The original cohort
The English Longitudinal Study of Ageing (ELSA) is an ongoing nationally representative sample of individuals aged 50 and older living in England.1 The study started in 2002, and participants are reassessed every 2 years with face-to-face interviews and self-completion questionnaires. Biomedical assessments take place every 4 years with a home visit from a study nurse who assesses functional capacity and anthropometry and takes blood samples for the extraction of biomarkers and DNA. More than 18 000 people have taken part in the study since its inception, and the data currently available span over 16 years (wave 9 took place in 2018/19). Refreshment samples of new participants have been recruited at waves 3, 4, 6, 7 and 9 to maintain the age profile and ensure that the study remains representative of the English population aged 50 and over. ELSA tracks multiple and complex characteristics of the same individuals as they move through middle age to older age and has collected a wide range of clinical, biological, psychological, economic and social measures, including biomarker and genetic data across the main waves. The study is a collaboration between epidemiology and behavioural science (University College London), economics (Institute for Fiscal Studies), social science (University of Manchester), clinical medicine (Norwich Medical School) and survey specialists (NatCen Social Research). For up-to-date information, see the study website [https://www.elsa-project.ac.uk/]. ELSA data are archived with the UK Data Service [https://ukdataservice.ac.uk] within a few months of completion of each wave, and are accessed by researchers and policy analysts around the world. The study informs policy across many aspects of ageing, including health and social care, retirement and pensions policy and social and civic participation. Internationally, ELSA is one of the sister studies of the Health and Retirement Study (HRS) in the USA, and part of a growing network of longitudinal population studies of ageing around the world. These surveys not only provide data for individual countries but also offer the opportunity for cross-national comparisons of harmonized datasets. For more information, see the Gateway to Global Ageing Data website [https://g2aging.org/].
Ethics Approval
The ELSA-HCAP Sub-study received ethical approval from the South Central-Berkshire National Health Services (NHS) Research Ethics Committee and was conducted in accordance with the ethical standards of the Declaration of Helsinki. Informed verbal consent was obtained from all participants or their guardians. For more information see Supplementary material available as Supplementary data at IJE online.
What is the reason for the new data collection?
One of the key epidemiological challenges in the 21st century is that non-communicable diseases, such as cardiovascular disease, stroke and dementia, have become the leading causes of mortality and morbidity.2 Among these, dementia represents one of the most debilitating conditions, with an increased prevalence in individuals aged 65 years and older and double risk with every 5 years’ increase in age after that. Attributable pathologies accounting for dementia at death suggest that age, brain atrophy, total volume loss, vascular changes and disease-related proteins such as TDP-43 and alpha-synuclein all contribute, with Alzheimer’s type pathology alone accounting for only around 20% of cases.3 Early detection and prevention are crucial but challenging, considering the interplay of the established risk factors operating at various points across the life course.4
The operationalization of diagnostic criteria for the ever-changing concepts of mild cognitive impairment and dementia, as well as their diagnostic boundaries, are complex. Yet in most cases, diagnosis involves a medical history and reported or measured changes in cognitive function, as well as behavioural and functional impairments that allow clinicians to gauge the severity of the symptoms presented. A significant challenge is to define what constitutes the normal spectrum of cognitive ageing in contrast to cognitive impairment while taking into consideration specific population norms.5 Despite our ability to identify certain brain pathologies in vivo using neuroimaging and biomarkers,6 a precise diagnostic algorithm remains problematic. The most significant difficulties encountered in diagnosing dementias stem principally from their shared heterogeneous classifications, influenced by multiple and mixed pathologies. Modern conceptualizations highlight preclinical stages reflecting changes that have already taken place in the brain before marked symptoms emerge.7 Crucially, individuals who are clinically diagnosed are only a subset of those with cognitive impairment and dementia, and it is estimated that dementia remains undetected in almost 50% of primary care patients in the UK,8 with even higher rates in other countries. In the absence of a single measurement instrument for dementia identification and classification, international efforts are being made to implement standardized neurocognitive assessments in various population studies.
The implementation of the international Harmonised Cognitive Assessment Protocol (HCAP) in the family of studies associated with the Health and Retirement Study offers an opportunity for investigating, in a comparable manner, measures relevant to dementia diagnosis including cognitive, sensory and psychological performance as well as functional abilities in large representative population samples of older adults in both high- and middle-income countries. The overall aim of HCAP is to ascertain and investigate mild cognitive impairment and dementia across the general population worldwide. HCAP involved much more detailed assessments of cognitive impairment and its correlates than was possible in the main waves of ELSA and so was carried out as a sub-study. ELSA-HCAP provides opportunities for identifying potential predictors of cognitive impairment and dementia, as well as the consequences of dementia, in the context of the longitudinal framework of ELSA main waves.
What will be the new areas of research?
The ELSA-HCAP protocol involves an extensive range of cognitive measures coupled with informant interviews on a stratified sub-sample of ELSA participants aged 65 and older. These cognitive measures will be included in an international algorithm aimed to classify dementia, mild cognitive impairment and healthy cognitive function in population studies, with benchmarking against the Cognitive Function and Ageing Study (CFAS), the Aging Demographics and Memory Study (ADAMS) and other studies of cognitive ageing and dementia around the world.
