Abstract
Dipyridamole is an old anti-platelet and coronary vasodilator agent that inhibits platelet phosphodiesterase and increases interstitial adenosine levels. Its use in coronary artery disease (CAD) has fallen out of practice in the modern era with the advent of new anti-platelet agents, and most modern guidelines on the management of CAD either neglect to comment on its utility or outright recommend against it. The majority of the studies used in these guidelines are outdated and took place in an era when high doses of aspirin were used and statins were not widely utilized. There is growing evidence in rat models of dipyridamole’s synergy with statins through adenosine modulation resulting in significant myocardial protection against ischemia–reperfusion injury and limitation of infract size. The data in human studies are limited but show a similar potential synergy between dipyridamole and statins. It would thus be prudent to reconsider the recommendations against the use of dipyridamole in CAD and to re-evaluate its possible role and potential benefits through well-designed randomized trials combining it with statins, low-dose aspirin, and/or other anti-platelet agents.
Keywords: Dipyridamole, Coronary artery disease, Adenosine, Statins, Infarct size, Platelets, Phosphodiesterase inhibitors
Introduction
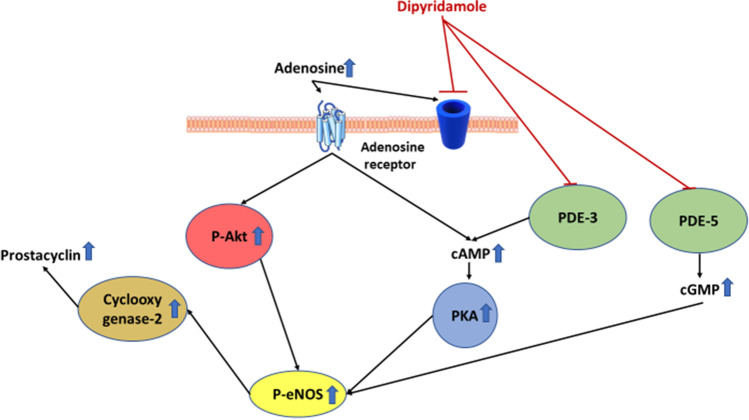
Dipyridamole is an old anti-platelet and coronary vasodilator agent that was first introduced in 1959 [1]. It decreases platelet aggregation by inhibiting platelet phosphodiesterase which in turn leads to the accumulation of cyclic adenosine monophosphate (cAMP) and cyclic guanine monophosphate (cGMP) [2]. Furthermore, dipyridamole increases interstitial adenosine levels by inhibiting its reuptake into the cells. Activation of the adenosine receptors stimulates adenylyl cyclase, further increasing the intracellular levels of cAMP. It is also thought to have vasodilatory effects through stimulation of prostacyclin production and potentiation of endothelial nitric oxide synthase activity downstream of cAMP (Fig. 1) [2].
Fig. 1.
The effects of dipyridamole on adenosine, cAMP and cGMP
Historically, dipyridamole was widely used as an anti-anginal and anti-thrombotic agent. It was frequently prescribed for a host of indications, including secondary prevention of myocardial infarction (MI), post coronary artery bypass surgery, and post coronary angioplasty [1]. It has slowly fallen out of favor over the years due to insufficient evidence to support its use for the various indications as well as the advent of newer anti-platelet agents with less frequent dosing.
In modern times, the primary utility of dipyridamole has been in coronary artery disease (CAD) diagnostics, namely its use as a coronary vasodilator in nuclear stress testing. Even for this indication, it has been replaced by adenosine and more recently by the A2A adenosine receptor agonist Regadenoson, as they are more potent vasodilators and the onset of action is faster [3, 4].
Currently, the only FDA-approved therapeutic indication for oral dipyridamole is as an adjunct to warfarin for thromboembolism prophylaxis in patients undergoing cardiac valve replacement. Dipyridamole is also used off label in addition to aspirin for secondary prevention of cerebral thromboembolism after the European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) showed that the dipyridamole-aspirin combination was superior to aspirin alone [5].
