Abstract
Context
Radiofrequency ablation (RFA) has only recently gained popularity in the United States for treatment of thyroid nodules (TNs), with a limited number of patients having undergone the procedure in this country.
Objective
To evaluate the safety and efficacy of RFA of TNs performed in an outpatient setting in the United States.
Methods
This is a retrospective, single-center study of 53 patients who underwent RFA of 58 TNs between November 2018 and January 2021. The reduction in volume of nodule, cosmetic and symptomatic improvement, effect on thyroid function, and complications following RFA were assessed.
Results
Eleven out of 53 patients were excluded from the analysis. A total of 47 benign TNs (23 nonfunctioning thyroid nodules [NFTNs] and 24 autonomously functioning thyroid nodules [AFTNs]), were assessed after RFA. The median reduction in volume was 70.8% after a median follow-up period of 109 days, with symptomatic and cosmetic improvement (P < 0.0001). Compared with larger nodules, smaller nodules had greater volume reduction (P = 0.0266). RFA improved thyrotropin (TSH) in AFTNs (P value = 0.0015) and did not affect TSH in NFTNs (P value = 0.23). There were no major complications; however, 1 patient had self-limited local bleeding and another had transient voice change that recovered in 6 months.
Conclusion
RFA is a safe and efficacious treatment for symptomatic NFTNs and AFTNs in our population and is especially effective for smaller nodules. RFA should be considered an alternative for TNs in patients who cannot or do not want to undergo surgery.
Keywords: radiofrequency ablation, thyroid nodules, autonomously functioning thyroid nodule
Thyroid nodules (TNs) are common and with the advent of high-resolution ultrasonography techniques, are detected in up to ~68% of the general population in the United States [1]. Most nodules are benign and asymptomatic; however, ~5% to 15% can grow and cause compressive symptoms or cosmetic issues, with palpable nodules being 5 times more prevalent in females compared with males [2-4]. In addition, ~5% to 10% of TNs are autonomously functioning and can result in symptoms or biochemical subclinical or overt hyperthyroidism [3-5]. The standard of care for symptomatic, benign, nonfunctioning thyroid nodules (NFTNs) in the United States is surgery, while for autonomously functioning thyroid nodules (AFTNs), radioactive iodine or surgery is preferred, although thionamides may also be used [6].
The overall risk of complications from thyroid surgery is estimated to be 2% to 20%, with total thyroidectomies associated with more complications than hemi-thyroidectomies [7]. In most cases, surgery is performed under general anesthesia with associated risks, and it results in scar formation [8, 9]. Both surgery and radioactive iodine ablation carry the risk of permanent hypothyroidism, resulting in lifelong thyroid hormone replacement [10-13]. To avoid these risks, minimally invasive thermal ablation techniques, including radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity-focused ultrasound are increasingly being used worldwide [14].
Although all thermal ablation techniques are relatively safe and effective, RFA has shown the most promise in treatment of benign TNs with long-term efficacy reported over 3 to 5 years [15-19]. Studies from South Korea and Italy have shown mean volume reduction of 50% to 93.4% in 6 to 12 months, with significant improvement in symptoms and cosmesis [20-27]. The data regarding the efficacy and safety of RFA from the United States are limited to a retrospective review of 14 patients from Mayo Clinic, Rochester, Minnesota, who were treated under general anesthesia and had a median volume reduction of 44.6% over a median follow-up period of 8.6 months; and a brief report of 24 nodules in 15 patients (excluding 1 patient with recurrent thyroid cancer) from Columbia University, New York, who were treated under local anesthesia and had a mean volume reduction of 52.9% over a mean follow-up period of 1.1 months [28, 29].
Therefore, we report our experience regarding the efficacy and safety of RFA of thyroid nodules performed in an outpatient setting, without general anesthesia, in the United States. We also compare outcomes, including complication rates, with those from already published studies to see if this technique is an acceptable alternative to surgery or other therapies in this population.
Methods
Study Population and Design
A retrospective chart review was conducted to identify all patients who underwent RFA of their thyroid nodules between November 2018 and January 2021, at The Thyroid Clinic, in Salt Lake City, Utah. A total of 53 patients with 58 nodules (30 NFTNs and 28 AFTNs) were identified.
The criteria for treatment with RFA was as follows:
NFTNs were treated if they were causing compressive symptoms or cosmetic concerns; and were predominantly solid or solid-cystic (>25% solid) on ultrasonographic imaging.
AFTNs were treated in cases of overt hyperthyroidism or subclinical hyperthyroidism with symptoms and/or risk factors for adverse outcomes, namely, advanced age, decreased bone density, and heart disease (heart failure, coronary artery disease, or atrial flutter/fibrillation); or in case of symptoms regardless of thyroid function.
In patients with more than one thyroid nodule, only the nodules that met the above criteria were treated with RFA. Nodules not treated with RFA were followed with ultrasonographic imaging.
Data were extracted from the electronic medical record to determine demographics of the patients, ultrasound characteristics of the nodules, compressive symptoms, cosmetic scores, preablation volume, postablation volume, complications, and effect on thyroid function studies.
Treatment success was defined as a volume reduction of more than 50% within 6 months after RFA with improvement in symptoms and/or cosmetic concerns, and in case of AFTNs, normalization of thyroid function tests and resolution of symptoms within 12 months after RFA.
The protocol of this study was reviewed and approved by the University of Texas Southwestern Institutional Review Board.
Preablation Assessment
All patients were evaluated clinically and had neck ultrasonography and laboratory blood tests prior to the RFA procedure. Patients with presence of metallic hardware/pacemaker, bleeding diathesis, pregnancy, or nodules that appeared suspicious on ultrasonographic imaging but had benign results on ultrasound-guided fine needle aspiration (UG-FNA) were not considered to be candidates for RFA. Written and verbal informed consent was obtained from all patients prior to performing RFA.
Thyroid function tests including serum thyrotropin (TSH; thyroid-stimulating hormone) and free thyroxine (T4) levels were performed, along with blood platelet count and coagulation tests (including prothrombin time and activated partial thromboplastin time) prior to RFA in all patients, with the exception of 1 patient who had a normal TSH level but did not have a free T4 level. Eighteen patients also underwent testing for thyroid peroxidase antibodies (TPO Ab) prior to RFA, as elevated levels of TPO Ab can increase risk of developing hypothyroidism [30].
All patients with suppressed TSH underwent a pretreatment radioactive iodine uptake and scan, and autonomously functioning thyroid nodules were identified. One patient had symptoms of hyperthyroidism in the presence of a thyroid nodule on ultrasonographic imaging but had normal levels of TSH and free T4. She also underwent a radioactive iodine uptake and scan that identified the AFTN.
Patients with NFTNs underwent 2 separate ultrasound-guided fine needle aspiration (UG-FNA) biopsies showing benign cytopathology (Bethesda II), with the exception of 1 patient who had one UG-FNA biopsy with benign cytopathology (Bethesda II) and a second UG-FNA biopsy with indeterminate cytopathology (Bethesda III) followed by negative molecular marker studies (Thyroseq v3 genomic classifier showed low probability of malignancy). Patients with AFTNs each underwent 1 UG-FNA biopsy that showed benign cytopathology (Bethesda II), except for 1 patient whose cytopathology showed atypia of undetermined significance (Bethesda III) followed by Thyroseq v3 genomic classifier showing low probability of malignancy.
