Abstract
Background
The role of smoking in nasopharyngeal carcinoma (NPC) remains uncertain, especially in endemic regions. We conducted an individual participant data (IPD) meta-analysis of prospective cohort studies to investigate the associations between smoking exposure and risk of NPC.
Methods
We obtained individual participant data of 334 935 male participants from six eligible population-based cohorts in NPC-endemic regions, including two each in Guangzhou and Taiwan, and one each in Hong Kong and Singapore. We used one- and two-stage approaches IPD meta-analysis and Cox proportional hazard models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of NPC for smoking exposure adjusting for age and drinking status.
Results
During 2 961 315 person-years of follow-up, 399 NPC evens were ascertained. Risks of NPC were higher in ever versus never smokers (HRone-stage = 1.32, 95% CI = 1.07-1.63, P = 0.0088; HRtwo-stage = 1.27, 1.01-1.60, 0.04). These positive associations appeared to be stronger in ever smokers who consumed 16+ cigarettes/day (HRone-stage = 1.67, 95% CI = 1.29-2.16, P = 0.0001), and in those who started smoking at age younger than 16 (2.16, 1.33-3.50, 0.0103), with dose-response relationships (P-values for trend = 0.0028 and 0.0103, respectively). Quitting (versus daily smoking) showed a small reduced risk (stopped for 5+ years: HRone-stage = 0.91, 95% CI = 0.60-1.39, P = 0.66; for former smokers: HRtwo-stage = 0.84, 0.61-1.14, 0.26).
Conclusions
This first IPD meta-analysis from six prospective cohorts in endemic regions has provided robust observational evidence that smoking increased NPC risk in men. NPC should be added to the 12–16 cancer sites known to be tobacco-related cancers. Strong tobacco control policies, preventing young individuals from smoking, would reduce NPC risk in endemic regions.
Keywords: Nasopharyngeal carcinoma, smoking, epidemiology, cohort study, individual data, meta-analysis
Key Messages
This first individual participant data (IPD) meta-analysis from six population-based prospective cohorts in endemic regions of nasopharyngeal carcinoma (NPC) assessed the associations between smoking exposure and risk of NPC in men.
Ever smokers had 32% higher risks of NPC than never smokers.
Smokers who consumed 16+ cigarettes per day had 67% higher risks of NPC.
Smokers who started smoking younger than age 16 showed the highest HR (hazard rato) of 2.16.
Introduction
Nasopharyngeal carcinoma (NPC) has a distinctive geographical variation,1,2 with over 70% of 129 000 new cases of NPC in 2018 diagnosed in East and South-East Asia.3 Despite its similar cell or tissue lineage, NPC presents an epidemiological pattern distinct from most types of head and neck cancer that have been confirmed to be smoking related.4–6 The 2012 International Agency for Research on Cancer (IARC) Monograph considered cigarette smoking to be causally related to NPC.7 However, the association between smoking and NPC has not been concluded to be causal in the 2014 US Surgeon General's Report.8
Previous epidemiological studies on the association between smoking and NPC have shown inconsistent results. Such association appeared to be stronger in case-control studies9–34 than cohort studies,35–39 probably because case-control studies are subject to recall bias. In addition, positive associations were mainly observed in non-endemic regions of NPC, where the major NPC histological type is squamous cell carcinoma.40,41 Prospective epidemiological data are very limited in endemic regions, where the major histological type is non-keratinizing undifferentiated carcinoma.42 Only two summary aggregate data meta-analyses have reported that smoking was associated with higher risks of NPC in non-endemic regions, but not in endemic regions.43,44 Individual participant data (IPD) meta-analysis is considered to be the ‘gold standard’ of systematic review and can provide the strongest evidence from observational studies.45 As smoking may have different roles in different subtypes of NPC, separate analyses would be ideal. However, information on subtypes was rarely collected by previous cohort studies and the numbers of NPC events were small, so pooling individual data restricting to studies in endemic regions, having over 95% of non-keratinizing undifferentiated carcinoma NPC, could reflect the association for non-keratinizing undifferentiated carcinoma. We conducted an IPD meta-analysis to assess the associations between smoking history and risk of NPC in endemic regions.
Methods
Ethics Approval
The Guangzhou Biobank Cohort Study has ethics approval from the Guangzhou Medical Ethics Committee of the Chinese Medical Association, Guangzhou, China (Co-Principal Investigator: Prof. Lam Tai-Hing). The Guangzhou Occupational Cohort Study obtained ethics approval from the Ethics Committee, Faculty of Medicine, and the University of Hong Kong. Permission to use data was granted by Guangzhou Occupational Diseases Prevention and Treatment Centre (Principal Investigator: Prof. Lam Tai-Hing). The Hong Kong Elderly Health Service Cohort Study obtained ethics approval from the University of Hong Kong–Hospital Authority Hong Kong West Cluster Joint Institutional Review Board (Principal Investigator: Prof. Lam Tai-Hing). The Singapore Chinese Health Study was approved by the Institutional Review Boards of the University of Southern California and the National University of Singapore (Principal Investigator: Prof. Yuan Jian-Min). The Taiwan Cohort conducted in 1984 was approved by the institutional review board of the College of Public Health National Taiwan University (Principal Investigator: Prof. Chen Chien-Jen). The Taiwan MJ Cohort was approved at the National Health Research Institutes and at China Medical University Hospital (Principal Investigator: Prof. Wen Chi-Pang).
Search strategy, cohort selection criteria and study sample
We identified prospective cohort studies in endemic regions published in Chinese or English from January 1970 to November 2019 from PubMed, Web of Science, CNKI and Wanfang. An endemic region was defined as having an age-standardized incidence rate (ASIR) greater than 8 per 100 000 person-years in men. Manual search was also done by reviewing references in relevant articles. Three published cohort studies from endemic regions were found in the literature review from Taiwan,38 Guangzhou39 and Singapore.37 Three other cohorts in endemic regions, including Taiwan,46 Guangzhou47 and Hong Kong,48 with ascertainment of NPC and smoking data, were identified by manual search, though they had no publication on NPC. The inclusion criteria were: (i) the cohort study was conducted in NPC-endemic regions with ASIR ≥8/100 000 persons-years in men; (ii) selection of participants was not based on history of any previous chronic disease; (iii) the cohort included sufficient NPC events with a male crude mortality ≥4/100 000 person-years or male crude event rate ≥10/100 000 person-years; (iv) the cohort had baseline information on sex, age and smoking and alcohol consumption; (v) the mean duration of follow-up of the cohort was ≥5 years; (vi) participants in the cohort were aged 18+; and (vii) the primary investigator of the eligible study agreed to provide individual-level data.
