Abstract
Background:
Mosquitos due to their role in the transmission of different pathogens to humans are considered as an important group in the phylum Arthropoda. According to the WHO and FAO guideline different groups of insecticide applied for controlling pests in both the agricultural and public health sectors.
Methods:
All the data published about resistant status of the mosquitoes Anopheles, Culex, Aedes and Culiseta species were searched on PubMed, Elsevier, Web of Science, Magiran and google scholar. The objectives of this study was to review the trend of resistance to insecticides during 2000–2020 in medically important mosquitoes in Iran. The criteria for resistant are followed according to WHO guideline.
Results:
The Results showed that there are widespread, multiple resistances in the country to different organochlorine, organophosphates, carbamate and pyrethroids insecticides in the mosquitoes.
Conclusion:
The effect of pesticide residues on the environment could be a cause for selection pressure on mosquitos and lead to insecticides resistance to them. Insecticides resistance is main challenge of the vector control program. Also result will provide a guideline for control of the mosquito-borne diseases in the country as well as the world.
Keywords: Resistance, Insecticide, Mosquito, Iran
Introduction
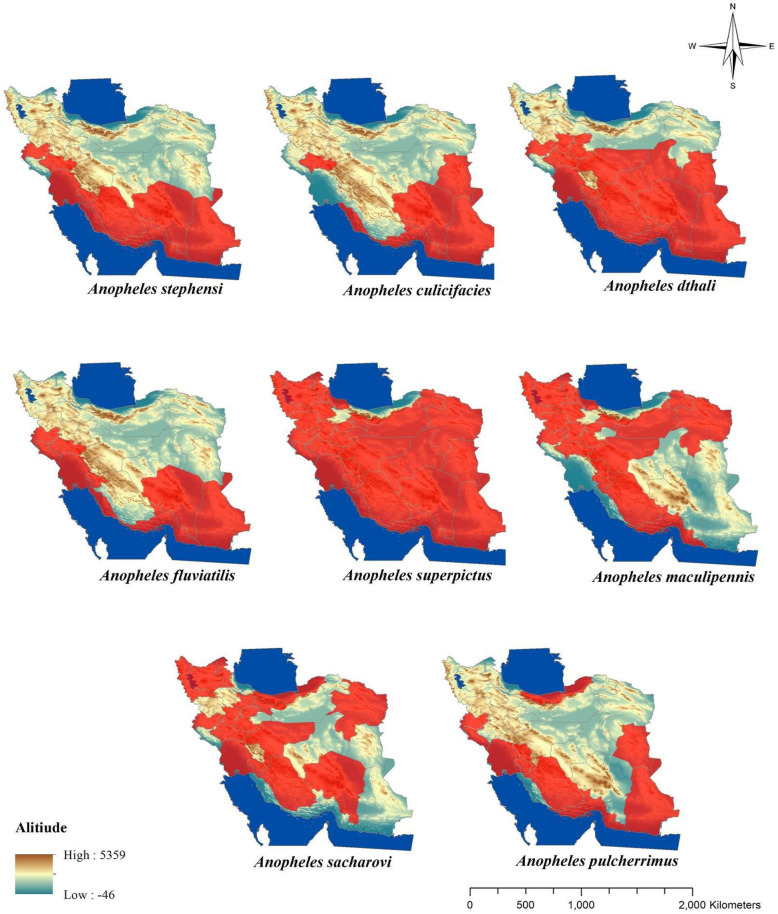
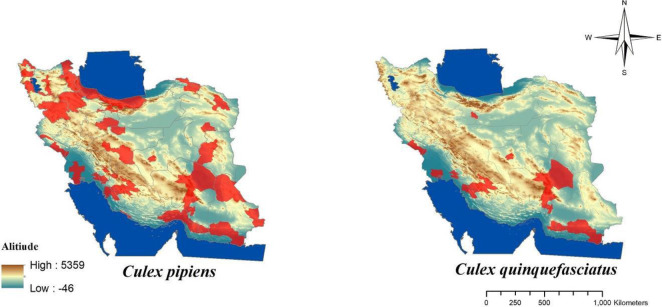
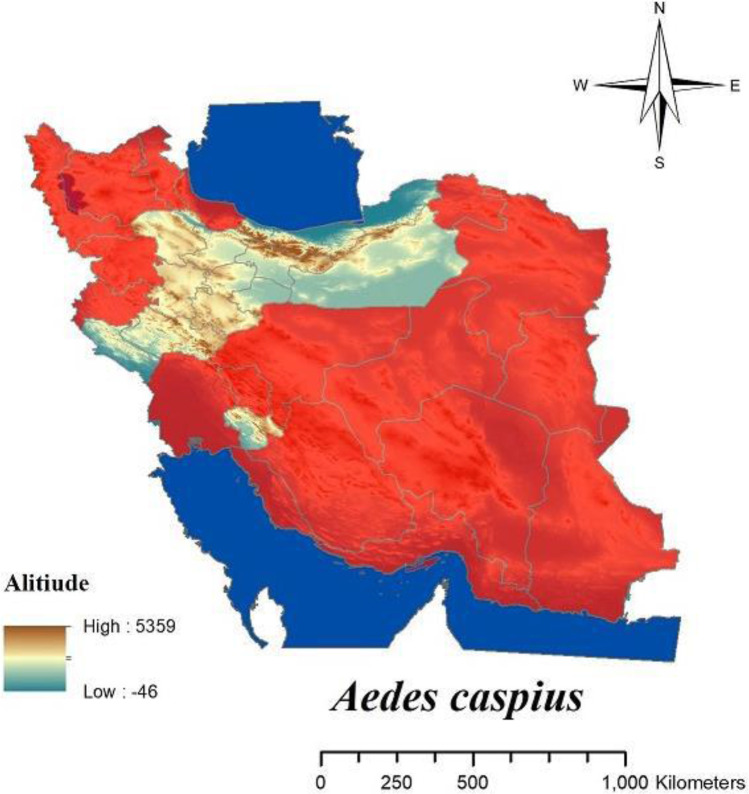
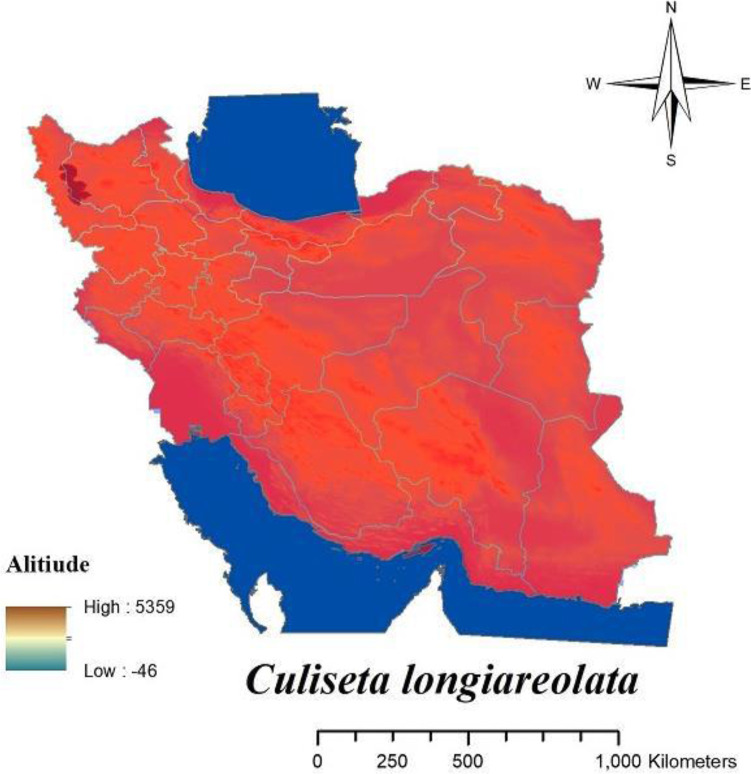
Malaria is one of the most important parasitic diseases transmitted by the genus Anopheles to human (1–3). In Iran malaria has been present for a long time ago (4, 5) and mainly mentioned as a big public health challenge in Sistan and Baluchistan, Hormozgan and Kerman Provinces in the south-eastern part of Iran also featured as refractory malaria (6). The favorable results obtained in reducing of malaria transmissions by application of DDT for controlling of mosquito in a hyperendemic area in Iran for the first time in 1947 and then spraying with DDT in combination with other vectors control interventions continued in the most malaria-endemic areas of the country until 1956, finally for first time in 1957 resistance to DDT was recorded in Anopheles stephensi as vector of malaria in Iran (4, 5). In Iran there are some species of malaria vectors (Fig. 1) including: An. stephensi, An. dthali, An. culicifacies s.l., An. fluviatilis s.l., An. superpictus s.l., An. sacharovi, An. maculipennis s.l. (3, 5). Culex pipiens pipiens and Culex. quinquefasciatus as complex members of Cx. pipiens are competent vectors for some filarial and arboviral disease and also Dirofilaria immitis moreover West Nile and Sindbis viruses have been detected from mentioned species in Iran (1, 7– 9). Culex pipiens habitat mainly is sewage system and there are different reagents and also residues of insecticides belong to several group of pesticides which have been previously used in agriculture and public health sectors (9, 10). For the first time resistance to insecticides was shown in 1975 in Cx. pipiens pipiens about DDT in the northern part of Iran (9, 11). Fig. 2 shows the distribution of Cx. pipiens and Cx. quinquefasciatus in Iran. After 20 years later from the first report of resistance to DDT in An. stephensi, this report was the second alarm related to resistance in medically important mosquitos in Iran. The presence of West Nile virus also has been reported in Aedes caspius or Ochlerotatus caspius from Iranian wetlands during recent years (12). Even though to date there is no report about the detection of pathogens among Culiseta longiareolata in Iran but, Ae. caspius (13) and Cs. longiaerolata (14) currently showed their resistance to DDT. Figs. 3 and 4 shows the distribution of Ae. caspius and Cs. longiareolata in Iran. Continuation of the previous report related to the appearance of insecticide resistance about DDT in An. stephensi and Cx. pipiens as vectors of important diseases in Iran. According to the latest studies only during 2012 to 2014 approximately 14,000 tons of pesticides consist of herbicides, insecticides, acaricides, and fungicides were used for agricultural pests (15). Due to the effect of pesticide residues on the environment it could be a cause for selection pressure on mosquitos which their breeding places are water and finally lead to