Ultrasonography was performed using the MyLab Gamma ultrasound system (Esaote North America) with a linear matrix array transducer (SL1543) operating at 4 to 14 MHz and included color Doppler imaging. Each nodule was measured in 3 dimensions and the nodule volume was calculated using the ellipsoid volume formula:
An objective cosmetic score was obtained using a 1 to 4 scale as reported in the 2017 thyroid RFA guidelines by the Korean Society of Thyroid Radiology, where 1 is no palpable mass; 2 is no cosmetic problem but presence of palpable mass; 3 is a cosmetic problem on swallowing only; and 4 is a readily visible cosmetic problem [31]. The presence or absence of compressive symptoms was also recorded in all patients.
Radiofrequency Ablation Procedure
RFA involves insertion of an internally cooled electrode into the target nodule. The electrode is connected to a generator that produces a high-frequency alternating current. This causes vibration of ions in the tissue in contact with the exposed tip, resulting in thermal injury and coagulative necrosis in the target nodule. The ablation is followed by shrinkage of the lesion over time.
The procedure was performed in an outpatient setting using standard aseptic techniques and local anesthesia for pain control. Patients were placed in supine position with hyperextended neck, and target nodule and vital cervical structures were visualized with ultrasonography in real time. To ensure safety and achieve maximum efficacy, the RFA was performed using limited hydrodissection, a trans-isthmic approach, and the ‘moving shot’ technique [31].
Anesthesia
Under ultrasound guidance, using a 25-gauge needle, 3 to 8 cc of 2% lidocaine without epinephrine was injected under the skin and into the thyroid capsule and perithyroidal area to provide adequate analgesia. Patients also had additional mild conscious sedation with midazolam and/or fentanyl, and 1 patient required ketamine, administered by an anesthesiologist who monitored the patients’ hemodynamics throughout the procedure. There were no problems reported with anesthesia. All patients remained alert, coherent, and verbal throughout the procedure, which is important for early detection of vocal cord paresis/paralysis during the procedure.
Hydrodissection
Hydrodissection is a technique used to separate the target lesion from surrounding structures in the neck, such as the carotid artery, recurrent laryngeal nerve, and anterior cervical muscles. It involves injecting either lidocaine (a total volume of 20 mL should not be exceeded in any patient to avoid lidocaine toxicity) or dextrose 5% in water in between the nodule and adjacent structures to create a safety margin that prevents thermal damage to these critical structures [31]. In our study, limited hydrodissection was performed for all patients. The 2% lidocaine without epinephrine used for anesthesia was injected to separate the anterior thyroid capsule (and anterior cervical muscles) from the thyroid parenchyma. Additional hydrodissection (eg, to separate the nodule from neurovascular bundle) was not required due to the location of the nodules.
Trans-isthmic approach
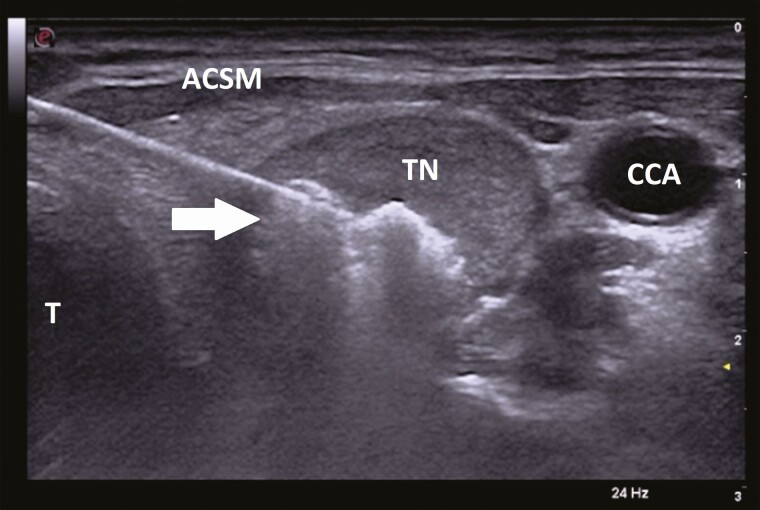
The trans-isthmic approach refers to inserting the electrode from the midline of the neck and advancing it laterally into the target nodule, thus limiting heat exposure to the recurrent laryngeal nerve located in the tracheoesophageal groove and/or esophagus (Fig. 1). In addition, this makes it harder for the electrode to change positions when the patient talks or swallows, compared with the lateral to medial approach, and also prevents leakage of hot fluid into the perithyroidal area [32].
Figure 1.
Ultrasonographic image (transverse view) of radiofrequency ablation electrode in thyroid nodule. White arrow pointing towards electrode inserted in the middle part of the thyroid nodule (TN) using the ‘trans-isthmic’ approach (from medial to lateral) with tip surrounded by hyperechoic area indicating ablated zone. In this approach, the electrode tip points away from the trachea (T) limiting heat exposure to the recurrent laryngeal nerve located between the trachea (T) and the inferior pole of the thyroid lobe. Inserting the electrode from medial to lateral, rather than lateral to medial also avoids the neurovascular bundle. Abbreviations: ACSM, anterior cervical strap muscles; CCA, common carotid artery.
Moving shot technique
In this technique, the nodule is ablated bit by bit, starting at the inferior-most posterolateral part and gradually moving medially and anteriorly, with the electrode pulled back along its longitudinal axis following the same track as the initial advancement as the ablation is continued [33]. As each area is ablated, it becomes hyperechoic on ultrasonography, indicating that the electrode must then be repositioned within the nodule to continue the ablation. The process is then repeated, with overlapping, to completely ablate the inferior part of the nodule, followed by the middle part and then the superior part, until the entire nodule is ablated. This technique was used for all patients.
Electrodes and generator
In 55 nodules, RFA was performed with an 18-gauge internally cooled electrode (STARMed, Seoul, South Korea), that was 7 cm in length with a 0.7-cm active tip and was powered by the VIVA RF generator (STARMed). In 3 nodules, similar 18-gauge internally cooled electrodes, 7 cm in length with 0.7-cm active tips from RF Medical powered by RF Ablation System V-1000 (RGS Healthcare), were used. For all patients, an initial power of 30 watts (W) was used for the ablation and this was increased in increments of 5 to 10 W every 10 seconds, up to a maximum of 55 W. The median power used was 35 W (range, 30-55 W), with median active ablation time of 3 minutes (range, 32 seconds to 11 minutes 13 seconds).
A total of 53 patients underwent a single RFA session for 58 nodules (5 patients had RFA of 2 separate nodules during the same treatment session on the same day). One patient with an AFTN required 2 RFA sessions 1 year apart.
Postablation Assessment
All patients were contacted by telephone 1 to 2 days after the RFA procedure to evaluate for immediate complications including pain, fever, hematoma or swelling, voice change (both immediate and after 24 hours), and onset of dysphagia.