All the six cohorts identified in the literature search were eligible. Each of the principal investigators has agreed to join the NPC Cohort Study Collaboration (NPC-CSC). With a data request sheet (Supplementary Figure S1, available as Supplementary data at IJE online), we obtained information on NPC event (fatal or non-fatal cases), demographic characteristics (sex, age and educational level), smoking and drinking status, medical history at baseline, duration of follow-up, and vital status (Supplementary Figure S2, available as Supplementary data at IJE online). All studies had obtained ethics approval and informed consent for their studies. All participants provided informed consent. This paper follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for Individual Patient Data reporting guidelines.49
Follow-up and outcome
Participants were followed from the baseline in each cohort to the date of first instance of non-fatal or fatal NPC event, or the date of death from other causes or the last follow-up date in each cohort (Table 1). All six studies classified NPC events by the International Classification of Disease (ICD) Revision 9 or 10. Malignant neoplasm of nasopharynx was coded as 147 in ICD-9 or C11 in ICD-10. We excluded deaths that occurred within 2 years from baseline. Missing duration of follow-up (one NPC event in the Taiwan Cohort 1984, 11 NPC events in the Taiwan MJ Cohort, and 104 participants in the Guangzhou Biobank Cohort Study) was imputed using the median follow-up years of each cohort.
Table 1.
Baseline characteristics of individual cohorts and the combined cohort in the meta-analyses (men only)
| Study | Taiwan Cohort 1984 (included men only) | Guangzhou Occupational Cohort | Singapore Chinese Health Study | Hong Kong Elderly Health Service Cohort | Guangzhou Biobank Cohort Study | Taiwan MJ Cohort | Combined cohort |
|---|---|---|---|---|---|---|---|
| Cohort reference number | 38 | 39 | 37 | 48 | 47 | 46 | 37–39 , 46–48 |
| Regions | Taiwan | Guangzhou | Singapore | Hong Kong | Guangzhou | Taiwan | Endemic regions |
| Enrolment/last follow-up date | 1984-86/2011 | 1988-92/1999 | 1993-99/2008 | 1998-2001/2012 | 2003-08/2017 | 1994-2006/2008 | 1984-2008/2017 |
| Median baseline survey year | 1985 | 1990 | 1996 | 1999 | 2005 | 1999 | 1996 |
| Population source | Population register | Health check-up | Population register | Health check-up | Health check-up | Health check-up | NA |
| Male participants | 9428 | 87 327 | 26 654 | 21 764 | 6952 | 182 810 | 334 935 |
| Mean (SD) age at baseline survey, years | 51.6 (12.5) | 41.2 (5.9) | 56.5 (7.9) | 71.9 (5.3) | 64.4 (6.7) | 40.6 (13.6) | 44.8 (14.2) |
| Mean (SD) follow-up, years | 21.4 (7.5) | 7.3 (0.7) | 12.2 (3.1) | 11.0 (3.2) | 11.3 (2.6) | 8.1 (3.3) | 8.8 (4.0) |
| No. of NPC case | 42 | 30 | 117 | 23 | 20 | 167 | 399 |
| Drinking status, no. (%) | |||||||
| Never | 7577 (80.4) | 72 205 (82.7) | 18 200 (68.3) | 15 771 (72.5) | 3530 (50.8) | 117 371 (64.2) | 234 654 (70.1) |
| Ever | 1851 (19.6) | 15 122 (17.3) | 8454 (31.7) | 5993 (27.5) | 3422 (49.2) | 65 439 (35.8) | 100 281 (29.9) |
| Smoking status, no. (%) | |||||||
| Never | 3277 (34.8) | 40 099 (45.9) | 11 342 (42.6) | 13 125 (60.3) | 2190 (31.5) | 87 678 (48.0) | 157 711 (47.1) |
| Ever | 6151 (65.2) | 47 228 (54.1) | 15 312 (57.5) | 8639 (39.7) | 4762 (68.5) | 95 132 (52.0) | 177 224 (52.9) |
| Smoking status, no. of NPC deaths | |||||||
| Never | 3 | 6 | 16 | 14 | 2 | 12 | 53 |
| Ever | 14 | 24 | 28 | 9 | 11 | 37 | 123 |
| Smoking status, no. of NPC new cases | |||||||
| Never | 9 | NA | 37 | NA | 1 | 47 | 94 |
| Ever | 16 | NA | 36 | NA | 6 | 71 | 129 |
Ever smokers included daily smokers and former smokers, and occasional smokers were excluded. Guangzhou Occupational Cohort and Hong Kong Elderly Health Service Cohort had mortality data only.
SD, standard deviation; NPC, nasopharyngeal carcinoma; NA, not applicable.
Smoking exposure assessment
Information on smoking was obtained from the baseline questionnaire. Smoking exposure in ever smokers (including daily smokers and former smokers) was classified into different categories and compared with never smokers. Cumulative consumption (pack-years) was calculated by multiplying the number of packs (20 cigarettes per pack) smoked per day by the number of years smoked. Smoking categories were first grouped into five-unit (e.g. five cigarettes per day/years/pack-years) intervals, then the intervals were regrouped if the number of events in a five-unit interval was too small for analysis. In the analyses of quitting, time since quitting was classified into four groups: daily smokers (reference group), quitters who had stopped smoking for <5, 5+ years and never smokers.
Statistical analyses
Participants with missing data on cigarette smoking or alcohol drinking at baseline were excluded. We also excluded participants with prevalent NPC or cancer at baseline because the disease status might have changed the subjects’ smoking habits. Due to the small number of ever smokers among women (7.6%; Supplementary Table S1, available as Supplementary data at IJE online) and missing data (95%) on occasional smokers, the present analysis excluded female participants and occasional smokers.
