Abstract
Background
Severe acute respiratory syndrome Coronavirus 2 has rapidly spread worldwide, with acute kidney injury (AKI) as one of the manifestations with unknown causal mechanisms. We aimed to investigate tubular injury by assessing tubular markers and their association with the severity of Coronavirus disease 2019 (COVID-19).
Methods
We examined the associations between laboratory markers and urinary levels of N-acetyl-β-d-glucosaminidase (uNAG), β2-microglobulin (u β2MG), α1-microglobulin (u α1MG), and liver-type fatty acid binding protein (L-FABP). We studied 18 COVID-19 patients without previous chronic kidney disease and analyzed the relationship between the urinary biomarkers and inflammatory markers in patients with severe (n = 7) or non-severe (n = 11) COVID-19, defined by requirements of supplemental oxygen.
Results
Fourteen patients (78%) showed abnormal urinalysis findings and two (11%) developed AKI. Patients with severe COVID-19 had significantly higher levels of proteinuria, uNAG, uβ2MG, uα 1MG, and L-FABP than those with non-severe disease. Serum levels of interleukin-6 (IL-6) were significantly higher on admission in all severe COVID-19 cases and correlated with the levels of L-FABP, uβ2MG, uα1MG, uNAG, and proteinuria. Moreover, the changes in serum IL-6 (ΔIL-6) levels from baseline to 7 days after admission significantly correlated with ΔL-FABP and Δuβ2MG.
Conclusions
Levels of tubular injury markers, especially L-FABP and uβ2MG, were significantly associated with IL-6 levels even in patients with no evident AKI. This suggests that L-FABP and uβ2MG could be useful as early detective biomarkers for COVID-19 associated renal injury.
Keywords: Acute tubular injury, Interleukin-6, COVID-19, L-FABP, β2MG
Introduction
Coronavirus disease 2019 (COVID-19) has rapidly spread worldwide since the novel Coronavirus, severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), was first detected in Wuhan, China, in December 2019 [1]. COVID-19-associated acute kidney injury (AKI) has been noted in several reports, and the incidence varies depending on criteria for hospitalization in different locales. In a meta-analysis of 25 Chinese papers published between the beginning of the epidemic and July 30, 2020, Zheng et al. found a 32.5% incidence of AKI in ICU patients and a 6.5% incidence of AKI in overall hospital patients [2]. Other recent meta-analyses showed AKI to be closely related to disease severity and mortality for COVID-19 patients [3, 4].
The detailed mechanisms for AKI caused by SARS-CoV-2 have not been elucidated; however, recent studies point to the possibility of direct viral infection to kidney cells via angiotensin-converting enzyme-2 (ACE2) [5–7]. Single-cell RNA-seq data analysis demonstrates that the ACE2 protein is enriched in the bronchus and lung parenchyma, ileum, heart, bladder, and kidney [8]. In the kidney, ACE2 is expressed on glomerular endothelial cells, podocytes, and proximal tubule epithelial cells [9].
In autopsies of 26 patients with COVID-19, electron microscopy found Coronavirus particles in both the proximal tubular epithelium and podocytes. The study reported that immunostaining of ACE2 in kidney tissue from the COVID-19 patients was prominent in proximal tubular cells, especially in areas with severe acute tubular injury with loss of brush border or acute tubular necrosis. Occasionally, weaker podocyte staining was also observed [10]. These data suggested that proximal tubules may be the main target of SARS-CoV-2 infection in the kidney.
Interleukin-6 (IL-6), an inflammatory cytokine that can induce cytokine storms, is found in high levels in critically ill COVID-19 patients [11–13]. A previous report found that renal damage in patients with severe acute respiratory syndrome (SARS) occurred because of hemodynamic changes caused by cytokine storms rather than by viral-induced tropism in the kidneys [14]. Thus, it is not known whether COVID-19 causes progressive kidney damage, in the proximal tubules in SARS-CoV-2, by direct viral infection or pro-inflammatory cytokine-mediated mechanisms.
In this study, we aim to investigate the relationships of tubular injury, severity of COVID-19, and inflammation markers, such as IL-6, to address the possible involvements of pro-inflammatory cytokine-mediated mechanisms on developing AKI.
Materials and methods
Patients
Our retrospective study used data from all inpatients with COVID-19 seen in April and May 2020 at Juntendo University Hospital, a 1051-bed university-affiliated hospital, Tokyo, Japan. The study included 21 Japanese adult (≥ 18) COVID-19 patients diagnosed by detecting SARS-CoV-2 RNA from nasopharyngeal swab specimens via reverse transcription-polymerase chain reaction (Ct value ≤ 40.0, LightCycler® 480 System II, Japan).
