Abstract
Introduction
For persons with hemophilia, optimization of joint outcomes is an important unmet need. The aim of this initiative was to determine use of ultrasound in evaluating arthropathy in persons with hemophilia, and to move toward consensus among hemophilia care providers regarding the preferred ultrasound protocols for global adaptation.
Methods
A global survey of hemophilia treatment centers was conducted that focused on understanding how and why ultrasound was being used and endeavored to move toward consensus definitions of both point‐of‐care musculoskeletal ultrasound (POC‐MSKUS) and full diagnostic ultrasound, terminology to describe structures being assessed by ultrasound, and how these assessments should be interpreted. Next, an in‐person meeting of an international group of hemophilia health care professionals and patient representatives was held, with the objective of achieving consensus regarding the acquisition and interpretation of POC‐MSKUS and full diagnostic ultrasound for use in the assessment of musculoskeletal (MSK) pathologies in persons with hemophilia.
Results
The recommendations were that clear definitions of the types of ultrasound examinations should be adopted and that a standardized ultrasound scoring/measurement system should be developed, tested, and implemented. The scoring/measurement system should be tiered to allow for a range of complexity yet maintain the ability for comparison across levels.
Conclusion
Ultrasound is an evolving technology increasingly used for the assessment of MSK outcomes in persons with hemophilia. As adoption increases globally for clinical care and research, it will become increasingly important to establish clear guidelines for image acquisition, interpretation, and reporting to ensure accuracy, consistency, and comparability across groups.
Keywords: consensus, hemophilia, musculoskeletal, surveys, ultrasonography
Essentials.
Ultrasound is an evolving technology gaining traction for assessment of persons with hemophilia.
To optimize outcomes, standardized ultrasound protocols should be adopted globally.
A tiered ultrasound scoring/measurement system allowing comparison across levels is recommended.
Guidelines for ultrasound acquisition, interpretation, and reporting will ensure consistency.
1. INTRODUCTION
Recurrent hemarthrosis and resultant hemophilic arthropathy continue to be a major cause of morbidity in persons with hemophilia 1 , 2 , 3 , 4 despite the rapidly advancing hemophilia treatment landscape and widespread introduction of prophylaxis with consequent improvement in bleed prevention and treatment strategies. 5 , 6 , 7 , 8 , 9 , 10 , 11 Hemophilia treatment center (HTC) providers have a growing interest in developing more accurate and objective methods for the assessment of acute musculoskeletal (MSK) episodes, joint health, and efficacy of novel hemostatic agents (factor, nonfactor, and gene therapy). Bleeding may be reduced with newer treatments, but any bleeding, including subclinical bleeding, can be deleterious to the joint. Optimization of long‐term joint outcomes is an important unmet need.
Musculoskeletal ultrasound (MSKUS) has emerged as a promising tool to serve as an adjunct to clinical evaluation of acute bleeding episodes. 12 , 13 MSKUS has also been used for longitudinal joint health assessment through assessment of disease activity (joint effusion and synovial proliferation) and, to a lesser extent, osteochondral derangement. 14 , 15 , 16 , 17 , 18 Several point‐of‐care musculoskeletal ultrasound (POC‐MSKUS) and full diagnostic MSKUS scanning protocols and scoring systems have been proposed over the past two decades, with varying degrees of validation, adoption, and implementation by HTCs. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Ideally, one key goal for MSKUS would be to detect clinically significant joint disease or predictors of joint disease at an early stage where intervention (e.g., administration or intensification of prophylaxis) is likely to be beneficial. 4 , 27
The aim of this initiative was to determine how ultrasound is currently used for the assessment of persons with severe inherited bleeding disorders, focusing on hemophilia, and to move toward establishing consensus among hemophilia care providers regarding the use of proposed ultrasound protocols, by conducting a survey and a 2‐day in‐person consensus meeting of relevant experts in the fields of hemophilia and imaging.
The objective of the survey was to determine the scope of use of MSKUS (POC‐MSKUS and full‐diagnostic MSKUS) for the management of persons with hemophilia as part of the comprehensive care model at HTCs, with focus on MSKUS definitions and terminology, image acquisition protocols, interpretation of findings, and grading or scoring.
Using data from the survey, existing literature, and expert opinion from the planning committee (PB, VB), the objectives of the 2018 Toronto Ultrasound Meeting were formulated to answer a series of questions listed in Table 1.
TABLE 1.
Summary of the meeting objectives, final recommendations from the clinical and technical workshops, and the unanimous recommendations from the group as a whole
|
Meeting Objectives:
|
| Source | Recommendations |
|---|---|
| High‐level summary from entire group | A basic understanding of ultrasound needs to be attained to increase user confidence. |
| Clear definitions of the various levels of ultrasound examinations should be developed. | |
| A standardized and harmonized ultrasound scoring system for assessment of MSK disease in persons with hemophilia should be developed, tested, and implemented for use both clinically and in the research setting. | |
| A tiered ultrasound scoring/measurement system that builds in complexity but allows for comparison across tiers should be adopted. | |
| Future endeavors should include standardization of ultrasound acquisition protocols for detection of MSK disease in the joints of persons with hemophilia, with a focus on the index joints (ankles, knees, and elbows). | |
| Clinical and technical workshops | A basic understanding of ultrasound and its potential role(s) needs to be established to increase confidence in its use. |
| A need exists to develop clear definitions of the various levels of ultrasound examinations. The group suggests dividing ultrasound examinations in persons with hemophilia into two categories: POC‐MSKUS and complete diagnostic ultrasound. | |
| There is an urgent need to standardize and globalize ultrasound scanning and scoring systems. Validation of ultrasound scanning and scoring protocols against the reference gold standard, MRI, is necessary. | |
| Unresolved controversies include the ability of ultrasound to detect hemosiderin and surface bone erosions and subchondral cysts, and the role (if any) of color and power Doppler and/or contrast Doppler in the evaluation of persons with hemophilia. | |
| A need exists for pediatric atlases detailing normal joint ossification and normal expected pediatric values for soft tissue, epiphyseal cartilage thickness, and vascularization at various ages. | |
| As ultrasound becomes more widely used, the group calls for guidelines addressing training and proficiency, credentialing and privileging, maintenance of competence, ultrasound management, and quality assurance. |
|
Next steps: Planned follow‐up meeting using a formal nominal groups process with a targeted group of experts to review available ultrasound (including point‐of‐care and full diagnostic ultrasound) and MRI protocols for acquisition and interpretation of musculoskeletal disease in persons with hemophilia and to develop an imaging algorithm suitable for clinical use when assessing patients with hemophilia, and possible acute/chronic MSK disease. |
Abbreviations: MRI, magnetic resonance imaging; MSK, musculoskeletal; POC‐MSKUS, point‐of‐care musculoskeletal ultrasound.