We examined the association between smoking exposure and NPC events by calculating hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazard models adjusting for age and drinking status. The Cox proportional hazard assumption was checked using Schoenfeld residuals, and no evidence of violation of the assumption was found. We conducted one-stage meta-analyses that analysed IPD from all cohorts simultaneously, and also used the two-stage random-effect approach to compare the associations for smoking (ever smokers versus never smokers) and quitting status (former smokers versus daily smokers).50 Smoking cumulative consumption (pack-years) was selected to examine any threshold of a great increase in the HR for smoking as it included both smoking amount and smoking duration. Never smokers were used as the referent to compare with 30 consecutive cut-off points (>1, >2, …… >29, >30 pack-years) in smoking cumulative consumption, and 30 HRs were calculated. The heterogeneity of HRs across the studies was measured by the I2 and Q statistics. Funnel plots were used to check for publication bias. Missing values for smoking exposure were coded as separate categories and included as indicator variables in the models, except for in dose-response analyses. To assess dose-response effects of smoking duration, smoking cumulative consumption, age at starting smoking and quitting duration, a test for trend was examined treating these factors as ordinal variables among ever smokers only. Statistical interactions by alcohol were assessed based on the likelihood ratio test that compared nested models with and without interaction terms.
Several sensitivity analyses were conducted. We repeated analyses in daily smokers (versus never smokers). We also conducted a sensitivity analysis excluding 116 participants with missing follow-up data for the associations between smoking exposure and NPC events, which did not substantially affect our results (Supplementary Table S2, available as Supplementary data at IJE online). Because the association of smoking with NPC mortality and incidence outcomes may be different, we examined the associations with fatal (NPC mortality) and non-fatal (NPC incidence) events separately, and the results were similar. All statistical analyses were conducted with Stata version 15.0 (StataCorp LLC, College Station, TX), and all tests were two-sided.
Results
Of 334 935 male participants (median follow-up of 8.8 years, standard deviation of 4.0) from six studies in regions endemic for NPC, 399 NPC events were ascertained (Table 1).
Risks of NPC were consistently higher in ever smokers, daily smokers and former smokers (versus never smokers) (Table 2). The corresponding adjusted HRs were, respectively, 1.44 (95% CI = 1.17-1.76, P = 0.0005), 1.49 (1.20-1.85, 0.0003) and 1.28 (0.94-1.74, 0.11) in Model 1 (adjusted for age), and 1.32 (1.07-1.63, 0.0088), 1.37 (1.10-1.71, 0.0058) and 1.19 (0.87-1.62, 0.28) in Model 2 (adjusted for age and drinking status). The risks of NPC for smoking were stronger in ever smokers (versus never) who consumed 16+ cigarettes per day (adjusted HR = 1.67, 95% CI = 1.29-2.16, P = 0.0001) and who started smoking at age younger than 16 (2.16, 1.33-3.50, 0.0103) with dose-response relationships (both P-values for trend < 0.05). The associations of smoking exposure with NPC incidence and mortality were similar (Supplementary Table S3, available as Supplementary data at IJE online) and remained in daily smokers (Supplementary Table S4, available as Supplementary data at IJE online). Quitting (versus daily smoking) showed a small reduced risk (for quitting duration < 5 years: adjusted HR = 1.22, 95% CI = 0.78-1.90, P = 0.38; 5+ years: 0.91, 0.60-1.39, 0.66). Figure 1 shows that HRs were consistently >1 and steadily increased with greater cut-off points of pack-year, suggesting no threshold effect.
Table 2.
Hazard ratios of NPC in male ever smokers in the one-stage approach IPD meta-analysis of the combined cohort
| Exposures and categories | Person-years (Total: 2 961 315) |
NPC events (n = 399) | Event rate of NPC per 100 000 person-years (95% CI) |
Model 1 (95% CI) | Model 2 (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never smokers | 1 360 051 | 147 | 10.8 | (9.2- | 12.7) | Ref | Ref | ||||||
| Smoking status 1 | |||||||||||||
| Ever smokers | 1 601 263 | 252 | 15.7 | (13.9- | 17.8) | 1.44 | (1.17- | 1.76) | *** | 1.32 | (1.07- | 1.63) | ** |
| Smoking status 2 | |||||||||||||
| Daily smokers | 1 250 684 | 190 | 15.2 | (13.2- | 17.5) | 1.49 | (1.20- | 1.85) | *** | 1.37 | (1.10- | 1.71) | ** |
| Former smokers | 345 257 | 60 | 17.4 | (13.5- | 22.4) | 1.28 | (0.94- | 1.74) | 1.19 | (0.87- | 1.62) | ||
| Smoking amount in ever smokers, cigarettes/day | |||||||||||||
| 1-15 | 912 741 | 116 | 12.7 | (10.6- | 12.2) | 1.20 | (0.94- | 1.53) | 1.11 | (0.87- | 1.43) | ||
| 16+ | 496 559 | 105 | 21.1 | (17.5- | 25.6) | 1.82 | (1.42- | 2.34) | **** | 1.67 | (1.29- | 2.16) | *** |
| P for trenda | 0.0022 | 0.0028 | |||||||||||
| Smoking duration in ever smokers, years | |||||||||||||
| Never | 1 213 532 | 133 | 11.0 | (9.2- | 13.0) | Ref | Ref | ||||||