their resistance to different group of insecticides indirectly (9 , 10, 16). In the public health sector also different groups of insecticide applied for controlling Anopheles mosquitoes in malarious areas of Iran such as DDT and Dieldrin belong to organochlorine compounds, Malathion and Pirimiphosmethyl (organophosphates), Propoxur (carbamates), Deltamethrin and Lamdacyhalothrin belong to pyrethroids compounds (4, 5). Previously for testing the insecticide susceptibility level among adult of mosquitos all bioassay studies have been performed referring to the test procedures of World Health Organization (WHO) recommended for insecticide resistance monitoring in mosquitos that suggested for each concentrations, six replicate samples of 20–25 adult female mosquitoes per tube (2 replicates as control) shroud be expose for one hour at diagnostic dose of each insecticide and the number of mortality determined 24 hours after recovery period, finally the mortality results divided in three categories include: 98–100% mortality indicates susceptibility, 80–97% mortality considered as tolerance and also requires confirmation of resistance with other methods and if mortality was less than 80% in tested samples, mosquitos considered as resistant to insecticide (17). Previous guideline was revised recently and considering the current WHO categories for susceptibility level, the following criteria have been used for interpretation of results related to mortality rate: higher than 98% was considered as susceptible, mortality between 90% to 97% considered as resistance candidate and more investigation is needed for the confirmation of resistance and finally mortality less than 90% demonstrated resistance (18). Here there are examples regarding resistance to DDT lonely in medically important mosquitos and its trend from the beginning of the resistance to insecticides. In the present paper, we aimed to review the trend of resistance to insecticides during 2000–2020 in medically important mosquitoes and also the distribution of resistant specimen plotted using Arc-GIS10.2 software (Redlands, CA) In Iran.
Fig. 1.
Distribution of malaria vectors in Iran
Fig. 2.
Distribution of Culex pipiens and Culex quiquefasciatus in Iran
Fig. 3.
Distribution of Aedes caspius in Iran
Fig. 4.
Distribution of Culiseta longiareolata in Iran
Materials and Methods
All the data published about resistant status of mosquitoes Anopheles, Culex, Aedes and Culiseta species were searched during 2000– 2020 on PubMed, Elsevier, Web of Science, Magiran and google scholar.
Results
Review of resistance to insecticides in Anopheles mosquitoes as malaria vectors
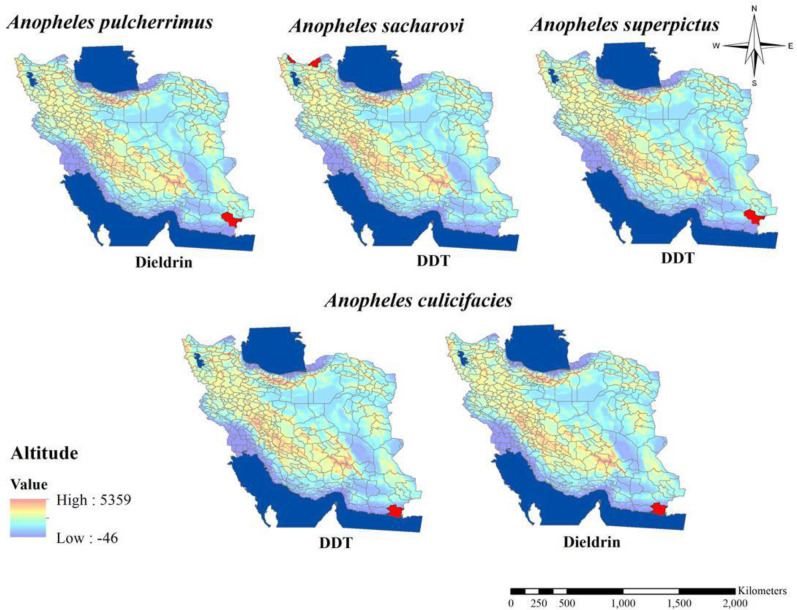
According to recent studies, seven species of Anopheles mosquitoes (Fig. 1) introduced as malaria vectors in Iran including: An. stephensi, An. culicifacies s.l., An. fluviatilis s.l., An. superpictus s.l., An. dthali, An. sacharovi and An. maculipennis s.l. while An. pulcherrimus is considered as a suspect vector moreover five of these vectors can be found in the southeast of the country, where the majority of malaria cases is reported also An. stephensi considered to be the main malaria vector in the same area (3, 19). The resistant status of Anopheles mosquitos to organochlorine compounds in Iran (Table 1 and Figs. 5–7) indicated that approximately all malaria vectors in Iran were resistant to the DDT and Dieldrin which have been used previously for control of mosquitoes (6, 13, 20–37). After the first report about resistance to DDT in An. stephensi in 1957 other malaria vectors gradually, showed their resistance to organochlorine compounds in Iran up to now. But about An. sacharovi and An. maculipennis s.l. as main malaria vectors in the northern part of the country, all studies which have been performed on the susceptibility level of these species to organochlorine compounds showed tolerance to dieldrin (21, 23, 30, 33). Similar to this finding about Dieldrin, tolerance to DDT in An. dthali in south eastern part of Iran also has been shown (13). Although in some districts in southern parts of the country in Jiroft District in Kerman Province An. stephensi was tolerant to DDT and dieldrin (27). Moreover, in Bashagard District in Hormozgan Province tolerant to dieldrin in An. stephensi as well as tolerant to DDT in An. culicifacies s.l. also have been shown (28, 29).
