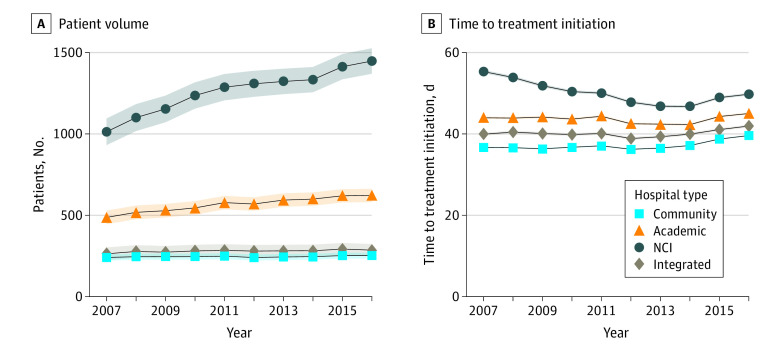
This cross-sectional study uses data from the National Cancer Database to investigate trends in patient volume by hospital type and the association of patient volume with time to treatment initiation for patients with cancer by hospital type.
Key Points
Question
Is the number of patients with cancer who are treated at referral centers increasing, and are more rapid increases in patient volume associated with treatment delays?
Findings
In this cross-sectional study of 4 218 577 patients treated for an incident cancer at 1351 hospitals from 2007 to 2016, patient volume increased more rapidly at National Cancer Institute and academic centers than at community hospitals, but this was not associated with clinically meaningful increases in time to treatment initiation.
Meaning
Faster patient volume growth at referral centers was not associated with clinically meaningful cancer treatment delays.
Abstract
Importance
Increasing demand for cancer care may be outpacing the capacity of hospitals to provide timely treatment, particularly at referral centers such as National Cancer Institute (NCI)–designated and academic centers. Whether the rate of patient volume growth has strained hospital capacity to provide timely treatment is unknown.
Objective
To evaluate trends in patient volume by hospital type and the association between a hospital’s annual patient volume growth and time to treatment initiation (TTI) for patients with cancer.
Design, Setting, and Participants
This retrospective, hospital-level, cross-sectional study used longitudinal data from the National Cancer Database from January 1, 2007, to December 31, 2016. Adult patients older than 40 years who had received a diagnosis of 1 of the 10 most common incident cancers and initiated their treatment at a Commission on Cancer–accredited hospital were included. Data were analyzed between December 19, 2019, and March 27, 2020.
Exposures
The mean annual rate of patient volume growth at a hospital.
Main Outcomes and Measures
The main outcome was TTI, defined as the number of days between diagnosis and the first cancer treatment. The association between a hospital’s mean annual rate of patient volume growth and TTI was assessed using a linear mixed-effects model containing a patient volume × time interaction. The mean annual change in TTI over the study period by hospital type was estimated by including a hospital type × time interaction term.
Results
The study sample included 4 218 577 patients (mean [SD] age, 65.0 [11.4] years; 56.6% women) treated at 1351 hospitals. From 2007 to 2016, patient volume increased 40% at NCI centers, 25% at academic centers, and 8% at community hospitals. In 2007, the mean TTI was longer at NCI and academic centers than at community hospitals (NCI: 50 days [95% CI, 48-52 days]; academic: 43 days [95% CI, 42-44 days]; community: 37 days [95% CI, 36-37 days]); however, the mean annual increase in TTI was greater at community hospitals (0.56 days; 95% CI, 0.49-0.62 days) than at NCI centers (−0.73 days; 95% CI, −0.95 to −0.51 days) and academic centers (0.14 days; 95% CI, 0.03-0.26 days). An annual volume growth rate of 100 patients, a level observed at less than 1% of hospitals, was associated with a mean increase in TTI of 0.24 days (95% CI, 0.18-0.29 days).
Conclusions and Relevance
In this cross-sectional study, from 2007 to 2016, across the studied cancer types, patients increasingly initiated their cancer treatment at NCI and academic centers. Although increases in patient volume at these centers outpaced that at community hospitals, faster growth was not associated with clinically meaningful treatment delays.
Introduction
Increasing demand for cancer care services1,2 has raised concern of an impending capacity crisis in oncology care.3,4 Although an increase in patient volume is expected nationally,1 this may be particularly true at referral centers, such as National Cancer Institute (NCI)–designated and academic cancer centers given trends in health care consolidation5 and ongoing calls for the regionalization of complex care.6,7,8,9,10,11 Although some studies suggest improved outcomes among patients with cancer treated at NCI and academic cancer centers,12,13,14,15 increased demand for care at these institutions might exceed their capacity to provide timely cancer treatment16 and result in treatment delays.
Population-based studies have suggested that delays in cancer treatment are increasingly common17,18 and may occur more frequently at NCI and academic cancer centers.17,19 Delays are also disproportionately experienced by individuals from underserved racial/ethnic minority groups17,20 and those with low income.21 Because delays may be associated with patient distress22,23 and worse outcomes,17,20 time to treatment initiation (TTI) has been used both as a patient-centered quality metric24,25 and as an outcome to evaluate the impact of health policies.18,26 However, the mechanisms of recent increases in TTI remain poorly characterized, and to our knowledge, the degree to which hospital capacity strain has contributed to these increases has not been investigated.
Because increases in demand without concurrent increases in capacity may be associated with longer wait times,27,28,29,30,31 increases in TTI at centers experiencing greater patient volume growth could provide an early indication of capacity strain.32,33 Therefore, the objective of this study was to evaluate hospitals’ patient volume growth rates by hospital type and the association between these growth rates and TTI trends. We hypothesized that patient volume growth would increase more rapidly at NCI and academic centers than at community hospitals and that there would be an association between more rapid patient volume growth and treatment delays.
