand Pain, Lyon, France). After incubation overnight in the primary antibody solution in a humid chamber at 4°C, the cells were washed 3 times with PBS. Then, cells were incubated with the secondary antibodies, Donkey Anti-mouse IGM-D488 (1:400, Jackson ImmunoResearch, West Grove,PA) and Alexa Fluor® 555 Donkey Anti-rabbit IgG (H+L) (1:500, Thermo Fisher Scientific, Waltham, MA), in a dark humid chamber for 1 h at room temperature. After washing with PBS, the nucleus was stained with TO-PRO-3 stain (1:500, T3605, Thermo Fisher Scientific, Waltham, MA), which is a far-red fluorescent (642/661) nuclear and chromosome counterstain. Coverslips were mounted on the slide with the application of 20 μL VECTASHIELD Mounting Media (H-1000, Vector Laboratories Inc., Burlingame, CA) and analysed by confocal microscopy (TCS SP8, Leica, Wetzlar, Germany). Negative controls were stained only with the secondary antibodies (Lázár et al., 2018).
Isolation, Synthesis of cDNA, and Quantitative Real-Time PCR
Total RNA from the established PGC lines was isolated using RNAqueous-Micro Total RNA Isolation Kit (Thermo Fisher Scientific) following the instructions of the manufacturer. The concentration of RNA was determined by NanoDrop Spectrophotometer (Thermo Fisher Scientific), and the samples were stored at −70°C until later use. The extracted RNA samples were reverse transcribed into cDNA with High Capacity cDNA Reverse Transcription Kit following the instructions of the manufacturer (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). RT Master Mix was used for cDNA writing. The cDNA was stored at −20°C. The synthesized cDNA was then used for quantitative real-time PCR. SYBR Green PCR Master Mix was applied for the qPCR as a double-stranded fluorescent DNA-specific dye according to the manufacturer's instructions (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). For each gene examined, triplicates were measured, fluorescence emission was detected, and relative quantification was calculated with the GenEx software (MultiD, Gothenburg, Sweden) (Lázár et al., 2018). Germ cell specific CVH and stem cell specific cPOUV genes were analyzed and cGAPDH was used as a housekeeping gene for calculating the relative expression levels. Primer sequences are shown in Table 1.
Cell Proliferation Assay.
Cell lines were analyzed for rate of proliferation. Proliferation was measured with the CCK-8 reagent (Sigma-Aldrich, MO, USA 96992). The strength of the CCK-8 reaction (bright orange color) is directly proportional with live cell number in the culture, therefore data was collected by measuring absorbance (optical density: O.D.) at 450 nm using a CLARIOstar® Microplate Reader (BMG Labtech, Ortenberg, Germany). PGC lines from the same breed were individually measured in the same experiment. The measurements were taken over 3 d, once per day. Triplicate wells were measured every day for every cell line on a 96-well plate (Lázár et al., 2018). Average absorbance values from each day was used to calculate doubling time (DT) for each cell line with the following formula: DT = d × log(2) / log(At) – log(A0), where d: duration in days; At: absorbance at the third day; A0: absorbance at the first day (Kong et al., 2018).
Fluorescent Labeling of Frozen/Thawed cells.
Cultured PGCs were marked with a vital cell surface fluorescent dye, which renders the injected cells visible in the embryo. PGCs were centrifuged and after washing in sterile PBS, the red dye (PKH26 Red Fluorescent Cell Linker, Sigma Aldrich, MO, USA, MIDI26-1KT) was applied according to the manufacturer's description. Fluorescence of freshly labeled cells was checked with confocal microscope (TCS SP8, Leica, Wetzlar, Germany), then the cells were put into CO2 incubator until subsequent use.
Assessing the Capability of PGC Migration Into the Gonad.
Fluorescently labeled cells were collected and counted (Arthur Cell Analyzer, NanoEnTek Inc., Seoul, Korea). After the centrifugation, the pellet was resuspended in mixture of DMEM and sterile water with ratio 2:1 (simplified media was used, because some components of the complete PGC media may affect the development of embryos). The recipient embryos were from the same breed. After the shell of recipient eggs were cleaned with alcohol, a hole was opened with a diameter of 1 cm, and then 1 µL of the prepared cell suspension (~3–5,000 PG cells/µL) was injected into the heart of 2.5-day-old embryos using a pulled glass microcapillary. After the injection, ~50 µL of preheated sterile 1xPBS was dropped on to the embryo, then the hole was closed with 2 layers of sterile laboratory parafilm and the individually marked eggs were put back into the hatchery.
Monitoring of Integration of Injected Cells Into the Gonads.
After the injection, the eggs were incubated for 6 d. Embryos were isolated and the gonads were dissected from the embryos. The gonads were washed in sterile 1× PBS, then fixed in 4% PFA for 1 h. The injected cells were identified in the fixed gonads with a fluorescence stereomicroscope and photo documentation was taken.
Recovering the Original Breed With Reintegration and Backcrossing
Injection of Donor Derived PGCs Into Recipient Embryos.