Patients were evaluated with repeat ultrasonography and thyroid function tests 6 to 10 weeks after the RFA procedure; and were asked to return for follow-up at 6- and 12-month intervals. The volume reduction percentage (VRP) for each nodule was calculated using the following equation:
An assessment was made with regards to presence or absence of compressive symptoms following the RFA procedure, and postablative cosmetic score was also recorded. Patients were also evaluated for late-onset complications, including hoarseness of voice, transient thyroiditis, and nodule rupture. Complications were classified as either minor (no or nominal therapy with no long-term consequence) or major (requiring therapy, leading to hospitalization, or causing permanent adverse sequelae) based on previously reported criteria [34].
Statistical Analysis
The categorical variables are presented by frequency (percentage) and continuous variables are presented by mean ± standard deviation for Gaussian distribution, and median (minimum and maximum values) for non-Gaussian distributed data. Comparisons between the AFTNs and NFTNs were made using the Fisher exact test for categorical variables, one-way analysis of variance (ANOVA) for Gaussian, and Kruskal-Wallis for non-Gaussian distributed data from continuous variables. The paired t test was applied to compare the pre- and post-RFA thyroid nodule volume changes, cosmetic score changes, and changes in laboratory values (TSH and free T4). A linear regression model was used to detect the association of baseline volume and volume changes after the procedure, adjusted by energy used, and Pearson correlation was used to see the relationship between volume changes and energy used.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). A P value of < 0.05 was considered statistically significant.
Results
A total of 58 thyroid nodules in 53 patients (50 female, 3 male) were treated with RFA. The patients were predominantly Caucasian (51 Caucasian, 1 African American, and 1 Asian patient). The median age was 45 years (range, 30-77 years) (Table 1). Eleven patients did not return for follow-up (10 female and 1 male, all Caucasian). The remaining 42 patients had a total of 23 NFTNs and 24 AFTNs that were analyzed after RFA. Thirty-nine nodules were predominantly solid (defined as more than 75% solid) and 8 were mixed solid-cystic nodules (defined as 25%-75% solid), of which 6 were >50% solid, and 2 were ~25% solid and 75% cystic. Baseline characteristics of the cohort are described in Table 1.
Table 1.
Baseline characteristics of patients with thyroid nodules included in the study
| All | NFTN | AFTN | |
|---|---|---|---|
| Patients n, (female/male)* | 42 (40/2) | 22 (22/0) | 20 (18/2) |
| Race (White/Black/Asian) | 40/1/1 | 21/0/1 | 19/1/0 |
| Median age (y) at time of RFA (range) | 45 (30-77) | 49.5 (35-70) | 42 (31-77) |
| Nodules, n | 47 | 23 | 24 |
| Single nodule/multinodular goiter, n | 17/25 | 8/14 | 9/11 |
| US characteristics of nodule (S/SC), n | 39/8 | 20/3 | 19/5 |
| On LT4 before RFA (Y/N), n | 7/35 | 7/15 | N/A |
| On MMI before RFA (Y/N), n | 8/34 | N/A | 8/12 |
Abbreviations: AFTN, autonomously functioning thyroid nodule; LT4, levothyroxine; MMI, methimazole; N/A, not applicable; NFTN, nonfunctional thyroid nodule; RFA, radiofrequency ablation; S, solid; SC, solid-cystic; US, ultrasound.
*Eleven patients with 11 nodules were lost to follow-up and therefore not included in the analysis.
On initial post-RFA evaluation, the median reduction in volume was 70.8% (range, 32.1%-96.3%) after a median follow-up period of 109 days (range, 31-654 days) (Figs. 2 and 3). Twenty-five patients had contralateral thyroid nodules that were not treated with RFA. The volume reduction in RFA treated nodules was significant compared to those that were not treated (P value <0.0001). Nodules that did not undergo RFA did not significantly change during the same follow-up period (Fig. 4).
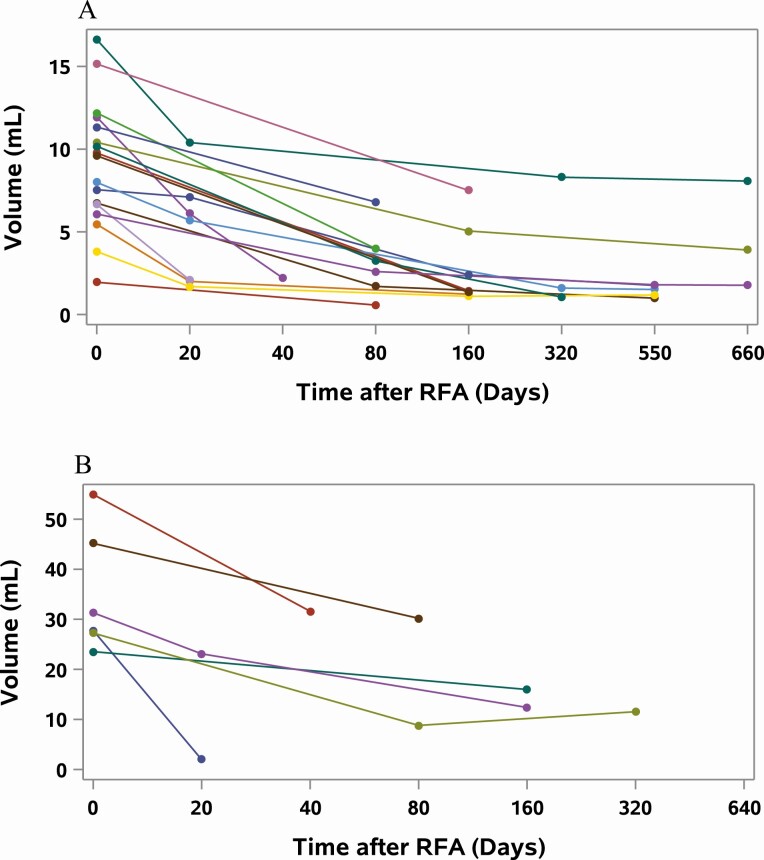
Figure 2.
Change in volume over time in nonfunctioning thyroid nodules (NFTN) after radiofrequency ablation (RFA). Each line represents an individual nodule; Time 0 days indicates the day of the RFA procedure, with the length of the line representing length of follow-up period. Each circle represents a point in time where volume of the nodule was measured by ultrasonography, and the points are connected by lines to give an approximate rate of volume reduction. A, Change in volume of NFTNs with initial volumes of less than 20 mL. B, Change in volume of NFTNs with initial volumes of more than 20 mL.