We excluded patients who lacked urinary data (n = 2) and a patient with chronic kidney disease (n = 1), resulting in 18 study patients. This study was approved by the ethics committee of Juntendo University Hospital, and informed written consent was obtained from all patients (Process No. 20–036). The authors have complied with the Declaration of Helsinki during this study.
Data collection
Clinical and laboratory information were collected at admission and on the seventh day. Laboratory parameters included serum creatinine (Cr), serum IL-6, urinary protein excretion, urinary liver-type fatty acid binding protein (L-FABP), urinary N-acetyl-β-d-glucosaminidase (uNAG), urinary alpha1-microglobulin (uα1MG), and urinary β2-microglobulin (uβ2MG). The urinary data contained a relatively small number of examples with missing values.
We analyzed the relationship between laboratory markers and respiratory status in patients with severe or non-severe COVID-19 as defined by requirements of supplemental oxygen referring to National Institutes of Health and Japanese guidelines [15, 16]. We also investigated the correlation analysis between the patients’ levels of urinary tubule markers (uNAG, uβ2MG, uα1MG, and L-FABP), laboratory markers, proteinuria, and the presence of urinary cast.
Definition of acute kidney injury
AKI was defined as follows: increase in serum Cr by ≥ 0.3 mg/dl within 48 h; or increase in serum Cr to ≥ 1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume of < 0.5 mL/kg/h for 6 h [17].
Statistical analyses
Data were expressed as median (interquartile range [IQR]) and n (%), respectively. Continuous and categorical variables were compared between groups using the Mann–Whitney U test or Fisher’s exact tests. The correlations between IL-6 and other variables were evaluated by Spearman correlation analysis. Statistical significance was defined as p < 0.05. These statistical analyses were conducted using the Prism software ver.8.4.2 (GraphPad Software, San Diego, CA, USA).
Results
Baseline demographic, clinical characteristics, and laboratory findings
We retrospectively evaluated 18 patients with COVID-19 whose age ranged from 26 to 78 years. Table 1 presents the clinical and laboratory characteristics and details about the COVID-19 cases (total, non-severe [n = 11] and severe [n = 7]). There were no significant differences in gender, hypertension, coronary heart disease, and respiratory disease; patients with severe COVID-19 were significantly older (median IQR: 76.0 years [66.5–76.0] vs. 56.0 years [37.5–65.5]) and more likely to have diabetes compared with non-severe cases.
Table 1.
Demographic, clinical, laboratory, and radiographic findings of study patients at admission and patient outcome
| Total (n = 18) | Non-severe (n = 11) | Severe (n = 7) | p value | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age, years | 64.0 (44.0–74.5) | 56.0 (37.5–65.5) | 76.0 (66.5–76.0) | 0.0059 |
| Gender (% male) | 66.7 | 54.6 | 85.7 | 0.32 |
| Comorbidity | ||||
| Hypertension | 5 (28%) | 2 (18%) | 3 (43%) | 0.33 |
| Diabetes | 6 (33%) | 2 (18%) | 4 (57%) | 0.14 |
| Coronary heart disease | 2 (11%) | 1 (9%) | 1 (14%) | 1 |
| Chronic kidney disease | 0 | 0 | 0 | |
| Respiratory disease | 0 | 0 | 0 | |
| Laboratory findings | ||||
| White blood cell count, × 109 per L | 5.70 (4.63–7.18) | 4.90 (4.35–6.55) | 6.40 (5.80–7.25) | 0.16 |
| Lymphocyte count, × 109 per L | 1.28 (0.74–1.49) | 1.48 (1.00–1.60) | 0.93 (0.65–1.28) | 0.13 |
| Hemoglobin, g/L | 144 (129–153) | 143 (128–152) | 145 (138–150) | 0.88 |
| Platelet count, × 109 per L | 221 (168–295) | 227 (212–287) | 175 (160–255) | 0.22 |