Methods
Study Design
We conducted a retrospective, hospital-level cross-sectional study of longitudinal data from January 1, 2007, to December 31, 2016, to evaluate patient volume growth rates by hospital type. We also evaluated the association between a hospital’s patient volume growth and TTI. The study was deemed exempt from review by the University of Pennsylvania institutional review board; informed consent was not required owing to the use of deidentified registry data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.34
Data Source
We used the National Cancer Database (NCDB), a hospital-based registry that captures patient-level data for those who receive either a cancer diagnosis or any part of the first course of treatment at a Commission on Cancer (CoC)–accredited facility. Jointly sponsored by the American College of Surgeons and American Cancer Society, the NCDB includes more than 1500 member institutions, representing approximately one-third of hospitals and nearly three-quarters of annual incident cancer cases in the US.35,36 Data from the NCDB are available to investigators upon application to and approval by the CoC.37
Population
The study population included adult patients who received a diagnosis of the following incident cancers from 2007 to 2016 and were treated within 365 days of diagnosis: breast, lung, prostate, colorectal, melanoma, bladder, non-Hodgkin lymphoma, kidney, uterine, or pancreatic. These represent the 10 most commonly diagnosed cancers in the US.38 Patients with cancer were identified using the International Classification of Diseases for Oncology, Third Edition site and histology codes (eMethods in the Supplement). We excluded individuals with noninvasive or in situ cancers, male breast cancer, small cell lung cancer, noncutaneous melanoma, or nonmuscle invasive bladder cancer. We also excluded patients for whom it was not possible to determine the type of hospital at which they initiated therapy (ie, those initiating treatment at a facility other than the reporting hospital and those who received a diagnosis when they were younger than 40 years, for whom the NCDB suppresses hospital type to prevent hospital identification).
Measures
The primary outcome was TTI, defined for each patient as the number of days between the date of diagnosis and the start of the first cancer treatment of any type. The TTI was derived from existing NCDB variables describing the number of days between diagnosis and treatment initiation for each of the following modalities: surgery, radiation therapy, and systemic therapy. The exposure was a hospital’s mean annual patient volume growth rate over the study period. Patient volume was defined annually as the total number of patients with an incident cancer diagnosis for whom the reporting hospital initiated a first course of treatment in the specified year. Each hospital was assigned 1 of 4 mutually exclusive designations as codified in the NCDB: community, academic, NCI, or integrated network.39
Covariates included age, sex, race/ethnicity (as reported to the NCDB), educational level, income, insurance type, distance from the patient’s residence to the treatment facility, rurality, Charlson-Deyo comorbidity score,40 history of cancer, cancer type, cancer stage, treatment modality, and whether care was transferred between the diagnosis and the start of treatment.
Statistical Analysis
We evaluated the association between a hospital’s mean annual patient volume growth rate and TTI using a linear mixed-effects model containing a patient volume × time interaction. The mean annual change in TTI over the study period by hospital type was estimated by including a hospital type × time interaction term. To estimate the mean annual patient volume growth rate by hospital type, we separately modeled patient volume as an outcome. Additional details of the model specifications are given in the eMethods in the Supplement. Our linear mixed-effects models contained a random time slope and a random intercept for each individual hospital (as identified by their facility number) to account for their differing growth rates. A hospital’s unique facility number was retained over time except in the case of hospital mergers or acquisitions, after which the consolidated entity adopted a new, distinct identification number. This approach allowed us to account for confounding owing to hospital mergers that would have otherwise produced large absolute increases in volume during the study period by essentially modeling separate entities before and after the merger or acquisition. We conducted secondary stratified analyses to examine trends by cancer type and initial treatment modality. Missing covariate data were imputed using multiple imputation by chained equations.41 The mean of 5 imputed data sets was calculated, and SEs were estimated using the Rubin formula.42
We were concerned a priori that increases in active surveillance of prostate cancer during the study period43,44 might result in a greater number of patients with low-risk prostate cancer managed with active surveillance rather than treatment initiation over time. Because the study population consisted of patients undergoing a first course of treatment, such changes could affect the total number of patients with prostate cancer included in the sample as well as the proportion of those patients with aggressive disease. Therefore, we designed a principal sensitivity analysis excluding patients with prostate cancer to test whether their inclusion biased our results. We also conducted the following additional sensitivity analyses to test key variable definitions and model specifications: an extreme case sensitivity analysis excluding patients with a TTI of 0, an analysis including only patients treated within 180 days of diagnosis, an analysis including only the 85% of hospitals continuously represented in the data set during all 10 years of our study (because hospitals may have gained or lost CoC accreditation), and an analysis including cases in which treatment was initiated outside the reporting facility. Although TTI for patients treated at another hospital was unlikely to be determined by the reporting hospital, such patients still contributed to annual patient volume, and therefore, their inclusion could have potentially altered the association between the mean annual patient volume growth and TTI.
Data were analyzed using Stata software, version 15 (StataCorp LLC) and RStudio, version 1.1.463 (RStudio Team) between December 19, 2019, and March 27, 2020. We evaluated our hypotheses using 2-tailed tests at a significance level of P = .02 (Bonferroni correction for testing 3 primary hypotheses45).
Results
Study Population
We identified 4 218 577 patients (mean [SD] age, 65.0 [11.4] years; 56.6% women) who received a diagnosis of an incident cancer from 2007 to 2016 and initiated a first course of treatment at 1351 hospitals (49.3% at 897 community hospitals, 23.2% at 177 academic hospitals, 13.8% at 50 NCI hospitals, and 13.7% at 227 integrated network hospitals). The study population flow diagram is shown in the eFigure in the Supplement. Table 1 presents patient demographic and clinical characteristics by hospital type. Compared with patients treated at community hospitals, those treated at academic and NCI centers more frequently were male, were non-White individuals, had higher income, had a higher educational level, were privately insured, were residents of urban areas, had a diagnosis of stage IV disease, received systemic therapy, and had care transferred to the treating hospital.
Table 1. Patient Demographic and Clinical Characteristics by Hospital Type From 2007 to 2016.