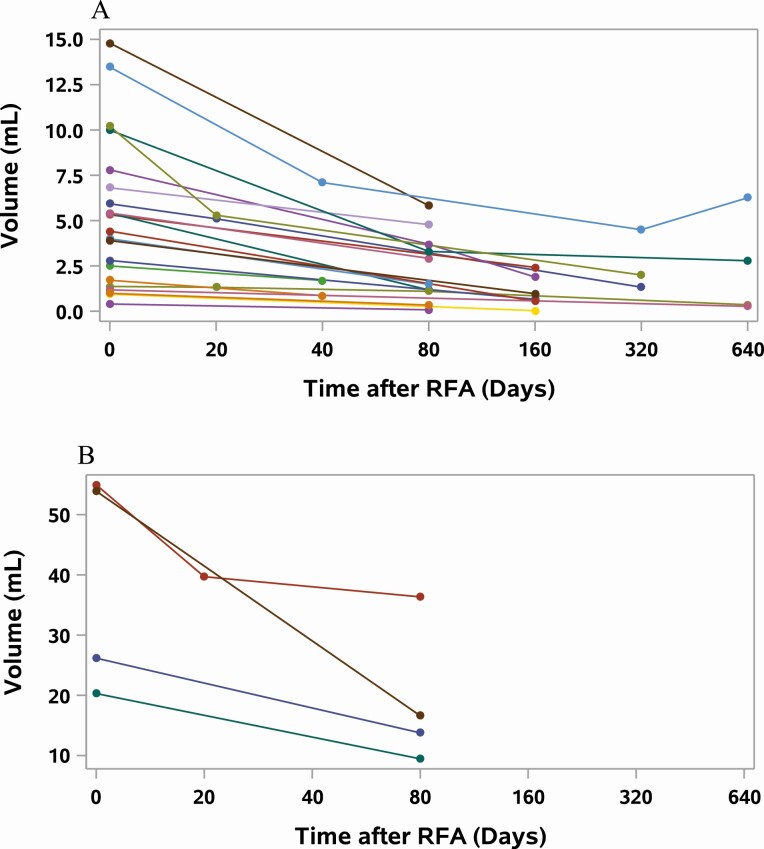
Figure 3.
Change in volume over time in autonomously functioning thyroid nodules (AFTN) after radiofrequency ablation (RFA). Each line represents an individual nodule; Time 0 days indicates the day of the RFA procedure, with the length of the line representing length of follow-up. Each circle represents a point in time where volume of the nodule was measured by ultrasonography, and the points are connected by lines to give an approximate rate of volume reduction. A, Change in volume of AFTNs with initial volumes of less than 20 mL. B, Change in volume of AFTNs with initial volumes of more than 20 mL.
Figure 4.
Change in volume over time of incidental thyroid nodules that did not undergo radiofrequency ablation (RFA). Each line represents an individual nodule; Time 0 days indicates the day RFA was performed on the contralateral nodule, with the length of the line representing length of follow-up. Each circle represents a point in time where volume of the nodule was measured by ultrasonography, and the points are connected by lines to give an approximate rate of volume reduction. A, Change in volume of incidental thyroid nodules in patients who underwent RFA of nodules with initial volume <20 mL. B, Change in volume of incidental thyroid nodules in patients who underwent RFA of nodules with initial volume >20 mL.
Volume reduction percentage (VRP) had an inverse relationship with the size of the nodule (P = 0.0266); with median VRP of 75.9% (range, 32.1%-96.3%) in nodules less than 10 mL in volume (n = 27 nodules); 61.6% (range, 40%-89.6%) in nodules 10 to 20 mL (n = 10); and 50.4% (range, 32.1%-92.9%) in nodules more than 20 mL (n = 10), respectively (Table 2). Overall, 38 out of 47 nodules (~80.9%) had a volume reduction of more than 50% during the total follow-up period (Figs. 2 and 3). A volume reduction of more than 50% within the first 6 months of the ablation could only be confirmed in 30 nodules, as post-RFA ultrasonography was performed after 6 months in some patients.
Table 2.
Response to radiofrequency ablation (RFA) expressed as a median volume reduction percentage based on initial volume of nodule
| Initial Volumes | All Nodules | n | NFTNs | n | AFTNs | n |
|---|---|---|---|---|---|---|
| All volumes | 70.8 (31.7-96.3) | 47 | 69 (32.1-86.1) | 23 | 71.1 (31.7-96.3) | 24 |
| < 5 mL | 73.1 (31.7-96.3) | 13 | 69.9 (69-70.7) | 2 | 75.2 (31.7-96.3) | 11 |
| 5 to < 10 mL | 77 (46-86.1) | 14 | 79.3 (68.7-86.1) | 8 | 74.4 (46-78.2) | 6 |
| 10-20 mL | 61.6 (40-89.6) | 10 | 62.6 (40-89.6) | 7 | 60.6 (53.5-80.4) | 3 |
| > 20 mL | 50.4 (33.1-92.9) | 10 | 50 (33.1-92.9) | 6 | 50.4 (33.8-69.2) | 4 |
Data shown as median with range in parentheses; n = number of nodules.
Abbreviations: AFTN, autonomous functional thyroid nodule; NFTN, nonfunctional thyroid nodule; VRP, volume reduction percentage.
The median VRP in predominantly solid nodules was 69% (range, 32.1%-96.3%), while the median VRP of mixed solid-cystic nodules was 77% (range, 47.4%-92.9%); however, this difference was not statistically significant (P = 0.249).
The initial median volume of NFTNs (n = 23) was 10.4 mL (range, 1.95-54.9 mL), which decreased to a median of 2.09 mL (range, 0.57-31.6 mL) after RFA, representing a median VRP of 69% (range, 32.1-86.1%) over a median follow-up period of 116 days (range, 40-654 days) (Fig. 2). The initial median volume of AFTNs (n = 24) was 5.4 mL (range, 0.4-53.9 mL), which decreased to a median of 1.8 mL (range, 0.08-36.4 mL) after RFA, representing a median VRP of 71.1% (range, 31.7%-96.3%) over a median follow-up period of 87 days (range, 31-613 days) (Fig. 3). There was a trend toward higher energy use resulting in smaller volume reduction (P = 0.1); however, there was no significant association between energy applied and volume reduction when adjusted for the initial volume of the nodule (P = 0.43). Both NFTNs and AFTNs showed similar volume reductions, and there was no statistical difference between the groups when adjusted for initial volume and energy applied.
Among the patients with NFTNs, 7 were already on levothyroxine for hypothyroidism prior to RFA, which was continued after the procedure. One of these patients did have an elevated TSH (9.6 mIU/L) after RFA while free T4 remained normal (1.2 ng/dL); however, this was attributed to the patient taking her levothyroxine inconsistently. None of the patients who were not on levothyroxine prior to RFA treatment developed thyroid dysfunction after the procedure. These patients had a median TSH of 1.3 mIU/L both before (range, 0.75-3.75 mIU/L), and after (range, 0.9-2.7 mIU/L) RFA; and a median free T4 of 0.94 ng/dL (range, 0.8-1.3 ng/dL) before RFA and 0.89 ng/dL (range, 0.74-1.1 ng/dL) after RFA. There was no significant difference between the pre- and post-RFA TSH (P = 0.09); however, the difference between the pre- and post-RFA free T4 was significant (P = 0.02). Of the 15 patients not on levothyroxine prior to RFA, 6 patients had TPO Ab checked, and 3 of these patients had elevated TPO Ab levels. Only 3 of the 7 patients already on levothyroxine had their TPO Ab checked, and all had elevated levels.