The HCAP instrument aims to use international algorithmic approaches to evaluate the prevalence of neurocognitive disorders in people aged over 65 years within each participating country including England, USA, Mexico, South Africa, China and India. Currently, the HCAP Network research group is developing a diagnostic algorithm that will make use of HCAP respondent and informant data to assign a research diagnosis of normal, mild cognitive impairment or dementia.
The HCAP project is being carried out in several countries around the world, and as a result, the international implementation of these assessments offers the opportunity for cross-national investigations and comparisons of the biological, medical, social and environmental factors that affect the risk of neurocognitive impairments. Therefore, HCAP represents a promising international platform for investigating and tracking changes in the prevalence of mild cognitive impairment and dementia over time, facilitating country-specific investigations of medical care systems and social policy interventions.
Who is in the cohort?
Participants were selected for the ELSA-HCAP Sub-study if they were an ELSA core member aged 65 and over at the start of fieldwork in January 2018 (born before 1 January 1953) and had completed an ELSA interview in person at either wave 8 (2016–17) or wave 7 (2014–15). Core members living in care or nursing homes were also eligible, if they had the capacity to consent, or if a consultee/family member agreed to participate on their behalf. Further selections were made based on a sample procedure related to their previous cognitive performance using the modified Telephone Interview Cognitive Screening (mTICS)9 and a diagnosis of Alzheimer’s disease or dementia previously reported (waves 1–8). Three groups were defined using the thresholds on the mTICS 27 items scale: group 1—low cognition (≤6 mTICS27 score) and/or a diagnosis of Alzheimer’s disease or dementia; group 2—moderate cognition (7–11 mTICS27 score) and had never reported a diagnosis of Alzheimer’s disease or dementia; group 3—normal cognition (≥12 mTICS27 score) or unknown for those with missing data on mTICS scores at most recent ELSA waves. ELSA-HCAP was administered to participants across all the range of cognitive abilities, but we oversampled those identified as having low cognitive scores in the most recent ELSA waves. Core members who had proxy interviews (indicating an inability to participate themselves) at both wave 7 and wave 8 were not eligible for ELSA-HCAP.
ELSA-HCAP dress rehearsal and fieldwork
The fieldwork for ELSA-HCAP took place between January 2018 and April 2018, between the ELSA main waves 8 and 9 (Supplementary Figure S1, available as Supplementary data at IJE online). To test the design, materials and procedure for the ELSA-HCAP Sub-study, a dress rehearsal was conducted between August and September 2017. For more information, see Supplementary material, available as Supplementary data at IJE online.
ELSA-HCAP participants selection and response rates
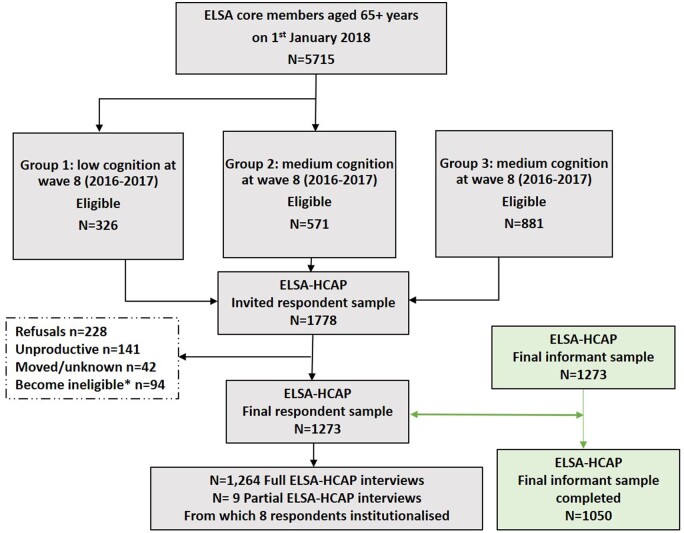
Based on the eligibility criteria, around 1800 individuals were sampled for ELSA-HCAP, with the expectation of a 60% response rate. Around half (n = 900) were sampled from cognitive groups 1 (low) and 2 (moderate). Since the number of people eligible for group 1 (n = 334) was small, all eligible members were invited, whereas for those eligible in group 3 (n = 4 610) only 1 in 5 people were invited. The response rate for the ELSA-HCAP study was higher than anticipated. From the 1684 eligible cases, 1273 completed the face-to-face interview, representing a final response rate of 75.6% (Figure 1). Table 1 shows that response rates were generally similar across age and sex, but with a slightly higher response within the 65–69 age group and lower in the 85+ age group.
Figure 1.
The flow of respondents and informant selection through the study in the English Longitudinal Study of Ageing Harmonised Cognitive Assessment Protocol (ELSA-HCAP)
Table 1.