Table 1.
List of Anopheles mosquitos resistant to organochlorine compounds in Iran using the WHO insecticide susceptibility tests (2000–2020)
| Species | Insecticides | Location (Province-District) | References |
|---|---|---|---|
| An. pulcherrimus | Dieldrin | Sistan and Baluchistan-Ghasreghand | 20 |
| An. sacharovi | DDT | West Azerbaijan-Poldasht | 21 |
| An. stephensi | DDT | Sistan and Baluchistan-Iranshahr | 22 |
| An. Sacharovi | DDT | East Azerbaijan-Kalibar | 23,30 |
| An. stephensi | DDT, Dieldrin | Kerman-Kahnooj | 24 |
| An. stephensi | DDT | Hormozgan-Siahoo, Geno and Bandar Abbas Hormozgan-Bandar Abbas | 25 |
| An. stephensi | DDT, Dieldrin | Sistan and Baluchistan-Iranshahr Fars-Kazeroon | 26 |
| An. maculipennis s.l. | DDT, Dieldrin | Gilan-Astara | 6 |
| An. stephensi | DDT | Hormozgan-Bashagard | 28,29 |
| An. stephensi | DDT | Sistan and Baluchistan-Chabahar | 13, 31, 34, 37 |
| An. superpictus s.l. | DDT | Sistan and Baluchistan-Sarbaz | 32 |
| An. stephensi | DDT | Hormozgan-Jask | 35 |
| An. culicifacies s.l. | DDT, Dieldrin | Sistan and Baluchistan-Chabahar | 36 |
Fig. 5.
Distribution of Anopheles mosquitos with resistance to organochlorine compounds in Iran (2000–2020)
Fig. 7.
Distribution of Anopheles maculipennis with resistance to different insecticides in Iran (2000–2020)
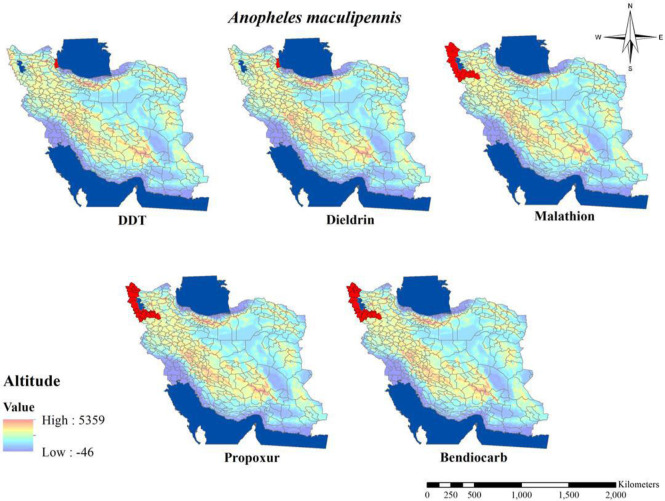
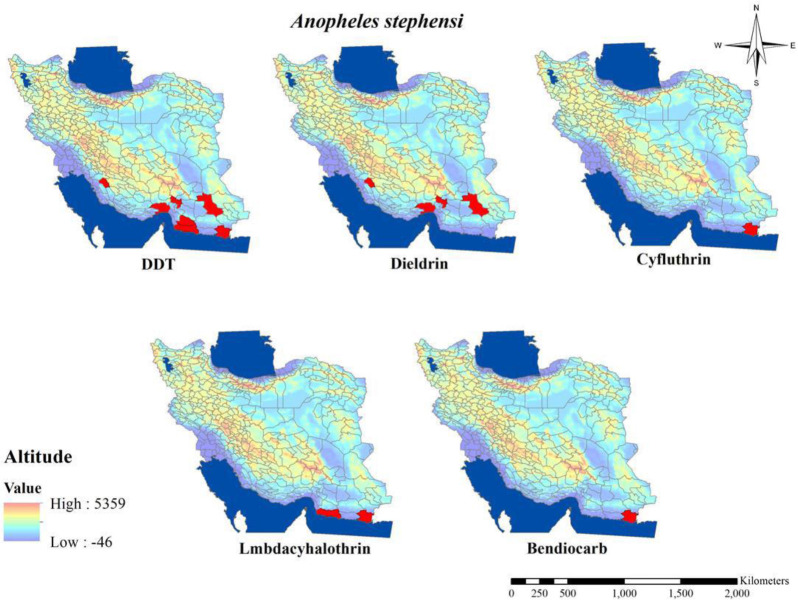
The susceptibility level of Anopheles mosquitos about pyrethroids compounds (Table 2 and Fig. 6) showed that, to date among malaria vectors in Iran, just in An. stephensi resistant to Cyfluthrin, and Lmbdacyhalothrin has been shown in Chabahar District in southeastern part of the country (31, 34, 35). Although tolerant to others pyrethroids insecticides such as deltamethrin, permethrin and etofenprox also have been reported in An. stephensi in this area (31, 34). Tolerant to deltamethrin also shown in An. stephensi, An. culicifacies s.l. and An. dthali in Bashagard District and An. stephensi in Jask District in Hormozgan Province (28, 35) and also in An. culicifacies s.l. in Chabahar District in Sistan and Baluchistan Province (36). Similar to mentioned finding about tolerant to pyrethroids insecticides in Anopheles mosquitos, tolerant to deltamethrin and permethrin also has been shown in An. maculipennis in the northern part of Iran (33).
Table 2.
List of Anopheles mosquitos resistant to pyrethroids compounds in Iran using WHO insecticide susceptibility tests (2000–2020)
Fig. 6.
Distribution of Anopheles stephensi with resistance to different insecticides in Iran (2000–2020)
The susceptibility level of Anopheles mosquitos to organophosphates compounds (Table 3 and Fig. 7) indicated that An. maculipennis s.l. in West Azarbaijan Province in the Northwestern part of the country has become resistant to Malathion (33) but about other Anopheles mosquitos tolerant to Malathion in southern part also in An. dthali in Hormozgan Province (28) and also in An. stephensi and An. culicifacies s.l. in Sistan and Baluchistan Province were shown (13).