Partridge Colored Hungarian chicken was chosen as donor breed, because the partridge color is a recessive trait; it is expressed only if both parents are pure partridge colored. In the recipient breed, the Black Transylvanian Naked Neck chicken, the gene for naked neck trait is dominant, thus if partridge colored covered neck chickens hatch after the backcrossing, they are derived from injected PGCs. PGCs were cultured as detailed above. PGCs were frozen and then thawed prior to injection. A male and a female cell line (No. PC101 and PC111 lines) were selected for injection (Lázár et al., 2018). Recipient eggs were incubated until stage HH15-16. About 3,000 to 5,000 male or female PGCs were injected into the heart of each embryo through a hole (5–6 mm in diameter) on the eggshell. After the injection, 50 µL of sterile 1× D-PBS was added and then 2 layers of parafilm were used to close the hole. The injected eggs were incubated at 37.8°C with 70% relative humidity.
Test Crossings of the Presumptive Germline Chimeras.
The presumptive chimera Black Transylvanian Naked Neck chickens were placed into individual cages for mating. For test crossing, Partridge Colored Hungarian mates were used as they were the donor breed, and were also kept in individual cages. Two Partridge Colored Hungarian hens were inseminated artificially with the sperm of each Black Transylvanian Naked Neck presumptive chimera cockerel. The Black Transylvanian Naked Neck presumptive chimera hens were inseminated with mixed sperm of 5 Partridge Colored Hungarian cockerels.
Statistical Analysis.
The following software were used to analyse the data: RStudio (1.0.136), R (R-3.2.2) and GeneEx (6.0). Levels of significance were applied as follows: ∗P < 005, ∗∗P < 001, and ∗∗∗P < 0001. In case of every qPCR run, expression changes of the target genes were calculated compared to the level of the housekeeping gene with the standard 2^(−ΔΔCt) method, where Ct = cycle threshold; ΔCt = Ct (target gene) − Ct (housekeeping gene) and ΔΔCt = ΔCt (test sample) − ΔCt (control sample).
RESULTS
Characteristics of the Species and Varieties Involved in the Crossing Experiments
Hungarian Chicken Breeds.
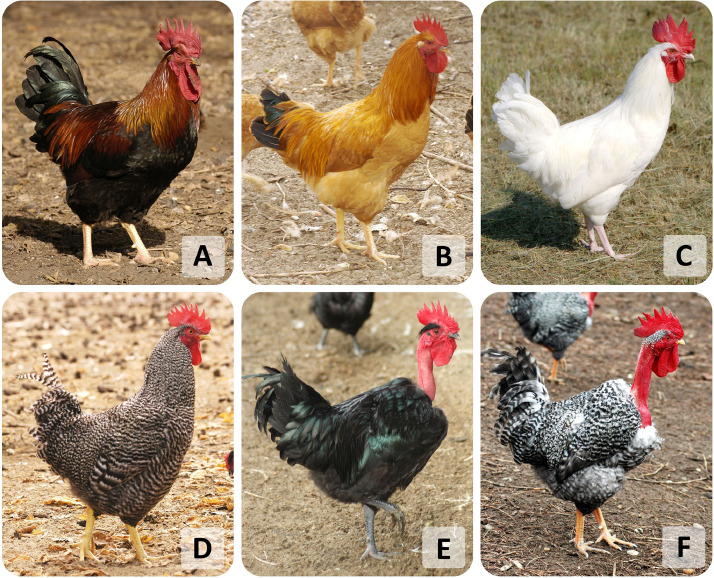
The progenitors of these birds were brought into the Carpathian Basin from Asia by the Hungarian conquerors at the end of the ninth century. Over the centuries of their formation, these breeds have become well adapted to the special climatic conditions and farming systems of the country, which made them very precious local varieties in the Carpathian Basin (Szalay, 2015). The following breeds were used during the investigations of this study: Yellow Hungarian, White Hungarian, Speckled Hungarian, and Partridge colored Hungarian (Figure 2.).
Figure 2.
Indigenous chicken breeds which were used in the experiments. (A) Partridge color Hungarian rooster. (B) Yellow Hungarian rooster. (C) White Hungarian rooster. (D) Speckled Hungarian rooster. (E) Black Transylvanian naked neck rooster. (F) Speckled Transylvanian naked neck rooster. (http://www.geneconservation.hu/content/old-hungarian-farm-animals).
Transylvanian Naked Neck Chicken Breeds.
The neck, partly the breast and the belly of Transylvanian Naked Neck chickens are characteristically featherless. The naked neck trait is controlled by an autosomal gene and dominant in relation to feathered neck trait. In the case of homozygous naked neck birds, the neck is completely naked, whereas heterozygous birds have a feather brush on the front, lower part of their neck, which can already be observed on day-old chicks. There is only a little plumage in the top of their head. They are extraordinarily hardy, firm and resistant, grow and feather fast, perform good egg production and the weight of the eggs can exceed 70 g, however, they have a weak brooding instinct (Szalay, 2015). More color variations can be distinguished; Black Transylvanian Naked Neck and the Speckled Transylvanian Naked Neck were used during these experiments (Figure 2.).
Isolation and Establishment of Stable PGC Lines
Blood and PGCs were isolated from 6 Hungarian native breeds and we managed to establish robust cell lines from all 6 breeds. The derivation rate was around 50% for 3 Hungarian breeds (Yellow, White and Speckled Hungarian), which were the most successful. Among the Hungarian varieties, Partridge color proved to be the worst; the derivation rate was only 28%. The derivation rate of 2 Transylvanian Naked Neck breeds was slightly below 40%. In case of every breed, at least 20 individual cell lines were created. Six parallel cryotubes from each cell line were placed in the gene bank, every vial containing approximately 5.0 × 104 cells (Table 2.). The number of isolations for each breed depended on egg availability, egg fertility, and the number of healthy embryos in the right developmental stage.