| 1-15 | 572 694 | 43 | 7.5 | (5.6- | 10.1) | 0.83 | (0.58- | 1.18) | 0.78 | (0.55- | 1.12) | ||
| 16-35 | 429 234 | 88 | 20.5 | (16.6- | 25.3) | 1.72 | (1.32- | 2.26) | *** | 1.59 | (1.20- | 2.09) | ** |
| 36+ | 280 254 | 72 | 25.7 | (20.4- | 32.4) | 1.66 | (1.22- | 2.27) | ** | 1.50 | (1.09- | 2.05) | * |
| P for trenda | 0.19 | 0.31 | |||||||||||
| Smoking cumulative consumption in ever smokers, pack-years | |||||||||||||
| Never | 1 213 532 | 133 | 11.0 | (9.2- | 13.0) | Ref | Ref | ||||||
| 1-5 | 337 593 | 25 | 7.4 | (5.0- | 11.0) | 0.82 | (0.53- | 1.26) | 0.78 | (0.51- | 1.21) | ||
| 6-25 | 619 062 | 93 | 15.0 | (12.3- | 18.4) | 1.36 | (1.05- | 1.78) | * | 1.26 | (0.96- | 1.65) | |
| 26+ | 301 034 | 82 | 27.2 | (21.9- | 33.8) | 1.80 | (1.35- | 2.42) | *** | 1.63 | (1.21- | 2.20) | ** |
| P for trenda | 0.05 | 0.10 | |||||||||||
| Age at starting smoking in ever smokers, years | |||||||||||||
| Never | 1 213 532 | 133 | 11.0 | (9.2- | 13.0) | Ref | Ref | ||||||
| 26+ | 353 338 | 37 | 10.5 | (7.6- | 14.5) | 1.00 | (0.69- | 1.44) | 0.96 | (0.66- | 1.38) | ||
| 16-25 | 991 233 | 165 | 16.6 | (14.3- | 19.4) | 1.40 | (1.11- | 1.76) | ** | 1.28 | (1.01- | 1.62) | * |
| <16 | 58 881 | 19 | 32.3 | (20.6- | 50.6) | 2.31 | (1.42- | 3.75) | ** | 2.16 | (1.33- | 3.50) | ** |
| P for trenda | 0.0069 | 0.0103 | |||||||||||
| Quitting duration in former smokers, years | |||||||||||||
| Daily smokers | 1 229 548 | 187 | 15.2 | (13.2- | 17.6) | Ref | Ref | ||||||
| <5 | 98 864 | 22 | 22.3 | (14.7- | 33.8) | 1.22 | (0.78- | 1.91) | 1.22 | (0.78- | 1.90) | ||
| ≥5 | 124 624 | 26 | 20.9 | (14.2- | 30.6) | 0.90 | (0.59- | 1.38) | 0.91 | (0.60- | 1.36) | ||
| Never smokers | 1 213 532 | 133 | 11.0 | (9.2- | 13.0) | 0.73 | (0.59- | 0.91) | ** | 0.79 | (0.63- | 0.99) | * |
| P for trenda | 0.60 | 0.61 | |||||||||||
Model 1 adjusted for age, Model 2 adjusted for age and drinking status.
IPD, individual participant data; NPC, nasopharyngeal carcinoma; CI, confidence interval.
Trend test in ever smokers, excluding never smokers; if trend tests in this table included never smokers, both would have yielded p < 0.05. Missing values for exposure were coded as separate categories and included as indicator variables in the models, except for in dose-response analyses.
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
Figure 1.

Adjusted hazard ratios of nasopharyngeal carcinoma for smoking cumulative consumption (pack-years) at each of 30 consecutive cut-off points in male ever smokers (daily smokers and former smokers combined)
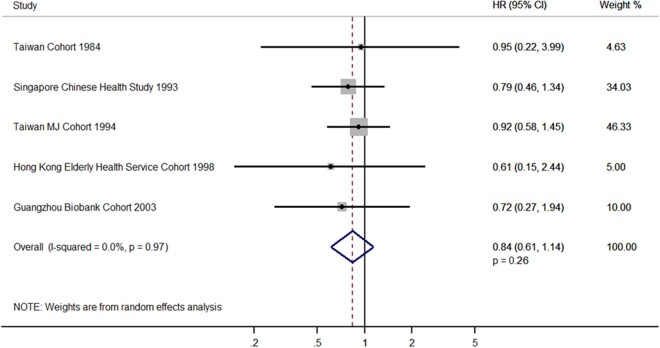
Risks of NPC were higher in ever smokers (versus never) in four individual cohorts, including the Taiwan Cohort (adjusted HR = 1.28, 95% CI = 0.64-2.53), Guangzhou Occupational Cohort 1988 (2.54, 1.00-6.50), Taiwan MJ Cohort 1994 (1.37, 0.98-1.91) and Guangzhou Biobank Cohort 2003 (2.57, 0.74-8.98), and in the pooled estimation (1.27, 1.01-1.60, P = 0.04). No heterogeneity was found in this meta-analysis (I2 = 7%, Pheterogeneity = 0.37) (Figure 2). These positive associations remained in daily smokers (versus never) (Supplementary Figure S3, available as Supplementary data at IJE online). However, no clear association was observed in former smokers (versus daily smokers) in each individual cohort or in the pooled analyses (adjusted HR = 0.84, 95% CI = 0.61-1.14, P = 0.26). No heterogeneity was found (I2 = 0%, Pheterogeneity = 0·97) (Figure 3). Visual inspection of funnel plots showed no publication bias in our overall analyses (Figure 4; Supplementary Figure S4, available as Supplementary data at IJE online).
Figure 2.
Adjusted hazard ratios of nasopharyngeal carcinoma in male ever smokers (daily smokers and former smokers combined) versus never smokers in individual cohort studies and two-stage approach individual participant data meta-analysis in random-effects model
Figure 3.
Adjusted hazard ratios of nasopharyngeal carcinoma in male former smokers (daily smokers and former smokers combined) versus daily smokers in individual cohort studies and two-stage approach individual participant data meta-analysis in random-effects model
Figure 4.
Funnel plots of the risk of nasopharyngeal carcinoma (log-adjusted hazard ratios) associated with A: ever smokers; B: former smokers (both versus never) in the two-stage approach individual participant data meta-analysis (men only)
Discussion
This is the first IPD meta-analysis of prospective cohort studies in endemic regions to evaluate the association between smoking exposure and NPC with detailed information on smoking. We found smoking consistently associated with increased risk of NPC. Ever smokers had 32% higher risks of NPC than never smokers. Smokers who consumed 16+ cigarettes per day had 67% higher risks of NPC. Smokers who started smoking younger than age 16 had over twice the risk of NPC compared with never smokers. Quitting was associated with a small reduced risk of NPC in this cohort. This is the largest study to show the harm of smoking and NPC, with dose-response relationships by different exposure indicators.