2. METHODS
2.1. Survey design, dissemination, and analysis
This survey is complementary to a recently published International Prophylaxis Study Group (IPSG) survey aimed at determining the global status of ultrasound use for the management of persons with hemophilia. 28 This second of two surveys focused on understanding how and why ultrasound was being used in various clinical contexts, and to begin to move toward consensus definitions of both POC‐MSKUS and full diagnostic MSKUS, terms used to describe structures being assessed by ultrasound, and how these assessments should be interpreted. This study was approved by the Research Ethics Board at the Hospital for Sick Children (SickKids), with participant consent implied upon completion of the survey.
The survey was developed by the Ultrasound Taskforce of the IPSG, with input from MSK radiologists and other hemophilia health care providers and was pilot tested by an international panel of subject matter experts. The complete survey can be found in Appendix A.
The survey was distributed from July to August 2018 using an HTC‐based approach; only one response was requested from each HTC surveyed. The list of HTCs was selected on the basis of the results of the first IPSG ultrasound survey, which specifically asked for contact information of health care providers who are currently using ultrasound at each HTC and also included the pooled email list used to distribute that survey. 28 The survey was distributed to 313 HTCs in 26 countries, spanning North America (Canada, United States), South America (Argentina, Brazil, Chile, Colombia, Venezuela), Europe (Czech Republic, Denmark, France, Finland, Germany, Greece, Italy, Norway, Spain, Sweden, the Netherlands, Turkey, and the United Kingdom), and the rest of the world (Australia, China, India, Israel, New Zealand, and Taiwan). The survey was hosted on a Research Electronic Data Capture system housed at SickKids. 29 , 30
Responses to the survey were summarized using descriptive statistics. All analyses were completed using R version 3.5.6 (R Foundation for Statistical Computing, Vienna, Austria).
2.2. Ultrasound consensus meeting
A 2‐day meeting and workshop was held in Toronto, Ontario, Canada, in September 2018. Invitees included a range of disciplines (pediatric and adult hematologists, MSK radiologists, physical therapists, orthopedist, etc.), and patient representatives, selected from among the survey respondents by the planning committee to ensure comprehensive, multi‐disciplinary representation of all relevant areas of expertise and patient experience in the use of ultrasound for the management, assessment, and surveillance of hemophilic arthropathy.
The goals of the Toronto 2018 Ultrasound Meeting are outlined in Table 1; the overall objective was to develop consensus regarding the acquisition and interpretation of ultrasound (POC‐MSKUS and full diagnostic MSKUS) for use in the assessment of MSK pathologies in persons with hemophilia and other severe inherited bleeding disorders. To accomplish this, the meeting was divided into two main components: a series of presentations and education sessions on the status and use of ultrasound at various global HTCs, including a review of the survey results, and breakout workshops as follows:
The Clinical Working Group (n = 27), which consisted primarily of hematologists, physical therapists, orthopedic surgeons, and patient representatives, aimed to establish the clinical questions that should be addressed with ultrasound, guided by the overall objectives of the meeting. In this workshop, presentations and discussions were held covering the following topics: the use of imaging in orthopedics/MSK system, lessons from clinical trials, perspectives from persons with hemophilia, and a final group discussion.
The Technical Working Group (n = 20), which consisted primarily of radiologists, sonographers, physicists, artificial/augmented intelligence specialists, and data scientists aimed to discuss and establish standardized protocols for ultrasound image acquisition, interpretation, and scoring with clear definitions. This group established a series of more specific questions to guide the ensuing discussions, including establishing the usefulness of ultrasound scoring systems for arthropathy versus the direct measurement of structures, how best to assess vascularity, and issues around existing normative data. The latter is particularly important for pediatric assessments, which pose additional challenges including the need for a sophisticated understanding of normal joint maturation, secondary ossification, and age‐based variations.
The Working Groups arrived at their recommendations through in‐depth discussion to reach a group consensus. Unanimous agreement of the overall group recommendations was achieved via a group discussion with a moderator with extensive experience in achieving group consensus (BF).
3. RESULTS
3.1. Global survey
Responses were received from 76 of 313 (24.3%) HTCs surveyed. Of those, 55% (42/76) reported routine use of full diagnostic ultrasound, and 52% (40/76) reported routine use of any type of POC‐MSKUS.
A proposed set of definitions for full diagnostic ultrasound and POC‐MSKUS that were developed during the first IPSG ultrasound survey were presented. 28 Most respondents agreed with the proposed definitions as written (76% for full diagnostic ultrasound and 90% for POC‐MSKUS), however, some suggestions were made as to how the definitions could be modified and improved (Table 2).
TABLE 2.