| Albumin, g/dL | 3.30 (3.10–3.80) | 3.50 (3.30–4.35) | 3.10 (2.85–3.20) | 0.01 |
| Ferritin, μg/L | 764 (433–973) | 469 (238–607) | 976 (932–1310) | 0.0008 |
| Creatinine, mg/dL | 0.68 (0.54–0.77) | 0.66 (0.49–0.81) | 0.71 (0.67–0.74) | 0.64 |
| eGFR, mL/min/1.73 m2 | 85.9 (76.1–97.3) | 88.5 (77.4–103.3) | 85.4 (79.5–87.3) | 0.60 |
| d-dimer, μg/mL | 2.05 (1.55–2.65) | 1.90 (1.40–2.05) | 2.70 (2.25–3.95) | 0.01 |
| Procalcitonin, ng/mL | 0.05 (0.01–0.11) | 0.03 (0.00–0.04) | 0.12 (0.08–0.15) | 0.0019 |
| IL-6, pg/mL | 14.3 (3.8–35.0) | 5.2 (2.6–12.8) | 40.5 (31.1–52.0) | 0.0004 |
| CRP, mg/L | 4.15 (0.28–8.39) | 0.44 (0.19–1.99) | 8.40 (8.00–11.88) | 0.0012 |
| Radiographic features | ||||
| Presence of pneumonia | 16 (89%) | 9 (82%) | 7 (100%) | 0.50 |
| Outcome | ||||
| Acute kidney injury | 2 (11%) | 0 | 2 (29%) | 0.14 |
| ICU admission | 4 (22%) | 0 | 4 (57%) | 0.011 |
| Invasive mechanical ventilation | 3 (17%) | 0 | 3 (43%) | 0.04 |
| Death | 1 (6%) | 0 | 1 (14%) | 0.39 |
Data are expressed as median (IQR); p values were assessed using a Mann–Whitney U test or Fisher’s exact test to compare differences between patients with severe and non-severe COVID-19
IL-6 interleukin-6, L-FABP liver-type fatty acid binding protein, uNAG N-acetyl-β-d-glucosaminidase); uβ2MG β2-microglobulin, uα1MG α1-microglobulin
When comparing the two groups, no significant differences were seen in the laboratory data, including white blood cell counts, lymphocyte counts, hemoglobin, platelet counts, creatinine, and estimated glomerular filtration rates; however, serum albumin was decreased, and the serum levels of ferritin, d-dimer, procalcitonin, IL-6, and C-reactive protein (CRP) were increased in the severe group compared with that in the non-severe group.
Urinary levels of tubular markers varied between non-severe and severe cases
Fourteen of the 18 patients (78%) had abnormal urinalysis findings. Patients with severe COVID-19 had significantly higher levels of proteinuria (0.42 g/gCr [0.36–0.78] vs. 0.07 g/gCr [0.05–0.29], p = 0.020); uNAG (32.5 U/L [26.8–42.0] vs. 5.3 U/L [3.0–18.4], p = 0.001); uβ2MG (10,516 μg/L [1649–29,237] vs. 160 μg/L [42–297], p = 0.003); uα1MG (65.8 mg/L [62.3–87.1] vs. 4.4 mg/L [3.1–15.0], p = 0.005); L-FABP (47.9 μg/gCr [29.4–75.8] vs. 3.3 μg/gCr [2.1–5.8], p = 0.001) (Fig. 1); and urinary cast (all; 86% vs. 18%, p = 0.013: epithelial cast; 86% vs. 9%, p = 0.003: granular cast; 43% vs. 9%, p = 0.24) when compared with non-severe cases. Regarding red blood cell and white blood cell cast, there was only 1 case among the 18 patients with COVID-19. We found only two cases (29%) of evident AKI, both in the severe group (Table 1).
Fig. 1.
The levels of L-FABP, uβ2MG, uα1MG, uNAG, proteinuria in patients with COVID-19 on admission. *p ≤ 0.05, **p ≤ 0.01. L-FABP liver-type fatty acid binding protein, uβ2MG β2-microglobulin, uα1MG α1-microglobulin), uNAG N-acetyl-β-d-glucosaminidase
Correlation between urinary tubular markers and laboratory markers
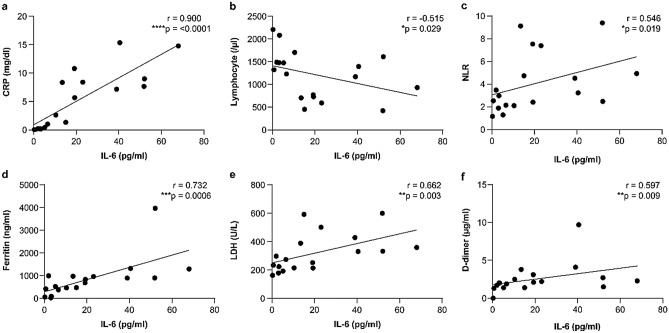
The baseline serum levels of IL-6 increased in all severe cases and in six non-severe cases. They were also significantly higher in severe cases than in non-severe cases (40.5 pg/mL [31.1–52.0] vs. 5.2 pg/mL [2.6–12.8], p = 0.0004) (Table 1). The IL-6 levels on admission were positively correlated with CRP (r = 0.9004, p < 0.0001), neutrophil-to-lymphocyte ratio (NLR) (r = 0.5459, p = 0.0191), ferritin (r = 0.7317, p = 0.0006), lactate dehydrogenase (LDH) (r = 0.6615, p = 0.0028), and D-dimer (r = 0.5971, p = 0.0089); IL-6 levels were negatively correlated with lymphocyte counts (r = − 0.515, p = 0.0287) (Fig. 2).