The findings from this IPD meta-analysis support previous research demonstrating an increased risk of NPC in ever smokers (versus never). Xue et al.al.43 reported an increased risk of NPC (odds ratio = 1.38, 95% CI = 0.96-1.98, P = 0.18) in an aggregate data-based meta-analysis of 399 975 participants with 328 NPC events including three cohorts from endemic regions (Guangzhou,51 Singapore37 and Taiwan38) and one cohort in a low-risk region of NPC (USA).35 They also reported a higher HR of 1.63 (95% CI = 1.38-1.92, P < 0.01) based on 28 case-control studies. Long et al.44 updated the meta-analysis including fofur recent studies (three case-control29–31 and one cohort39) and showed that ever smokers (versus never) had a 56% higher risk of NPC, based on 17 case-control studies and four cohort studies. Whereas Long et al.44 reported a null association based on two cohort studies37,38 (OR for ever versus never smoking = 1.11, 95% CI = 0.84-1.48, P = 0.83), an increased risk of NPC was observed in current smokers (2.19, 1.02-4.72) based on three cohort studies37–39 including our recent study.39 Another cohort study in 34 439 male British doctors with four NPC deaths also showed a positive association for smoking.36
A dose-response effect for age at starting smoking was first observed in our study. Participants who started smoking younger than 16 years showed the highest HR of 2.16. A relative risk (RR) of greater than 2 means that the attributable fraction in the exposed is greater than 50% [(RR-1)/RR]. This indicates that in NPC patients who started smoking at a young age, about half of the NPC cases can be attributed to smoking. Friborg et al. reported a suggestive association between age at smoking initiation and NPC (smokers started smoking at age <15 years: RR = 1.5, 95% CI = 0.8-2.8, P for trend = 0.08). Our findings of increased risk of NPC associated with heavy and chronic smoking (higher smoking amount, smoking duration and cumulative consumption in ever smokers) are consistent with previous studies in Singapore,37 Taiwan38 and Guangzhou.39 We did not find dose-response relationships for smoking duration and cumulative consumption in ever smokers. Dose-response relationships were observed for smoking duration in Singapore (P = 0.04)37 and for smoking cumulative consumption in Guangzhou (P = 0.014),39 but they both included never smokers in the trend test, which would also have shown dose-response relationships in our analyses.
Tobacco has been classified as a group 1 carcinogen by the IARC since 1992.52 As tobacco can cause laryngeal cancer53 and pharyngeal cancer,54 there is no plausible explanation why it cannot cause cancer in the nasopharyngeal region, which is also directly exposed to the carcinogens from smoking, and all were not associated with ionizing radiation exposure.55–57 The main reason for the limited evidence to support causation is probably because NPC is rare and individual cohort studies did not have sufficient number of NPC events.
There may be several explanations for our findings of increased risk of NPC associated with smoking exposure in men. One possibility is that the association between smoking and NPC was mediated through Epstein-Barr virus (EBV) reactivation.34,58,59 EBV is closely associated with the occurrence and development of NPC, and its reactivation is associated with smoking. Whereas one study in subjects with elevated IgA antibodies against EBV viral capsid antigen (VCA/IgA) found a null association between smoking and EBV,60 several large studies in healthy subjects showed that both smoking61,62 and cotinine63 were associated with higher seropositivity for several biomarkers of EBV reactivation and subsequently with higher risk of NPC.34,59 Another possibility is formaldehyde, a constituent of cigarette smoke which causes squamous cell carcinoma of the nasal cavities upon inhalation exposure of rats, and formaldehyde is considered a cause of nasopharyngeal cancer in humans by IARC.64 A study demonstrated a 10-fold higher level of the formaldehyde-DNA adduct N6-hydroxymethyl deoxyadenosine in leukocytes of smokers than never smokers, suggesting its possible involvement in NPC in smokers.65 Moreover, tobacco smoke contains more than 70 carcinogens66 and some of them may also contribute to the mechanism of how tobacco causes NPC.
By using the IPD meta-analysis design, our study has the largest number of NPC events (n = 399) and of total participants (n = 334 935) in NPC-endemic regions and the world. With the IPD data, we have provided more reliable and robust results and improved the potentially important limitations of reviews based on published aggregated data. We used one- and two-stage approach meta-analysis to evaluate the reliability of the results.50 IPD allowed us to conduct sensitivity and subgroup analyses by sex, cohorts and smoking status categories of each individual cohort, and used the same adjustment for potential confounders before the combined analysis. Compared with previous studies, we have enhanced generalizability by combining findings from all six eligible cohort studies across NPC endemic regions.67
We recognize the limitations of the short follow-up (<10 years), lack of detailed information on alcohol consumption, and missing data of smoking duration, age at starting smoking and quitting duration in one cohort.48 More NPC events would be available if all cohorts can further follow up and update the data. Limited by the data we collected, another concern is confounding since our analyses have only adjusted for age and alcohol consumption, but not other potential confounders, such as salted fish intake and EBV reactivation. Previous studies in Guangdong and Guangxi, China, showed that associations between smoking and NPC did not alter substantially after adjusting for consumption of salted fish.32,34 Our case-control study in Hong Kong, China, also reported similar association between smoking and NPC with and without adjusting for salted fish intake (data not shown).68 Although our results may be influenced by EBV infection and activation, EBV may not be a confounder but a mediator of the association between smoking and NPC. We did not collect information on reasons for quitting (whether stopped by choice or because of illness). The protective effects of quitting cannot be assessed straightforwardly.69 Cessation for 5 years or longer appeared to reduce NPC risk, but a larger dataset in future research is needed for confirmation. As the present analysis included Chinese men only, our findings may not be generalized to women and non-Chinese who are not in endemic regions. Future studies with detailed information on quitting and in women are recommended.
In conclusion, this first IPD meta-analysis from six prospective cohorts in endemic regions has provided robust observational evidence that smoking increased NPC risk in men. NPC should be added to the 12–16 cancer sites known to be tobacco-related cancers. Strong tobacco control policies, preventing young individuals from smoking, would reduce NPC risk in endemic regions.