Patients with AFTNs had predominantly subclinical hyperthyroidism, with only 1 patient out of 20 with overt hyperthyroidism, and 1 patient who had normal thyroid function tests with AFTN diagnosed on radioactive iodine uptake and scan. Eight patients were on methimazole prior to RFA; it was continued in 6 patients immediately after the procedure and discontinued in 2 patients. Of the 6 patients who continued methimazole, 3 patients had discontinued it after 1 month, 6 months, and 7 months, respectively, and 3 patients were on a lower dose (2.5-5 mg by mouth daily) at the last follow-up appointment. Of note, out of the 8 patients on methimazole prior to RFA, 5 patients had TPO Ab checked; 2 patients had elevated levels, whereas 3 patients did not have TPO Ab. Of the 3 patients who were on methimazole at the last follow-up, only 1 had positive TPO Ab. Thyroid-stimulating immunoglobulin and thyroglobulin antibodies were not checked.
Thyroid function tests significantly improved after RFA of AFTNs in patients not on methimazole, with a median TSH of 0.1 mIU/L (range, 0.01-0.32 mIU/L) before the procedure, compared with a median TSH of 0.63 mIU/L (range, 0.01-1.2 mIU/L) after RFA (P = 0.0015); and a median free T4 of 1.15 ng/dL (range, 0.9-1.9 ng/dL) before RFA and 0.9 ng/dL (range, 0.7-1.8 ng/dL) after the procedure (P = 0.01). All patients had normal free T4 levels (normal range, 0.7-1.8 ng/dL) after RFA; and 15 of 20 patients (75%) had their TSH normalize (normal range, 0.4-4.5 mIU/L) within 12 months of the procedure; however, 3 of the 15 patients were still on methimazole. These 3 patients chose to continue methimazole rather than get definitive therapy with radioactive iodine or surgery.
The patient with normal thyroid function tests in the presence of a confirmed AFTN reported resolution of symptoms after the procedure and her TSH remained normal. One patient had 2 RFA procedures on the same AFTN 1 year apart and only the first ablation was used in the volume reduction calculations. She had a volume reduction of 76.3% after her first ablation; however, her TSH remained suppressed so she elected to have a second RFA, with a further volume reduction of 29.6% over 58 days. Her TSH did normalize after the second procedure; the level was 0.76 mIU/L with the patient off methimazole at the last follow-up. Her cosmetic score improved from 4 to 2 after the first RFA and from 2 to 1 after the second.
The median cosmetic score before RFA was 4 (range, 0-4), and this improved significantly to a median of 2 (range, 0-4) after the procedure (P < 0.0001). NFTNs caused more cosmetic concern with a median cosmetic score of 4 (range, 3-4) before RFA, whereas patients with AFTNs had a median cosmetic score of 3.5 (range, 0-4). After RFA, the median cosmetic score decreased significantly to 2 (range, 0-4) and 1 (range, 0-3) in NFTNs and AFTNs, respectively. One patient with a NFTN and pretreatment cosmetic score of 4 was not seen physically in clinic so posttreatment cosmetic score was not assessed. Compressive symptoms (if present prior to RFA) improved in all patients. The response to RFA is summarized in Table 3.
Table 3.
Response of thyroid nodules to radiofrequency ablation (RFA)
| Pre RFA | n | Post RFA | n | P value | |
|---|---|---|---|---|---|
| All Nodules | |||||
| Volume (mL) | 8 (0.4-54.95) | 47 | 1.89 (0.03-36.35) | 47 | < 0.0001 |
| Cosmetic score | 4 (0-4) | 47 | 2 (0-4) | 45 | < 0.0001 |
| NFTNs | |||||
| Volume (mL) | 10.4 (1.96-31.3) | 23 | 2.09 (0.57-30.2) | 23 | < 0.0001 |
| Cosmetic score | 4 (3-4) | 23 | 2 (0-4) | 21 | < 0.0001 |
| TSH (mIU/L) | 1.3 (0.75-3.75) | 13 | 1.3 (0.73-2.7) | 13 | 0.23 |
| Free T4 (ng/dL) | 0.97 (0.8-1.3) | 11 | 0.89 (0.74-1.1) | 11 | 0.02 |
| On levothyroxine, n | 7 | 22 | 7 | 22 | 1 |
| AFTNs | |||||
| Volume (mL) | 5.4 (0.4-54.95) | 24 | 1.7 (0.03-36.35 | 24 | 0.0001 |
| Cosmetic score | 3.5 (0-4) | 24 | 1 (0-3) | 24 | < 0.0001 |
| TSH mIU/L | 0.1 (0.01-0.32) | 12 | 0.63 (0.01-1.2) | 12 | 0.0015 |
| Free T4 (ng/dL) | 1.15 (0.9-1.9) | 12 | 0.9 (0.7-1.8) | 12 | 0.01 |
| On methimazole, n | 8 | 20 | 6 | 20 | 0.74 |
Data are shown as median with minimum and maximum values in parentheses; n = number of patients. Abbreviations: AFTN, autonomous functional thyroid nodule; NFTN, nonfunctional thyroid nodule; T4, thyroxine; TSH, thyroid-stimulating hormone.
No major complications were noted after RFA. Among minor complications, 1 patient developed a hematoma that resolved spontaneously within a day; and 1 patient developed hoarseness of voice during the procedure and 10 mL of cold dextrose 5% in water was injected in the tracheoesophageal groove with partial improvement in symptoms at the time. She did not have any predisposing factors for hoarseness of voice (nonsmoker, not a singer, and no vocal polyps confirmed on laryngoscopy). Her voice returned to normal in 6 months. Vocal cord recovery was documented by laryngoscopy and ultrasonography. The total complication rate was 4.2%.
Discussion
Conservative estimates indicate that 100 000 to 150 000 thyroidectomies are performed in the United States annually [35, 36]. An estimated 53 000 patients developed thyroid cancer in 2020 [37], indicating that most thyroidectomies are for benign disease [35]. It is well established that high-volume thyroid surgeons have lower complication rates [36, 38]; however, 69% to 86% of the thyroidectomies in the United States are not performed by high-volume surgeons [39-41]. Risks include hypoparathyroidism, both transient (8.2%-39%) and persistent (0.7%-3%); severe hypocalcemia necessitating hospitalization (0.5%-12.5%); recurrent laryngeal nerve injury, both transient (3%-8%) and persistent (0.2%-6.6%); and hemorrhage (0.7%-2.1%) [4, 42-47]. Up to 40% of patients also report transient minor symptoms, including voice change, dysphagia, or choking sensation postoperatively [42]. Conventional thyroidectomy and minimally invasive video–assisted thyroidectomy (MIVAT) both result in a permanent scar on the neck [9]. Clinicians tend to underestimate the negative perceptions that patients have regarding scarring and may not realize that most patients prefer a “scarless” approach [48]. This is especially true among non-White patients who can experience a significant decline in quality of life because of scar-related issues [48, 49].