In this article, we will review current and recent cardiovascular guidelines recommendations against the use of dipyridamole for acute coronary syndrome and stable coronary artery disease. We will then analyze the studies upon which these recommendations are based and explore their limitations leading to sub-optimal conclusions regarding the drug. Finally, we will review and present the evidence in animal and human models that show potential benefits of dipyridamole and thus the need to reconsider the general recommendations against it in the guidelines.
Methods
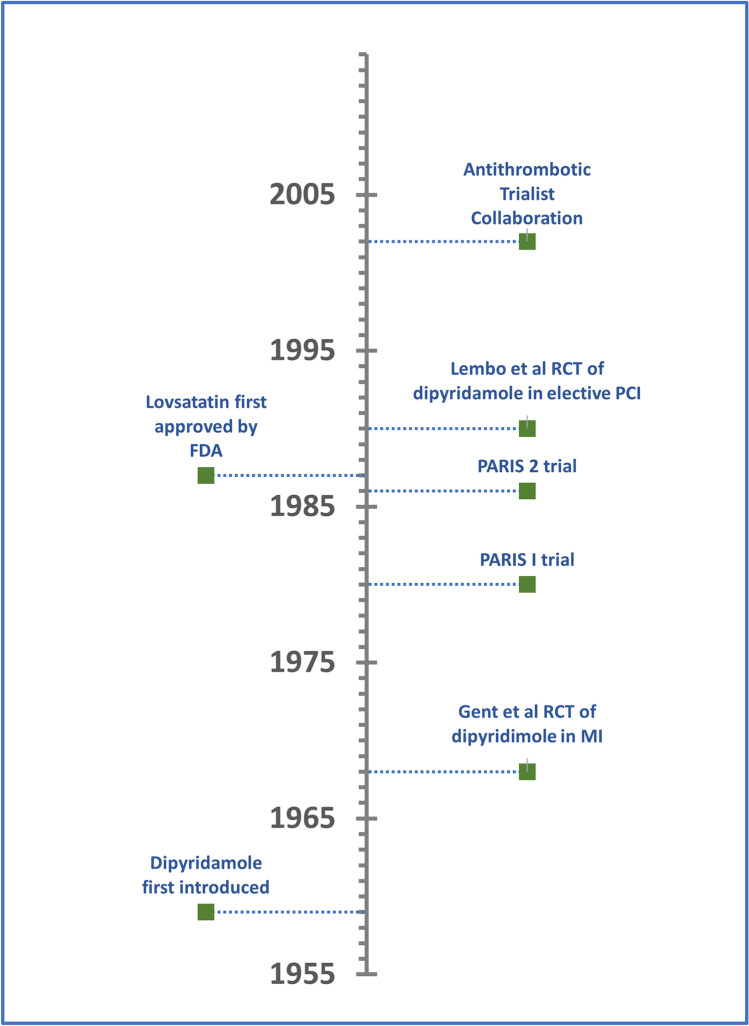
To prepare this review article, the authors first collected background information regarding the history of dipyridamole and its use in cardiovascular disease (Fig. 2). Next, the most recent management guidelines from prominent cardiovascular disease professional societies were identified and reviewed for recommendations pertaining to dipyridamole. In addition, we reviewed and summarized the clinical trials upon which these recommendations were based in said guidelines. We then searched the basic science literature including animal studies for studies and trials exploring the mechanism of action of dipyridamole and its various effects on cardiovascular disease. Finally, we searched clinicaltrials.gov for active human clinical trials exploring the role of dipyridamole of cardiovascular disease (Table 1).
Fig. 2.
Timeline of major events and clinical trials pertaining to dipyrdidamole use in cardiovascular disease
Table 1.