The response among ELSA-HCAP respondents and informants (eligible and completed interviews)
| ELSA-HCAP respondent |
ELSA-HCAP informant |
|||||
|---|---|---|---|---|---|---|
| Group | Category | Eligible | Completed interviews |
Completed interviews |
||
| n | n | % | n | % | ||
| Sex | Male | 742 | 573 | 77.2 | 371 | – |
| Female | 942 | 700 | 74.3 | 679 | – | |
| Age | <65 | – | – | – | 375 | – |
| 65–69 | 376 | 301 | 80.1 | 174 | – | |
| 70–74 | 391 | 299 | 76.5 | 183 | – | |
| 75–79 | 339 | 259 | 76.4 | 154 | – | |
| 80–84 | 328 | 249 | 75.9 | 108 | – | |
| 85–89 | 162 | 114 | 70.4 | 27 | – | |
| 90 and over | 88 | 51 | 57.9 | – | – | |
| Missing | – | – | – | 23 | – | |
| Cognitive grouping selection | 1: Low | 282 | 179 | 63.5 | 154 | 86.0* |
| 2: Moderate | 540 | 419 | 77.6 | 364 | 86.9* | |
| 3: Normal | 862 | 675 | 78.3 | 618 | 91.5* | |
| 1684 | 1273 | 75.6 | 1050 | 82.5* | ||
ELSA-HCAP: English Longitudinal Study of Ageing-Harmonised Cognitive Assessment Protocol.
Percentage response rates from the completed number of respondents.
1273 represents the total sample of ELSA-HCAP respondents and 1050 represents the total sample of ELSA-HCAP informants.
ELSA-HCAP informants selection and response rates
A total of 1050 informant interviews were conducted, representing a response rate of 82.5% of all the eligible sample (n = 1273) contacted. Of these, 194 (18.5%) informant interviews were completed by telephone, and 856 (81.5%) by self-completion questionnaire. The informant relationships with the respondent included spouse/partner (56%), child (22%), grandchild (1%), sibling (3%), parent (3%), friend (10%), carer (0.3%), neighbour (1%) or other (3%). A small number refused to answer (0.4%). The average period an informant reported knowing the respondent was 43 years [standard deviation (SD) = 18; range = 1–82]. Table 2 also shows the response rates among the informant sampling groups (eligible and completed interviews), which were similar across age and sex, with slightly lower rates for informants aged 80 and over.
Table 2.
The battery of tests included in the ELSA-HCAP respondent and informant interviews with the correspondence between ELSA main waves
| Neuropsychological domain | ELSA-HCAP | ELSA main waves | |
|---|---|---|---|
| ELSA-HCAP respondent interview | |||
| Mini-Mental State Examination (MMSE) | Multiple | ✓ | X |
| HRS Telephone Interview for Cognitive Status (HRS-TICS) | ✓ | ✓ | |
| CERAD word list recall-immediate | Memory | ✓ | ✓ (alternative list) |
| Retrieval fluency | Language | ✓ | ✓ |
| Letter cancellation | Visuospatial | ✓ | ✓ |
| Backward count | Attention | ✓ | ✓ |
| 10/66 (Community Screening Instrument for Dementia, CSI-D) | Executive functioning | ✓ | X |
| CERAD word list recall–delayed | Memory | ✓ | ✓ (alternative list) |
| East Boston memory test–immediate | Memory | ✓ | x |
| Wechsler memory scale-IVa–immediate | Memory | ✓ | X |
| CERAD word list recognition | Memory | ✓ | X |
| Constructional praxis–immediate | Memory | ✓ | X |
| Symbol-digit modalities test | Executive functioning | ✓ | X |
| Constructional praxis–delayed | Visuospatial | ✓ | X |
| Wechsler memory scale-IV–delayed | Memory | ✓ | X |
| East Boston memory test–delayed | Memory | ✓ | x |
| Wechsler memory scale-IV–recognition | Memory | ✓ | X |
| Number series | Executive functioning | ✓ | X |
| Raven’s standard progressive matrices | Executive functioning | ✓ | X |
| Trail making A & B | Executive functioning | ✓ | X |
| Center for Epidemiological Studies Depression Scale (CES-D) | Depression | ✓ (11 items) | ✓ (8 items) |
| Smell test | Olfaction | ✓ | X |
| ELSA-HCAP informant interview | Functional domain | ||
| Jorm informant questionnaire on cognitive decline in the elderly (IQCODE) | Informant evaluation | ✓ | ✓ |
| Blessed dementia rating scale-part 2 | Informant evaluation | ✓ | x |
| HRS activities questionnaire | Informant evaluation | ✓ | x |
| Community Screening Instrument for Dementia (CSI-D) cognitive activities questionnaire | Informant evaluation | ✓ | x |
| 10/66 dementia research group informant questionnaire | Informant evaluation | ✓ | X |
| Blessed dementia rating scale–part 1 | Informant evaluation | ✓ | X |
ELSA-HCAP: English Longitudinal Study of Ageing-Harmonised Cognitive Assessment Protocol; HRS, Health Retirement Study; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease.
Wechsler memory scale-IV story administered in this study was ‘Anna Thompson’.
Sample weights
A weighting procedure was derived for the ELSA-HCAP Sub-study in order to adjust for non-response bias within each of the three cognition groups, especially for the low cognition group, which had the lowest response rate. It involved three components: design weights, non-response weights and a calibration procedure accounting for differential selection probabilities and adjusting for non-response. The final weight for this sub-study represents a combination of the design and non-response weights, which is made available to the users with the ELSA-HCAP data (Supplementary data and Supplementary Table S1, available at IJE online).
How often have they been followed up?
At the time of this report, only wave 1 of the ELSA-HCAP Sub-study has been completed. However, participants will continue to be included in the ELSA main waves, so it is expected that they will be followed up as part of the study. It is planned that ELSA will continue with assessments every 2 years, nurse visits for biomarkers measurements every 4 years and linkage with registry data, including mortality statistics.