Table 2.
Derivation rates of established cell lines and number of stored samples in the gene bank.
| Hungarian indigenous breeds | No. of isolations | No. of cell cultures | No. of male cell lines (%) | No. of female cell lines (%) | Derivation rate (%) | No. of cryotubes in genebank |
|---|---|---|---|---|---|---|
| Yellow Hungarian | 80 | 40 | 29 (72,5) | 11 (27,5) | 50,0 | 240 |
| White Hungarian | 42 | 20 | 14 (70,0) | 6 (30,0) | 47,6 | 120 |
| Speckled Hungarian | 76 | 37 | 27 (73,0) | 10 (27,0) | 48,7 | 222 |
| Partridge colour | 74 | 21 | 17 (81) | 4 (19,0) | 28,4 | 126 |
| Black Transylvanian naked neck | 68 | 27 | 23 (85,2) | 4 (14,8) | 39,7 | 162 |
| Speckled Transylvanian naked neck | 56 | 21 | 11 (52,4) | 10 (47,6) | 37,5 | 126 |
| Σ (%) | 396 | 166 (42,0) | 121 (72,3) | 45 (27,7) | - | 996 |
In Vitro Characterization of the PGC Lines
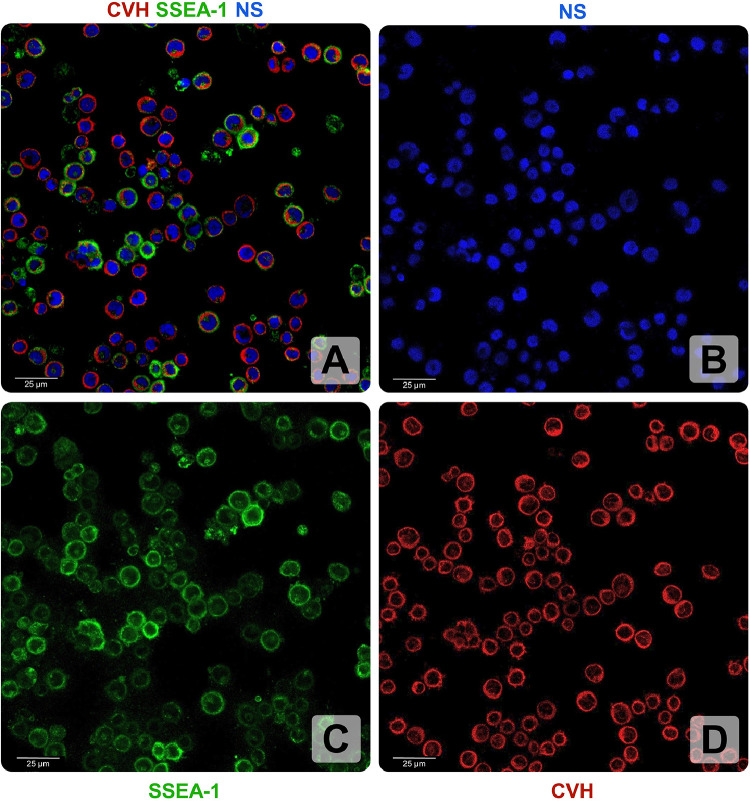
The established PGC lines were submitted to in vitro validation in order to define the essential characteristics and ensure that the cell populations are actually homogenous and have the specific characteristics of this cell type. Immunohistochemistry of PGCs was performed on cell lines of all 6 breeds. Two cell lines, one male and one female were selected randomly from each breed. Cells were collected after culturing and freeze-thawing the cell lines. Each of the tested cell lines showed germ cell-specific CVH and stem cell-specific SSEA1 staining (Figure 3.). CVH is a cytoplasmic while SSEA1 is a cell surface marker. TO-PRO-3 (blue) was used as a nuclear stain. The stem cell marker SSEA-1 stained with green and the germ cell specific marker CVH stained with red color. Figure 3 shows immunostained picture of the WH1102 male PGCs. This PGC line was derived from White Hungarian chicken breed.
Figure 3.
Germ cell and stem cell specific immunohistochemistry of PGC lines. (A) Merged image of CVH (red), SSEA-1 (green) and nuclear staining (blue). (B) Nuclear staining (NS), TO-PRO-3® was used (blue). (C) SSEA-1 appears in the cell membrane of the immunostained PG cells. (D) CVH is localized in the cytoplasm.; In this figure, the WH1102 (male) PGC line is shown which is originated from the White Hungarian breed. (Scale bar: 25μm).