Summary of major clinical trials evaluating use of dipyridamole in cardiovascular disease
| Trial name | Year | Summary |
|---|---|---|
| Dipyridamole: a Controlled Trial of its Effect in Acute Myocardial Infarction [6] | 1968 | Dipyridamole vs. placebo in patients with acute MI. No statistically significant difference found in mortality or complications |
| PARIS 1 [7] | 1980 | Aspirin and dipyridamole vs. aspirin alone for secondary prevention of MI. No statistically significant difference found in mortality or non-fatal MIs |
| PARIS 2 [8] | 1986 | Aspirin and dipyridamole vs. placebo in patients with history of MI 1–4 months prior. A statistically significant reduction in the incidence of coronary events was seen with the treatment vs. the placebo group |
| Effect of pretreatment with aspirin versus aspirin plus dipyridamole on frequency and type of acute complications of percutaneous transluminal coronary angioplasty [9] | 1990 | Pretreatment with aspirin and dipyridamole vs. aspirin alone in patients undergoing elective PCI. No statistically significant difference found in clinical success or need for CABG |
| Antithrombotic Trialists Collaboration [10] | 2002 | Meta-analysis that pooled data from 25 trials comparing combination of dipyridamole plus aspirin vs. aspirin alone. There was a slight reduction in serious vascular events with the combination group, but it was not statistically significant |
Dipyridamole in Cardiovascular Disease Guidelines
Over the past 20 years, major cardiovascular disease guidelines have generally either recommended against the use of dipyridamole or did not provide any recommendation regarding its use.
The American College of Cardiology (ACC)/American Heart Association (AHA) 2012 guideline for the diagnosis and management of patients with stable ischemic heart disease makes a class III recommendation (no benefit) against the use of dipyridamole as anti-platelet therapy in patients with stable disease (level of evidence: B) [11].
The most recent AHA/ACC 2014 guideline for the management of patients with non-ST-elevation acute coronary syndromes does not make any recommendation regarding dipyridamole [12]. However, the previous ACC/AHA 2007 guideline for the management of patients with unstable angina (UA)/non-ST-elevation myocardial infarction (NSTEMI) makes a class III recommendation (no benefit) against the use of dipyridamole as an anti-platelet agent in post-UA/NSTEMI patients “because it has not been shown to be effective” (level of evidence: B) [13]. The 2012 focused update to the same guideline maintained the same recommendation [14].
For patients with ST-elevation myocardial infarction (STEMI), the most recent ACC/AHA 2013 guideline does not provide any recommendations regarding the use of dipyridamole [15]. The previous ACC/AHA STEMI guideline from 2004 recommends the use of dipyridamole and aspirin for STEMI patients with ischemic stroke not of cardioembolic source who do not undergo percutaneous intervention (PCI) [16]. The 1999 ACC/AHA STEMI guideline allows for the substitution of dipyridamole in lieu of aspirin in patients with aspirin allergy [17].
The American College of Chest Physicians 2008 clinical practice guideline for the primary and secondary prevention of CAD takes a more aggressive stance against dipyridamole use. The guideline makes a grade 1B recommendation for the use of a thienopyridine derivative rather than dipyridamole in aspirin-intolerant patients undergoing PCI. Furthermore, the guideline makes a grade 1A recommendation against the addition of dipyridamole to aspirin in patients undergoing coronary artery bypass grafting (CABG). It also contends that the addition of dipyridamole to aspirin provides “little incremental benefit over aspirin alone” for the prevention of early complications after PCI [18].
The 2016 European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice only recommend the use of dipyridamole for secondary prevention in patients with non-cardioembolic ischemic strokes [19]. There is no mention of dipyridamole in the 2017 European STEMI guidelines, and it is only mentioned in the scope of stress testing in the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation [20, 21].
Clinical Trials
To understand the context in which the aforementioned practice guidelines made their recommendations on the use of dipyridamole for CAD, we examined the evidence upon which these recommendations were based. Randomized control trials (RTC) evaluating the use of dipyridamole in CAD are actually quite limited in the literature. Common among all these trials is that they are all took place well before the modern era of widespread statin use. Furthermore, almost all of them involved the co-administration of high doses of aspirin by modern standards.
Two of the most prominent RTCs referenced in the guidelines on the use of dipyridamole are the Persantine-Aspirin Reinfarction Study (PARIS) I and PARIS II trials performed in 1980 and 1986, respectively. In Paris I, 2026 patients with CAD were randomized to either the combination of 324 mg of aspirin and 75 mg of dipyridamole three times daily or the same dose of aspirin alone to evaluate their role in the secondary prevention of myocardial infarction (MI). There was no statistically significant difference in the two groups for the primary endpoints of total mortality, coronary mortality, and fatal plus non-fatal myocardial infarction [7].