When radioactive iodine ablation is used for AFTNs (toxic adenomas or toxic multinodular goiters), up to 40% of patients become hypothyroid within 5 to 8 years, and ~21% have persistence or recurrence of hyperthyroidism [11-13]. Furthermore, a higher dose of radioactive iodine (131I) is typically needed to achieve a higher cure rate, especially in toxic multinodular goiters, and this results in a higher rate of hypothyroidism [50]. In a small subset of patients with AFTNs, administration of 131I may result in development of TSH-receptor antibodies and Graves-like hyperthyroidism [51]. A trend toward increase in solid organ malignancies is also noted in hyperthyroid patients treated with radioactive iodine ablation [52].
Radiofrequency ablation offers a therapeutic approach that can mitigate some of the above-mentioned disadvantages of surgical treatment or radioactive iodine ablation. The volume reduction achieved in our study (70.8%) is comparable to other studies published in the literature (50%-93.3%), with a similar low complication rate (reported to be 2.4%-3.5% in larger studies), indicating that this is a viable treatment modality in the United States [23-26, 53-70]. It is noted that centers with higher experience have greater volume reductions [20, 21, 71, 72], although it is promising that our study compares favorably with the retrospective reviews from the Mayo Clinic and Columbia University, which are the only other studies that record the US experience [28, 29].
In keeping with previously published data, smaller nodules responded better to RFA, although there was no significant difference in the volume reduction percentage between NFTNs and AFTNs [73, 74]. The data indicates the response is on a continuum which precludes defining a size or volume beyond which RFA would not be effective. Large nodules may require more than one RFA session, although most did respond after a single session with corresponding symptomatic and cosmetic improvement, which is also in keeping with the experience of others [75]. The available data suggests that there is an advantage of performing RFA sooner rather than later in case of symptomatic thyroid nodules for best results.
We did not note any significant differences in volume reduction based on the composition (more solid vs more cystic) on ultrasonography, which is somewhat contrary to the findings from a multicenter study in Italy [57]. However, this may be because of low sample size and the somewhat nonuniform interpretation of ultrasound features across different studies.
In our study, more than 50% of patients became euthyroid, in keeping with results from Italy and South Korea [27, 76-78], with significant improvement in thyroid function tests. RFA of AFTNs may be of value to patients who do not want to risk the hypothyroidism that may occur after surgery or radioactive iodine ablation—a reasonable success rate (~75%) is achieved in our study as well as in the literature [78, 79]. Of interest, the free T4 decreased significantly in the NFTN group after RFA (although remained within normal range), whereas the TSH did not significantly change. This could be either an early trend toward hypothyroidism after RFA, or a result of a smaller sample size. An increased risk of hypothyroidism secondary to RFA has not been demonstrated in previous studies. Long-term studies with larger samples would be needed to assess whether RFA can cause hypothyroidism in the long term.
Complication rates are also noted to be quite low compared to surgery and are likely to be operator dependent [69]. Fortunately, there were no major complications in our study; however, this may be simply because of relatively low sample size. In addition, a small margin of normal thyroid tissue was preserved while ablating the nodules, which would have helped avoid complications, but conversely would result in lower volume reductions. The regrowth of thyroid nodules after ablation, resulting in the need for multiple procedures, remains a concern; however, no significant regrowth was noted in our study. Two nodules (1 NFTN and 1 AFTN) were noted to have a small increase in volume after previous decrease (Figs. 2 and 3); this may be attributed to either differences in measurements due to intra-operator variability, or regrowth. A longer follow-up period may give insight into the relative rates of regrowth and the factors associated with this.
RFA of thyroid nodules is a well-established practice in some countries, with clinical guidelines issued by academic societies in Korea, Italy, Austria, and the United Kingdom, as well the recent recommendations from the European Thyroid Association and Asian Conference on Tumor Ablation Task Force [31, 80-84]. In the United States, RFA is mentioned as a treatment option for benign symptomatic thyroid nodules in the 2016 American Association of Clinical Endocrinologists guidelines [85] but is only considered as second-line therapy for metastatic lymph nodes in patients who are not surgical candidates in the American Thyroid Association 2015 guidelines [2]. RFA can be performed in the outpatient setting in the United States under local anesthesia with results similar to those in more experienced international centers as demonstrated by our study.
Barriers to widespread availability of RFA of thyroid nodules in the United States include both a lack of awareness regarding the procedure and a lack of trained operators to perform the procedure. An additional barrier is the cost of the procedure (currently without a CPT code and thus not reimbursed by insurance) which often needs to be paid out-of-pocket by the patient. However, the absolute cost of the procedure (average US cost: $3500-$6000) is favorable when compared with surgery (estimated national average: ~$19 500), which has the additional burden of possible lifelong thyroid hormone replacement and monitoring, along with perioperative risks and complications [86]. In our study, ~65% of patients paid out-of-pocket for their procedure, whereas the rest were able to get a varying amount reimbursed by their insurance company. The main motivating factors for patients choosing RFA over surgery despite the cost included avoiding a surgical scar and thyroid hormone replacement therapy.
To safely perform the procedure, appropriate training programs need to be established in the United States where skills can be practiced under the supervision of experts. Prerequisites to learning how to perform this procedure include a thorough understanding of the anatomy of the neck, ability to perform and interpret neck ultrasonography, and adequate skill in performing fine needle aspiration of the thyroid using the parallel technique. As with other procedural skills, RFA also has a steep learning curve. Physicians who routinely treat thyroid disease, perform diagnostic ultrasound imaging themselves, and routinely do fine needle aspiration biopsies of neck lesions are ideally placed to add this procedure to the list of treatment options they can offer. More awareness regarding thermal ablation techniques and their use in thyroid nodular disease will ultimately lead to greater access and decreased costs for patients. As the procedure becomes more widespread and more data become available, it will become easier to compare long-term outcomes of RFA and surgery to determine which approach may be better.
Conclusion
Our study demonstrates that outpatient RFA is a safe and effective treatment for NFTNs and AFTNs in a US population sample, with smaller nodules responding better. Advantages of RFA include no surgical scars or general anesthesia, low complication rates, and avoiding lifelong thyroid hormone replacement.
We propose that RFA by skilled operators be considered a viable therapy for the treatment of thyroid nodules in the United States and recognize the need for further studies with long-term follow-up [74].
Acknowledgment
We would like to thank Dr. Abhimanyu Garg for his comments and advice.