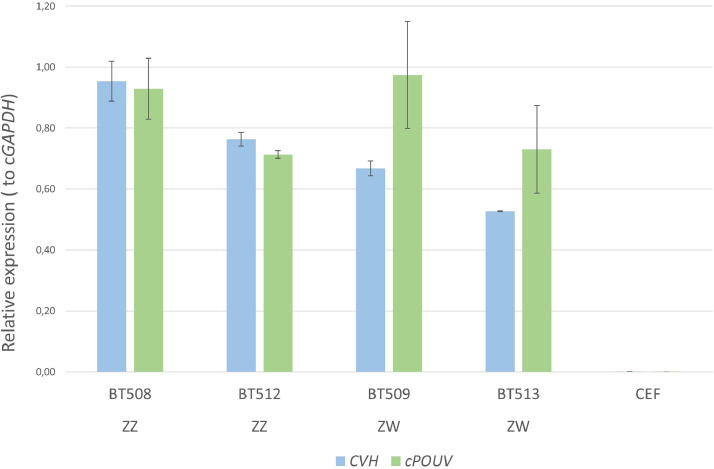
PGC lines were analysed from all 6 breeds for marker expression analysis using qPCR. A male and a female cell line was chosen randomly from every chicken breed. All tested cell lines showed expression of the germ cell-specific CVH and the stem cell-specific cPOUV markers. Figure 4 presents the CVH and cPOUV expression in 2 male (BT508, BT512) and 2 female (BT509, BT513) PGC lines derived from Black Transylvanian Naked Neck (BT) embryos. All PGC lines highly expressed the stem and germ cell-specific markers. Chicken embryonic fibroblast cells were used as negative control.
Figure 4.
Germ cell and stem cell specific marker expression of PGC lines. Expression levels of CVH and cPOUV genes compared to cGAPDH housekeeping gene were high in all the examined PGC lines in all breeds. In this figure, four PGC lines (male (ZZ): BT508, BT512 and female (ZW): BZ509, BT513) derived from Black Transylvanian Naked neck embryos are shown. Expression levels from chicken embryonic fibroblast (CEF) cells are present as negative control. Relatively higher CVH expression in the male (ZZ) cell lines is due to CVH gene is located on the Z chromosome.
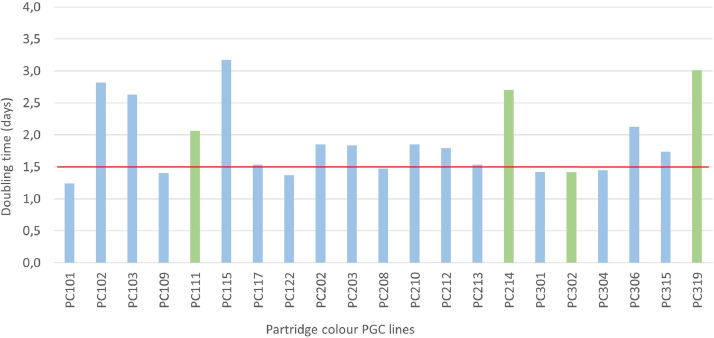
Next, we calculated the doubling time (days) for different PGC lines. In general, all the analysed PGC cultures showed appropriate proliferation, which means relatively shorter doubling times, therefore they were considered as valuable lines for gene banking purposes. There was no significant difference between the breeds. The speed of cell proliferation is an important factor for cell lines established for gene banking purposes. Cell lines with relatively fast proliferation (shorter doubling time) are easier and quicker to work with, but on the other hand the genetic value is not necessarily linked to proliferation. Since the main purpose was to preserve as much genetic diversity as possible, we did not set a strict doubling time threshold for the indigenous PGC lines. If any given cell line could produce enough cells for the in vitro experiments and gene bank samples, it was cryopreserved. Nevertheless, general assumptions were made based on the collected data. Cell lines with 1.5 d or less doubling times considered as ‘fast’, between 1.5 and 2.5 d as ‘average’, between 2.5 and 3.5 d as ‘slow’ and above 3.5 d usually means ‘not appropriate for gene banking’. Figure 5 shows doubling time data for the 21 PG cell lines established from the Partridge color Hungarian breed. All the cell lines are in the ‘fast’, ‘average’ or ‘slow’ category therefore gene bank samples were collected successfully.
Figure 5.
Proliferation measurements for one of the indigenous Hungarian breeds. The doubling time of a cell line was calculated from the absorbance (optical density) data acquired from the CCK-8 assays. Average values of the first and third days of the absorbance measurements were used to calculate the doubling time for each PGC line. Cell lines with 1.5 d or less doubling times considered as ‘fast’, between 1.5 and 2.5 d as ‘average’, between 2.5 and 3.5 d as ‘slow’ and above 3.5 d usually means ‘not appropriate for gene banking’. (The red horizontal line indicates the 1.5 d doubling time. Green columns represent female, blue columns show male cell lines.)