| Variable | Patientsa | ||||
|---|---|---|---|---|---|
| Community hospital (n = 2 078 051) | Academic hospital (n = 979 318) | NCI hospital (n = 583 994) | Integrated hospital (n = 577 214) | Total (N = 4 218 577) | |
| Age, mean (SD), y | 65.8 (11.5) | 64.2 (11.2) | 63.3 (10.9) | 65.0 (11.4) | 65.0 (11.4) |
| Sex | |||||
| Male | 865 016 (41.6) | 430 780 (44.0) | 294 081 (50.4) | 241 124 (41.8) | 1 831 001 (43.4) |
| Female | 1 213 035 (58.4) | 548 538 (56.0) | 289 913 (49.6) | 336 090 (58.2) | 2 387 576 (56.6) |
| Race | |||||
| White | 1 818 997 (87.5) | 774 035 (79.0) | 487 788 (83.5) | 487 886 (84.5) | 3 568 706 (84.6) |
| Black | 186 504 (9.0) | 149 189 (15.2) | 63 963 (11.0) | 66 508 (11.5) | 466 164 (11.1) |
| Asian | 43 967 (2.1) | 32 357 (3.3) | 17 137 (2.9) | 10 589 (1.8) | 104 050 (2.5) |
| American Indian | 7367 (0.4) | 1612 (0.2) | 1895 (0.3) | 833 (0.1) | 11 707 (0.3) |
| Other | 9496 (0.5) | 10 720 (1.1) | 5195 (0.9) | 4458 (0.8) | 29 869 (0.7) |
| Missing | 11 720 (0.6) | 11 405 (1.2) | 8016 (1.4) | 6940 (1.2) | 38 081 (0.9) |
| Ethnicity | |||||
| Non-Hispanic | 1 902 354 (91.5) | 869 020 (88.7) | 543 230 (93.0) | 522 444 (90.5) | 3 837 048 (91.0) |
| Hispanic | 77 450 (3.7) | 59 226 (6.0) | 25 360 (4.3) | 24 467 (4.2) | 186 503 (4.4) |
| Missing | 98 247 (4.7) | 51 072 (5.2) | 15 404 (2.6) | 30 303 (5.2) | 195 026 (4.6) |
| Incomeb | |||||
| Quartile 1 | 361 532 (17.4) | 180 533 (18.4) | 85 470 (14.6) | 82 909 (14.4) | 710 444 (16.8) |
| Quartile 2 | 508 735 (24.5) | 183 914 (18.8) | 116 274 (19.9) | 119 498 (20.7) | 928 421 (22.0) |
| Quartile 3 | 543 412 (26.2) | 232 049 (23.7) | 133 417 (22.8) | 156 536 (27.1) | 1 065 414 (25.3) |
| Quartile 4 | 658 604 (31.7) | 380 273 (38.8) | 246 984 (42.3) | 216 514 (37.5) | 1 502 375 (35.6) |
| Missing | 5768 (0.3) | 2549 (0.3) | 1849 (0.3) | 1757 (0.3) | 11 923 (0.3) |
| Educational levelc | |||||
| Quartile 1 | 357 873 (17.2) | 193 626 (19.8) | 86 261 (14.8) | 74 793 (13.0) | 712 553 (16.9) |
| Quartile 2 | 546 606 (26.3) | 242 737 (24.8) | 130 183 (22.3) | 138 133 (23.9) | 1 057 659 (25.1) |
| Quartile 3 | 673 007 (32.4) | 278 269 (28.4) | 176 353 (30.2) | 196 961 (34.1) | 1 324 590 (31.4) |
| Quartile 4 | 495 960 (23.9) | 262 555 (26.8) | 189 629 (32.5) | 165 874 (28.7) | 1 114 018 (26.4) |
| Missing | 4605 (0.2) | 2131 (0.2) | 1568 (0.3) | 1453 (0.3) | 9757 (0.2) |
| Insurance | |||||
| Uninsured | 43 181 (2.1) | 37 407 (3.8) | 10 410 (1.8) | 10 615 (1.8) | 101 613 (2.4) |
| Private | 846 780 (40.7) | 425 105 (43.4) | 273 302 (46.8) | 248 678 (43.1) | 1 793 865 (42.5) |
| Medicaid | 88 692 (4.3) | 66 032 (6.7) | 27 641 (4.7) | 27 025 (4.7) | 209 390 (5.0) |
| Medicare | 1 050 978 (50.6) | 424 020 (43.3) | 235 265 (40.3) | 279 725 (48.5) | 1 989 988 (47.2) |
| Other government | 25 139 (1.2) | 10 919 (1.1) | 9060 (1.6) | 6378 (1.1) | 51 496 (1.2) |
| Missing | 23 281 (1.1) | 15 835 (1.6) | 28 316 (4.8) | 4793 (0.8) | 72 225 (1.7) |
| Charlson-Deyo comorbidity score | |||||
| 0 | 1 502 788 (72.3) | 733 013 (74.8) | 453 972 (77.7) | 418 045 (72.4) | 3 107 818 (73.7) |
| 1 | 417 813 (20.1) | 179 081 (18.3) | 96 595 (16.5) | 114 434 (19.8) | 807 923 (19.2) |
| 2 | 111 789 (5.4) | 46 015 (4.7) | 23 062 (3.9) | 31 180 (5.4) | 212 046 (5.0) |
| ≥3 | 45 661 (2.2) | 21 209 (2.2) | 10 365 (1.8) | 13 555 (2.3) | 90 790 (2.2) |
| Prior cancer | |||||
| No | 1 723 818 (83.0) | 816 725 (83.4) | 474 573 (81.3) | 476 094 (82.5) | 3 491 210 (82.8) |
| Yes | 354 135 (17.0) | 162 542 (16.6) | 109 409 (18.7) | 101 038 (17.5) | 727 124 (17.2) |
| Missing | 98 (0.0) | 51 (0.0) | 12 (0.0) | 82 (0.0) | 243 (0.0) |
| Distance to hospital, median (IQR), mi | 8.9 (4.0-19.7) | 10.0 (4.6-23.4) | 23.7 (9.2-62.9) | 8.6 (4.2-17.1) | 10.1 (4.5-23.6) |
| Transfer of care | |||||
| No | 1 511 776 (72.7) | 627 456 (64.1) | 243 245 (41.7) | 393 567 (68.2) | 2 776 044 (65.8) |
| Yes | 566 275 (27.3) | 351 862 (35.9) | 340 749 (58.3) | 183 647 (31.8) | 1 442 533 (34.2) |
| Geographic location | |||||
| Metropolitan | 1 617 679 (77.8) | 856 553 (87.5) | 483 607 (82.8) | 505 543 (87.6) | 3 463 382 (82.1) |
| Nonmetropolitan | 410 826 (19.8) | 102 638 (10.5) | 86 922 (14.9) | 48 841 (8.5) | 649 227 (15.4) |
| Missing | 49 546 (2.4) | 20 127 (2.1) | 13 465 (2.3) | 22 830 (4.0) | 105 968 (2.5) |
| Cancer type | |||||
| Breast | 654 927 (31.5) | 252 221 (25.8) | 119 379 (20.4) | 169 845 (29.4) | 1 196 372 (28.4) |
| Lung | 319 384 (15.4) | 148 390 (15.2) | 89 665 (15.4) | 84 292 (14.6) | 641 731 (15.2) |
| Prostate | 344 016 (16.6) | 181 829 (18.6) | 127 272 (21.8) | 101 910 (17.7) | 755 027 (17.9) |
| Colon | 270 978 (13.0) | 92 056 (9.4) | 32 755 (5.6) | 68 508 (11.9) | 464 297 (11.0) |