Data quality
To ensure data quality, two researchers scrutinized the data collected, by comparing the results between various tests and the overall performance on the Mini-Mental State Examination (MMSE). The concordance between the distributions of ELSA-HCAP cognitive scores with those published in similar age-matched population studies was also examined. When outliers were identified (±3 standard deviations from the mean), further checks of the paper tests and interview records were made, correcting any errors in data entry (n = 48).
What has been measured?
The HCAP comprises two interviews, one with the respondent and one with an informant nominated by the respondent.
The HCAP respondent interview covers a broad range of cognitive domains (memory, language, executive function, psychomotor speed, problem solving and numeracy) known to be affected by the ageing process. Table 2 summarizes these tests, the majority of which are new to ELSA, although they all are well-established neurocognitive assessments such as Mini-Mental State Examination (MMSE), CERAD (Consortium to Establish a Registry for Alzheimer’s Disease), Word List Memory, Backward Counting Task, Community Screening Interview for Dementia, Logical memory (East Boston Memory Test and ‘Anna Thompson’ story of the Wechsler Memory Scale), CERAD Constructional Praxis (shape drawing), Symbol Digit Modalities Test, HRS Number Series, Raven’s Standard Progressive Matrices Test, and Trail Making. For more detail and references, see Supplementary Table S2, available as Supplementary data at IJE online. The 11-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) was also administered, together with an optional test of olfaction (smell), evaluating odour identification and detection. The duration of the ELSA-HCAP respondent interview was approximately 1 h (range 17.6–186.2 min, median 72.8 min).
The ELSA-HCAP informant interview was conducted with a nominated family member or knowledgeable friend who was asked to describe the general health of the respondent and evaluate any changes in their cognitive abilities. The interview measured the functional status of the respondent, such as their ability to engage in activities of daily living and social, and leisure activities. It also determined whether the respondent had been previously diagnosed with a stroke, Alzheimer's disease, other forms of dementia or Parkinson’s disease. Further measures included were the Informant Questionnaire on Cognitive Decline in the Elderly, Blessed Dementia Rating Scale, Community Screening Instrument for Dementia (CSI-D) Cognitive Activities Questionnaire, 10/66 Dementia Research Group Informant Questionnaire and HRS Activities Questionnaire, comprising questions related to the amount of time spent watching TV, reading, doing household chores, yoga or other exercises (see Table 2 and Table S2). The length of time for which the informant had known the respondent was also recorded. The informant interview was completed either via a paper self-completion questionnaire or by a telephone interview using computer-assisted telephone interviewing. The ELSA-HCAP informant interview took approximately 20–25 min to complete.
What has it found? Key findings and publications
The ELSA-HCAP Sub-study provides a detailed neuropsychological and clinical assessment of a selected sample of individuals aged 65 and over which can be extrapolated to the rest of the ELSA population and, by extension, to those living in the community in England. Table 3 shows the sociodemographic characteristics of the ELSA-HCAP respondent sample. Gender differences were observed in educational attainment and wealth, with more men attaining a higher level of education and achieving higher wealth than women. Men were more likely than women to be married or living with a partner and to smoke or drink frequently. In contrast, women were more likely to feel lonely and experience difficulties in the Instrumental Activities of Daily Living, particularly using a map, shopping or working around the house/gardening. Table 4 presents the weighted means and standard deviation for each cognitive test included in the ELSA-HCAP respondent interview. The performance of men was on average better than women on the MMSE, verbal fluency, backwards counting, constructional praxis, logical reasoning (number series) and solving intelligence tests (Ravens). Women outperformed men in memory and visual-spatial scanning abilities (letter cancellation).
Table 3.
Sociodemographic, lifestyle and health characteristics of the ELSA-HCAP sample
| Overall sample | Men | Women | ||
|---|---|---|---|---|
| Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | P-value | |
| n/variables | 1273 | 573 (45%) | 700 (55%) | |
| Age | 75.3 (6.6) | 75.1 (6.4) | 75.4 (6.7) | 0.83 |
| Educational attainment | ≤0.001 | |||
| No qualification | 37.5% | 33.2% | 40.1% | |
| Education to age 16 | 17.2% | 15.7% | 18.5% | |
| Education to age 18 | 33.5% | 34.4% | 32.8% | |