Table 3.
List of Anopheles mosquitos resistant to organophosphates compound’s in Iran using WHO insecticide susceptibility tests (2000–2020)
| Species | Insecticides | Location (Province-District) | References |
|---|---|---|---|
| An. maculipennis s.l. | Malathion | West Azerbaijan | 33 |
The status of Anopheles mosquito resistant to carbamates compound’s (Table 4 and Figs. 6, 7) indicated that among all malaria vectors around the country resistance to carbamates insecticides such as propoxur and bendiocarb is shown in An. maculipennis s.l. in Northwestern part and resistance to bendiocarb in An. stephensi is in the southern part of the country (33, 37). Although in An. stephensi, An. culicifacies s.l. and An. dthali tolerant to propoxur in some parts of the country has been reported (13, 22, 28). By reviewing the resistance status of Anopheles mosquitos during 2000 to 2020 it is concluded that approximately all Anopheles mosquitos found resistance to organochlorine insecticides in Iran and although about other classes of insecticides there are a few reports for resistance about some malaria vectors to one or more insecticides, but considering the modification of the guideline for susceptibility test in mosquitos From 2013 which demonstrated resistance in mosquitos with mortality less than 90%, susceptible species in previous studies can be categories as resistant to insecticides (36).
Table 4.
List of Anopheles mosquitos resistant to Carbamates compound’s in Iran using WHO insecticide susceptibility tests (2000–2020)
Review of resistance to insecticides in Culex mosquitos
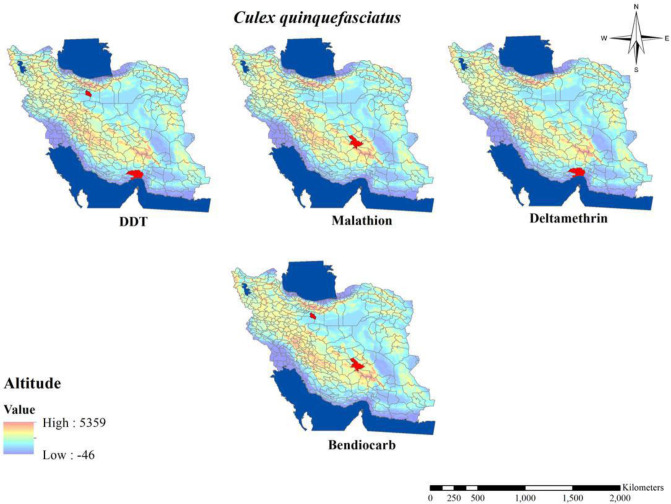
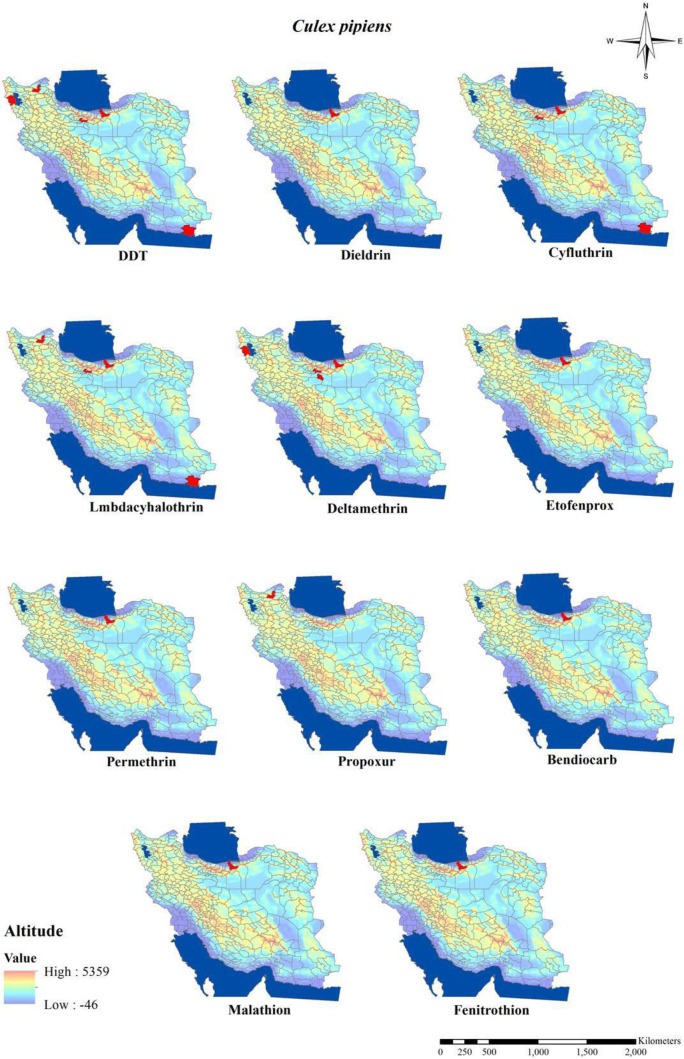
The susceptibility level of Culex mosquitos against organochlorine compounds in Iran (Table 5 and Figs. 8–10) revealed that Cx. pipiens, Cx. quinquefasciatus and Cx. theileri in most area of the country showed their highly level of insecticide resistance to DDT and deildrin (9 , 13, 14, 16, 38, 39, 41, 42). Although, Cx. quinquefasciatus in a study in Southeast area showed its 90% mortality rate to DDT and according to the WHO considered as tolerant or candidate for resistance (40), similar Anopheles species, resistance to DDT in Culex mosquitos around the country have been developed during recent years after the first report for resistance to DDT in the northern part of Iran about Cx. pipiens pipiens in 1975 (9, 11).
Table 5.
List of Culex mosquitos resistant to organochlorine compounds in Iran using WHO insecticide susceptibility tests (2000–2020)
| Species | Insecticides | Location (Province-District) | References |
|---|---|---|---|
| Cx .theileri, Cx. pipiens | DDT | Tehran-Tehran | 38 |
| Cx. quinquefasciatus | DDT | Tehran-Varamin | 16 |
| Cx. pipiens | DDT | Sistan and Baluchistan-Chabahar | 13 |
| Cx. theileri and Cx. pipiens | DDT | East Azarbaijan-Ahar | 14 |
| Cx. pipiens | DDT | West Azerbaijan-Urmia | 39 |
| Cx. pipiens | DDT | Tehran-Tehran | 9 |
| Cx. pipiens | DDT, Dieldrin | Mazandaran-Sari | 41 |
| Cx. quinquefasciatus | DDT | Hormozgan-Suru | 42 |
Fig. 8.
Distribution of Culex quinquefasciatus with resistance to different insecticides in Iran (2000–2020)
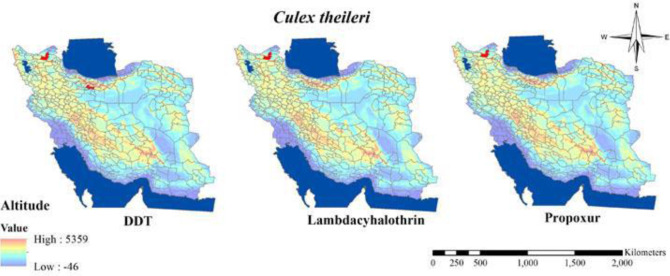
Fig. 10.