In PARIS II, 3128 patients who had survived a myocardial infarction 1 to 4 months earlier were randomized to aspirin 330 mg and dipyridamole 75 mg combination three times a day or to placebo. There was no aspirin-only treatment group. A statistically significant reduction in the incidence of coronary events was seen with the treatment vs. the placebo group. However, due to the lack of an aspirin-only treatment group, it was not clear whether the benefit was due to the combination of aspirin/dipyridamole or just aspirin [8].
Another often-cited source for the argument against dipyridamole is The Antithrombotic Trialists’ Collaboration 2002 meta-analysis of randomized trials of anti-platelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. The meta-analysis pooled data from 25 trials occurring before 1997 that compared the combination of dipyridamole plus aspirin vs. aspirin alone. There was a slight reduction in serious vascular events with the combination group, but it was not statistically significant [10].
Probably the oldest trial looking at dipyridamole in CAD was performed by Gent et al. in 1968. Patients admitted with acute MI were randomized to dipyridamole alone or placebo. No statistically significant difference in mortality or complications rate was found [6]. Of note, aspirin was not given to either the treatment or placebo group. This study is the basis on which the recommendation against dipyridamole is made in the 2007 ACC/AHA UA/NSTEMI guideline [13].
The 2008 American College of Chest Physicians guideline bases its aforementioned recommendations against dipyridamole partly on a 1990 prospective randomized trial performed by Lembo et al. [18]. In this study, 232 patients were randomly assigned to aspirin 325 mg TID plus dipyridamole 75 mg TID or aspirin alone before undergoing elective coronary angioplasty. No statistically significant differences were seen in the primary end points of clinical success, Q-wave MI frequency, or the need for emergency CABG [9].
Discussion
Almost all of the aforementioned studies took place in the 1960s–1980s, well before the widespread use of statins or the establishment of the non-inferiority of low-dose aspirin compared to higher doses. The high doses of aspirin used in most of these older studies likely confound the results and make it difficult to extrapolate them to modern times. Even more limiting is the occurrence of these studies before the era of statins.
In 1987, the FDA first approved Lovastatin for the treatment of hypercholesteremia [22]. Lovastatin was the first hydroxymethyl glutaryl coenzyme A (HMG-CoA) reductase inhibitor approved for this indication and it was followed by several other statins over the next decade, most notably Atorvastatin in 1997 and Rosuvastatin in 2003. It was not until the 2000s that statins started being widely used and accessible to a large segment of the population especially those with CAD.
Statins have been shown to activate the adenosine-producing enzyme ecto-5′-nucleotidase, which in turn plays a role in restoring the infarct size-limiting effect of ischemic preconditioning, an effect that is known to be blunted by hypercholesterolemia. Ueda et al. illustrated this effect with Pravastatin in rabbit model of myocardial infarction [23]. This phenomenon was also described by Sanada et al. in canine models using several different statins, postulating the existence of an optimal dose for each statin to produce this effect irrespective of the extent of their cholesterol-lowering effect [24]. Obata and Nakashima further described the underlying mechanism of statin-induced adenosine production in rats ventricular myocardium through activation of ecto-5′-nucleotidase which in turn stimulates a1-adrenoceptors of protein kinase C resulting in increased adenosine concentration [25]. The myocardial protective effects of statins depend on adenosine receptor activation. Adenosine receptor inhibitors, including caffeinated coffee, blunt the infarct size-limiting effects of statins [26].
This is relevant to dipyridamole because of its hypothesized synergistic effects with statins in reducing myocardial infarct size in animal models [27]. As discussed above, statins increase the release of adenosine via activation of ecto-5′-nucleotidase, and dipyridamole is thought to potentiate this effect through prevention of adenosine reuptake [28]. In fact, dipyridamole has been found to confer a chronic preconditioning-like cardio-protection in guinea pig models and this effect seems to pers for 3 days after discontinuation of dipyridamole. This effect is mediated through adenosine A1 receptors along with increased levels of activated Akt and endothelial nitric oxide synthase [29].