| Degree | 11.8% | 16.8% | 7.8% | |
| Wealth | ≤0.001 | |||
| Lowest | 33.6% | 28.2% | 38.1% | |
| Medium | 33.1% | 33.4% | 32.9% | |
| Highest | 33.3% | 38.5% | 29.3% | |
| Geographical region | 0.91 | |||
| North | 5.8% | 5.8% | 5.9% | |
| Yorkshire | 10.5% | 10.3% | 10.6% | |
| East Midlands | 13.0% | 12.6% | 13.4% | |
| East Anglia | 11.1% | 10.8% | 11.3% | |
| South East | 11.9% | 12.0% | 11.7% | |
| South West | 12.6 | 13.8% | 11.6% | |
| West Midlands | 7.1% | 7.0% | 7.1% | |
| North West | 17.1% | 18.0% | 16.4% | |
| Wales | 11.0% | 9.8% | 12% | |
| Depressive symptomsa | 2.9% | 2.3% | 3.4% | 0.22 |
| Poor eyesight | 4.1% | 4.1% | 4.2% | 0.91 |
| Poor hearing | 7.0% | 8.2% | 6.0% | 0.13 |
| Poor olfaction | 7.3% | 7.5% | 7.2% | 0.83 |
| Poor general health | 10.1% | 10.0% | 10.1% | 0.86 |
| Cardiovascular condition | 4.8% | 5.8% | 4.0% | 0.15 |
| Diabetes | 5.3% | 5.4% | 5.2% | 0.84 |
| Hypertension | 15.0% | 15.0% | 15.0% | 0.99 |
| Stroke | 2.1% | 2.8% | 1.6% | 0.13 |
| Cancer | 4.8% | 5.2% | 4.4% | 0.51 |
| Respiratory disease | 4.6% | 5.8% | 3.6% | 0.06 |
| Activities of daily living | ||||
| Difficulty dressing | 11.8% | 11.3% | 12.2% | 0.65 |
| Difficulty walking | 6.9% | 5.9% | 7.8% | 0.24 |
| Difficulty bathing | 10.9% | 9.3% | 12.2% | 0.10 |
| Difficulty eating | 2.0% | 2.6% | 1.4% | 0.13 |
| Difficulty getting out of bed | 4.9% | 4.4% | 5.3% | 0.44 |
| Difficulty using toilet | 3.9% | 3.3% | 4.4% | 0.30 |
| Instrumental activities of daily living | ||||
| Difficulty using map | 7.2% | 5.6% | 8.5% | 0.05 |
| Difficulty recognizing danger | 1.7% | 2.1% | 1.4% | 0.37 |
| Difficulty preparing a meal | 6.7% | 5.9% | 7.3% | 0.33 |
| Difficulty shopping | 12.9% | 9.6% | 15.6% | ≤0.001 |
| Difficulty making calls | 4.2% | 4.9% | 3.6% | 0.25 |
| Difficulty communicating | 4.7% | 5.4% | 4.2% | 0.29 |
| Difficulty taking medication | 4.5% | 4.2% | 4.7% | 0.64 |
| Difficulty working around | 17.8% | 12.9% | 21.8% | ≤0.001 |
| house/gardening | ||||
| Difficulty managing money | 7.2% | 6.6% | &0 .6% | 0.51 |
| Sedentary lifestyle | 10.6% | 10.3% | 11.0% | 0.67 |
| Smoking | 8.2% | 8.6% | 7.9 | ≤0.001 |
| Drinking (daily) | 20.7% | 27.2% | 15.2% | ≤0.001 |
| Married/living with partner | 64.6% | 75.0% | 54.3% | ≤0.001 |
| Feels lonely | 6.8% | 4.3% | 9.1% | ≤0.001 |
ELSA-HCAP: English Longitudinal Study of Ageing-Harmonised Cognitive Assessment Protocol.
Depressive symptoms were ascertained using the threshold of 9 on the 11-item version of the Centre for Epidemiologic Studies Depression Scale (CES-D);
Table 4.
Descriptive statistics presenting weighted means and standard deviation of the cognitive tests included in the ELSA-HCAP respondent interview
| Overall sample |
Men |
Women |
|||||
|---|---|---|---|---|---|---|---|
| Cognitive test | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | P-value |
| MMSE | 1273 | 26.8 (3.3) | 573 | 27.2 (3.0) | 700 | 26.5 (3.5) | ≤0.001 |
| HRS-TICS | 1271 | 2.8 (0.5) | 572 | 2.8 (0.5) | 699 | 2.7 (0.6) | 0.10 |
| CERAD immediate | 1263 | 18.4 (5.1) | 569 | 17.7 (4.9) | 694 | 18.9 (5.2) | ≤0.001 |
| CERAD delayed | 1264 | 5.5 (2.6) | 568 | 5.2 (2.4) | 696 | 5.8 (2.6) | ≤0.001 |
| CERAD recognition | 1261 | 18.6 (2.5) | 567 | 18.6 (2.2) | 694 | 18.5 (2.7) | 0.57 |
| Retrieval fluency | 1270 | 19.2 (8.5) | 572 | 20.2 (8.8) | 698 | 18.3 (8.2) | ≤0.001 |
| Letter cancellation | 1186 | 269.1 (84.5) | 542 | 258.6 (76.3) | 644 | 278.3 (85.5) | ≤0.001 |
| Backwards counting | 1253 | 31.5(10.9) | 563 | 33.2(10.8) | 690 | 30.1(10.9) | ≤0.001 |
| CSID | 1271 | 4.0 (0.3) | 572 | 4.0 (0.2) | 699 | 3.9 (0.3) | 0.01 |
| EBMT story | |||||||
| immediate | 1264 | 4.1 (1.5) | 568 | 4.1 (1.5) | 696 | 4.2 (1.5) | 0.43 |
| delayed | 1217 | 2.9 (1.9) | 546 | 2.8 (1.9) | 671 | 2.9 (1.9) | 0.22 |
| Wechsler story | |||||||
| immediate | 1254 | 9.3 (4.7) | 565 | 9.2 (4.5) | 689 | 9.4 (4.8) | 0.28 |
| delayed | 1210 | 7.2 (4.7) | 544 | 7.0 (4.6) | 666 | 7.4 (4.8) | 0.17 |
| recognition | 1222 | 11.3 (2.8) | 549 | 11.4 (2.7) | 673 | 11.3 (2.9) | 0.57 |
| CERAD Praxis | |||||||
| drawing | 1250 | 9.5 (1.8) | 564 | 9.9 (1.6) | 686 | 9.2 (1.9) | ≤0.001 |
| delayed recall | 1161 | 7.8 (2.8) | 531 | 8.3 (2.7) | 630 | 7.3 (2.8) | ≤0.001 |
| Symbol digit | 1196 | 33.8 (12.6) | 546 | 33.7 (11.9) | 650 | 33.9 (13.1) | 0.64 |
| Number series | 1153 | 530.4 (31.4) | 528 | 536.3 (29.9) | 625 | 525.3 (31.7) | ≤0.001 |
| Ravens | 1258 | 13.6 (3.5) | 567 | 14.1 (3.2) | 691 | 13.2 (3.7) | ≤0.001 |
| Trail making A | 1209 | 55.0 (34.9) | 548 | 53.9 (35.8) | 661 | 55.9 (34.1) | 0.33 |
| Trail making B | 1038 | 116.1 (57.5) | 487 | 115.1 (54.1) | 551 | 116.9 (60.5) | 0.65 |
ELSA-HCAP: English Longitudinal Study of Ageing-Harmonised Cognitive Assessment Protocol. MMSE, Mini-Mental Status Examination; HRS-TICS, Health Retirement Study-Telephone Interview for Cognitive Status; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CSI-D, Community Screening Instrument for Dementia; EBMT, East Boston Memory Test.