Definitions as proposed in the global survey for full diagnostic ultrasound and POC‐MSKUS with suggested modifications for consideration based on participant responses
| Proposed definitions | Proposed modifications by some respondents |
|---|---|
| Full diagnostic ultrasound | |
| Referring to the use of ultrasound in radiology departments to diagnose and follow pathologic findings throughout the extent of the joint/muscle that is amenable to visualization of anatomic structures by conventional ultrasound transducers (typical frequency range: 3.5‐15 MHz) using a 360‐degree coverage approach. | Full diagnostic ultrasound can be performed outside of a radiology department by trained providers |
| POC‐MSKUS | |
| An ultrasound examination performed by a health care professional in which the purpose is to identify the presence or absence of a limited number of specific findings; examples of such findings in persons with hemophilia are (i) presence or absence of fluid in a joint consistent with a recent joint bleed (hemarthrosis); and (ii) presence or absence of synovial hypertrophy in a joint. The POC‐ MSKUS examination can be performed by a practitioner other than a radiologist and in a site other than a diagnostic imaging center (ie, at the bedside in an inpatient ward or outpatient clinic). | POC‐MSKUS must be defined as an adjunct to a detailed joint/muscle physical examination by an experienced and trained health care provider, which is also crucial to differentiating POC‐MSKUS from full diagnostic ultrasound. |
Of note, a consensus regarding an accepted version of the definitions was not achieved in this work.
Abbreviation: POC‐MSKUS, point‐of‐care musculoskeletal ultrasound.
Of the HTCs using full diagnostic ultrasound, 74% reported direct tissue measurements; of the HTCs using POC‐MSKUS, 58% reported results in a standardized way (e.g., a score), with 32% using direct measurements. POC‐MSKUS was used primarily to support clinical decision making (85%) and as an educational tool (78%). At the time of the survey, only 40% of HTCs using POC‐MSKUS reported using it to support research.
For full diagnostic ultrasound, >70% of scans were both acquired and interpreted by radiologists, and the maximum acceptable scan time per joint was up to 30 minutes. Conversely, for POC‐MSKUS, the majority of scans were being performed and interpreted by physical therapists (70%) and/or hematologists (35%), and the maximum acceptable scan time per joint was up to 10 minutes. Less than 10% of HTCs felt that it was acceptable to scan a joint for longer than 60 minutes for full diagnostic ultrasound or 20 minutes for POC‐MSKUS.
Ultrasound examination was completed most commonly to evaluate joint effusions (85%), followed by assessment of soft‐tissue structures (82%), cartilage (75%), osteochondral surfaces (71%), and muscle integrity (65%). There was less agreement regarding the use of Doppler technology to assess for increased vascularity, 30% of HTCs do not use any Doppler in their ultrasound evaluations, while the remainder reported using primarily color Doppler (28%), primarily power Doppler (27%), or both color and power Doppler about equally (15%).
There was a high level of agreement in terms of nomenclature to report soft‐tissue (86%) and osteochondral (83%) findings. Figure 1 shows the items that HTCs felt should be considered for assessment in the soft‐tissue domain. A majority agreed that assessment of effusion/hemarthrosis, simple/complex effusion, and synovial hypertrophy/thickening should be mandatory components of an ultrasound evaluation.
FIGURE 1.
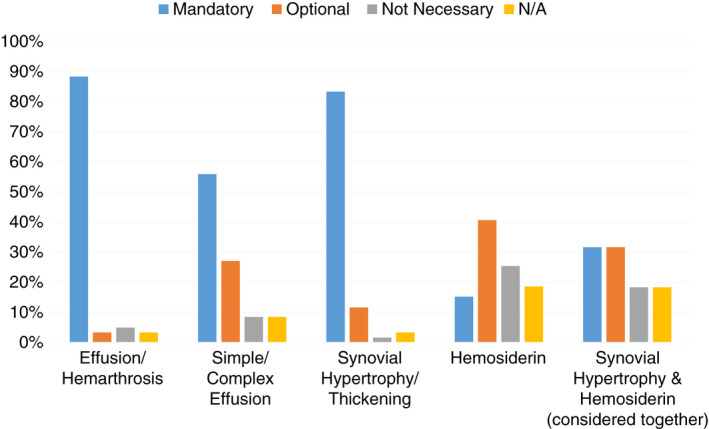
Soft‐tissue domains that survey respondents felt should be considered for assessment with ultrasound; N/A indicated respondent did not feel they were qualified to answer the question
In contrast, there was very little consensus on what structures can and should be assessed from the osteochondral domain. Figure 2 shows a breakdown of HTC opinions regarding what can be seen on ultrasound for the assessment of cartilage loss, subchondral cysts, and surface erosions. The highest proportion of respondents (ranging from 24% to 43% per question) indicated that they felt they did not have the expertise required to answer these questions.
FIGURE 2.
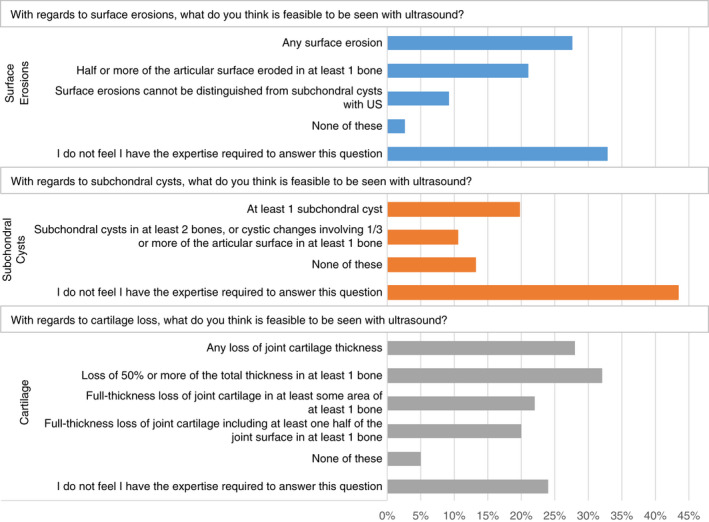
Summary of pathologies in the osteochondral domain that survey respondents felt could be seen on ultrasound
3.2. Ultrasound consensus meeting
Forty‐seven of 50 (94%) invited individuals attended the consensus meeting and workshop. Attendees included a global representation of pediatric and adult hematologists, radiologists, physical therapists, orthopedic surgeons, data scientists, and patient representatives (Figure 3).