Validation of PG Cell Lines In Vivo
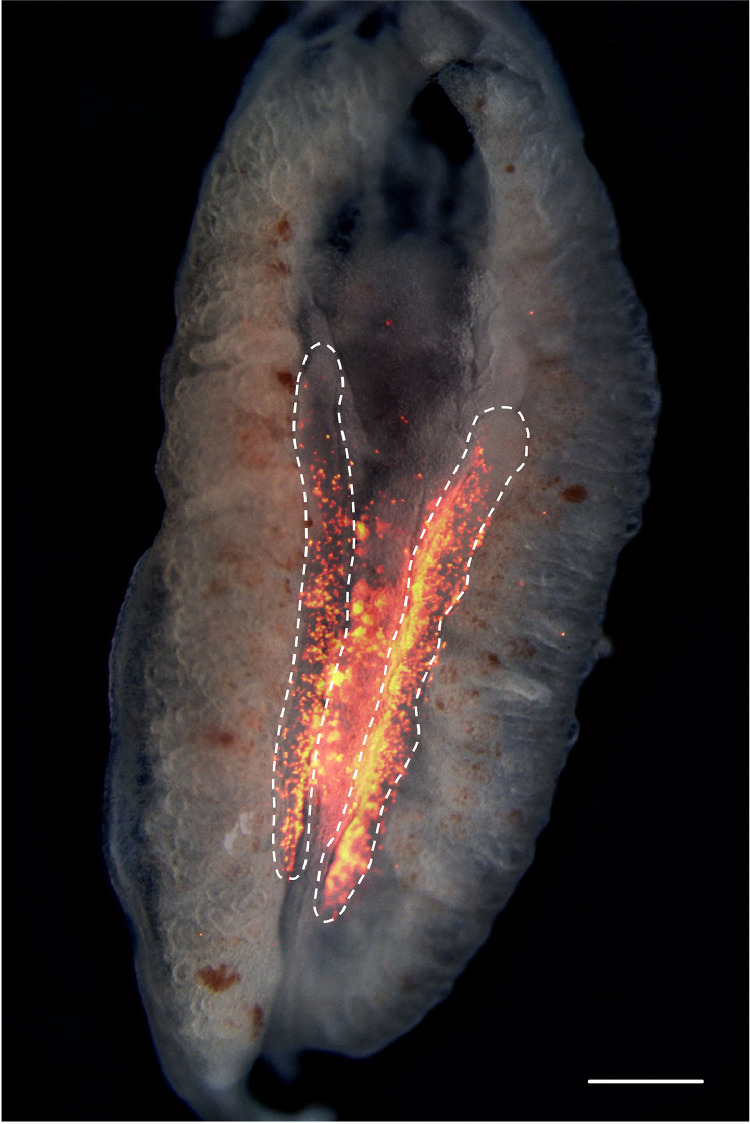
The cultured PGCs were characterized in vivo to investigate the cell function, and their ability to migrate into the gonads. An in vivo colonization test was done on PGCs from 4 Hungarian chicken breeds. In total 103 injections were performed and 61 embryos (59.2%) survived the procedure. In the case of 52 embryos, donor derived PGCs were found in the gonads which is an 85.3% average colonization rate (Table 3.). In the Yellow Hungarian and Black Transylvanian Naked Neck breeds, fluorescently labeled donor cells were found in 100% of the surviving embryos (Figure 6.). The Partridge color Hungarian and the Speckled Transylvanian Naked Neck varieties showed colonization rates between 76 and 78%. From this data we would expect the highest germ line transmission rates from the Yellow Hungarian and Black Transylvanian Naked Neck breeds.
Table 3.
Results of the in vivo colonization test of four Hungarian indigenous chicken breeds.
| Hungarian indigenous breeds | No. of injections | No. of live embryos (%) | No. of colonizations | Colonization rate (%) |
|---|---|---|---|---|
| Yellow Hungarian | 20 | 13 (65.0) | 13 | 100.0 |
| Partridge color | 32 | 21 (65.6) | 16 | 76.2 |
| Black Transylvanian naked neck | 19 | 9 (47.4) | 9 | 100.0 |
| Speckled Transylvanian Naked Neck | 32 | 18 (56.3) | 14 | 77.8 |
| Σ (%) | 103 | 61 (59.2) | 52 (85.3) | - |
Figure 6.
In vivo validation of the cultured and frozen-thawed PGC lines. Fluorescently labeled (Sigma PKH26) PG cells were injected into the heart of 2.5-day-old recipient embryos. Embryonic gonads were screened at embryonic day 6 for the presence of injected cells. Injected PGCs from the Black Transylvanian naked neck breed (red color) are present in the recipient gonads (highlighted with white dashed lines), showing the proper functionality and migration capability of the stored cell lines. (Scale bar: 500 μm).
Recovering the Original Breed With Injection and Crossing
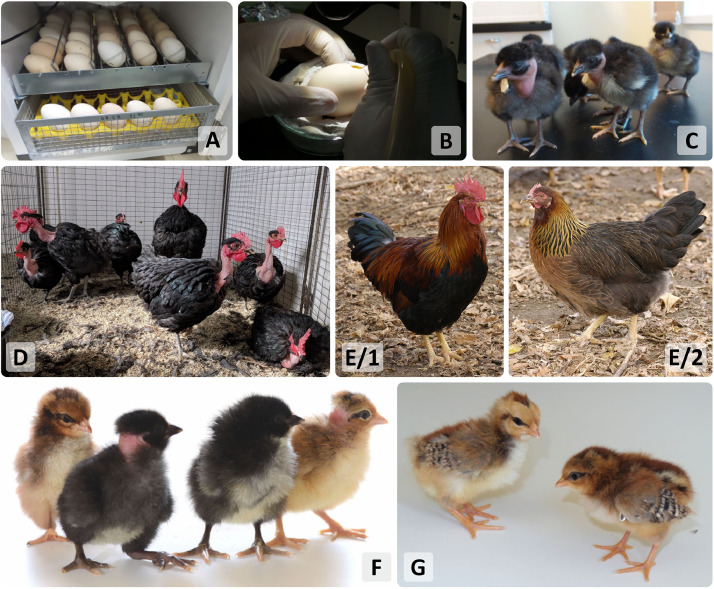
From 5 experiments, 52 Black Transylvanian Naked Neck Chicken embryos were injected as recipients on the 2.5th d of incubation with frozen/thawed PG cells of Partridge Colored Hungarian chicken (Figure 7.). A mixture of a male (No. PC101) and a female (No. PC111) Partridge Colored PG cell lines in ratio 1:1 was used for injection. From 52 injected eggs, 29 chicks were hatched and 24 of them were raised until sexual maturity (Figure 7.). Thirteen males and 11 females were produced. During the mating experiments, 795 eggs were incubated over a 9-wk period. Three hundred and forty chicks were hatched and 17 of them (17/340, 5.4%) were pure partridge colored with a covered neck (Figure 7.). Based on the mating results 4 out of 24 presumptive chimeras (16.6%) were proven to be germline chimeras: 1 hen and 3 roosters. Therefore, we have proven that the original breed can be recovered from both male and female PGCs which are stored in the gene bank.