Data Availability
Due to ethical restrictions protecting patient privacy, data may be available on request from the Guangzhou Biobank Cohort Study Data Access Committee. Please contact us at [gbcsdata@hku.hk] for fielding data accession requests. The data of the Guangzhou Occupational Cohort and the Hong Kong Elderly Health Service Cohort Study underlying this article will be shared on reasonable request to the corresponding author, and the principal investigator Prof. Tai-Hing Lam [hrmrlth@hku.hk]. Data are from the Singapore Chinese Health Study, and the authors did not seek approval from the IRB to make the data publicly available. According to the Singapore Personal Data Protection Act, the authors could not release the data without approval from IRB. Researchers who meet the criteria for access to confidential data may contact the principal investigators of Singapore Chinese Health Study at Prof. Jian-Min Yuan [yuanj@upmc.edu] and Prof. Woon Puay Koh [woonpuay.koh@duke-nus.edu.sg] to seek approval from the National University of Singapore IRB. The Taiwan Cohort (conducted in 1984) data underlying this article will be shared on reasonable request to the principal investigator at Prof. Chen Chien-Jen [cjchen@ntu.edu.tw]. The data that support the findings of this study are available from MJ Health Research Foundation, but restrictions apply to the availability of these data, which were used under licence for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of MJ Health Research Foundation.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This study was funded by the Hong Kong RGC Area of Excellence Scheme (AoE/M-06/08), World Cancer Research Fund UK (WCRF UK) and Wereld Kanker Onderzoek Fonds (WCRF NL), as part of the WCRF International grant programme (2011/460). This revision was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. The Guangzhou Biobank Cohort Study was funded by the University of Hong Kong Foundation for Educational Development and Research, Hong Kong, the Guangzhou Public Health Bureau and the Guangzhou Science and Technology Bureau, Guangzhou, China, and the University of Birmingham, UK. The Guangzhou Occupational Cohort was funded by the Hong Kong Research Grants Council (HKU 466/96 M), Hong Kong Health Services Research Committee (531036), Guangdong Province Public Health Bureau Five One Project (96–186), Guangzhou Municipal Science and Technology Commission (96-Z-65). The Hong Kong Elderly Health Service Cohort Study was funded by the Health Services Research Fund in Hong Kong (grant no. HSRF#S111016). The Singapore Chinese Health Study was funded by National Institutes of Health grants RO1 CA55069, R35 CA53890, and R01 CA80205, from the National Cancer Institute, Bethesda, MD. The Taiwan Cohort conducted in 1984 was funded by DOH 75–0203-18 and DOH 76–0203-17 from the Department of Health, Executive Yuan, Taipei, Taiwan. The Taiwan MJ Cohort was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212–114004), MOST Clinical Trial Consortium for Stroke (MOST 109–2321-B-039–002), China Medical University Hospital (DMR-109–231), Tseng-Lien Lin Foundation, Taichung, Taiwan.
Supplementary Material
Acknowledgements
The chief acknowledgments are to the participants who provided information for these studies and the research staff. We thank the Guangzhou Centre for Disease Control and Prevention (Professors G Z Lin, H Z Liu and M Wang), the Medical Insurance Administration Bureau of Guangzhou, and the Guangzhou Municipal Public Security Bureau for assisting follow-up and record linkage. We also thank the Guangzhou Health and Happiness Association for the Respectable Elders for convoking the subjects. We thank Dr Ye Guo-Xiong, Director of Guangzhou Public Health Bureau, adviser of the study, Guangzhou Public Security Bureau Population Information Centre and street police stations, District Public Health Bureaux, Guangzhou Funeral Home and other staff of the Guangzhou Occupational Diseases Prevention and Treatment Centre. We wish to thank the staff of the Elderly Health Service, Department of Health, particularly Shelley Chan, and the Hospital Authority, the Government of Hong Kong Special Administrative Region, for their assistance in data collection and entry. We would also like to acknowledge the contribution of Dr P Y Leung, when at the Department of Health, in facilitating the creation of the cohort. We thank Siew-Hong Low of the National University of Singapore for overseeing the fieldwork of the Singapore Chinese Health Study and Renwei Wang for the development and maintenance of the cohort study database. We also thank Mimi C Yu for being the founding and longstanding principal investigator of the Singapore Chinese Health Study. We thank Dr Elizabeth K Cahoon and Dr Lindsay M Morton for revising the manuscript.
Author Contributions
All the authors participated in individual cohort design, data collection and data cleaning. J.H.L. conducted the combined statistical analysis and drafted the manuscript under T.H.L.’s and C.P.W.’s supervision. Z.M.M. re-analysed the data, conducted additionally analyses and revised the manuscript substantially based on reviewers’ comments, and is the guarantor for the paper. All authors helped to draft the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Conflict of interest
None declared.
References
- 1. Ferlay J, Ervik M, Lam F. et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2018. [Google Scholar]
- 2. Tang L-L, Chen W-Q, Xue W-Q. et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett 2016;374:22–30. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. Chang ET, Adami H-O.. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765–77. [DOI] [PubMed] [Google Scholar]
- 5. Jia W-H, Qin H-D.. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol 2012;22:117–26. [DOI] [PubMed] [Google Scholar]
- 6. Mai Z-M, Lin J-H, Ip DKM, Ho S-Y, Chan Y-H, Lam T-H. . Epidemiology and Population Screening. Nasopharyngeal Carcinoma. Elsevier, 2019; pp. 65–84. [Google Scholar]
- 7.Cancer IAfRo. A Review of Human Carcinogens: Personal Habits and Indoor Combustions. World Health Organization. Report No.: 928321322X. Geneva: WHO, 2012. [Google Scholar]
- 8. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, 2014. [Google Scholar]
- 9. Shanmugaratnam K, Tye CY, Goh EH, Chia KB.. Etiological factors in nasopharyngeal carcinoma: a hospital-based, retrospective, case-control, questionnaire study. IARC Sci Publ 1978;199–212. [PubMed] [Google Scholar]
- 10. Mabuchi K, Bross DS, Kessler II.. Cigarette smoking and nasopharyngeal carcinoma. Cancer 1985;55:2874–76. [DOI] [PubMed] [Google Scholar]
- 11. Lin TM, Chang HJ, Chen CJ. et al. Risk factors for nasopharyngeal carcinoma. Anticancer Res 1986;6:791–96. [PubMed] [Google Scholar]