| Rectal | 48 204 (2.3) | 21 506 (2.2) | 13 025 (2.2) | 11 691 (2.0) | 94 426 (2.2) |
| Melanoma | 64 361 (3.1) | 38 973 (4.0) | 40 446 (6.9) | 20 477 (3.5) | 164 257 (3.9) |
| Bladder | 22 944 (1.1) | 12 578 (1.3) | 10 631 (1.8) | 5939 (1.0) | 52 092 (1.2) |
| NHLd | 85 043 (4.1) | 44 027 (4.5) | 36 221 (6.2) | 20 871 (3.6) | 186 162 (4.4) |
| Kidney | 115 368 (5.6) | 67 353 (6.9) | 45 456 (7.8) | 36 390 (6.3) | 264 567 (6.3) |
| Uterine | 111 300 (5.4) | 87 429 (8.9) | 38 329 (6.6) | 43 487 (7.5) | 280 545 (6.7) |
| Pancreatic | 41 526 (2.0) | 32 956 (3.4) | 30 815 (5.3) | 13 804 (2.4) | 119 101 (2.8) |
| Cancer stage | |||||
| I | 847 390 (40.8) | 401 617 (41.0) | 218 190 (37.4) | 246 066 (42.6) | 1 713 263 (40.6) |
| II | 656 528 (31.6) | 301 344 (30.8) | 179 625 (30.8) | 182 014 (31.5) | 1 319 511 (31.3) |
| III | 337 202 (16.2) | 158 073 (16.1) | 98 817 (16.9) | 90 929 (15.8) | 685 021 (16.2) |
| IV | 236 931 (11.4) | 118 284 (12.1) | 87 362 (15.0) | 58 205 (10.1) | 500 782 (11.9) |
| First treatment | |||||
| Surgery | 1 497 871 (72.1) | 692 898 (70.8) | 387 950 (66.4) | 437 582 (75.8) | 3 016 301 (71.5) |
| Radiation | 230 853 (11.1) | 103 854 (10.6) | 50 130 (8.6) | 62 649 (10.9) | 447 486 (10.6) |
| Systemic | 349 327 (16.8) | 182 566 (18.6) | 145 914 (25.0) | 76 983 (13.3) | 754 790 (17.9) |
Abbreviations: IQR, interquartile range; NCI, National Cancer Institute; NHL, non-Hodgkin lymphoma.
Data are presented as number (percentage) of patients unless otherwise specified.
Quartile at the zip code level. From 2007 to 2012, quartile 1 was less than $38 000; quartile 2, $38 000 to $47 999; quartile 3, $48 000 to $62 999; and quartile 4, greater than $63 000. From 2013 to 2016, quartile 1 was less than $40 227; quartile 2, $40 227 to $50 353; quartile 3, $50 354 to $63 332; and quartile 4, greater than $63 333.
Quartiles at the zip code level for the percentage of adults who did not graduate high school. From 2007 to 2012, quartile 1 was 21% or more; quartile 2, 13% to 20.9%; quartile 3, 7% to 12.9%; and quartile 4, less than 7%. From 2013 to 2016, quartile 1 was 17.6% or more; quartile 2, 10.9% to 17.5%; quartile 3, 6.3% to 10.8%; and quartile 4, less than 6.3%.
Includes chronic lymphocytic leukemia and small lymphocytic lymphoma.
Patient Volume Growth by Hospital Type
In 2007, the mean number of patients with incident cancer diagnoses who had treatment initiated at NCI hospitals was 1027 (95% CI, 960-1093 patients), at academic hospitals was 505 (95% CI, 470-540 patients), and at community hospitals was 232 (95% CI, 217-248 patients) (Table 2). Figure 1A shows trends in patient volume by hospital type. From 2007 to 2016, patient volume increased by 40% at NCI hospitals, 25% at academic hospitals, and 8% at community hospitals; the mean annual patient volume growth rate was 45.2 patients (95% CI, 38.1-52.2 patients) at NCI hospitals and 13.9 patients (95% CI, 10.2-17.5 patients) at academic hospitals compared with 2.0 patients (95% CI, 0.4-3.7 patients) at community hospitals (Table 2). In analyses stratified by cancer type, NCI and academic hospitals experienced significantly higher mean annual patient volume growth compared with community hospitals across all cancer types except prostate cancer (Figure 2 and eTable in the Supplement). Growth rates were proportionally greatest at NCI centers for patients with non-Hodgkin lymphoma and pancreatic cancer; for both cancer types, the volume increased 69% over the study period. Increases in volume were lower for bladder (30%) and kidney (38%) cancers. When stratified by treatment modality, growth rates were higher at NCI and academic centers regardless of whether patients were initially treated with surgery or systemic therapy.
Table 2. Level and Annual Growth Rate of Patient Volume and Adjusted TTI by Hospital Type, 2007-2016.
| Variable | Communitya | Academic | NCI | Integrated |
|---|---|---|---|---|
| Patients treated, mean (95% CI), No. | ||||
| 2007 | 232 (217 to 248) | 505 (470 to 540)b | 1027 (960 to 1093)b | 248 (217 to 279) |
| 2016 | 251 (229 to 272) | 630 (582 to 678)b | 1433 (1342 to 1524)b | 278 (235 to 321) |
| Mean annual patient volume growth rate, mean (95% CI), No. | 2.0 (0.4 to 3.7) | 13.9 (10.2 to 17.5)b | 45.2 (38.1 to 52.2)b | 3.3 (0.0 to 6.6) |
| Adjusted TTI, mean (95% CI), d | ||||
| 2007 | 37 (36 to 37) | 43 (42 to 44)b | 50 (48 to 52)b | 38 (37 to 39) |
| 2016 | 42 (41 to 42) | 44 (43 to 45)c | 43 (42 to 45) | 42 (41 to 43) |
| Mean annual TTI growth rate, mean (95% CI), d | 0.56 (0.49 to 0.62) | 0.14 (0.03 to 0.26)b | −0.73 (−0.95 to −0.51)b | 0.51 (0.39 to 0.63) |
Abbreviations: NCI, National Cancer Institute; TTI, time to treatment initiation.
Reference.
P < .001 compared with reference.