FIGURE 3.

Summary of attendees at 2018 Ultrasound Meeting in Toronto
A summary of the recommendations from both the clinical and technical working groups is outlined in Table 1. In response to these recommendations, a moderated discussion occurred, where unanimous consensus was reached on a series of high‐level recommendations, detailed in Table 1.
4. DISCUSSION
Hemophilic arthropathy continues to occur in patients with inherited bleeding disorders. This arthropathy persists despite advances in management and a multimodal approach to joint health assessment, based on the International Classification of Functioning, Disability, and Health developed by the World Health Organization. 1 , 2 , 3 , 4 , 8 , 27 , 31 , 32 , 33 , 34
It is widely accepted that joint bleeding drives the progression of hemophilic arthropathy, but the pathophysiology of blood‐induced joint/muscle damage in persons with hemophilia is complex, and the multifactorial process that results in bone damage is not yet fully elucidated. 35 A need exists for a more accurate serial joint status assessment, which may provide evidence of hemostatic agent or gene therapy efficacy and allow for personalization of prophylactic regimens when used as part of a set of outcome measures. 5 , 15 , 17 , 28 , 36 , 37 , 38
Hemophilic arthropathy shares some clinical and sonographic features of rheumatoid arthritis (RA), a disease model that has successfully implemented high‐resolution ultrasound for research and patient management. 39 In hemophilia, synovitis and osteochondral damage are markers of disease progression and clinical/subclinical joint bleeding. 1 , 11 , 35 , 40 Ultrasound, especially POC‐MSKUS, is a promising tool in hemophilia that is being used globally, irrespective of HTC size or resources in the assessment of MSK pathologies in individuals with inherited bleeding disorders. 28 Adoption rates are expected to increase as POC‐MSKUS and full diagnostic ultrasound definitions are established, and awareness of guidelines and demonstration of clinical utility of this imaging tool increases, while the cost of ultrasound scanners decreases. 41
The survey respondents agreed to the proposed definitions of POC‐MSKUS and full diagnostic ultrasound but suggested minor modifications to make them crisp and more differentiated based on scope of use, question(s) being answered, scanning times, and reporting of findings. This will facilitate a better uniformity irrespective of who is scanning (radiologist vs other trained provider). This survey showed sustained POC‐MSKUS implementation rates in clinical practice, relative to the first IPSG ultrasound survey, 28 primarily by HTC physical therapists and hematologists as a real‐time, quick scan that serves as an adjunct to physical examination to support clinical decision making by answering yes/no questions, as an educational tool for HTC staff and patients, and potentially as a research tool to monitor joint outcomes over time.
Proposed roles of MSKUS include, but are not limited to, screening for early detection of hemophilic arthropathy in index joints (elbows, knees, and ankles), 16 , 17 , 42 following progression of hemophilic arthropathy in serial examinations, evaluation of painful MSK episodes, 12 , 13 , 25 , 43 and interventional (image‐guided joint aspiration with or without joint injection). 44 , 45 , 46 , 47 , 48 Well‐designed prospective multicenter studies evaluating these roles in this population are needed.
Several full diagnostic and POC‐MSKUS scanning and scoring systems have been proposed, 18 , 19 , 20 , 22 some validated and others in various stages of validation. However, a unifying globally standardized protocol is not yet agreed on. Reaching a consensus on the list of pathological findings to include and their assigned weight in a scoring system is necessary to meet this end and allow for comparison to magnetic resonance imaging (MRI) examinations. 49 Pertaining to POC‐MSKUS, the working groups acknowledge that more than one protocol might be needed to satisfy different needs, and a tiered system that builds in complexity but allows for comparison across tiers may be useful with simplified, detailed, and expanded protocols for bedside, detailed clinical, and research purposes, respectively. Semiquantitative, simplified, POC‐MSKUS scanning protocols that rely on pattern recognition and use little to no measurements appear best matched for typical clinical use and real‐time joint assessment (Hemophilia Early Arthropathy Detection by Ultrasound and Universal Simplified Ultrasound). 19 , 22 Conversely, quantitative, more detailed scanning protocols that report soft‐tissue and osteochondral measurements and include color and/or power Doppler imaging may be better suited for more comprehensive joint evaluation, research, and clinical trials (Joint Activity and Damage Exam and full diagnostic protocols). 18 , 20 The choice of scanning protocol and scoring system is best determined by the user, based on local health care environment, extent of joint disease, and information sought from the scan.