Ye et al. demonstrated the synergistic effects of dipyridamole and statins in a rat model. In the first protocol, rats were treated with 3 days of water, atorvastatin alone, dipyridamole alone, or combination atorvastatin-dipyridamole. In the second protocol, rats were treated with 3 days of aminophylline alone or aminophylline plus atorvastatin-dipyridamole combination. Rats were subjected to ligation of coronary arteries for 30 min followed by 4 h of reperfusion [28].
The combination of atorvastatin and dipyridamole was found to induce a statistically significant decrease in infarct size compared to each of the medications alone. This combination was also found to cause a statistically significant increase in adenosine levels. As expected, the addition of the nonselective adenosine receptor inhibitor aminophylline to the combination completely blocked this effect, further suggesting that the synergistic effects of atorvastatin and dipyridamole were attained through potentiation of adenosine [28].
Ye et al. further demonstrated this phenomenon in a second study in a rat model. In this study, rats were pretreated with 3 days of simvastatin before they underwent 30 min of myocardial ischemia followed by 4 h of reperfusion using the same process described above. Five minutes after ischemia was induced, rats were treated with intravenous dipyridamole alone, high- or low-dose aspirin alone, or combination of dipyridamole with high- or low-dose aspirin. Low-dose aspirin alone did not attenuate the infarct size-limiting effect of simvastatin, whereas high-dose aspirin completely blocked it. Dipyridamole alone or in combination with low-dose aspirin did not attenuate the infarct size-limiting effect of simvastatin. In fact, the combination of dipyridamole, simvastatin, and low-dose aspirin led to the smallest measured infarct size in this experiment [30].
As the aforementioned study shows, aspirin is thought to attenuate the infract size-limiting cardio-protective properties of statins by inhibiting cyclooxygenase-2, whereas dipyridamole appears to augment these effects by increasing the availability of adenosine. Furthermore, adding dipyridamole to low-dose aspirin in the setting of statins appears to limit the attenuation of statins cardio-protective properties seen with high-dose aspirin [30].
There are limited data in human studies that show a similar potential myocardial protection against ischemia–reperfusion injury of dipyridamole when combined with statins. Meijer et al. showed that rosuvastatin augmented the forearm vasodilator response to intra-arterial dipyridamole in healthy volunteers [31]. Additionally, there is some evidence in human studies showing benefit of dipyridamole alone on ischemia–reperfusion injury. Riksen et al. showed that oral dipyridamole limits ischemia–reperfusion injury in human forearm skeletal muscle [32].
In addition to their potential benefit when combined with statins, there are many important clinical applications of dipyridamole in cardiovascular disease, particularly in heart failure. Akhtar et al. showed in a pilot study of ischemic cardiomyopathy patients that sustained-release dipyridamole improved hyperemic myocardial blood flow and left ventricular systolic function [33]. Sanada et al. performed a randomized clinical trial examining potential benefit of 1 year of dipyridamole use in patients with chronic heart failure. The results showed statistically significant improvement in echocardiographic ejection fraction (32.4 ± 2.7 to 43.9 ± 3.9%, p < 0.01), plasma B-type natriuretic peptide level (282 ± 60 to 121 ± 35 pg/mL, p < 0.01), and specific activity scale score (5.1 ± 0.72 to 6.2 ± 0.44, p < 0.05), among others [34].
Dipyridamole may play a potential role for treatment of microvascular angina. The presence of adenosine resistance in the regulation in blood flow was reported previously and may serve as one of the mechanisms of abnormal coronary flow reserve [35]. Moreover, there might also be some benefits of dipyridamole in small vessel disease as Kihara et al. showed in a 1990 Japanese study of hypertrophic cardiomyopathy patients [36]. The lack of state-of-the-art treatment for abnormal coronary flow reserve and slow coronary blood flow may serve as a rationale to use dipyridamole as one of the treatments. Treatment with 2 weeks of dipyridamole 150 mg/day was found to improve subjective symptoms, left ventricular function, exercise tolerance, and arrhythmias, effects that are thought to be attained through improvement in myocardial perfusion [36].