P = .003.
Figure 1. Trends in Patient Volume and Time to Treatment Initiation Among All Cancer Types by Hospital Type From 2007 to 2016.
Shaded areas represent 95% CIs. NCI indicates National Cancer Institute.
Figure 2. Trends in Patient Volume by Hospital Type and Cancer Type From 2007 to 2016.
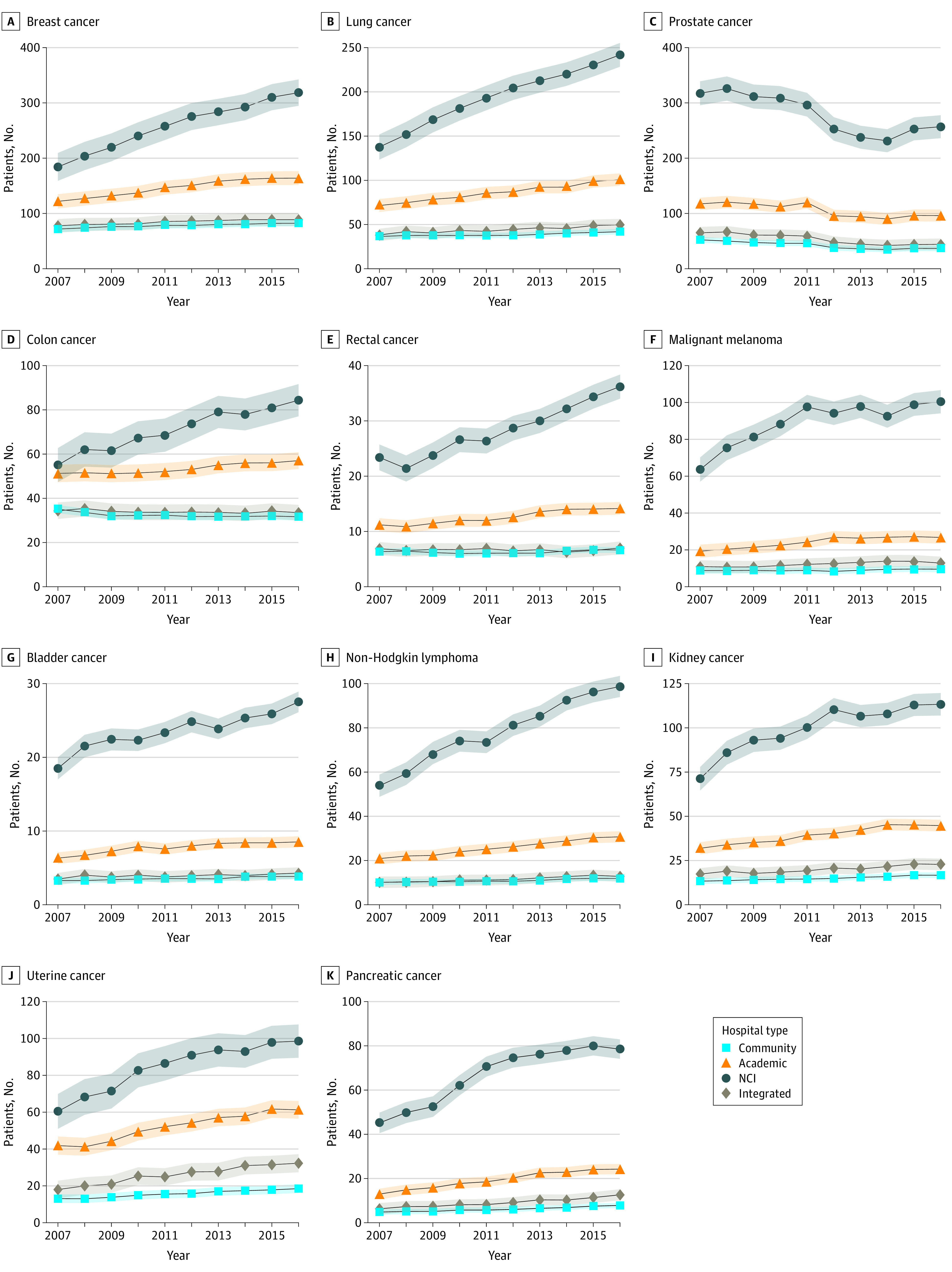
Shaded areas represent 95% CIs. NCI indicates National Cancer Institute.
Trends in TTI by Hospital Type
Figure 1B shows trends in TTI by hospital type before adjustment for patient, tumor, and treatment factors. Figure 3 shows unadjusted TTI trends stratified by cancer type. Unadjusted TTI was longer at NCI and academic hospitals than at community hospitals throughout the study period (Figure 1B). The adjusted TTI in 2007 and 2016 by hospital type and the mean annual TTI growth rates by hospital type are shown in Table 2. After adjustment, in 2007, the mean TTI was significantly longer at NCI (50 days; 95% CI, 48-52 days) and academic (43 days; 95% CI, 42-44 days) hospitals than at community hospitals (37 days; 95% CI, 36-37 days). However, the adjusted annual mean TTI growth rate was greater at community hospitals (0.56 days; 95% CI, 0.49-0.62 days) than at NCI centers (−0.73 days; 95% CI, −0.95 to −0.51 days), where there was a mean annual reduction in TTI, and academic hospitals (0.14 days; 95% CI, 0.03-0.26 days). Therefore, by 2016, the difference in the adjusted TTI between academic and community hospitals had attenuated (44 days [95% CI, 43 to 45 days] vs 42 days [95% CI, 41 to 42 days]), and there was no statistically significant difference in the mean adjusted TTI between NCI hospitals (43 days; 95% CI, 42-45 days) and community hospitals (42 days; 95% CI, 41-42 days) (Table 2).
Figure 3. Trends in Unadjusted Time to Treatment Initiation by Hospital Type and Cancer Type From 2007 to 2016.