Regarding structures routinely examined by ultrasound, the majority of meeting participants agreed on the nomenclature used to report soft tissue findings with effusion (simple vs. complex) and synovial hypertrophy or thickening being mandatory components of the MSKUS evaluation. However, very little consensus was achieved with what can be feasibly and accurately assessed from an osteochondral domain, the utility of Doppler (color/power) and/or contrast in the evaluation of hemophilic arthropathy, and hemosiderin detection. This was not surprising, as available evidence is contradictory and inconclusive. Ultrasound remains inferior to MRI in the evaluation of the central aspect of the cartilage and subchondral bone; it appears to be limited to the peripheral aspect of the joint, as most of the ultrasound beam is reflected over the bony surfaces. 16 , 21 , 50
MRI remains the gold standard to assess joint changes in hemophilia. The clinical relevance of MRI findings was recently highlighted in a single‐center, prospective cohort study, which showed that in persons with hemophilia with limited arthropathy, joints with synovial hypertrophy on MRI had significantly higher 5‐year bleeding rates; those joints bled sooner and more often. 51 Currently, there is fair evidence (grade B) to recommend MSKUS as an accurate technique for early diagnosis of hemophilic arthropathy with particular regard to soft‐tissue abnormalities. POC‐MSKUS may be most suitable for routine joint assessment given the limited availability and high cost of MRI, and the insensitivity of radiographs to early joint changes. 18 , 21 Interestingly, the application of artificial intelligence/machine learning (AIML) to pattern recognition in medical images has recently been proven to be very successful in enhancing clinical decision support software solutions, especially in situations that require the standardization and processing of very complex problems. 52 By coupling these advances with the relatively inexpensive and readily available ultrasound modality, one may achieve an efficient and yet diagnostically accurate solution. A standardized and harmonized scoring system for ultrasound protocols would be essential for informing the development and validation of such an AIML solution.
In RA, Doppler technology and contrast‐enhanced ultrasound (CEUS) are routinely used to assess synovial hypertrophy and hypervascularity indicating acute inflammation and active disease. 39 , 53 , 54 , 55 , 56 , 57 However, Doppler imaging is operator dependent and has a high rate of interequipment variability, making its utility and applicability in the hemophilia disease model challenging. 42 , 58 , 59 The frequency of highly vascular synovitis in patients with hemophilia is dependent upon the prevalence and intensity of prophylactic regimens; therefore, the usefulness of Doppler technology may vary by patient population and will emerge over time as more studies are conducted.
Color Doppler ultrasound enables assessment of the velocity and direction of blood flow within the synovial vessels, making it suboptimal for assessment of small vessels and slow flow. Conversely, power Doppler ultrasound is more sensitive to detect slow, nondirectional flow but is not generally used as a first option imaging technique for pediatric patients, as it is more sensitive to “flash” artifacts related to movement. 60 Recent technological advances, such as ultrafast Doppler imaging and coherent flow power Doppler, may overcome limitations of conventional power/color Doppler ultrasound once they become more broadly available. 61
Recently, CEUS was found to be safe and effective and demonstrated higher sensitivity in detecting synovial hypertrophy and vascularity than conventional gray‐scale ultrasound and color flow Doppler imaging in patients with hemophilic arthropathy. 62 Its potential to influence the clinical management of subclinical bleeding in persons with hemophilia is yet to be determined but has been demonstrated in childhood arthritis. 63 Blood flow signal detected by CEUS may predict risk for recurrent bleeding from disturbed angiogenesis seen in hemophilic arthropathy. 64
The ability of ultrasound to detect hemosiderin deposition independent of synovial hypertrophy remained debatable at the time of this initiative. 21 , 65 , 66 , 67 Intra‐articular blood‐derived hemosiderin deposition may be useful in detection of early hemophilic arthropathy, as it acts as a marker for blood‐induced synovial proliferation and associated articular cartilage surface destruction. 66 The value of detecting and/or reporting hemosiderin as an independent item by ultrasound is not substantiated with current data, as it is frequently a concurrent finding with synovial proliferation on joint MRI. 51
In the first IPSG global ultrasound survey, barriers to POC‐MSKUS implementation included the need for protected time for training and scanning, access to equipment, local regulations, and scarcity of cases, making maintenance of competency a challenge, especially for smaller HTCs. 28 The meeting attendees agreed that the potential benefits of using POC‐MSKUS in the evaluation and management of persons with hemophilia were numerous, and included improved diagnostic accuracy, personalized image‐guided management of joint disease, and enhanced patient engagement, education, and adherence. Ultrasound is rapid, efficient, and allows for screening and serial monitoring of pediatric patients without the need for sedation. However, the need for specialized ultrasound training for use in pediatric patients was identified, as these require a sophisticated understanding of the immature skeleton, growth plates, secondary ossification centers, and areas with abundant normal periarticular fat. The paucity of available literature coupled with this need for specialized training results in the underutilization of ultrasound for pediatric patients. Atlases on expected soft‐tissue and epiphyseal cartilage thickness and vascularization and normal ossification of the joints of maturing healthy children are needed. Awareness of normal variation and contralateral comparison with the other joint, if normal, would also be helpful. 68 , 69 The use of ultrasound in this population may prove beneficial, especially if pediatric atlases become available, acquisition protocols are standardized, and guidelines for formal training, proper use, maintenance of competence, and quality assurance are established. 70
Finally, ultrasound may advance our understanding of the natural history of hemophilic arthropathy, which has thus far been evaluated primarily by MRI and radiographs, neither of which are ideally suited for the serial evaluation of asymptomatic joints. While MRI is the gold standard for joint evaluations, there is emerging evidence that ultrasound may be useful in identifying structural changes in certain joint compartments due to its high resolution and, if given with contrast, to distinguish synovium from fluid and to determine if effusions are bloody or not. 67 , 71 , 72 It is yet to be determined if ultrasound‐detectable findings are responsive or sensitive to changes in therapy; available evidence is insufficient in both quality and quantity. 18
There are some limitations to be considered when reviewing this work. The results of this survey may not be representative of all stakeholders who might have been surveyed. While the response rate was relatively low, potentially due to the low uptake of ultrasound at the time of the survey (given that just over 50% of respondents reported having experience with ultrasound), the sample is likely representative due to the breadth of the global hemophilia community represented, and therefore has a low risk of nonresponse bias. The field of ultrasonography is rapidly evolving, and the speed at which it is advancing is another limitation of the present work. The survey and meeting were conducted in the second half of 2018, and while they represent an important step in the process of moving toward standardization in the use of ultrasound for the assessment of MSK status in persons with hemophilia, the results reported here must be interpreted within this context.