The longitudinal nature of ELSA provides opportunities for the investigation of precursors of cognitive impairment and dementia over the 16 years of data, including the role of demographic, economic, social, cognitive, behavioural, biological and health factors. These in-depth phenotype data provide important avenues to address the timing, duration and multifactorial nature of the many biological, medical and social factors that influence the onset of clinical symptoms of dementia.
There have been >30 peer-reviewed articles published using ELSA data on cognitive functioning and dementia outcomes, covering a wide range of issues: see [https://www.elsa-project.ac.uk/publications]. However, these analyses preceded the implementation of HCAP Sub-study and we have, therefore, outlined a selection of findings to illustrate the broad spectrum of factors that can be related to cognitive functioning and dementia.
In ELSA, information about dementia has to date been captured through a self-reported physician diagnosis of dementia or Alzheimer’s disease and informant (proxy interviews) evaluations via the Informant Questionnaire on Cognitive Decline in the Elderly.10 A proxy interview is usually carried out with a nominated person (e.g. a family member) when the core member is not able or declines to take part but consents that someone else can complete a shorter interview on their behalf. These measures have been used in a number of studies to model future trends of dementia incidence11 and to investigate socioeconomic determinants12 and other modifiable risk factors associated with dementia13 such as social support,14 loneliness,15 digital literacy16 and social and cultural engagement,17 as well as a range of non-modifiable risk factors such as stroke,18 sensory impairments,19 physical capability20 and frailty.21 Over the different waves of data collection, an array of cognitive tests has been administered including measures of verbal memory, orientation, verbal fluency and letter cancellation, in addition to the assessment of basic cognitive abilities such as numerical ability and literacy. These have been used to investigate the relationship between cognitive performance and depressive symptoms,22 level of neighbourhood deprivation,23 diabetes,24 sensory impairments25 and mortality.26 Furthermore, international comparisons of cognitive performance have been conducted across middle-aged and older adults in England and the USA.27
What are the main strengths and weaknesses?
The ELSA-HCAP used identical measures to the HRS-HCAP, providing enhanced opportunities for international comparisons of the broader biopsychosocial context of cognitive impairment and algorithmic approaches to dementia definition. Furthermore, the ELSA-HCAP battery of tests has considerable overlap with other relevant studies such as the Cognitive Function and Ageing Studies (CFAS),28 Aging, Demographics, and Memory Study (ADAMS), Rush Memory and Aging Project29 and 10/66 dementia study.30
Several limitations should be acknowledged. The ELSA-HCAP Sub-study is currently cross-sectional, and a follow-up would be valuable to assess patterns of change in this detailed extended neuropsychological battery. Adding neuroimaging to the study would also greatly enhance the research possibilities of discriminating structural brain changes related to healthy ageing from the level of abnormal atrophy and neuronal loss associated with various neurocognitive disorders. Combined with detailed functional imaging, highlighting specific biochemical and molecular processes could inform and refine diagnostic accuracy. An important limitation is that the study sample is predominantly of White European ancestry. ELSA was designed to be representative of the population of older people in England in 2002 when only 3.2% of the older population in England were from ethnic minorities, according to the national census. Last, the number of ELSA-HCAP participants who were institutionalized at the time of the interview was very low (n = 8), restricting generalization to more fragile sectors of the population.
Some features that make the HCAP Sub-study distinctive arise from it being embedded in ELSA. Several biomarkers have been assessed in the study sample every 4 years since 2004. Study nurses have collected venous blood for the determination of lipids (total cholesterol, HDL cholesterol, triglycerides), glucose and glycated haemoglobin (HbA1c), inflammatory markers including C-reactive protein, fibrinogen, and white blood cell counts, ferritin and haemoglobin, insulin-like growth factor 1 (IGF1), vitamin D and other biomarkers that could be analysed longitudinally in relation to many ageing outcomes.