Distribution of Culex theileri with resistance to different insecticides in Iran (2000–2020)
The status of Culex mosquitoes resistant to pyrethroids compound’s (Table 6 and Figs. 8–10) showed that they are almost resistant to most insecticides belong to pyrethroids (9, 13, 14, 39, 41–43). For example, in the north of Iran, Cx. pipiens in Sari District was highly resistance to all tested pyrethroids insecticides including Cyfluthrin, Lambda-cyhalothrin, Deltamethrin, Etofenprox and Permethrin (41).
Table 6.
List of Culex mosquitos resistant to pyrethroids compound’s in Iran using WHO insecticide susceptibility tests (2000–2020)
| Species | Insecticides | Location (Province-District) | References |
|---|---|---|---|
| Cx. pipiens | Cyfluthrin and Lambdacyhalothrin | Sistan and Baluchistan-Chabahar | 13 |
| Cx. theileri, Cx. pipiens | Lambdacyhalothrin | East Azarbaijan-Ahar | 14 |
| Cx. pipiens | Deltamethrin | West Azerbaijan-Urmia | 39 |
| Cx. pipiens | Cyfluthrin, Lambdacyhalothrin, Deltamethrin | Tehran-Tehran | 9 |
| Cx. pipiens | Cyfluthrin, Lambdacyhalothrin, Deltamethrin, Etofenprox, Permethrin | Mazandaran-Sari | 41 |
| Cx. quinquefasciatus | Deltamethrin | Hormozgan-Suru | 42 |
| Cx. pipiens complex | Deltamethrin | Tehran-Gharchak | 43 |
Resistance to organophosphates compounds in Culex mosquitos (Table 7 and Figs. 8, 9) indicated that members of Cx. pipiens complex were found resistant to Malathion and Fenitrothion (40, 41) although in Ahar District in East Azarbaijan tolerance to malathion in Cx. pipiens and Cx. theileri have been reported (14).
Table 7.
List of Culex mosquitos resistant to organophosphates compound’s in Iran using WHO insecticide susceptibility tests (2000–2020)
Fig. 9.
Distribution of Culex pipiens with resistance to different insecticides in Iran (2000–2020)
The susceptibility level of Culex mosquitos to carbamates compounds (Table 8 and Figs. 8–10) revealed that Cx. quinquefasciatus, Cx. theileri and Cx. pipiens were found to be resistant to all tested insecticides belong to carbamates compounds (14, 16, 40, 41).
Table 8.
List of Culex mosquitos resistant to carbamates compounds in Iran using WHO insecticide tests (2000–2020)
Comparing the resistance status of Culex mosquitos with Anopheles mosquitos during the past 20 years ago revealed that Culex species almost were found resistant to most insecticides belong to pyrethroids and also about other classes of insecticides the number of species which showed resistance to insecticides are more than Anopheles mosquitos.
Review of resistance to insecticides in Aedes caspius
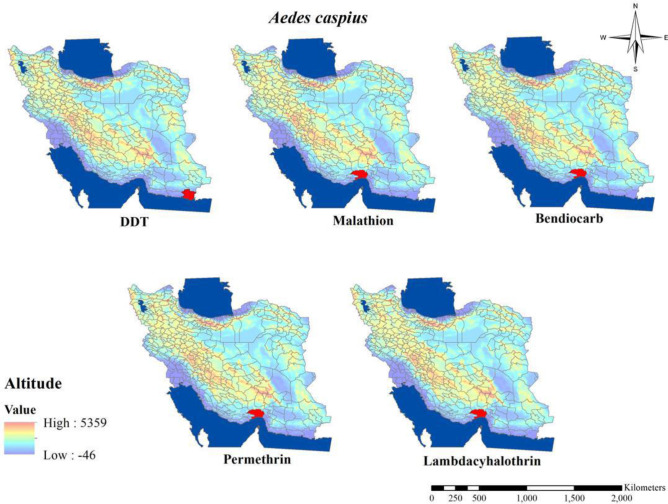
The resistance status of Ae. caspius to different groups of insecticides in Iran (Table 9 and Fig. 11) showed that Ae. caspius is resistant to DDT, Bendiocarb, Malathion, Permethrin and Lambdacyhalothrin (13, 44). Actually this species was found to be resistant to all classes of insecticides although in some part this species was susceptible to deltamethrin (44).
Table 9.
Resistance status of Aedes caspius to different groups of insecticides in Iran using WHO insecticide susceptibility tests (2000–2020)
Fig. 11.
Distribution of Aedes caspius with resistance to different insecticides in Iran (2000–2020)
Review of resistance to insecticides in Culiseta longiareolata
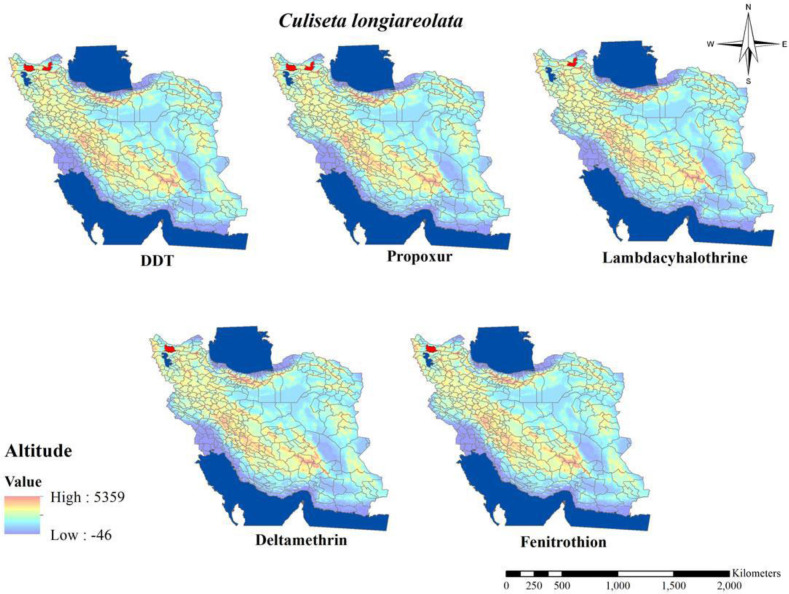
The susceptibility level of Cs. longiareolata to different groups of insecticides (Table 10 and Fig. 12) indicated that this species similar Ae. caspius was resistant to all classes of insecticides (14, 45). There are a few studies about the susceptibility level of Cs. longiareolata and Ae. caspius in Iran, but both of them were found to be resistant to all groups of insecticides. In the following resistance to insecticides in medically important mosquitos in Iran resistance to insecticides in mentioned species also can be considered as a problem in the vector control program.
Table 10.
Resistance status of Culiseta longiareolata to different groups of insecticides in Iran using WHO insecticide susceptibility tests (2000–2020)
Fig. 12.