5. CONCLUSION
The results from this survey suggest that full diagnostic ultrasound and POC‐MSKUS are rapidly evolving technologies that are gaining traction for the assessment of MSK outcomes in persons with hemophilia. As more HTCs begin to use ultrasound for a variety of contexts spanning from clinical decision‐making support to research and clinical trials, it will become increasingly important to establish clear guidelines for the acquisition, interpretation, and reporting of the images. A basic understanding of ultrasound needs to be established, including clear definitions of the various levels of ultrasound examinations. A standardized and harmonized scoring system that ideally builds in complexity but allows for comparison should be adopted. Finally, future research should focus on how ultrasound protocols can be standardized, validated, and implemented across various clinical/research contexts.
RELATIONSHIP DISCLOSURE
PB is a member of the International Prophylaxis Study Group and cochair of the Imaging Expert Working Group. AvD has received fees from Bayer, Biomarin, Bioverativ/Sanofi‐Genzyme, CSL Behring, Novo Nordisk, Pfizer, UniQure, and Takeda for participation in industry‐sponsored education events and advisory boards. She is the cofounder and a member of the board of directors of Hematherix Inc. She holds a patent for an activated superFactor V (superFVa) and is the inventor/physician lead for the Joint Tissue Activity and Damage Exam (JADE) ultrasound measurement tool. JADE is copyrighted and commercialized through a partnership between the University of California, University of San Diego School of Medicine, and the Hemophilia and Thrombosis Treatment Center at the University of San Diego, California, of which AvD is the medical director. IB has received lecture fees from Axon. AD has received fees from Genentech, Uniqure, and Medscape, and nonfinancial support from the World Federation of Hemophilia. SF has received fees from Sanofi‐Genzyme, Pfizer, Alnylam, and Bayer Inc. RK‐J has received personal fees from Pfizer and grants from Genentech/Roche. MM‐J has received fees from Bayer HealthCare, CSL Behring, Freeline, BioMarin, Novo Nordisk, and Sanofi‐Genzyme. CM has received personal fees and nonfinancial support from Pfizer, and personal fees from Novo Nordisk, Sobi, and Takeda. FQ has received teaching fees from Bayer, Baxter/Baxalta/Shire/Takeda, Pfizer, Novo Nordisk, Roche, and Sobi, for teaching educational courses to health care professionals in Spain and Latin America related to hemophilic arthropathy and the use of ultrasound in the diagnosis and treatment of musculoskeletal complications as a result of hemophilia. KS has received honoraria/consulting fees from Pfizer Canada, Bayer, Shire, and Roche, and a grant from Pfizer Canada. PT has received grants and personal fees from Novo Nordisk. LV receives employment income from LT Imaging Inc. VB has received personal fees from Amgen, Bayer, Novo Nordisk, Pfizer, Roche, Shire/Takeda, and Octapharma; grants from Novo Nordisk, Bioverativ/Sanofi‐Genzyme, and Shire/Takeda; and is the Chair of the International Prophylaxis Study Group, a cooperative study group funded by education grants from Bayer Healthcare, Bioverativ/Sanofi‐Genzyme, Novo Nordisk, Pfizer, Shire/Takeda, and Spark Therapeutics to the Hospital for Sick Children Foundation. The remaining authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
NB assisted with data interpretation and wrote the first draft of the manuscript. SD was on the Symposium Steering Committee, conducted data analysis and interpretation, and assisted with the first draft of the manuscript. PB was the cochair of the Symposium Steering Committee, assisted with data interpretation, and critically revised the manuscript. BF facilitated the symposium, assisted with data interpretation, and critically revised the manuscript. DI was on the Symposium Steering Committee and critically revised the manuscript. VB was the principal investigator for the study, cochair of the Symposium Steering Committee, assisted with data interpretation, and critically revised the manuscript. The remaining authors critically revised the manuscript. All authors approved the final version to be considered for publication.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study, including the travel costs associated with attending the consensus meeting in Toronto, was provided by Novo Nordisk Healthcare AG in the form of an unrestricted education grant held at the SickKids Foundation. The funders had no input into the design of the study, the content and outcomes of the consensus meeting, or the writing of the manuscript. The authors thank all those who participated in the process described in this work.
Handling Editor: Dr Suzanne Cannegieter.