In addition to economic, psychosocial and health measures, genome-wide genotyping was carried out on 7412 ELSA participants of European ancestry, using the same Illumina HumanOmni2.5 Beadchip as used in the HRS. Polygenic risk scores for Alzheimer’s disease (AD) and dementia have been created using results from the genome-wide association study conducted by the International Genomics of Alzheimer’s Project, and have been made available through the UK Data Service. This offers a rich resource of data for various analyses involving genome-wide gene-environment or gene-gene interactions as well as the potential for Mendelian randomization analyses and sub-phenotyping patient-stratification investigations.
Moreover, because participants in ELSA-HCAP will be followed up in future waves of data collection, it will be possible to quantify the consequences of severe cognitive impairment for family income and expenditure, financial decision making, social connectivity, mental well-being and physical health. This information has the potential to strengthen the evidence base for health and social care provision to this vulnerable sector of the population, and other vital policy initiatives related to older fragile men and women.
Can I get hold of the data? Where can I find out more?
The ELSA-HCAP data, including the individual items and the derived scores for each of the respondent and informant interviews, have been made available via UK Data Service [https://ukdataservice.ac.uk]. The ELSA-HCAP study number (SN) is 8502; http://doi.org/10.5255/UKDA-SN-8502-2. The main ELSA study is SN 5050 and is held under [http://doi.org/10.5255/UKDA-SN-5050-17]. The person to contact for the ELSA-HCAP study is Dr Dorina Cadar (e-mail: d.cadar@ucl.ac.uk).
Profile in a nutshell
The Harmonised Cognitive Assessment Protocol Sub-study of the English Longitudinal Study of Ageing (ELSA-HCAP), comprising 1273 men and women aged 65 and older, was set up in 2018 to ascertain the prevalence of neurocognitive disorders such as cognitive impairment and dementia and to investigate the risk factors and regional variations in these conditions across England.
The ELSA-HCAP protocol represents a more extensive range of cognitive measures than those collected in regular waves of the English Longitudinal Study of Ageing (ELSA), covering a broad range of cognitive domains such as memory, language, executive function, psychomotor speed, problem solving and numeracy, which are known to be affected by the ageing process.
The performance of men was on average better than women on the Mini-Mental State Examination, verbal fluency, backwards counting, constructional praxis, logical reasoning (number series), and solving intelligence tests (Ravens). Women outperformed men in memory and visual-spatial scanning abilities (letter cancellation). However, it should be noted that levels of educational attainment were higher among men than women.
The harmonized cognitive measures will contribute to an international algorithm aimed at classifying mild cognitive impairment and dementia, benchmarking against the Cognitive Function and Ageing Study (CFAS), Aging Demographics and Memory Study (ADAMS) and other cognitive ageing studies around the world. For new collaborative projects and enquiries about data sharing, please contact Dorina Cadar at [d.cadar@ucl.ac.uk].
Supplementary data
Supplementary data are available at IJE online.
Funding
The English Longitudinal Study of Ageing Harmonised Cognitive Assessment Protocol (ELSA-HCAP) is funded by the National Institute on Aging (grant R01AG017644) and performed at the Institute of Epidemiology and Health Care, University College London. The English Longitudinal Study of Ageing is funded by the National Institute on Aging (grant R01AG017644) and by a consortium of UK government departments coordinated by the Economic and Social Research Council (ESRC) and, since 2018, by the National Institute for Health Research. The English Longitudinal Study of Ageing (ELSA) was developed by a team of researchers based at University College London, the Institute for Fiscal Studies, University of Manchester, and NatCen Social Research. The ELSA-HCAP Sub-study has been being carried out by NatCen and the Institute of Epidemiology and Health Care at University College London, with collaborators from University of Cambridge, University of Exeter and Newcastle University. The National Institute on Aging had no role in preparing this manuscript.
Supplementary Material
Acknowledgements
We wish to thank ELSA-HCAP participants for their voluntary contribution to the study and the NatCen team for the technical support. We also want to thank all our collaborators, in particular, Professor David Weir, Professor Kenneth Langa, Dr Lyndsay Ryan and Eva Leissou from the HRS team, University of Michigan USA, for their continuous support in ensuring a similar administration protocol across studies; Professor Martha McClintock, University of Chicago, and Professor David Kern, Northeastern Illinois University, for their permission to use the Olfaction test; Linda Barnes and CFAS team for their consultancy on interview training; Professor Carol Brayne, University of Cambridge, Professor Fiona Matthews, Newcastle University, Professor Ian Deary, University of Edinburgh and Professor Martin Prince, King’s College London, for their scientific input during this project; Sheema Ahmed and Dr Nina Rogers, University College London, for the administrative support.
Author contributions
D.C., A.S., G.D.B., D.J.L. and NatCen team designed and oversaw the project. C.B. and F.E.M. provided scientific advice at different stages of the project implementation. D.C. oversaw the implementation of the harmonized cognitive protocol in ELSA-HCAP, ensuring a similar administration as in the HRS-HCAP. D.C. and A.S. acquired all the licensing material and purchased the tests. D.C. and J.A. contributed to the fieldwork implementation strategy and were in charge of data quality control and preliminary checks of the data and its documentation. A.S., D.J.L., G.D.B. and C.B. were responsible for the funding of this project and oversaw its development. D.C. prepared the first draft of the manuscript, and all authors reviewed and refined the paper.