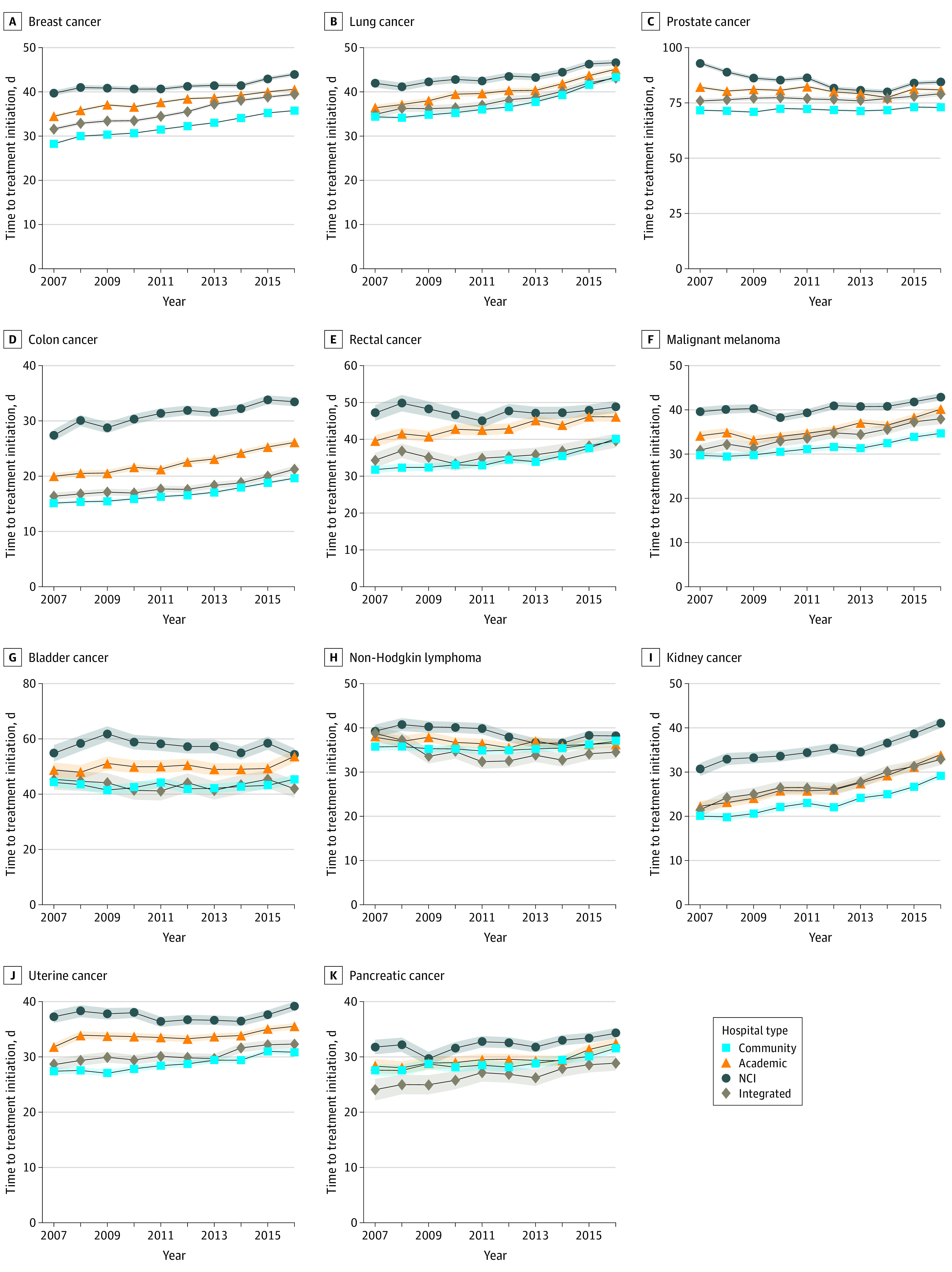
Shaded areas represent 95% CIs. NCI indicates National Cancer Institute.
Association of Patient Volume Growth Rate With Changes in TTI and Results of Sensitivity Analyses
Mean annual volume growth of 100 patients, a level observed in less than 1% of hospitals in the sample, was associated with an annual mean increase in TTI of 0.24 days (95% CI, 0.18-0.29 days). The association between this level of annual patient volume growth and changes in TTI was similar across hospital types (NCI centers: 0.55 days [95% CI, 0.09 to 1.01 days]; academic hospitals: −0.04 days [95% CI, −0.72 to 0.64 days]; community hospitals: 0.17 days [95% CI, −0.38 to 0.72 days]). The results of models stratified by cancer type did not demonstrate an association between greater volume growth and increases in TTI. The results of our sensitivity analyses excluding prostate cancer, excluding patients with a TTI of 0, including only patients treated within 180 days of diagnosis, including only continuously represented hospitals, and including cases in which treatment was initiated outside the reporting facility were similar to those of the primary analysis.
Discussion
From 2007 to 2016, NCI and academic centers experienced greater patient volume growth than did community hospitals for all but 1 of the 10 cancer types included in our study. However, over the same period, TTI increased most rapidly at community hospitals, while the mean TTI decreased over time at NCI centers. We found no evidence that receipt of treatment at hospitals with greater patient volume growth was associated with clinically meaningful treatment delays. Our study is, to our knowledge, the first to investigate whether rapid patient volume growth is associated with capacity constraints leading to cancer treatment delays. The findings suggest that NCI and academic centers had or developed sufficient capacity during this period to treat an increasing number of patients with newly diagnosed cancer without incurring delays in treatment.
Increases in the demand for cancer care in the US have not been evenly distributed, with NCI and academic centers treating an increasing share of the country’s incident cancer cases across cancer types. Greater increases in patient volume at referral centers may reflect increasing health care consolidation over the past decade5 and an increasing tendency for community hospitals that are affiliated with NCI and academic centers to refer patients centrally for oncologic care. Although there is some evidence that health systems–based centralization of cancer care has occurred,46 it remains unclear whether acquisitions and affiliations are associated with improvement in either care processes or patient outcomes.47,48 Financial incentives may also contribute to patient volume growth given that high revenues in oncology49 have been shown to incentivize hospitals to expand their cancer treatment capacity.50 Also, increased volume at referral centers might be the result of specific efforts aimed at regionalizing cancer care, but these efforts have focused primarily on specific complex cancer surgeries, such as pancreatic, esophageal, lung, and rectal cancer resections,51,52 and our analysis demonstrated higher patient volume growth rates at NCI and academic hospitals across cancer types and for both surgical and systemic therapy.