Figure 7.
Producing presumptive germline chimeras with PGC injection and donor-derived progeny with backcrossing. (A) Incubation of Black Transylvanian naked neck recipient eggs for injection of donor PGCs. (B) Injection of Partridge color Hungarian donor PGCs into 2.5-day-old recipient embryos. (C) The hatched Black Transylvanian naked neck presumptive germline chimera chickens. (D) Mature Black Transylvanian naked neck presumptive chimaera chickens. E: Partridge color Hungarian donor breed. They were kept in individual cages for the backcrossing experiment. (E/1) Partridge color Hungarian rooster. (E/2) Partridge color Hungarian hen. F: Hatched chicks from the mating experiments. All four possible varieties are shown. (G) One-week-old donor-derived Partridge color chickens. (Photos by B. Lázár, E. Patakiné Várkonyi, I. Lehoczky and http://www.geneconservation.hu).
Proposed Protocol for PGC-Based Gene-Banking of Chicken Genetic Resources
Along with establishing a PGC-based gene bank for the Hungarian indigenous chicken breeds, we aimed to propose a general protocol for efficiently producing and storing high quality PGC lines to meet preservation goals. After reviewing the established methods and considering our own experiences, we propose a simple protocol for culturing and quality control of PGC lines. After completing these steps, it will be safe to assume that the cell lines match the in vitro and in vivo characteristics of a high quality PGC line and they will be useful for future regeneration of the breed/species. The proposed steps and criteria to be met are summarized in Table 4. Further information about the techniques can be found in the Materials and Methods section.
Table 4.
Proposed general protocol for establishing and testing PGC lines for gene banking purposes.
| Steps | Criteria | |
|---|---|---|
| 1 | Isolation of blood from donor embryos | Min. 50 isolations |
| 2 | Establishing PGC cultures in feeder-free conditions | 1.0 × 105 or more PGCs in 3 weeks |
| 3 | Sex determination of cell lines | 4–6 female cell lines or more |
| 4 | Proliferation test of cell lines | Doubling time 3–3.5 d or less |
| 5 | Immunohistochemistry of selected cell lines | CVH and SSEA1 positive cells |
| 6 | Gene expression analysis of selected cell lines | CVH and cPOUV expression |
| 7 | In vivo migration assay with selected fluorescently labeled cell lines | Colonization rate 60% or above |
| 8 | Freezing of established and tested cell lines | Min. 6 duplicate vials, min. 5.0 × 104 cells/vial |
DISCUSSION
According to the IUCN Red list there are currently 11,147 avian species on the planet, of which 1,486 species (13.3%) are considered critically endangered, endangered, or vulnerable (IUCN Red List version 2020-1: Table 4a, https://www.iucnredlist.org/resources/summary-statistics#Summary%20Tables). There are 3,689 avian breed populations recorded worldwide (of which 2,222 are indigenous breeds) and 28% of these breeds are endangered, vulnerable or already extinct (FAO, DAD-IS, http://www.fao.org/3/CA0121EN/ca0121en.pdf). For the ~8,800 recorded breeds of 40 domesticated animals species, 7% of the breeds are already extinct and 24% are at risk of extinction (FAO 2019, http://www.fao.org/resources/infographics/ infographics-details/en/c/174199/). Looking at this data, it is evident that wild birds and native poultry breeds alike are at great risk. Preservation of the genetic resources in local chicken breeds not only helps to maintain the biodiversity in poultry, but also provides valuable material that we might seek and use in crossbreeding strategies in the future (Wang et al., 2017).