Conflict of interest
None of the authors declares any conflict of interest regarding the data and materials presented in this paper.
References
- 1. Steptoe A, Breeze E, Banks J, Nazroo J.. Cohort Profile: The English longitudinal Study of Ageing. Int J Epidemiol 2013;42:1640–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office for National Statistics. Causes of Death Over 100 years. 2017. https://www.ons.gov.uk (October 2020, date last accessed).
- 3.Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001;357:169–75. [DOI] [PubMed] [Google Scholar]
- 4. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C.. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- 5. Matthews FE, Stephan BC, Robinson L. et al.;Cognitive Function and Ageing Studies (CFAS) Collaboration. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun 2016;7:11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Staffaroni AM, Cobigo Y, Goh SM. et al. Individualized atrophy scores predict dementia onset in familial frontotemporal lobar degeneration. Alzheimers Dement 2019;16:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKhann GM, Knopman DS, Chertkow H. et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connolly A, Gaehl E, Martin H, Morris J, Purandare N.. Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health 2011;15:978–84. [DOI] [PubMed] [Google Scholar]
- 9. Brandt J, Spencer M, Folstein M.. The telephone interview for cognitive status. Neuropsychiatry, Neuropsychol Behav Neurol 1988;1:111–17. [Google Scholar]
- 10. Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994;24:145–53. [DOI] [PubMed] [Google Scholar]
- 11. Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P. et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ 2017;358:j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cadar D, Lassale C, Davies H, Llewellyn DJ, Batty GD, Steptoe A.. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English Longitudinal Study of Ageing. JAMA Psychiatry 2018;75:723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deckers K, Cadar D, van Boxtel MPJ, Verhey FRJ, Steptoe A, Kohler S.. Modifiable risk factors explain socioeconomic inequalities in dementia risk: evidence from a population-based prospective cohort study. J Alzheimers Dis 2019;71:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khondoker M, Rafnsson SB, Morris S, Orrell M, Steptoe A.. Positive and negative experiences of social support and risk of dementia in later life: an investigation using the English Longitudinal Study of Ageing. J Alzheimers Dis 2017;58:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rafnsson SB, Orrell M, d’Orsi E, Hogervorst E, Steptoe A.. Loneliness, social integration, and incident dementia over 6 years: prospective findings from the English Longitudinal Study of Ageing. J Gerontol Ser B Sci Soc Sci 2020;75:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. d'Orsi E, Xavier AJ, Rafnsson SB, Steptoe A, Hogervorst E, Orrell M.. Is use of the internet in midlife associated with lower dementia incidence? Results from the English Longitudinal Study of Ageing. Aging Ment Health 2018;22:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fancourt D, Steptoe A, Cadar D.. Cultural engagement and cognitive reserve: museum attendance and dementia incidence over a 10-year period. Br J Psychiatry 2018;213:661–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dregan A, Wolfe CD, Gulliford MC.. Does the influence of stroke on dementia vary by different levels of prestroke cognitive functioning?: a cohort study. Stroke 2013;44:3445–51. [DOI] [PubMed] [Google Scholar]
- 19. Davies-Kershaw HR, Hackett RA, Cadar D, Herbert A, Orrell M, Steptoe A.. Vision impairment and risk of dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc 2018;66:1823–29. [DOI] [PubMed] [Google Scholar]
- 20. Hackett RA, Davies-Kershaw H, Cadar D, Orrell M, Steptoe A.. Walking speed, cognitive function, and dementia risk in the English Longitudinal Study of Ageing. J Am Geriatr Soc 2018;66:1670–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers NT, Steptoe A, Cadar D.. Frailty is an independent predictor of incident dementia: evidence from the English Longitudinal Study of Ageing. Sci Rep 2017;7:15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng F, Zhong B, Song X, Xie W.. Persistent depressive symptoms and cognitive decline in older adults. Br J Psychiatry 2018;213:638–44. [DOI] [PubMed] [Google Scholar]
- 23. Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D.. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J Am Geriatr Soc 2008;56:191–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng F, Yan L, Yang Z, Zhong B, Xie W.. HbA1c, diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia 2018;61:839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N; Sense-Cog WP1 group . Visual and hearing impairments are associated with cognitive decline in older people. Age Ageing 2018;47:575–81. [DOI] [PubMed] [Google Scholar]
- 26. Batty GD, Deary IJ, Zaninotto P.. Association of cognitive function with cause-specific mortality in middle and older age: follow-up of participants in the English Longitudinal Study of Ageing. Am J Epidemiol 2016;183:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langa KM, Llewellyn DJ, Lang IA. et al. Cognitive health among older adults in the United States and in England. BMC Geriatr 2009;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brayne C, McCracken C, Matthews FE.. Cohort Profile: The Medical Research Council Cognitive Function and Ageing Study (CFAS). Int J Epidemiol 2006;35:1140–45. [DOI] [PubMed] [Google Scholar]
- 29. Bennett DA, Schneider JA, Buchman AS, Mendes de LC, Bienias JL, Wilson RS.. The Rush memory and aging project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005;25:163–75. [DOI] [PubMed] [Google Scholar]
- 30. Prince M, Ferri CP, Acosta D, et al. The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health 2007;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.