REFERENCES
- 1. Manco‐Johnson MJ, Abshire TC, Shapiro AD et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535‐544. [DOI] [PubMed] [Google Scholar]
- 2. Mazepa MA, Monahan PE, Baker JR, Riske BK, Soucie JM, Network USHTC. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood. 2016;127(24):3073‐3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambert T, Auerswald G, Benson G et al. Joint disease, the hallmark of haemophilia: what issues and challenges remain despite the development of effective therapies? Thromb Res. 2014;133:967‐971. [DOI] [PubMed] [Google Scholar]
- 4. van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: Pathophysiology, pain and imaging. Haemophilia. 2018;24(suppl 6):44‐49. [DOI] [PubMed] [Google Scholar]
- 5. Spadarella G, Di Minno A, Milan G et al. Paradigm shift for the treatment of hereditary haemophilia: towards precision medicine. Blood Rev. 2020;39:100618. [DOI] [PubMed] [Google Scholar]
- 6. Pelland‐Marcotte MC, Carcao MD. Hemophilia in a changing treatment landscape. Hematol Oncol Clin North Am. 2019;33:409‐423. [DOI] [PubMed] [Google Scholar]
- 7. Weyand AC, Pipe SW. New therapies for hemophilia. Blood. 2019;31(133):389‐398. [DOI] [PubMed] [Google Scholar]
- 8. Arruda VR, Doshi BS, Samelson‐Jones BJ. Emerging therapies for hemophilia: controversies and unanswered questions. F1000Res. 2018;7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nienhuis AW, Nathwani AC, Davidoff AM. Gene therapy for hemophilia. Mol Ther. 2017;25(5):1163‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathwani AC, Davidoff AM, Tuddenham EGD. Gene therapy for hemophilia. Hematol Oncol Clin North Am. 2017;31:853‐868. [DOI] [PubMed] [Google Scholar]
- 11. Warren BB, Thornhill D, Stein J et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the Joint Outcome Continuation Study. Blood Adv. 2020;4:2451‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidder W, Nguyen S, Larios J, Bergstrom J, Ceponis A, von Drygalski A. Point‐of‐care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful haemophilic arthropathy. Haemophilia. 2015;21:530‐537. [DOI] [PubMed] [Google Scholar]
- 13. Ceponis A, Wong‐Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19:790‐798. [DOI] [PubMed] [Google Scholar]
- 14. Maclachlan J, Gough‐Palmer A, Hargunani R, Farrant J, Holloway B. Haemophilia imaging: a review. Skeletal Radiol. 2009;38:949‐957. [DOI] [PubMed] [Google Scholar]
- 15. Fischer K, Poonnoose P, Dunn A et al. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia. 2017;23:11‐24. [DOI] [PubMed] [Google Scholar]
- 16. Di Minno MN, Ambrosino P, Quintavalle G et al. Assessment of hemophilic arthropathy by ultrasound: where do we stand? Semin Thromb Hemost. 2016;42:541‐549. [DOI] [PubMed] [Google Scholar]
- 17. Bakeer N, Shapiro AD. Merging into the mainstream: the evolution of the role of point‐of‐care musculoskeletal ultrasound in hemophilia. F1000Res. 2019;8:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ligocki C, Abadeh A, Wang K, Adams‐Webber T, Blanchette V, Doria A. A systematic review of ultrasound imaging as a tool for evaluating haemophilic arthropathy in children and adults. Haemophilia. 2017;23:598‐612. [DOI] [PubMed] [Google Scholar]
- 19. Kandagaddala M, Sundaramoorthy M, Keshava SN et al. A new and simplified comprehensive ultrasound protocol of haemophilic joints: the Universal Simplified Ultrasound (US‐US) protocol. Clin Radiol. 2019;74(897):e9‐e16. [DOI] [PubMed] [Google Scholar]
- 20. Volland LM, Zhou JY, Barnes RF et al. Development and reliability of the joint tissue activity and damage examination for quantitation of structural abnormalities by musculoskeletal ultrasound in hemophilic joints. J Ultrasound Med. 2019;38:1569‐1581. [DOI] [PubMed] [Google Scholar]
- 21. Doria AS, Keshava SN, Mohanta A et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am J Roentgenol. 2015;204:W336‐W347. [DOI] [PubMed] [Google Scholar]
- 22. Martinoli C, Della Casa Alberighi O, Di Minno G et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD‐US). Thromb Haemost. 2013;109:1170‐1179. [DOI] [PubMed] [Google Scholar]
- 23. Muça‐Perja M, Riva S, Grochowska B, Mangiafico L, Mago D, Gringeri A. Ultrasonography of haemophilic arthropathy. Haemophilia. 2012;18:364‐368. [DOI] [PubMed] [Google Scholar]
- 24. Melchiorre D, Linari S, Innocenti M et al. Ultrasound detects joint damage and bleeding in haemophilic arthropathy: a proposal of a score. Haemophilia. 2011;17:112‐117. [DOI] [PubMed] [Google Scholar]
- 25. Querol F, Rodriguez‐Merchan EC. The role of ultrasonography in the diagnosis of the musculo‐skeletal problems of haemophilia. Haemophilia. 2012;18:e215‐e226. [DOI] [PubMed] [Google Scholar]
- 26. Klukowska A, Czyrny Z, Laguna P, Brzewski M, Serafin‐Krol M, Rokicka‐Milewska R. Correlation between clinical, radiological and ultrasonographical image of knee joints in children with haemophilia. Haemophilia. 2001;7:286‐292. [DOI] [PubMed] [Google Scholar]
- 27. Manco‐Johnson MJ, Soucie JM, Gill JC, Joint Outcomes Committee of the Universal Data Collection USHTCN . Prophylaxis usage, bleeding rates, and joint outcomes of hemophilia, 1999 to 2010: a surveillance project. Blood. 2017;129(17):2368‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ignas DM, Doria AS, von Drygalski A et al. Use of ultrasound for assessment of musculoskeletal disease in persons with haemophilia: Results of an International Prophylaxis Study Group global survey. Haemophilia. 2020;26(4):685‐693. [DOI] [PubMed] [Google Scholar]
- 29. Harris PA, Taylor R, Minor BL et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warren BB, Jacobson L, Kempton C et al. Factor VIII prophylaxis effects outweigh other hemostasis contributors in predicting severe haemophilia A joint outcomes. Haemophilia. 2019;25:867‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manco‐Johnson MJ, Lundin B, Funk S et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15:2115‐2124. [DOI] [PubMed] [Google Scholar]