- 12. Yu MC, Garabrant DH, Huang TB, Henderson BE.. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer 1990;45:1033–39. [DOI] [PubMed] [Google Scholar]
- 13. Chen CJ, Liang KY, Chang YS. et al. Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res 1990;10:547–53. [PubMed] [Google Scholar]
- 14. Sriamporn S, Vatanasapt V, Pisani P, Yongchaiyudha S, Rungpitarangsri V.. Environmental risk factors for nasopharyngeal carcinoma: a case-control study in northeastern Thailand. Cancer Epidemiol Biomarkers Prev 1992;1:345–48. [PubMed] [Google Scholar]
- 15. Nam JM, McLaughlin JK, Blot WJ.. Cigarette smoking, alcohol, and nasopharyngeal carcinoma: a case-control study among U.S. whites. J Natl Cancer Inst 1992;84:619–22. [DOI] [PubMed] [Google Scholar]
- 16. West S, Hildesheim A, Dosemeci M.. Non-viral risk factors for nasopharyngeal carcinoma in the Philippines: results from a case-control study. Int J Cancer 1993;55:722–27. [DOI] [PubMed] [Google Scholar]
- 17. Zhu K, Levine RS, Brann EA, Gnepp DR, Baum MK.. A population-based case-control study of the relationship between cigarette smoking and nasopharyngeal cancer (United States). Cancer Causes Control 1995;6:507–12. [DOI] [PubMed] [Google Scholar]
- 18. Cheng YJ, Hildesheim A, Hsu MM. et al. Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control 1999;10:201–07. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong RW, Imrey PB, Lye MS, Armstrong MJ, Yu MC, Sani S.. Nasopharyngeal carcinoma in Malaysian Chinese: occupational exposures to particles, formaldehyde and heat. Int J Epidemiol 2000;29:991–98. [DOI] [PubMed] [Google Scholar]
- 20. Zou J, Sun Q, Akiba S. et al. A case-control study of nasopharyngeal carcinoma in the high background radiation areas of Yangjiang, China. J Radiat Res 2000;41(Suppl):53–62. [DOI] [PubMed] [Google Scholar]
- 21. Chelleng PK, Narain K, Das HK, Chetia M, Mahanta J.. Risk factors for cancer nasopharynx: a case-control study from Nagaland, India. Natl Med J India 2000;13:6–8. [PubMed] [Google Scholar]
- 22. Yuan JM, Wang XL, Xiang YB, Gao YT, Ross RK, Yu MC.. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer 2000;85:364–69. [PubMed] [Google Scholar]
- 23. Feng BJ, Khyatti M, Ben-Ayoub W. et al. Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Br J Cancer 2009;101:1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo X, Johnson RC, Deng H. et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer 2009;124:2942–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nesic V, Sipetic S, Vlajinac H, Stosic-Divjak S, Jesic S.. Risk factors for the occurrence of undifferentiated carcinoma of nasopharyngeal type: a case-control study. Srp Arh Celok Lek 2010;138:6–10. [DOI] [PubMed] [Google Scholar]
- 26. Turkoz FP, Celenkoglu G, Dogu GG. et al. Risk factors of nasopharyngeal carcinoma in Turkey - an epidemiological survey of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev 2011;12:3017–21. [PubMed] [Google Scholar]
- 27. Polesel J, Franceschi S, Talamini R. et al. Tobacco smoking, alcohol drinking, and the risk of different histological types of nasopharyngeal cancer in a low-risk population. Oral Oncol 2011;47:541–45. [DOI] [PubMed] [Google Scholar]
- 28. Ji X, Zhang W, Xie C, Wang B, Zhang G, Zhou F.. Nasopharyngeal carcinoma risk by histologic type in central China: impact of smoking, alcohol and family history. Int J Cancer 2011;129:724–32. [DOI] [PubMed] [Google Scholar]
- 29. Fachiroh J, Sangrajrang S, Johansson M. et al. Tobacco consumption and genetic susceptibility to nasopharyngeal carcinoma (NPC) in Thailand. Cancer Causes Control 2012;23:1995–2002. [DOI] [PubMed] [Google Scholar]
- 30. Lye MS, Visuvanathan S, Chong PP, Yap YY, Lim CC, Ban EZ.. Homozygous wildtype of XPD K751Q polymorphism is associated with increased risk of nasopharyngeal carcinoma in Malaysian population. PLoS One 2015;10:e0130530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie SH, Yu IT, Tse LA, Au JS, Lau JS.. Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: a case-referent study in Hong Kong Chinese. Cancer Causes Control 2015;26:913–21. [DOI] [PubMed] [Google Scholar]
- 32. Chang ET, Liu Z, Hildesheim A. et al. Active and passive smoking and risk of nasopharyngeal carcinoma: a population-based case-control study in Southern China. Am J Epidemiol 2017;185:1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yong SK, Ha TC, Yeo MC, Gaborieau V, McKay JD, Wee J.. Associations of lifestyle and diet with the risk of nasopharyngeal carcinoma in Singapore: a case-control study. Chin J Cancer 2017;36:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu F-H, Xiong D, Xu Y-F. et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein–Barr virus activation. J Natl Cancer Inst 2012;104:1396–410. [DOI] [PubMed] [Google Scholar]
- 35. Chow WH, McLaughlin JK, Hrubec Z, Nam JM, Blot WJ.. Tobacco use and nasopharyngeal carcinoma in a cohort of US Veterans. Int J Cancer 1993;55:538–40. [DOI] [PubMed] [Google Scholar]
- 36. Doll R, Peto R, Boreham J, Sutherland I.. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer 2005;92:426–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Friborg JT, Yuan JM, Wang R, Koh WP, Lee HP, Yu MC.. A prospective study of tobacco and alcohol use as risk factors for pharyngeal carcinomas in Singapore Chinese. Cancer 2007;109:1183–91. [DOI] [PubMed] [Google Scholar]
- 38. Hsu W-L, Chen J-Y, Chien Y-C. et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev 2009;18:1218–26. [DOI] [PubMed] [Google Scholar]
- 39. Lin J-H, Jiang C-Q, Ho S-Y. et al. Smoking and nasopharyngeal carcinoma mortality: a cohort study of 101,823 adults in Guangzhou, China. BMC Cancer 2015;15:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marks JE, Phillips JL, Menck HR.. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 1998;83:582–88. [DOI] [PubMed] [Google Scholar]
- 41. Lee AW, Lung ML, Ng WT.. Nasopharyngeal Carcinoma: From Etiology to Clinical Practice. Cambridge, MA, United Kingdom: Academic Press, 2019. [Google Scholar]
- 42. Nicholls J, Niedobitek G, Histopathological Diagnosis of Nasopharyngeal Carcinoma: Looking beyond the Blue Book. New York, NY: Springer, 2013; pp. 10–22. [Google Scholar]
- 43. Xue WQ, Qin HD, Ruan HL, Shugart YY, Jia WH.. Quantitative association of tobacco smoking with the risk of nasopharyngeal carcinoma: a comprehensive meta-analysis of studies conducted between 1979 and 2011. Am J Epidemiol 2013;178:325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Long M, Fu Z, Li P, Nie Z.. Cigarette smoking and the risk of nasopharyngeal carcinoma: a meta-analysis of epidemiological studies. BMJ Open 2017;7:e016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simmonds M, Stewart G, Stewart L.. A decade of individual participant data meta-analyses: A review of current practice. Contemp Clin Trials 2015;45:76–83. [DOI] [PubMed] [Google Scholar]