The fact that dipyridamole increases intracellular cAMP could have a potential effect in diabetes too. Activation of the GLP-1 receptors with direct GLP-1 agonists or dipeptidyl-peptidase-4 (DPP4) inhibitors increases intracellular cAMP [36]. A synergistic effect between a GLP-1 agonist or DPPP4 inhibitor and cilostazol (another PDE-3 inhibitor) has been shown in a rat model of ischemia–reperfusion injury [37].
Analysis of the aforementioned animal models and limited human studies presents an interesting question that may be worth further exploring in future human trials: How significant would the potential benefit be of adding dipyridamole to the currently standard regimen of aspirin and statin? Furthermore, are current guidelines selling dipyridamole short when we seem to have reasonable suspicion of the existence of such cardio-protective benefits?
Conclusion
Major cardiovascular disease guidelines over the past 20 years have generally recommended against the use of dipyridamole for CAD. Upon review of the evidence on which these recommendations are based, it is clear that the majority of the trials are outdated and took place in an era when high doses of aspirin were used, and statins were not widely utilized. Despite this, most of the studies on dipyridamole from that period were either neutral or slightly positive.
One possible reason for this theme of recommending against the use dipyridamole in the various guidelines is the fact that high-dose intravenous dipyridamole induced ischemia in stress tests. However, it is faulty to assume that the effects of high dose intravenous form are applicable to low-dose oral administration of dipyridamole.
There is decent evidence in animal and human models of possible synergy of dipyridamole and statins through adenosine modulation resulting in significant myocardial protection against ischemia–reperfusion injury and limiting infract size. Aspirin is well known to attenuate that effect of statins, and there is a well-established advantage of ticagrelor over clopidogrel or prasugrel in the literature, which has been shown in animal trials to be likely related to the adenosine reuptake inhibition effects of ticagrelor [38–40]. As discussed earlier, dipyridamole also inhibits adenosine reuptake and could provide similar benefit to ticagrelor or even potentially augment its effects with used in combination.
Clinicians should usually follow the recommendations set by the guidelines and the approved indications of each drug. However, in light of the aforementioned studies and discussion, it would be prudent to critically re-examine the recommendations against the use of dipyridamole in CAD and to re-evaluate its possible role and potential benefits through well-designed clinical trials combining it with statins, low-dose aspirin, or other anti-platelet agents such as ticagrelor. Furthermore, it might be worthwhile to conduct clinical trials for the use of dipyridamole for special indications such as hypertrophic cardiomyopathy and angina with slow coronary flow, among others. Current ongoing clinical trials evaluating dipyridamole use in cardiovascular disease are summarized in Table 2.
Table 2.
Summary of active clinical trials evaluating dipyridamole use in cardiovascular disease
| NCT number | Trial name | Phase | Year | Summary |
|---|---|---|---|---|
| NCT04424901 | Trial of Open Label Dipyridamole- In Hospitalized Patients With COVID-19 | Phase 2 | 2020 | Evaluate dipyridamole in treating respiratory tract infection and circulatory dysfunction due to SARS-CoV-2 coronavirus in hospitalized patients |
| NCT04391179 | Dipyridamole to Prevent Coronavirus Exacerbation of Respiratory Status (DICER) in COVID-19 | Phase 2 | 2020 | Evaluate whether 14 days of treatment with dipyridamole will reduce excessive blood clotting in COVID-19 |
| NCT04666454 | BROKEN-SWEDEHEART-Optimized Pharmacological Treatment for Broken Heart (Takotsubo) Syndrome | Phase 2 | 2020 | Adenosine + dipyridamole vs. apixaban vs. ESC standard of care vs. placebo for treating takotsubo syndrome |
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of Interest
Mahmoud Allahham, MD: None.
Amir Lerman, MD: consulting fee—Philips/Volcano and Itamar medical.
Dan Atar, MD: None.