The alteration in the diverse use of PGCs occurred after 2006, when it became possible to proliferate and maintain PGCs in cell culture (van de Lavoir et al., 2012). However, in the “Cryoconservation of animal genetic resources” Guidelines published by the FAO in 2012 (FAO, 2012, http://www.fao.org/3/i3017e/i3017e00.pdf), gene conservation with PGCs still exists only at a theoretical level. FAO did not include any protocols that could be used for that purpose. Therefore, in this study, our aim was not only to present our results on establishing the first Hungarian PGC-based gene bank, but also propose a uniformly recommended protocol for the isolation, establishment, cryopreservation and testing of PGC lines used for gene banking in chicken. To date several research groups have already succeeded in recovering indigenous chicken breeds from PGCs (Nakamura et al., 2010; Rikimaru et al., 2011; Tonus et al., 2016; Wang et al., 2017; Yu et al., 2019), but most of them did not use cell lines or if cell lines were involved they propagated them with feeder cells and/or only male cell lines were established. To our knowledge only Woodcock and colleagues used stable PGC lines from both sexes derived from a specialised breed and applied feeder free culturing conditions (Woodcock et al., 2019). Our study is unique in how we used feeder free culturing conditions for both sexes and also using multiple indigenous breeds which cover most of the Hungarian local chicken breed spectrum. The advantage of the in vitro culturing method we have adapted (Whyte et al. 2015) to the Hungarian indigenous breeds is that female cell lines of all breeds could be established and maintained in this selective medium and succeeded in obtaining female germline chimeras and donor-derived offspring. A further advantage of the recommended cell line establishment and maintenance protocol is that it is easy to apply as it does not use feeder cells. In the current study, we successfully isolated PGCs from 6 Hungarian indigenous breeds and maintained both sexes in vitro. The average establishment rate of the PGC lines (42.0%) is similar to other studies based on specialised breeds and we obtained more male PGC lines than female lines (Nandi et al., 2016; Woodcock et al., 2019). The derived PGC lines were positive for SSEA-1, and CVH, and expressed the germ-cell specific genes CVH and cPOUV. Donor-derived offspring were produced with 5% efficiency which is comparable to the results presented in previous experiments (Nakamura et al., 2010; Nakamura et al., 2013b; Miyahara et al., 2014; Yu et al., 2019). The in vitro characterization and in vivo validation of PGC lines described above can be used to ensure that PGC samples placed in the gene bank will be suitable for recovering the endangered, rare or native breeds. Also, this proves that the germ cell lines established by this method and placed in the gene bank are suitable for the recovery of the original breed.
Acknowledgments
ACKNOWLEDGMENTS
We acknowledge Luca Pataki for assistance in the preparation of Figure 1. and István Lehoczky for the photos. This work was supported by the European Union's Horizon 2020 Research and Innovation Program (grant number: n°677353 IMAGE); VEKOP-2.3.2-16-2016-00012; and the EFOP-3.6.3-VEKOP-16-2017-00008 grant, co-financed by the European Union and the European Social Fund.
Ethics Approval: Animals were kept and maintained according to general animal welfare prescriptions of the Hungarian Animal Protection Law (1998; XXVIII). All experimental methods described herein were approved by the Institutional Ethics Review Board of the Institute for Farm Animal Gene Conservation (No. 7/2011).
Authors' Contributions
B. L., E. P. V., and E. G. conceived and designed the experiments. M. M., N. Sz., B. V., and Á. D. performed the backcrossing experiments. B. L. and M. M. isolated and established the six PGC lines. E. P. V., M. M., and B. L. analyzed the data. B. L. performed the immunostaining of PGCs, R. T. and N. T. Sz. performed the marker expression analysis and sex determination. E. G. performed the proliferation assay of PGCs and GenEX analysis of qPCR runs. E. P. V., B. L., M. J. M., and E. G. wrote the paper.
DISCLOSURES
The authors declare that they have no conflict of interest.
REFERENCES
- Bakst M.R., Dymond J.S. Artificial insemination in poultry. In Success in Artificial Insemination - Quality of Semen and Diagnostics Employed. A. Lemma, ed. 2013 [Google Scholar]
- Báldy B. Mezőgazda Kiadó; Budapest, Hungary: 1954. A Baromfi Tenyésztése. [Google Scholar]
- Biszkup F., Beke L. A magyaróvári sárga magyar tájfajta tyúk kitenyésztésének módszerei és eredményei. Agrártudomány. 1951;III:461–467. [Google Scholar]
- Blesbois E. Current status in avian semen cryopreservation. Worlds. Poult. Sci. J. 2007;63:213–222. [Google Scholar]
- Burrows W.H., Quinn J.P. A method of obtaining spermatozoa from the domestic fowl. Poult. Sci. 1935;14:251–253. [Google Scholar]
- Griffiths R., Double M.C., Orr K., Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim D.H., Han J.Y., Choi S.B., Ko Y.-G., Do Y.J., Seong H.-H., Kim S.W. The effect of modified cryopreservation method on viability of frozen-thawed primordial germ cell on the Korean native chicken (Ogye) J. Anim. Sci. Technol. 2013;40:207–216. [Google Scholar]