In contrast to our hypothesis, we observed the greatest increase in TTI among community hospitals, where the slowest increases in patient volume occurred, whereas TTI decreased at NCI centers despite higher mean annual volume growth. The lack of association between the increasing number of patients treated at referral centers and treatment delays might reflect efficiency gains, expanded capacity, or quality improvement efforts during the study period. Previous efforts have shown that hospitals can increase their efficiency through interventions to improve communication, enhance coordination, and leverage technology.53
Although referral centers appear to have met the increased demand for cancer care without delaying treatment, our results suggest several areas for improvement. First, patients treated at NCI and academic centers waited longer to start treatment than did patients at community hospitals before adjustment for patient population. However, when adjusting for case mix and facility transfer, this difference was eliminated or attenuated, respectively, in later years. This may reflect a number of factors, such as a need to gather multidisciplinary input into treatment decisions, to reevaluate data that were obtained at another site, or to repeat tissue sample obtainment.54 Second, for most of the cancers studied, TTI increased regardless of hospital type. Although this trend may partially reflect the increasing complexity of treatment decisions and a desire to consider the results of molecular testing when making frontline treatment decisions, previous work has shown that hospitals can successfully implement programs that reduce treatment delays.53 Because of the potential association of such delays with psychosocial distress22,23 and survival,17,20 continued efforts to ensure timely cancer treatment are warranted.
Limitations
This study has limitations. First, this was an observational study, and although our linear mixed-effects model accounted for both observed changes in hospital case mix over time and hospital-specific factors, we could not rule out bias owing to unobserved changes in a hospital’s patient population over time. We performed several sensitivity analyses to test the robustness of our findings to potentially time-varying characteristics, and each yielded results that were similar to those of the primary analysis. A second limitation was that the NCDB is a hospital-based registry, and inclusion in the database depends on CoC accreditation. Hospitals without CoC accreditation may have had trends that differed from those we observed. However, because our hypotheses focused on changes over time at the hospital level, a hospital-based registry was well suited for the present analysis. Furthermore, the NCDB contains nearly three-quarters of all incident cancer diagnoses in the US and is likely to reflect the circumstances in which most patients receive their cancer care. In addition, although the findings of our study are representative of only the cancer types that we selected and that were treated in the first-line setting during the study period, these cancer types collectively represented more than half of incident cancer diagnoses in the US. Future studies should seek to understand whether the results are generalizable to patients with other cancer types and to those seeking second- or later-line therapy. Further investigation is also needed to understand whether referral centers will continue to be able to expand their capacity at the rates observed in our study.
Conclusions
In this cross-sectional study, patient volume growth was most rapid at NCI and academic centers across cancer types, but more rapid growth was not associated with clinically meaningful delays in cancer treatment during the study period. Additional investigation is needed to determine whether the observed patient volume growth rates are sustainable.
eMethods. ICD-O-3 Site and Histology Codes Used to Define Cancer Types
eFigure. Patient Flow Diagram
eTable. Level and Annual Growth Rate of Patient Volume by Cancer Type
References
- 1.Yang W, Williams JH, Hogan PF, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract. 2014;10(1):39-45. doi: 10.1200/JOP.2013.001319 [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takvorian SU, Balogh E, Nass S, et al. Developing and sustaining an effective and resilient oncology careforce: opportunities for action. J Natl Cancer Inst. 2020;112(7):663-670. doi: 10.1093/jnci/djz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shulman LN, Sheldon LK, Benz EJ. The future of cancer care in the United States-overcoming workforce capacity limitations. JAMA Oncol. 2020;6(3):327-328. doi: 10.1001/jamaoncol.2019.5358 [DOI] [PubMed] [Google Scholar]
- 5.Fulton BD. Health care market concentration trends in the United States: evidence and policy responses. Health Aff (Millwood). 2017;36(9):1530-1538. doi: 10.1377/hlthaff.2017.0556 [DOI] [PubMed] [Google Scholar]
- 6.Tandstad T, Kollmannsberger CK, Roth BJ, et al. Practice makes perfect: the rest of the story in testicular cancer as a model curable neoplasm. J Clin Oncol. 2017;35(31):3525-3528. doi: 10.1200/JCO.2017.73.4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halm EA, Anderson LD Jr, Gerber DE. Understanding the relationship between care volume and clinical outcomes in multiple myeloma. J Clin Oncol. 2017;35(6):580-582. doi: 10.1200/JCO.2016.70.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein AM. Volume and outcome–it is time to move ahead. N Engl J Med. 2002;346(15):1161-1164. doi: 10.1056/NEJM200204113461512 [DOI] [PubMed] [Google Scholar]
- 9.Fong Y, Patti MG. Volume standards for high-risk cancer surgery. JAMA Surg. 2019;154(11):1012-1013. doi: 10.1001/jamasurg.2019.3018 [DOI] [PubMed] [Google Scholar]
- 10.Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business School Press; 2006. [Google Scholar]
- 11.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128-2137. doi: 10.1056/NEJMsa1010705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shulman LN, Palis BE, McCabe R, et al. Survival as a quality metric of cancer care: use of the National Cancer Data Base to assess hospital performance. J Oncol Pract. 2018;14(1):e59-e72. doi: 10.1200/JOP.2016.020446 [DOI] [PubMed] [Google Scholar]
- 13.Wolfson JA, Sun C-L, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: results from a population-based study. Cancer. 2015;121(21):3885-3893. doi: 10.1002/cncr.29576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfister DG, Rubin DM, Elkin EB, et al. Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol. 2015;1(9):1303-1310. doi: 10.1001/jamaoncol.2015.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birkmeyer NJO, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103(3):435-441. doi: 10.1002/cncr.20785 [DOI] [PubMed] [Google Scholar]
- 16.Raphael MJ, Siemens DR, Booth CM. Would regionalization of systemic cancer therapy improve the quality of cancer care? J Oncol Pract. 2019;15(7):349-356. doi: 10.1200/JOP.18.00671 [DOI] [PubMed] [Google Scholar]