Distribution of Culiseta longiaerolata with resistance to different insecticides in Iran (2000–2020)
Discussion
The trend of insecticides resistance in medially important mosquitos in Iran revealed that among specimens of Anopheles mosquitoes as malaria vectors, approximately all of them have been found resistant to one or more insecticides and also most malaria vectors are resistant to the organochlorine compounds in Iran. It cannot conclude certainly that all the malaria vectors are resistant to insecticide because there are no definite report about resistance to insecticides in An. fluviatilis s.l. and An. dthali although there are some reports about tolerant in An. dthali to some insecticides. In Iran, An. stephensi as the main malaria vector has been found resistant to most classes of insecticides during recent years and this resistance may be caused by others intervention for controlling of malaria vectors such as Indoor Residual Spraying (IRS) and Insecticide Treated Nets (ITNs) or usage of insecticide in the agriculture sector (34). Resistance to all classes of insecticides also reported in other countries in An. stephensi for example in Afghanistan, An. stephensi has been found resistant to DDT, malathion, bendiocarb, deltamethrin, and permethrin and in Ethiopia, it was highly resistant to deltamethrin, permethrin pirimiphosmethyl, malathion, DDT, propoxur, and bendiocarb (46, 47). Similar finding of Anopheles mosquitos, Culex specimens also were resistant to several insecticides belong to each class moreover Cx. pipiens in the north of Iran, in Mazandaran Province was high resistance to all tested insecticides of all major classes (41) around the world some studies also reported a high level of resistance in members of Cx. pipiens complex to many groups of insecticides (48–50). Based on the literature, there were no reports available on monitoring the susceptibility level of Ae. caspius and Cs. longiaerolata to insecticides which recommended by WHO around the world. Recent studies about baseline susceptibility of mentioned species in Iran revealed that both of these mentioned species were found to be resistant to all classes of insecticides in the study areas of Ae. caspius and Cs. longiaerolata (13, 14, 44 , 45). During recent years the development of resistance to insecticides in mosquitos as vectors of important diseases in Iran were increased. So that almost all medially important mosquitos were found to be resistant to all different classes of insecticides. The use of alternative insecticide which is made from natural products and some biological agents can be appropriates method for vectors control programs (45, 51, 52). Secondary metabolites of plants such as essential oils are candidates for the discovery of new compounds against vector mosquitoes. Insecticide-based plants have the advantage of exhibiting novel modes of action against mosquito vectors that could lessen the risk of resistance (45, 52–61). Moreover, for controlling of mosquito populations, Wolbachia as an intracellular organism that infect different groups of arthropods, also introduced as a bioagent due to its environmentally friendly feature (62, 63).
Conclusion
Relevant studies about resistance to insecticides during the quarter of a century about medially important mosquitos in Iran indicated that the development of resistance to all classes of insecticides in mosquitos is happening gradually, so alternative and efficient intervention methods should be used to preventing the development of resistance to insecticides in mosquitos.
Acknowledgements
This research is partially supported by Ministry of Health and Medical Education under NIMAD code numbers of 963262, 983984 and 995633. The authors declare that there is no conflict of interest.
References
- 1.Mullen G, Durden L. (2009) Medical and Veterinary Entomology, 2ed edition, Mosquitoes (Culicidae) Woodbridge Foster A., Walter Edward D. Vol. 2. Elsevier, Burlington. [Google Scholar]
- 2.Service MW. (2003) Medical Entomology for Students. Vol. 3. United Kingdom: Cambridge. University Press, Cambridge. [Google Scholar]
- 3.Vatandoost H, Raeisi A, Saghafipour A, Nikpour F, Nejati J. (2019) Malaria situation in Iran: 2002–2017. Malar J. 18 (1): 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edrissian GH. (2006) Malaria past and present situation in Iran. Iran J Parasitol. 1 (1): 1–14. [Google Scholar]
- 5.Yousef Mogaddam M, Motevalli-Haghi F, Fazeli-Dinan M, Hosseini-Vasoukolaei N, Enayati AA. (2016) A review of insecticide resistance in malaria vectors of Iran. J Mazandaran Univ Med Sci. 25 (134): 394–411. [Google Scholar]
- 6.Vatandoost H, Zahirnia AH. (2010) Responsiveness of Anopheles maculipennis to different imagicides during resurgent malaria. Asian Pacific J Trop Med. 3: 360–363. [Google Scholar]
- 7.Naficy K, Saidi S. (1970) Serological survey on viral antibodies in Iran. Trop Geogr Med. 22: 183–188. [PubMed] [Google Scholar]
- 8.Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE. (2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 23(2): 111–21. [DOI] [PubMed] [Google Scholar]
- 9.Salim-Abadi Y, Oshaghi MA, Enayati AA, Abai MR, Vatandoost H, Eshraghian MR. (2016) High insecticides resistance in Culex pipiens (Diptera: Culicidae) from Tehran, capital of Iran. J Arthropod Borne Dis. 10(4): 483–492. [PMC free article] [PubMed] [Google Scholar]
- 10.Nikookar SH, Fazeli-Dinan M, Ziapour SP, Ghorbani F, Salim-Abadi Y, Vatan-doost H, Enayati AA. (2019) First report of biochemical mechanisms of insecticide resistance in the field population of Culex pipiens (Diptera: Culicidae) from Sari, Mazandaran, north of Iran. J Arthropod Borne Dis. 13(4): 378–390. [PMC free article] [PubMed] [Google Scholar]
- 11.Lotfi MD, Manouchehri AV, Yazdanpanah H. (1975) Resistance of Cx. pipiens pipiens to DDT in northern Iran, 1973. Bull Soc Pathol Exot Filiales. 68(1): 91–93. [PubMed] [Google Scholar]
- 12.Bagheri M, Terenius O, Oshaghi MA, Motazakker M, Asgari S, Dabiri F, Vatandoost H, Mohammadi Bavani M, Chavshin AR. (2015) West Nile Virus in mosquitoes of Iranian wetlands. Vector Borne Zoonotic Dis. 15(12): 750–754. [DOI] [PubMed] [Google Scholar]
- 13.Fathian M, Vatandoost H, Moosa-Kazemi SH, Raeisi A. (2015) Susceptibility of Culicidae mosquitoes to some insecticides recommended by WHO in a malaria endemic area of southeastern Iran. J Arthropod Borne Dis. 9(1): 22–34. [PMC free article] [PubMed] [Google Scholar]