- 46. Wu X, Tsai SP, Tsao CK. et al. Cohort Profile: The Taiwan MJ Cohort: half a million Chinese with repeated health surveillance data. Int J Epidemiol 2017;46:1744–1744g. [DOI] [PubMed] [Google Scholar]
- 47. Jiang C, Thomas GN, Lam TH. et al. Cohort Profile: The Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int J Epidemiol 2006;35:844–52. [DOI] [PubMed] [Google Scholar]
- 48. Schooling CM, Chan WM, Leung SL. et al. Cohort Profile: Hong Kong Department of Health Elderly Health Service Cohort. Int J Epidemiol 2016;45:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart L, Clarke M, Rovers M. et al. ; PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. 2015;313:1657–65. [DOI] [PubMed] [Google Scholar]
- 50. Burke DL, Ensor J, Riley RD.. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med 2017;36:855–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang W, Jiang C, Hing LT. et al. A prospective cohort study on the comparison of risk of occupational dust exposure and smoking to death [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 2004;25: 748–752. [CVOCROSSCVO] [PubMed] [Google Scholar]
- 52. IARC.Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Lyon: International Agency for Research on Cancer, 1993. [Google Scholar]
- 53. Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchi A.. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Health 2006;29:193–98. [CVOCROSSCVO] [PMC free article] [PubMed] [Google Scholar]
- 54. Blot WJ, McLaughlin JK, Winn DM. et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282–87. [PubMed] [Google Scholar]
- 55. Cahoon EK, Preston DL, Pierce DA. et al. Lung, laryngeal and other respiratory cancer incidence among Japanese atomic bomb survivors: an updated analysis from 1958 through 2009. Radiat Res 2017;187:538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zou J, Sun Q, Akiba S. et al. A case-control study of nasopharyngeal carcinoma in the high background radiation areas of Yangjiang. J Radiat Res 2000;41:53–S62. [DOI] [PubMed] [Google Scholar]
- 57. Sakata R, Preston DL, Brenner AV. et al. Radiation-related risk of cancers of the upper digestive tract among Japanese atomic bomb survivors. Radiat Res 2019;192:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu T, Lin CY, Xie SH. et al. Smoking can increase nasopharyngeal carcinoma risk by repeatedly reactivating Epstein‐Barr Virus: an analysis of a prospective study in southern China. Cancer Med 2019;8:2561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsu WL, Chien YC, Huang YT. et al. ; GEV-NPC Study Group. Cigarette smoking increases the risk of nasopharyngeal carcinoma through the elevated level of IgA antibody against Epstein‐Barr virus capsid antigen: a mediation analysis. Cancer Med 2020;9:1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen Y, Xu Y, Zhao W. et al. Lack of association between cigarette smoking and Epstein Barr virus reactivation in the nasopharynx in people with elevated EBV IgA antibody titres. BMC Cancer 2018;18:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. He Y-Q, Xue W-Q, Xu F-H. et al. The relationship between environmental factors and the profile of Epstein-Barr virus antibodies in the lytic and latent infection periods in healthy populations from endemic and non-endemic nasopharyngeal carcinoma areas in China. EBioMedicine 2018;30:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. He Y-Q, Liao X-Y, Xue W-Q. et al. Association between environmental factors and oral Epstein-Barr virus DNA Loads: a multicenter cross-sectional study in China. J Infect Dis 2019;219:400–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang Q-Y, He Y-Q, Xue W-Q. et al. Association between serum cotinine level and serological markers of Epstein–Barr virus in healthy subjects in South China where nasopharyngeal carcinoma is endemic. Front Oncol 2019;9:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.IARC. Formaldehyde, 2-Butoxyethanol and 1-Tert-Butoxypropan-2-ol. Lyon,France: International Agency for Research on Cancer, 2006. [PMC free article] [PubMed] [Google Scholar]
- 65. Wang M, Cheng G, Balbo S, Carmella SG, Villalta PW, Hecht SS.. Clear differences in levels of a formaldehyde-DNA adduct in leukocytes of smokers and nonsmokers. Cancer Res 2009;69:7170–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.IARC. Personal Habits and Indoor Combustions. Lyon: International Agency for Research on Cancer, 2012. [Google Scholar]
- 67. Stewart LA, Clarke M.. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med 1995;14:2057–79. [DOI] [PubMed] [Google Scholar]
- 68. Mai Z-M, Lin J-H, Ngan RK-C. et al. Solar ultraviolet radiation and vitamin D deficiency on Epstein-Barr Virus reactivation: observational and genetic evidence from a nasopharyngeal carcinoma-endemic population. Open Forum Infect Dis 2020;7:ofaa426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen Z, Peto R, Zhou M. et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 2015;386:1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to ethical restrictions protecting patient privacy, data may be available on request from the Guangzhou Biobank Cohort Study Data Access Committee. Please contact us at [gbcsdata@hku.hk] for fielding data accession requests. The data of the Guangzhou Occupational Cohort and the Hong Kong Elderly Health Service Cohort Study underlying this article will be shared on reasonable request to the corresponding author, and the principal investigator Prof. Tai-Hing Lam [hrmrlth@hku.hk]. Data are from the Singapore Chinese Health Study, and the authors did not seek approval from the IRB to make the data publicly available. According to the Singapore Personal Data Protection Act, the authors could not release the data without approval from IRB. Researchers who meet the criteria for access to confidential data may contact the principal investigators of Singapore Chinese Health Study at Prof. Jian-Min Yuan [yuanj@upmc.edu] and Prof. Woon Puay Koh [woonpuay.koh@duke-nus.edu.sg] to seek approval from the National University of Singapore IRB. The Taiwan Cohort (conducted in 1984) data underlying this article will be shared on reasonable request to the principal investigator at Prof. Chen Chien-Jen [cjchen@ntu.edu.tw]. The data that support the findings of this study are available from MJ Health Research Foundation, but restrictions apply to the availability of these data, which were used under licence for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of MJ Health Research Foundation.