Yochai Birnbaum, MD: research grant—AstraZeneca.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gibbs CR, Lip GY. Do we still need dipyridamole? Br J Clin Pharmacol. 1998;45(4):323–328. doi: 10.1046/j.1365-2125.1998.t01-1-00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HH, Liao JK. Translational therapeutics of dipyridamole. Arterioscler Thromb Vasc Biol. 2008;28(3):s39–s42. doi: 10.1161/ATVBAHA.107.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers [published correction appears in J Nucl Cardiol 2016 Jun;23(3):640–2] J Nucl Cardiol. 2016;23(3):606–639. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 4.Rossen JD, Quillen JE, Lopez JG, Stenberg RG, Talman CL, Winniford MD. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18(2):485–491. doi: 10.1016/0735-1097(91)90604-8. [DOI] [PubMed] [Google Scholar]
- 5.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367(9523):1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 6.Gent AE, Brook CGD, Foley TH, Miller TN. Dipyridamole: a controlled trial of its effect in acute myocardial infarction. Bmj. 1968;4(5627):366–368. doi: 10.1136/bmj.4.5627.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persantine and aspirin in coronary heart disease The Persantine-Aspirin Reinfarction Study Research Group. Circulation. 1980;62(3):449–461. doi: 10.1161/01.CIR.62.3.449. [DOI] [PubMed] [Google Scholar]
- 8.Klimt CR, Knatterud GL, Stamler J, Meier P. Persantine-aspirin reinfarction study. Part II. Secondary coronary prevention with persantine and aspirin. J Am Coll Cardiol. 1986;7(2):251–269. doi: 10.1016/S0735-1097(86)80489-2. [DOI] [PubMed] [Google Scholar]
- 9.Lembo NJ, Black AJ, Roubin GS, et al. Effect of pretreatment with aspirin versus aspirin plus dipyridamole on frequency and type of acute complications of percutaneous transluminal coronary angioplasty. Am J Cardiol. 1990;65(7):422–426. doi: 10.1016/0002-9149(90)90804-A. [DOI] [PubMed] [Google Scholar]
- 10.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients [published correction appears in BMJ 2002 Jan 19;324(7330):141] BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126(25). [DOI] [PubMed]
- 12.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes. Circulation. 2014;130(25). [DOI] [PubMed]
- 13.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction. Circulation. 2007;116(7).
- 14.Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non–ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update) Circulation. 2012;126(7):875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 15.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. Circulation. 2013;127(4). [DOI] [PubMed]
- 16.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 17.Ryan TJ, Antman EM, Brooks NH, et al. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations. Circulation. 1999;100(9):1016–1030. doi: 10.1161/01.CIR.100.9.1016. [DOI] [PubMed] [Google Scholar]
- 18.Becker RC, Meade TW, Berger PB, et al. The primary and secondary prevention of coronary artery disease. Chest. 2008;133(6). [DOI] [PubMed]
- 19.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 21.Collet J-P, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal. 2020;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 22.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(5):484–493. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda Y, Kitakaze M, Komamura K, et al. Pravastatin restored the infarct size-limiting effect of ischemic preconditioning blunted by hypercholesterolemia in the rabbit model of myocardial infarction. J Am Coll Cardiol. 1999;34(7):2120–2125. doi: 10.1016/S0735-1097(99)00440-4. [DOI] [PubMed] [Google Scholar]
- 24.Sanada S, Asanuma H, Minamino T, et al. Optimal windows of statin use for immediate infarct limitation. Circulation. 2004;110(15):2143–2149. doi: 10.1161/01.CIR.0000143830.59419.73. [DOI] [PubMed] [Google Scholar]