- 14.Ataie A, Moosa-Kazemi SH, Vatandoost H, Yaghoobi-Ershadi MR, Bakhshi H, Anjomruz M. (2015) Assessing the susceptibility status of mosquitoes (Diptera: Culicidae) in a dirofilariasis focus, northwestern Iran. J Arthropod Borne Dis. 9 (1): 7–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Morteza Z, Mousavi SB, Baghestani MA, Aitio A. (2017) An assessment of agricultural pesticide use in Iran, 2012–2014. J Environ Health Sci Eng. 15(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vatandoost H, Ezeddinloo L, Mahvi AH, Abai MR, Kia EB, Mobedi I. (2004) Enhanced tolerance of house mosquito to different insecticides due to agricultural and household pesticides in sewage system of Tehran, Iran. Iran J Environ Health Sci Eng. 1(1): 42–45. [Google Scholar]
- 17.World Health Organization (WHO) (1998) Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces: report of the WHO informal consultation, Geneva, 28–30 September 1998. p. 46. [Google Scholar]
- 18.World Health Organization (WHO) (2016) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2nd edition. p. 56. [Google Scholar]
- 19.Gorouhi MA, Oshaghi MA, Vatandoost H, Enayati AA, Raeisi A, Abai MR. (2018) Biochemical basis of cyfluthrin and DDT resistance in Anopheles stephensi (Diptera: Culicidae) in malarious area of Iran. J Arthropod Borne Dis. 12 (3): 310–320. [PMC free article] [PubMed] [Google Scholar]
- 20.Zahirnia A, Vatandoost H, Nateghpour M, Djavadian E. (2002) Insecticide resistance/ susceptibility monitoring in Anopheles pulcherrimus (Diptera: Culicidae) in Ghasreghand District, Sistan and Baluchistan Province, Iran. Iran J Public Health. 31(1–2): 11–14. [Google Scholar]
- 21.Lak SS, Vatandoost H, Entezarmahdi M, Ashraf H, Abai M, Nazari M. (2002) Monitoring of insecticide resistance in Anopheles sacharovi (Favre, 1903) in borderline of Iran, Armenia, Naxcivan and Turkey, 2001. Iran J Public Health. 31 (3–4): 96–99. [Google Scholar]
- 22.Borhani N, Vatandoost H. (2004) Susceptibility and irritability levels of main malaria vectors to synthetic pyrethroids in the endemic areas of Iran. Acta Med Iran. 42(4): 240–247. [Google Scholar]
- 23.Vatandoost H, Abdoljabari Boonab R, Abai MR, Oshaghi MA. (2005) Entomological survey in Kalibar, a resurgent malaria focus in East-Azerbaijan, Iran. Pak J Biol Sci. 8(10): 1466–1471. [Google Scholar]
- 24.Vatandoost H, Mashayekhi M, Abai MR, Aflatoonian M, Hanafi-Bojd AA, Sharifi I. (2005) Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj District, Kerman Province, southeastern Iran. J Vector Borne Dis. 42(3): 100–108. [PubMed] [Google Scholar]
- 25.Vatandoost H, Oshaghi MA, Abai MR, Shahi M, Yaaghoobi F, Baghaii M, Hanafi-Bojd AA, Zamani G, Townson H. (2006) Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, Southern Iran, 2002. Acta Trop. 97(2): 196–203. [DOI] [PubMed] [Google Scholar]
- 26.Davari B, Vatandoost H, Ladonni H, Shaeghi M, Oshaghi MA, Basseri HR, Enayati AA, Rassi Y, Abai MR, Hanafi-Bojd AA, Akbarzadeh K. (2006) Comparative efficacy of different imagicides against different strains of Anopheles stephensi in the malaroius areas of Iran, 2004–2005. Pakistan J Biol Sci. 9(5): 885–892. [Google Scholar]
- 27.Abai MR, Mehravaran A, Vatandoost H, Oshaghi MA, Javadian E, Mashayekhi M, Mosleminia A, Piyazak N, Edallat H, Mohtarami F, Jabbari H, Rafi F. (2008) Comparative performance of imagicides on Anopheles stephensi, main malaria vector in a malarious area, southern Iran. J Vector Borne Dis. 45(4): 307–312. [PubMed] [Google Scholar]
- 28.Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Sedaghat MM, Abedi F, Yeryan M, Pakari A. (2012) Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 121(2): 85–92. [DOI] [PubMed] [Google Scholar]
- 29.Soleimani-Ahmadi M, Vatandoost H, Shayeghi M, Raeisi A, Abedi F, Eshraghian MR, Madani A, Safari R, Shahi M, Mojahedi A, Poorahmad-Garbandi F. (2012) Vector ecology and susceptibility in a malaria endemic focus in southern Islamic Republic of Iran. East Mediterr Health J. 18: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 30.Vatandoost H, Abai MR. (2012) Irritability of malaria vector, Anopheles sacharovi to different insecticides in a malaria–prone area. Asian Pac J Trop Med. 5 (2): 113–116. [DOI] [PubMed] [Google Scholar]
- 31.Vatandoost H, Hanafi-Bojd AA. (2012) Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac J Trop Med. 5(9): 722–726. [DOI] [PubMed] [Google Scholar]
- 32.Nejati J, Vatandoost H, Oshaghi MA, Salehi M, Mozafari E, Moosa-Kazemi SH. (2013) Some ecological attributes of malarial vector Anopheles superpictus Grassi in endemic foci in Southeastern Iran. Asian Pac J Trop Biomed. 3(12): 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavshin AR, Dabiri F, Vatandoost H, Mohammadi-Bavani MM. (2015) Susceptibility of Anopheles maculipennis to different classes of insecticides in West Azarbaijan Province, Northwestern Iran. Asian Pac J Trop Biomed. 5(5): 403–406. [Google Scholar]
- 34.Gorouhi MA, Vatandoost H, Oshaghi MA, Raeisi A, Enayati AA, Mirhendi H. (2016) Current susceptibility status of Anopheles stephensi (Diptera: Culicidae) to different imagicides in a malarious area, southeastern of Iran. J Arthropod Borne Dis. 10 (4): 493–500. [PMC free article] [PubMed] [Google Scholar]
- 35.Zare M, Soleimani-Ahmadi M, Davoodi SH, Sanei-Dehkordi A. (2016) Insecticide susceptibility of Anopheles stephensi to DDT and current insecticides in an elimination area in Iran. Parasit Vectors. 9 (1): 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vatandoost H, Hanafi-Bojd AA, Raeisi A, Abai MR, Nikpour F. (2017) Ecology, monitoring and mapping of insecticide resistance of malaria vector, Anopheles culicifacies (Diptera: Culicidae) to different imagicides in Iran. Asian Pac J Trop Dis. 7(1): 53–56. [Google Scholar]
- 37.Vatandoost H, Abai MR, Akbari M, Raeisi A, Yousefi H, Sheikhi S, Bagheri A. (2019) Comparison of CDC bottle bio-assay with WHO standard method for assessment susceptibility level of malaria vector, Anopheles stephensi to three imagicides. J Arthropod Borne Dis. 13 (1): 17–26. [PMC free article] [PubMed] [Google Scholar]
- 38.Nazari M, Janbakhsh B. (2000) A survey of the susceptibility level of Culex theileri and Cx. pipiens to DDT, Dieldrin, Propoxur and Malathion in the southern area of Tehran. J Uromia Univ Med Sci. 11(1): 13–19. [Google Scholar]
- 39.Naseri-Karimi N, Vatandoost H, Bagheri M, Chavshin AR. (2015) Susceptibility status of Culex pipiens against deltamethrin and DDT, Urmia County, West Azerbaijan Province, northwestern Iran. Asian Pac J Trop Dis. 5(Suppl 1): S77–S79. [Google Scholar]
- 40.Salim-Abadi Y, Asadpour M, Sharifi I, Sanei-Dehkordi A, Gorouhi MA, Paksa A. (2017) Baseline susceptibility of filarial vector Culex quinquefasciatus (Diptera: Culicidae) to five insecticides with different modes of action in southeast of Iran. J Arthropod Borne Dis. 11(4): 453–462. [PMC free article] [PubMed] [Google Scholar]