Fig. 2.
Correlations between serum IL-6 levels and other variables. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. IL-6 interleukin-6, CRP C-reactive protein, NLR neutrophil-to-lymphocyte ratio, LDH lactate dehydrogenase
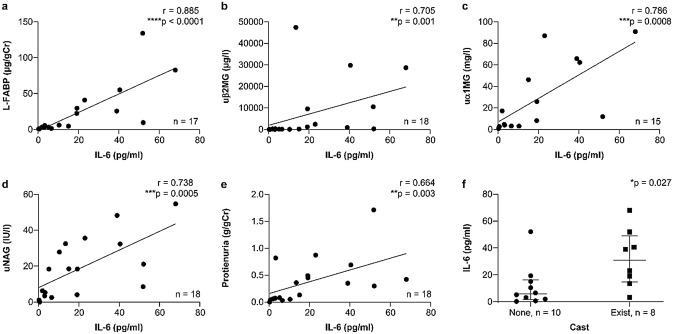
Serum levels of IL-6 significantly correlated with the levels of L-FABP on admission (r = 0.89, p < 0.0001), uβ2MG (r = 0.71, p = 0.001), uα1MG (r = 0.79, p = 0.0008), uNAG (r = 0.74, p = 0.0005), and proteinuria (r = 0.66, p = 0.003) (Fig. 3a–e); the changes in serum IL-6 (ΔIL-6) from baseline to 7 days after admission also significantly correlated with ΔL-FABP (r = 0.67, p = 0.005) and Δuβ2MG (r = 0.79, p = 0.003) (Fig. 4a–e). Serum IL-6 and ∆IL-6 were significantly higher in the patients who exhibited the urinary cast at admission and on the seventh day (Fig. 3f, 4f).
Fig. 3.
Correlations between serum IL-6 levels and urinary biomarker values. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. IL-6 interleukin-6, L-FABP liver-type fatty acid binding protein, uβ2MG β2-microglobulin, uα1MG α1-microglobulin, uNAG N-acetyl-β-d-glucosaminidase
Fig. 4.
Correlation of change from baseline in serum IL-6 levels and urinary biomarker values. *p ≤ 0.05, **p ≤ 0.01. IL-6 interleukin-6, L-FABP liver-type fatty acid binding protein, uβ2MG β2-microglobulin, uα1MG α1-microglobulin, uNAG N-acetyl-β-d-glucosaminidase
Discussion
The salient findings in this study were as follows: (1) our subjects had an 11% AKI incidence during hospitalization; (2) even if they did not have evident AKI, patients with severe COVID-19 had significantly elevated tubular injury markers, such as L-FABP, uβ2MG, uα1MG, and uNAG; (3) urinary epithelial casts, which may indicate tubular injury, were also observed in cases with severe COVID-19 infections; and (4) IL-6 was significantly associated with tubular markers, and its reduction correlated to a decrease in L-FABP and uβ2MG. These observations suggest that severe systemic inflammatory condition, such as cytokine storms could be involved in tubular injury, and L-FABP and uβ2MG levels could be useful early diagnostic markers for COVID-19 associated renal injury.
The incidence of evident AKI in our COVID-19 patients during hospitalization was similar to the findings in previous reports [2]. In a previous paper reporting proximal tubular injury in COVID-19, 67% of the 49 patients had elevated urinary levels of uβ2MG, and 85% had an increase in proteinuria [18]. In our study, 18 COVID-19 patients had abnormalities in the following markers: proteinuria, 56% (10); uβ2MG, 61% (11); L-FABP; 47% (8 of 17), uNAG 61% (11); and uα1MG, 53% (8 of 15). Consistent with our report, elevated tubular markers were observed even in cases without evident AKI in both studies. These observations may suggest that the elevation of tubular markers, such as L-FABP and uβ2MG, could reflect even the modest tubular injury and be early detection markers for COVID-19-mediated renal injury. Additionally, the group of patients with severe disease and worsening respiratory status had high levels of L-FABP, uβ2MG, uα1MG, uNAG, proteinuria, the presence of urinary casts, mainly epithelial cast, and serum levels of IL-6.