Funding Source: None
Glossary
Abbreviations
- AFTN
autonomously functioning thyroid nodule
- NFTN
nonfunctioning thyroid nodule
- RFA
radiofrequency ablation
- T4
thyroxine
- TN
thyroid nodule
- TPO Ab
thyroid peroxidase antibodies
- TSH
thyrotropin (thyroid-stimulating hormone)
- UG-FNA
ultrasound-guided fine needle aspiration
- VRP
volume reduction percentage
Additional Information
Disclosures: The authors have no financial relationships relevant to this article to disclose. The authors have no conflict of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kant R, Davis A, Verma V. Thyroid nodules: advances in evaluation and management. Am Fam Physician. 2020;102(5):298-304. [PubMed] [Google Scholar]
- 2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313(9):926-935. [DOI] [PubMed] [Google Scholar]
- 4. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: a review. JAMA. 2018;319(9):914-924. [DOI] [PubMed] [Google Scholar]
- 5. Chami R, Moreno-Reyes R, Corvilain B. TSH measurement is not an appropriate screening test for autonomous functioning thyroid nodules: a retrospective study of 368 patients. Eur J Endocrinol. 2014;170(4):593-599. [DOI] [PubMed] [Google Scholar]
- 6. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421. [DOI] [PubMed] [Google Scholar]
- 7. Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. 2014;21(12):3844-3852. [DOI] [PubMed] [Google Scholar]
- 8. Snyder SK, Roberson CR, Cummings CC, Rajab MH. Local anesthesia with monitored anesthesia care vs general anesthesia in thyroidectomy: a randomized study. Arch Surg. 2006;141(2):167-173. [DOI] [PubMed] [Google Scholar]
- 9. Sahm M, Otto R, Pross M, Mantke R. Minimally invasive video-assisted thyroidectomy: a critical analysis of long-term cosmetic results using a validated tool. Ann R Coll Surg Engl. 2019;101(3):180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97(7):2243-2255. [DOI] [PubMed] [Google Scholar]
- 11. Azizi F, Takyar M, Madreseh E, Amouzegar A. Treatment of toxic multinodular goiter: comparison of radioiodine and long-term methimazole treatment. Thyroid. 2019;29(5):625-630. [DOI] [PubMed] [Google Scholar]
- 12. Porterfield JR Jr, Thompson GB, Farley DR, Grant CS, Richards ML. Evidence-based management of toxic multinodular goiter (Plummer’s disease). World J Surg. 2008;32(7):1278-1284. [DOI] [PubMed] [Google Scholar]
- 13. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282. [DOI] [PubMed] [Google Scholar]
- 14. Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20(1):11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label, trial (LARA trial). Thyroid. 2020;30(6):847-856. [DOI] [PubMed] [Google Scholar]
- 16. Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korkusuz Y, Gröner D, Raczynski N, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol. 2018;28(3):929-935. [DOI] [PubMed] [Google Scholar]
- 18. Deandrea M, Trimboli P, Garino F, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: a longitudinal 5-year observational study. J Clin Endocrinol Metab. 2019;104(9):3751-3756. [DOI] [PubMed] [Google Scholar]
- 19. Volpi E. Thermal treatment options for benign thyroid nodules—the role of radio-frequency ablation and laser therapy. Clin Thyroidol. 2021;33:17-20. [Google Scholar]
- 20. Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044-1049. [DOI] [PubMed] [Google Scholar]
- 22. Spiezia S, Garberoglio R, Di Somma C, et al. Efficacy and safety of radiofrequency thermal ablation in the treatment of thyroid nodules with pressure symptoms in elderly patients. J Am Geriatr Soc. 2007;55(9):1478-1479. [DOI] [PubMed] [Google Scholar]
- 23. Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19(3):219-225. [DOI] [PubMed] [Google Scholar]
- 24. Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16(4):361-367. [DOI] [PubMed] [Google Scholar]
- 25. Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244-1250. [DOI] [PubMed] [Google Scholar]
- 26. Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34(5):784-791. [DOI] [PubMed] [Google Scholar]
- 27. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33(9):1971-1977. [DOI] [PubMed] [Google Scholar]
- 28. Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a Mayo Clinic case series. Mayo Clin Proc. 2018;93(8):1018-1025. [DOI] [PubMed] [Google Scholar]
- 29. Kuo JH, Lee JA. The adoption of ultrasound-guided radiofrequency ablation of thyroid nodules in the United States. Ann Surg. 2021;273(1):e10-e12. [DOI] [PubMed] [Google Scholar]
- 30. Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab. 2005;19(1):1-15. [DOI] [PubMed] [Google Scholar]
- 31. Kim JH, Baek JH, Lim HK, et al. ; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology . 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee J, Shin JH, Hahn SY, Park KW, Choi JS. Feasibility of adjustable electrodes for radiofrequency ablation of benign thyroid nodules. Korean J Radiol. 2020;21(3):377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611-618. [DOI] [PubMed] [Google Scholar]
- 35. Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271(3):e21-e93. [DOI] [PubMed] [Google Scholar]
- 36. Al-Qurayshi Z, Robins R, Hauch A, Randolph GW, Kandil E. Association of surgeon volume with outcomes and cost savings following thyroidectomy: a national forecast. JAMA Otolaryngol Head Neck Surg. 2016;142(1):32-39. [DOI] [PubMed] [Google Scholar]
- 37. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 38. Meltzer C, Hull M, Sundang A, Adams JL. Association between annual surgeon total thyroidectomy volume and transient and permanent complications. JAMA Otolaryngol Head Neck Surg. 2019;145(9):830-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sosa JA, Mehta PJ, Wang TS, Boudourakis L, Roman SA. A population-based study of outcomes from thyroidectomy in aging Americans: at what cost? J Am Coll Surg. 2008;206(6):1097-1105. [DOI] [PubMed] [Google Scholar]
- 40. Gourin CG, Tufano RP, Forastiere AA, Koch WM, Pawlik TM, Bristow RE. Volume-based trends in thyroid surgery. Arch Otolaryngol Head Neck Surg. 2010;136(12):1191-1198. [DOI] [PubMed] [Google Scholar]
- 41. Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. 2013;123(8):2056-2063. [DOI] [PubMed] [Google Scholar]
- 42. Minuto MN, Reina S, Monti E, Ansaldo GL, Varaldo E. Morbidity following thyroid surgery: acceptable rates and how to manage complicated patients. J Endocrinol Invest. 2019;42(11):1291-1297. [DOI] [PubMed] [Google Scholar]
- 43. Bin Saleem R, Bin Saleem M, Bin Saleem N. Impact of completion thyroidectomy timing on post-operative complications: a systematic review and meta-analysis. Gland Surg. 2018;7(5):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kazaure HS, et al. Severe hypocalcemia after thyroidectomy: an analysis of 7366 patients. Ann Surg. Published online December 5, 2019. doi:10.1097/SLA.0000000000003725 [DOI] [PubMed] [Google Scholar]
- 45. Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393(5):667-673. [DOI] [PubMed] [Google Scholar]
- 46. Xie QP, Xiang C, Wang Y, et al. The patterns and treatment of postoperative hemorrhage and hematoma in total endoscopic thyroidectomy via breast approach: experience of 1932 cases. Endocrine. 2019;63(3):422-429. [DOI] [PubMed] [Google Scholar]