- 41.Ghorbani F, Vatandoost H, Hanafi-Bojd AA, Abai MR, Nikoobar H, Enayati AA. (2018) High resistance of vector of West Nile Virus, Culex pipiens Linnaeus (Diptera: Culicidae) to different insecticides recommended by WHO in northern Iran. J Arthropod Borne Dis. 12(1): 24–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Shemshadian A, Abai MR, Vatandoost H, Dinparast-Djadid N, Oshaghi MA, Mojahedi A. (2020) Assessment the changing trend of susceptibility to two insecticides mmong field-population Culex quinquefasciatus compared with the same population undergoing to multiple colonization. J Arthropod Borne Dis. 14(2): 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeidabadinezhad R, Vatandoost H, Abai MR, Dinparast-Djadid N, Raz A, Sedaghat MM, Oshaghi MA, Raeisi A, Adibi N. (2019) Target site insensitivity detection in deltamethrin resistant Culex pipiens complex in Iran. Iran J Public Health. 48 (6): 1091–1098. [PMC free article] [PubMed] [Google Scholar]
- 44.Hassandoust S, Moosa-Kazemi SH, Vatandoost H, Sedaghat MM, Akbarzadeh K. (2020) Evaluation of susceptibility of Aedes caspius (Diptera: Culicidae) to insecticides in a potent arboviral-prone area, southern Iran. J Arthropod Borne Dis. 14 (2): 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazratian T, Paksa A, Sedaghat MM, Vatandoost H, Moosa-Kazemi SH, Sanei-Dehkordi A, Salim-Abadi Y, Pirmohammadi M, Yousefi S, Amin M, Oshaghi MA. (2019) Baseline Susceptibility of Culiseta longiareolata (Diptera: Culicidae) to Different Imagicides, in Eastern Azerbaijan, Iran. J Arthropod Borne Dis. 13 (4): 407–415. [PMC free article] [PubMed] [Google Scholar]
- 46.Safi NH, Ahmadi AA, Nahzat S, Warusavithana S, Safi N, Valadan R, Shemshadian A, Sharifi M, Enayati A, Hemingway J. (2019) Status of insecticide resistance and its biochemical and molecular mechanisms in Anopheles stephensi (Diptera: Culicidae) from Afghanistan. Malar J. 18(1): 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yared S, Gebressielasie A, Damodaran L, Bonnell V, Lopez K, Janies D, Carter TE. (2020) Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar J. 19: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben Cheikh II, Ben Ali-Haouas Z, Marquine M, Pasteur N. (1998) Resistance to Organophosphorus and Pyrethroid Insecticides in Culex pipiens (Diptera: Culicidae) from Tunisia. J Med Entomol. 35(3): 251–260. [DOI] [PubMed] [Google Scholar]
- 49.Corbel V, N'Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Akogbéto M, Hougard JM, Rowland M. (2007) Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 101: 207–216. [DOI] [PubMed] [Google Scholar]
- 50.Bisset JA, Rodriguez MM, Diaz C, Ortiz E, Marquetti MC, Hemingway J. (1990) The mechanisms of organophosphate and carbamate resistance in Culex quinquefasciatus (Diptera: Culicidae) from Cuba. Bull Entomol Res. 80(3): 245–250. [Google Scholar]
- 51.Salim Abadi Y, Vatandoost H, Rassi Y, Abai MR, Sanei-Dehkordi AR, Paksa A. (2010) Evaluation of biological control agents for mosquitoes control in artificial breeding places. Asian Pac J Trop Med. 3(4): 276–277. [Google Scholar]
- 52.Vatandoost H, Nikpour F, Hanafi-Bojd AA, Abai MR, Khanavi M, Hajiiakhondi A, Raesi A, Nejati J. (2019) Efficacy of extractions of Iranian native plants against main malaria vector, Anopheles stephensi in Iran for making appropriate formulation for disease control. J Arthropod Borne Dis. 13(4): 344–352. [PMC free article] [PubMed] [Google Scholar]
- 53.Isman MB. (2004) Plant essential oils for pest and disease management. Crop Prot. 19(8–10): 603–608. [Google Scholar]
- 54.Sedaghat MM, Sanei-Dehkordi AR, Khanavi M, Abai MR, Mohtarami F, Vatandoost H. (2011) Chemical composition and larvicidal activity of essential oil of Cupressus arizonica E.L. Greene against malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Pharmacognosy Res. 3 (2): 135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sedaghat MM, Sanei-Dehkordi AR, Abai M, Khanavi M, Mohtarami F, Abadi YS, Rafi F, Vatandoost H. (2011) Larvicidal activity of essential oils of apiaceae plants against malaria vector, Anopheles stephensi. Iran J Arthropod Borne Dis. 5 (2): 51–59. [PMC free article] [PubMed] [Google Scholar]
- 56.Vatandoost H, Sanei-Dehkordi A, Sadeghi S, Davari B, Karimian F, Abai M. (2012) Identification of chemical constituents and larvicidal activity of Kelussia odoratissima Mozaffarian essential oil against two mosquito vectors Anopheles stephensi and Culex pipiens (Diptera: Culicidae). Exp Parasitol. 132(4): 470–474. [DOI] [PubMed] [Google Scholar]
- 57.Sanei-Dehkordi A, Soleimani-Ahmadi M, Akbarzadeh K, Salim Abadi Y, Paksa A, Gorouhi MA. (2016) Chemical composition and mosquito larvicidal properties of essential oil from leaves of an Iranian indigenous plant Zhumeria majdae. J Essent Oil Bear Pl. 19(6): 1454–1461. [Google Scholar]
- 58.Soleimani-Ahmadi M, Sanei-Dehkordi AR, Turki H, Madani A, Abadi YS, Paksa A. (2017) Phytochemical properties and insecticidal potential of volatile oils from Tanacetum persicum and Achillea kellalensis against two medically important mosquitoes. J Essent Oil Bear Pl. 20(5): 1254–1265. [Google Scholar]
- 59.Soleimani-Ahmadi M, Abtahi SM, Madani A, Paksa A, Abadi YS, Gorouhi MA. (2017) Phytochemical profile and mosquito larvicidal activity of the essential oil from aerial parts of Satureja bachtiarica Bunge against malaria and lymphatic filariasis vectors. J Essent Oil Bear Pl. 20 (2): 328–336. [Google Scholar]
- 60.Soleimani-Ahmadi M, Gorouhi MA, Mohammadi Azani S, Salim-Abadi Y, Paksa A, Rashid G. (2017) Larvicidial effects of essential oil and methanol extract of Achillea wilhelmsii C. Koch (Asteraceae) against Anopheles stephensi Liston (Diptera: Culicidae), a malaria vector. J Kerman Univ of Med Sci. 24(1): 58–67. [Google Scholar]
- 61.Sanei-Dehkordi A, Soleimani-Ahmadi M, Salim-Abadi Y, Paksa A. (2019) Wild chive oil is an extremely effective larvicide against malaria mosquito vector Anopheles stephensi. Asian Pac J Trop Med. 12(4): 170–174. [Google Scholar]
- 62.Karami M, Moosa-Kazemi SH, Oshaghi MA, Vatandoost H, Sedaghat MM, Rajabnia R, Hosseini M, Maleki-Ravasan N, Yahyapour Y, Ferdosi-Shahandashti E. (2016) Wolbachia Endobacteria in natural populations of Culex pipiens of Iran and its phylogenetic congruence. J Arthropod Borne Dis. 10(3): 347–363. [PMC free article] [PubMed] [Google Scholar]
- 63.Yen PS, Failloux AB. (2020) A Review: Wolbachia-based population replacement for mosquito control shares common points with genetically modified control Approaches. Pathogens. 9(5): 404. [DOI] [PMC free article] [PubMed] [Google Scholar]