- 17.Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS One. 2019;14(4):e0215108. doi: 10.1371/journal.pone.0215108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takvorian SU, Oganisian A, Mamtani R, et al. Association of Medicaid expansion under the Affordable Care Act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Netw Open. 2020;3(2):e1921653. doi: 10.1001/jamanetworkopen.2019.21653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779-785. doi: 10.1097/SLA.0b013e318211cc0f [DOI] [PubMed] [Google Scholar]
- 20.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330-339. doi: 10.1001/jamaoncol.2015.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graboyes EM, Halbert CH, Li H, et al. Barriers to the delivery of timely, guideline-adherent adjuvant therapy among patients with head and neck cancer. JCO Oncol Pract. 2020;16(12):e1417-e1432. doi: 10.1200/OP.20.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RE, Fitch MI, Phillips C, Labrecque M, Klotz L. Presurgery experiences of prostate cancer patients and their spouses. Cancer Pract. 1999;7(3):130-135. doi: 10.1046/j.1523-5394.1999.07308.x [DOI] [PubMed] [Google Scholar]
- 23.Jones RVH, Greenwood B. Breast cancer: causes of patients’ distress identified by qualitative analysis. Br J Gen Pract. 1994;44(385):370-371. [PMC free article] [PubMed] [Google Scholar]
- 24.The Commission on Cancer . The National Accreditation Program for Rectal Cancer Standards Manual: 2017 Edition. American College of Surgeons; 2017. [Google Scholar]
- 25.The Commission on Cancer . National Accreditation Program For Breast Centers Standards Manual: 2018 Edition. American College of Surgeons; 2018. [Google Scholar]
- 26.Adamson BJS, Cohen AB, Estevez M, et al. Affordable Care Act (ACA) Medicaid expansion impact on racial disparities in time to cancer treatment. J Clin Oncol. 2019;37(18):LBA1. doi: 10.1200/JCO.2019.37.18_suppl.LBA1 [DOI] [Google Scholar]
- 27.Pines JM, Shofer FS, Isserman JA, Abbuhl SB, Mills AM. The effect of emergency department crowding on analgesia in patients with back pain in two hospitals. Acad Emerg Med. 2010;17(3):276-283. doi: 10.1111/j.1553-2712.2009.00676.x [DOI] [PubMed] [Google Scholar]
- 28.Pines JM, Localio AR, Hollander JE, et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007;50(5):510-516. doi: 10.1016/j.annemergmed.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 29.Terwiesch C, Diwas KC, Kahn JM. Working with capacity limitations: operations management in critical care. Crit Care. 2011;15(4):308. doi: 10.1186/cc10217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palvannan RK, Teow KL. Queueing for healthcare. J Med Syst. 2012;36(2):541-547. doi: 10.1007/s10916-010-9499-7 [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee P, Cucchiara BL, Lazarciuc N, Shofer FS, Pines JM. Emergency department crowding and time to care in patients with acute stroke. Stroke. 2011;42(4):1074-1080. doi: 10.1161/STROKEAHA.110.586610 [DOI] [PubMed] [Google Scholar]
- 32.Mant J, Hicks NR. Assessing quality of care: what are the implications of the potential lack of sensitivity of outcome measures to differences in quality? J Eval Clin Pract. 1996;2(4):243-248. doi: 10.1111/j.1365-2753.1996.tb00054.x [DOI] [PubMed] [Google Scholar]
- 33.Mant J, Hicks N. Detecting differences in quality of care: the sensitivity of measures of process and outcome in treating acute myocardial infarction. BMJ. 1995;311(7008):793-796. doi: 10.1136/bmj.311.7008.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 35.Mallin K, Browner A, Palis B, et al. Incident cases captured in the National Cancer Database compared with those in US population based central cancer registries in 2012-2014. Ann Surg Oncol. 2019;26(6):1604-1612. doi: 10.1245/s10434-019-07213-1 [DOI] [PubMed] [Google Scholar]
- 36.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722-1728. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 37.American College of Surgeons . Participant user files. Accessed April 14, 2021. https://www.facs.org/quality-programs/cancer/ncdb/puf
- 38.National Cancer Institute . Cancer stat facts: common cancer sites. Accessed February 2, 2020. https://seer.cancer.gov/statfacts/html/common.html
- 39.American College of Surgeons. About cancer program categories. Accessed October 7, 2019. https://www.facs.org/quality-programs/cancer/coc/accreditation/categories
- 40.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 41.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 42.Rubin D. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987.. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 43.Ingimarsson JP, Celaya MO, Laviolette M, Rees JR, Hyams ES. Trends in initial management of prostate cancer in New Hampshire. Cancer Causes Control. 2015;26(6):923-929. doi: 10.1007/s10552-015-0574-8 [DOI] [PubMed] [Google Scholar]
- 44.Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC; Michigan Urological Surgery Improvement Collaborative . Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67(1):44-50. doi: 10.1016/j.eururo.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 45.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310(6973):170. doi: 10.1136/bmj.310.6973.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheetz KH, Dimick JB, Nathan H. Centralization of high-risk cancer surgery within existing hospital systems. J Clin Oncol. 2019;37(34):3234-3242. doi: 10.1200/JCO.18.02035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaulieu ND, Dafny LS, Landon BE, Dalton JB, Kuye I, McWilliams JM. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020;382(1):51-59. doi: 10.1056/NEJMsa1901383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resio BJ, Hoag JR, Chiu AS, et al. Variations in surgical safety according to affiliation status with a top-ranked cancer hospital. JAMA Oncol. 2019;5(9):1359-1362. doi: 10.1001/jamaoncol.2019.1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conti RM, Nikpay SS, Buntin MB. Revenues and profits from Medicare patients in hospitals participating in the 340B drug discount program, 2013-2016. JAMA Netw Open. 2019;2(10):e1914141. doi: 10.1001/jamanetworkopen.2019.14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai S, McWilliams JM. Consequences of the 340B drug pricing program. N Engl J Med. 2018;378(6):539-548. doi: 10.1056/NEJMsa1706475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheetz KH, Chhabra KR, Smith ME, Dimick JB, Nathan H. Association of discretionary hospital volume standards for high-risk cancer surgery with patient outcomes and access, 2005-2016. JAMA Surg. 2019;154(11):1005-1012. doi: 10.1001/jamasurg.2019.3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373(15):1388-1390. doi: 10.1056/NEJMp1508472 [DOI] [PubMed] [Google Scholar]
- 53.Khorana AA, Bolwell BJ. Reducing time-to-treatment for newly diagnosed cancer patients. February 14, 2019. Accessed August 20, 2019. https://catalyst.nejm.org/time-to-treatment-cancer-patients/
- 54.Kruger S, Schirle K, Haas M, et al. Prolonged time to treatment initiation in advanced pancreatic cancer patients has no major effect on treatment outcome: a retrospective cohort study controlled for lead time bias and waiting time paradox. J Cancer Res Clin Oncol. 2020;146(2):391-399. doi: 10.1007/s00432-019-03061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. ICD-O-3 Site and Histology Codes Used to Define Cancer Types
eFigure. Patient Flow Diagram
eTable. Level and Annual Growth Rate of Patient Volume by Cancer Type