- 47. Khan AA, Koch CA, Van Uum S, et al. Standards of care for hypoparathyroidism in adults: a Canadian and International Consensus. Eur J Endocrinol. 2019;180(3):P1-P22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arora A, Swords C, Garas G, et al. The perception of scar cosmesis following thyroid and parathyroid surgery: a prospective cohort study. Int J Surg. 2016;25:38-43. [DOI] [PubMed] [Google Scholar]
- 49. Chaung K, Duke WS, Oh SJ, et al. Aesthetics in thyroid surgery: the patient perspective. Otolaryngol Head Neck Surg. 2017;157(3):409-415. [DOI] [PubMed] [Google Scholar]
- 50. Sonmez B, Erem C, Dogan I, Ersoz HO, Sonmez M. Efficacy of low and high fixed dose radioactive iodine therapy in patients with toxic nodular goiter. Minerva Endocrinol. 2011;36(2):117-121. [PubMed] [Google Scholar]
- 51. Nygaard B, Faber J, Veje A, Hegedüs L, Hansen JM. Transition of nodular toxic goiter to autoimmune hyperthyroidism triggered by 131I therapy. Thyroid. 1999;9(5):477-481. [DOI] [PubMed] [Google Scholar]
- 52. Kitahara CM, Berrington de Gonzalez A, Bouville A, et al. Association of radioactive iodine treatment with cancer mortality in patients with hyperthyroidism. JAMA Intern Med. 2019;179(8):1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab. 2017;61(2):173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015;25(8):890-896. [DOI] [PubMed] [Google Scholar]
- 55. Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turtulici G, Orlandi D, Corazza A, et al. Percutaneous radiofrequency ablation of benign thyroid nodules assisted by a virtual needle tracking system. Ultrasound Med Biol. 2014;40(7):1447-1452. [DOI] [PubMed] [Google Scholar]
- 57. Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol. 2019;180(1):79-87. [DOI] [PubMed] [Google Scholar]
- 58. Ha EJ, Baek JH, Lee JH. The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2011;18(5):310-314. [DOI] [PubMed] [Google Scholar]
- 59. Familiar Casado C, Merino Menendez S, Ganado Diaz T, et al. Single-session treatment of benign thyroid nodules with radiofrequency ablation: Results at 6 months in 24 patients. Endocrinol Diabetes Nutr (Engl Ed). 2020;67(3):164-171. [DOI] [PubMed] [Google Scholar]
- 60. Jawad S, Morley S, Otero S, Beale T, Bandula S. Ultrasound-guided radiofrequency ablation (RFA) of benign symptomatic thyroid nodules - initial UK experience. Br J Radiol. 2019;92(1098):20190026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vuong NL, Dinh LQ, Bang HT, Thuy TTM, Bac NH, Vy TT. Radiofrequency ablation for benign thyroid nodules: 1-year follow-up in 184 patients. World J Surg. 2019;43(10):2447-2453. [DOI] [PubMed] [Google Scholar]
- 62. Rabuffi P, Spada A, Bosco D, et al. Treatment of thyroid nodules with radiofrequency: a 1-year follow-up experience. J Ultrasound. 2019;22(2):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dobnig H, Amrein K. Monopolar radiofrequency ablation of thyroid nodules: a prospective Austrian single-center study. Thyroid. 2018;28(4):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cappelli C, Franco F, Pirola I, et al. Radiofrequency ablation of functioning and non-functioning thyroid nodules: a single institution 12-month survey. J Endocrinol Invest. 2020;43(4):477-482. [DOI] [PubMed] [Google Scholar]
- 65. Doros A, Reismann P, Huszty G, et al. Treatment of benign thyroid nodules by radiofrequency thermal ablation. Orv Hetil. 2020;161(27):1131-1136. [DOI] [PubMed] [Google Scholar]
- 66. Aldea Martínez J, Aldea Viana L, López Martínez JL, Ruiz Pérez E. Radiofrequency ablation of thyroid nodules: a long-term prospective study of 24 patients. J Vasc Interv Radiol. 2019;30(10):1567-1573. [DOI] [PubMed] [Google Scholar]
- 67. Baek JH, Lee JH, Sung JY, et al. ; Korean Society of Thyroid Radiology . Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335-342. [DOI] [PubMed] [Google Scholar]
- 68. Kim C, Lee JH, Choi YJ, Kim WB, Sung TY, Baek JH. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128-3137. [DOI] [PubMed] [Google Scholar]
- 69. Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920-930. [DOI] [PubMed] [Google Scholar]
- 70. Muhammad H, Santhanam P, Russell JO, Kuo JH. RFA and benign thyroid nodules: review of the current literature. Laryngoscope Investig Otolaryngol. 2021;6(1):155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cui D, Ding M, Tang X, et al. Efficacy and safety of a combination of hydrodissection and radiofrequency ablation therapy for benign thyroid nodules larger than 2 cm: a retrospective study. J Cancer Res Ther. 2019;15(2):386-393. [DOI] [PubMed] [Google Scholar]
- 72. Hong MJ, Sung JY, Baek JH, et al. Safety and efficacy of radiofrequency ablation for nonfunctioning benign thyroid nodules in children and adolescents in 14 patients over a 10-year period. J Vasc Interv Radiol. 2019;30(6):900-906. [DOI] [PubMed] [Google Scholar]
- 73. Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460-466. [DOI] [PubMed] [Google Scholar]
- 74. Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency ablation for the management of thyroid nodules: a critical appraisal of the literature. Clin Endocrinol (Oxf). 2017;87(6):639-648. [DOI] [PubMed] [Google Scholar]
- 75. Guang Y, He W, Luo Y, et al. Patient satisfaction of radiofrequency ablation for symptomatic benign solid thyroid nodules: our experience for 2-year follow up. BMC Cancer. 2019;19(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25(1):112-117. [DOI] [PubMed] [Google Scholar]
- 77. Faggiano A, Ramundo V, Assanti AP, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab. 2012;97(12):4439-4445. [DOI] [PubMed] [Google Scholar]
- 78. Cesareo R, Palermo A, Benvenuto D, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20(1):37-44. [DOI] [PubMed] [Google Scholar]
- 79. de Boer H, Bom W, Veendrick P, Bom E, van Borren M, Joosten F. Hyperactive thyroid nodules treated by radiofrequency ablation: a Dutch single-centre experience. Neth J Med. 2020;78(2):64-70. [PubMed] [Google Scholar]
- 80. Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015;18(4):423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dobnig H, Zechmann W, Hermann M, et al. Radiofrequency ablation of thyroid nodules: “Good Clinical Practice Recommendations” for Austria: an interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr. 2020;170(1-2):6-14. [DOI] [PubMed] [Google Scholar]
- 83. National Institute for Health and Care Excellence (NICE) . Ultrasound-guided percutaneous radiofrequency ablation for benign thyroid nodules. Interventional procedures guidance [IPG562]. June 22, 2016.. Accessed February 2, 2021. https://www.nice.org.uk/guidance/ipg562
- 84. Ha EJ, Baek JH, Che Y, et al. Radiofrequency ablation of benign thyroid nodules: recommendations from the Asian Conference on Tumor Ablation Task Force. Ultrasonography. 2021;40(1):75-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gharib H, Papini E, Garber JR, et al. ; AACE/ACE/AME Task Force on Thyroid Nodules . American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(5):622-639. [DOI] [PubMed] [Google Scholar]
- 86.MDsave Incorporated. Thyroidectomy. How much does a thyroidectomy cost? Accessed February 2, 2021. https://www.mdsave.com/procedures/thyroidectomy/d78bfdca
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.