We confirmed a correlation between IL-6, CRP, NLR, ferritin, LDH, and d-dimer, consistent with earlier reports [19, 20], which is considered a parameter of disease aggravation. Interestingly, serum levels of IL-6 significantly correlated with urinary biomarker values, such as L-FABP, uβ2MG, uα1MG, uNAG, proteinuria, and urinary cast. Changes in L-FABP and uβ2MG had a strong positive correlation with the IL-6 changes. L-FABP, which is predominantly expressed in the proximal tubules, is known to be a predictive and sensitive early diagnostic biomarker for AKI [21]. Additionally, L-FABP can reflect defective renal microcirculation as opposed to other tubular markers, such as uNAG, uα1MG, and uβ2MG [21]. These results suggest that IL-6, one of key mediators for cytokine storm, could be strongly involved in developing our subjects’ tubular injuries.
Most abnormalities in the urinary biomarker levels might be primarily due to acute proximal tubular damage; however, it is unclear whether proteinuria reflected tubular injury or glomerular impairment with podocyte damages. Aside from ACE2 expression in podocytes, the finding that IL-6 levels were significantly associated with the presence of nephritic sediments could indicate that SARS-CoV-2 might cause glomerular injury. Further study is needed to elucidate this issue.
The present study has several limitations. First, because we only studied patients from a single center, we had a small population; second, patients with severe COVID-19 were significantly older and more likely to have diabetes, which might confound the results; third, we lacked clinical and pathological data that would suggest decisive evidence for acute renal tubular damage; fourth, though there are various inflammatory cytokines, this study assessed only IL-6, known as one of the representative inflammatory cytokines; finally, our study could not investigate the involvement of direct viral infection to the kidney cells. Large-scale studies are needed to focus on tubular damage and elucidate underlying mechanisms of renal complication in COVID-19 infection.
The present study suggests that acute tubular injury can occur depending on the severity of COVID-19 infection, and L-FABP and uβ2MG can be useful early diagnostic biomarkers for COVID-19-associated renal injury.
Acknowledgements
The authors would like to thank all the clinicians for their day-to-day clinical care, Dr. Naotake Yanagisawa for statistical consultation, and Enago (www.enago.jp) for the English review.
Author contributions
YF, HN, and YN wrote the original draft and contributed equally. MH, TN, MK, TG, SU, and YS reviewed and edited the manuscript.
Funding
A Japanese rock band, named GLAY, donated funds to this research.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the ethics committee of Juntendo University Hospital (Process No. 20-036). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institution at which the studies were conducted and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng X, Zhao Y, Yang L. Acute kidney injury in COVID-19: The Chinese experience. Semin Nephrol. 2020;40:430–442. doi: 10.1016/j.semnephrol.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansrivijit P, Qian C, Boonpheng B, Thongprayoon C, Vallabhajosyula S, Cheungpasitporn W, Ghahramani N. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68:1261–1270. doi: 10.1136/jim-2020-001407. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang L, Gong Y, Zhu Y, Gong J. Association of acute kidney injury with the severity and mortality of SARS-CoV-2 infection: a meta-analysis. Am J Emerg Med. 2020;43:149–157. doi: 10.1016/j.ajem.2020.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 7.Danilczyk U, Sarao R, Remy C, et al. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting Enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nihei Y, Nagasawa H, Fukao Y, et al. Continuous extracorporeal treatments in a dialysis patient with COVID-19. CEN Case Rep. 2020;10:1–6. doi: 10.1007/s13730-020-00538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons EM, Himmelfarb J, Sezer MT, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu KH, Tsang WK, Tang CS, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines, 2021. https://www.covid19treatmentguidelines.nih.gov/. Accessed date 14 Mar 2021 [PubMed]
- 16.Ministry of Health, Labour and Welfare of Japan. Clinical Management of Patients with COVID-19: a guide for front-line healthcare workers, version 2.1, 2020. https://www.mhlw.go.jp/content/000646531.pdf. Accessed date 17 June 2020
- 17.McMurray JJ, Parfrey PS, Adamson JW, et al. Kidney disease: improving global outcomes (KDIGO) AKI work group. Clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:279–335. doi: 10.1038/kisup.2012.37. [DOI] [Google Scholar]
- 18.Werion A, Belkhir L, Perrot M, et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98:1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12:e12421. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui K, Kamijo-Ikemori A, Sugaya T, Yasuda T, Kimura K. Usefulness of urinary biomarkers in early detection of acute kidney injury after cardiac surgery in adults. Circ J. 2012;76:213–220. doi: 10.1253/circj.CJ-11-0342. [DOI] [PubMed] [Google Scholar]