- Kong L., Qiu L., Guo Q., Chen Y., Zhang X., Chen B., Zhang Y., Chang G. Long-term in vitro culture and preliminary establishment of chicken primordial germ cell lines. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár B., Anand M., Tóth R., Várkonyi E.P.E.P., Liptói K., Gócza E. Comparison of the MicroRNA expression profiles of male and female avian primordial germ cell lines. Stem Cells Int. 2018;2018:1–17. doi: 10.1155/2018/1780679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J., Glover J.D., Taylor L., Sang H.M., McGrew M.J. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS One. 2010;5:e15518. doi: 10.1371/journal.pone.0015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara D., Mori T., Makino R., Nakamura Y., Oishi I., Ono T., Nirasawa K., Tagami T., Kagami H. Culture conditions for maintain propagation, long-term survival and germline transmission of chicken primordial germ cell-like cells. J. Poult. Sci. 2014;51:87–95. [Google Scholar]
- Miyahara D., Oishi I., Makino R., Kurumisawa N., Nakaya R., Ono T., Kagami H., Tagami T. Chicken stem cell factor enhances primordial germ cell proliferation cooperatively with fibroblast growth factor 2. J. Reprod. Dev. 2016;62:143–149. doi: 10.1262/jrd.2015-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár M., Lázár B., Sztán N., Végi B., Drobnyák Á., Tóth R., Liptói K., Marosán M., Gócza E., Nandi S., McGrew M.J., Várkonyi E.P. Investigation of the Guinea fowl and domestic fowl hybrids as potential surrogate hosts for avian cryopreservation programmes. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-50763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M., Tajima A., Tagami T., Yasuda Y., Kuwana T. Preservation of chick primordial germ cells in liquid nitrogen and subsequent production of viable offspring. J. Reprod. Fertil. 1994;102:321–325. doi: 10.1530/jrf.0.1020321. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Kagami H., Tagami T. Development, differentiation and manipulation of chicken germ cells. Dev. Growth Differ. 2013;55:20–40. doi: 10.1111/dgd.12026. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tasai M., Takeda K., Nirasawa K., Tagami T. Production of functional gametes from cryopreserved primordial germ cells of the Japanese Quail. J. Reprod. Dev. 2013;59:580–587. doi: 10.1262/jrd.2013-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Usui F., Miyahara D., Mori T., Ono T., Takeda K., Nirasawa K., Kagami H., Tagami T. Efficient system for preservation and regeneration of genetic resources in chicken: concurrent storage of primordial germ cells and live animals from early embryos of a rare indigenous fowl (Gifujidori) Reprod. Fertil. Dev. 2010;22:1237–1246. doi: 10.1071/RD10056. [DOI] [PubMed] [Google Scholar]
- Nandi S., Whyte J., Taylor L., Sherman A., Nair V., Kaiser P., Mcgrew M.J. Cryopreservation of specialized chicken lines using cultured primordial germ cells. Poult. Sci. 2016;95:1905–1911. doi: 10.3382/ps/pew133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain B., Clark M.E., Shen M., Nakazawa H., Sakurai M., Samarut J., Etches R.J. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development. 1996;122:2339–2348. doi: 10.1242/dev.122.8.2339. [DOI] [PubMed] [Google Scholar]
- Rikimaru K., Ito N., Nakamura Y., Takahashi D., Ono M., Komatsu M., Matsubara K. Identification of germline chimeric chickens produced by transfer of primordial germ cells using a hinai-dori-specific microsatellite marker. J. Poult. Sci. 2011;48:281–291. [Google Scholar]
- Rikimaru K., Nakamura Y., Takahashi D., Komatsu M., Ito N., Matsubara K., Tagami T. Production of pure hinai-dori with normal reproductive capability from transferred primordial germ cells. J. Poult. Sci. 2014;51:297–306. [Google Scholar]
- Szalay István. Regi Magyar Baromfifajtak a XXI. században (Old Hungarian Poultry in the 21st Century) Mezőgazda Kiadó; Budapest, Hungary: 2015. A régi magyar baromfifajták kialakulásáról és tenyésztéséről (Formation and breeding of the old Hungarian poultry breeds) pp. 26–44. [Google Scholar]
- Tajima A., Naito M., Yasuda Y., Kuwana T. Production of germ line chimera by transfer of primordial germ cells in the domestic chicken (Gallus domesticus) Theriogenology. 1993;40:509–519. doi: 10.1016/0093-691x(93)90404-s. [DOI] [PubMed] [Google Scholar]
- Tonus C., Cloquette K., Ectors F., Piret J., Gillet L., Antoine N., Desmecht D., Vanderplasschen A., Waroux O., Grobet L. Long term-cultured and cryopreserved primordial germ cells from various chicken breeds retain high proliferative potential and gonadal colonisation competency. Reprod. Fertil. Dev. 2016;28:628–639. doi: 10.1071/RD14194. [DOI] [PubMed] [Google Scholar]
- van de Lavoir M.C., Collarini E.J., Leighton P.A., Fesler J., Lu D.R., Harriman W.D., Thiyagasundaram T.S., Etches R.J. Interspecific germline transmission of cultured primordial germ cells. PLoS One. 2012;7:e35664. doi: 10.1371/journal.pone.0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Lavoir M.C., Diamond J.H., Leighton P.A., Mather-Love C., Heyer B.S., Bradshaw R., Kerchner A., Hooi L.T., Gessaro T.M., Swanberg S.E., Delany M.E., Etches R.J. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- Wang L., Chen M.J., Chen D.Y., Peng S.F., Zhou X.L., Liao Y.Y., Yang X.G., Xu H.Y., Lu S.S., Zhang M., Lu K.H., Lu Y.Q. Derivation and characterization of primordial germ cells from Guangxi yellow-feather chickens. Poult. Sci. 2017;96:1419–1425. doi: 10.3382/ps/pew387. [DOI] [PubMed] [Google Scholar]
- Whyte J., Glover J.D., Woodcock M., Brzeszczynska J., Taylor L., Sherman A., Kaiser P., McGrew M.J. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Rep. 2015;5:1171–1182. doi: 10.1016/j.stemcr.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock M.E., Gheyas A.A., Mason A.S., Nandi S., Taylor L., Sherman A., Smith J., Burt D.W., Hawken R., Mcgrew M.J. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. PNAS. 2019;116:20930–20937. doi: 10.1073/pnas.1906316116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Zhu Z., Chen X., Huang J., Jia R., Pan J. Isolation, characterization and germline chimera preparation of primordial germ cells from the Chinese Meiling chicken. Poult. Sci. 2019;98:566–572. doi: 10.3382/ps/pey410. [DOI] [PubMed] [Google Scholar]