- 25.Obata T, Nakashima M. Fluvastatin, an HMG-CoA reductase inhibitor, facilitate adenosine production in the rat hearts via activation of ecto-5′-nucleotidase. Microvasc Res. 2016;107:1–5. doi: 10.1016/j.mvr.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Ye Y, Abu Said GH, Lin Y, et al. Caffeinated coffee blunts the myocardial protective effects of statins against ischemia-reperfusion injury in the rat. Cardiovasc Drugs Ther. 2008;22(4):275–282. doi: 10.1007/s10557-008-6105-z. [DOI] [PubMed] [Google Scholar]
- 27.Ye Y, Perez-Polo JR, Birnbaum Y. Protecting against ischemia-reperfusion injury: antiplatelet drugs, statins, and their potential interactions. Ann N Y Acad Sci. 2010;1207(1):76–82. doi: 10.1111/j.1749-6632.2010.05725.x. [DOI] [PubMed] [Google Scholar]
- 28.Ye Y, Lin Y, Perez-Polo R, et al. Enhanced cardioprotection against ischemia-reperfusion injury with a dipyridamole and low-dose atorvastatin combination. Am J Physiol Heart Circ Physiol. 2007;293(1). [DOI] [PubMed]
- 29.Figueredo VM, Okusa C, Kaneda K, Inamura Y, Miyamae M. Regular dipyridamole therapy produces sustained protection against cardiac ischemia–reperfusion injury: is it time to revisit PARIS? Int J Cardiol. 2014;176(3):822–827. doi: 10.1016/j.ijcard.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Ye Y, Long B, Qian J, Perez-Polo JR, Birnbaum Y. Dipyridamole with low-dose aspirin augments the infarct size-limiting effects of simvastatin. Cardiovasc Drugs Ther. 2010;24(5–6):391–399. doi: 10.1007/s10557-010-6252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer P, Oyen WJ, Dekker D, et al. Rosuvastatin increases extracellular adenosine formation in humans in vivo. Arterioscler Thromb Vasc Biol. 2009;29(6):963–968. doi: 10.1161/ATVBAHA.108.179622. [DOI] [PubMed] [Google Scholar]
- 32.Riksen N, Oyen W, Ramakers B, et al. Oral therapy with dipyridamole limits ischemia-reperfusion injury in humans. Clin Pharmacol Ther. 2005;78(1):52–59. doi: 10.1016/j.clpt.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Akhtar M, Ordovas K, Martin A, Higgins CB, Michaels AD. Effect of chronic sustained-release dipyridamole on myocardial blood flow and left ventricular function in patients with ischemic cardiomyopathy. Congest Heart Fail. 2007;13(3):130–135. doi: 10.1111/j.1527-5299.2007.06047.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanada S, Asanuma H, Koretsune Y, et al. Long-term oral administration of dipyridamole improves both cardiac and physical status in patients with mild to moderate chronic heart failure: a prospective open-randomized study. Hypertens Res. 2007;30(10):913–919. doi: 10.1291/hypres.30.913. [DOI] [PubMed] [Google Scholar]
- 35.Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol. 2006;101(6):1678–1684. doi: 10.1152/japplphysiol.00546.2006. [DOI] [PubMed] [Google Scholar]
- 36.Koga Y, Kihara K, Yamaguchi R, Wada T, Toshima H. Therapeutic effect of oral dipyridamole on myocardial perfusion and cardiac performance in patients with hypertrophic cardiomyopathy. Am Heart J. 1992;123(2):433–438. doi: 10.1016/0002-8703(92)90658-I. [DOI] [PubMed] [Google Scholar]
- 37.Birnbaum Y, Castillo AC, Qian J, et al. Phosphodiesterase III inhibition increases cAMP levels and augments the infarct size limiting effect of a DPP-4 inhibitor in mice with type-2 diabetes mellitus. Cardiovasc Drugs Ther. 2012;26(6):445–456. doi: 10.1007/s10557-012-6409-x. [DOI] [PubMed] [Google Scholar]
- 38.Kubisa MJ, Jezewski MP, Gasecka A, Siller-Matula JM, Postuła M. Ticagrelor - toward more efficient platelet inhibition and beyond. Ther Clin Risk Manag. 2018;14:129–140. doi: 10.2147/TCRM.S152369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Y, Nylander S, Birnbaum Y. Unraveling the interaction of aspirin, ticagrelor, and rosuvastatin on the progression of atherosclerosis and inflammation in diabetic mice. Cardiovasc Drugs Ther. 2017;31(5–6):489–500. doi: 10.1007/s10557-017-6763-9. [DOI] [PubMed] [Google Scholar]
- 40.Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovascular Drugs and Therapy. 2016;30(6):539–550. doi: 10.1007/s10557-016-6701-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.