- 33. Young G. New challenges in hemophilia: long‐term outcomes and complications. Hematology. 2012;2012:362‐368. [DOI] [PubMed] [Google Scholar]
- 34. De la Corte‐Rodriguez H, Rodriguez‐Merchan E. The ICF (International Classification of Functioning, Disability and Health) developed by the WHO for measuring function in hemophilia. Exp Rev Hematol. 2016;9:661‐668. [DOI] [PubMed] [Google Scholar]
- 35. Melchiorre D, Manetti M, Matucci‐Cerinic M. Pathophysiology of hemophilic arthropathy. J Clin Med. 2017;6(7):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Recht M, Konkle BA, Jackson S, Neufeld EJ, Rockwood K, Pipe S. Recognizing the need for personalization of haemophilia patient‐reported outcomes in the prophylaxis era. Haemophilia. 2016;22:825‐832. [DOI] [PubMed] [Google Scholar]
- 37. van den Berg HM, Feldman BM, Fischer K, Blanchette V, Poonnoose P, Srivastava A. Assessments of outcome in haemophilia ‐ what is the added value of QoL tools? Haemophilia. 2015;21:430‐435. [DOI] [PubMed] [Google Scholar]
- 38. Stephensen D, Drechsler WI, Scott OM. Outcome measures monitoring physical function in children with haemophilia: a systematic review. Haemophilia. 2014;20:306‐321. [DOI] [PubMed] [Google Scholar]
- 39. do Prado AD, Staub HL, Bisi MC et al. Ultrasound and its clinical use in rheumatoid arthritis: where do we stand? Adv Rheumatol. 2018;2(58):19. [DOI] [PubMed] [Google Scholar]
- 40. Kraft J, Blanchette V, Babyn P et al. Magnetic resonance imaging and joint outcomes in boys with severe hemophilia A treated with tailored primary prophylaxis in Canada. J Thromb Haemost. 2012;10:2494‐2502. [DOI] [PubMed] [Google Scholar]
- 41. Zhou JY, Rappazzo KC, Volland L et al. Pocket handheld ultrasound for evaluation of the bleeding haemophilic joint: a novel and reliable way to recognize joint effusions. Haemophilia. 2018;24:e77‐e80. [DOI] [PubMed] [Google Scholar]
- 42. Di Minno MND, Pasta G, Airaldi S et al. Ultrasound for early detection of joint disease in patients with hemophilic arthropathy. J Clin Med. 2017;6(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strike K, Uy M, Lawson W et al. Point‐of‐care ultrasonography in haemophilia care: training and competency for muscular haematomas. Haemophilia. 2018;24:335‐337. [DOI] [PubMed] [Google Scholar]
- 44. Rodriguez‐Merchan EC. Cartilage damage in the haemophilic joints: pathophysiology, diagnosis and management. Blood Coagul Fibrinolysis. 2012;23:179‐183. [DOI] [PubMed] [Google Scholar]
- 45. Martin EJ, Cooke EJ, Ceponis A et al. Efficacy and safety of point‐of‐care ultrasound‐guided intra‐articular corticosteroid joint injections in patients with haemophilic arthropathy. Haemophilia. 2017;23(1):135‐143. [DOI] [PubMed] [Google Scholar]
- 46. Rezende MU, Andrusaitis FR, Silva RT et al. Joint lavage followed by viscosupplementation and triamcinolone in patients with severe haemophilic arthropathy: objective functional results. Haemophilia. 2017;23(2):e105‐e115. [DOI] [PubMed] [Google Scholar]
- 47. Rodriguez‐Merchan EC. Intra‐articular injections of fat‐derived mesenchymal stem cells in knee osteoarthritis: are they recommended? Hosp Pract (1995). 2018;46(4):172–174. [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez‐Merchan EC. Intra‐articular corticosteroid injections in haemophilic arthropathy: are they recommended? Hosp Pract. 2018;46(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 49. Lundin B, Manco‐Johnson M, Ignas D et al. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia. 2012;18:962‐970. [DOI] [PubMed] [Google Scholar]
- 50. Sierra Aisa C, Lucia Cuesta JF, Rubio Martinez A et al. Comparison of ultrasound and magnetic resonance imaging for diagnosis and follow‐up of joint lesions in patients with haemophilia. Haemophilia. 2014;20:e51‐e57. [DOI] [PubMed] [Google Scholar]
- 51. Foppen W, van der Schaaf IC, Beek FJ, Mali WP, Fischer K. MRI predicts 5‐year joint bleeding and development of arthropathy on radiographs in hemophilia. Blood Adv. 2020;4:113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shortliffe EH, Sepúlveda MJ. Clinical decision support in the era of artificial intelligence. JAMA. 2018;320(21):2199. [DOI] [PubMed] [Google Scholar]
- 53. Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers. 2015;2015:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheung PP, Kong KO, Chew LC et al. Achieving consensus in ultrasonography synovitis scoring in rheumatoid arthritis. Int J Rheum Dis. 2014;17:776‐781. [DOI] [PubMed] [Google Scholar]
- 55. D'Agostino MA, Terslev L, Aegerter P et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR‐OMERACT ultrasound taskforce‐Part 1: definition and development of a standardised, consensus‐based scoring system. RMD Open. 2017;3:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Terslev L, Naredo E, Aegerter P et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR‐OMERACT ultrasound taskforce‐Part 2: reliability and application to multiple joints of a standardised consensus‐based scoring system. RMD Open. 2017;3(1):e000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ohrndorf S, Hensch A, Naumann L et al. Contrast‐enhanced ultrasonography is more sensitive than grayscale and power Doppler ultrasonography compared to MRI in therapy monitoring of rheumatoid arthritis patients. Ultraschall Med. 2011;32:E38‐E44. [DOI] [PubMed] [Google Scholar]
- 58. Acharya SS, Schloss R, Dyke JP et al. Power Doppler sonography in the diagnosis of hemophilic synovitis–a promising tool. J Thromb Haemost. 2008;6:2055‐2061. [DOI] [PubMed] [Google Scholar]
- 59. Bhat V, Olmer M, Joshi S et al. Vascular remodeling underlies rebleeding in hemophilic arthropathy. Am J Hematol. 2015;90(11):1027‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mitchell DG. Color Doppler imaging: principles, limitations, and artifacts. Radiology. 1990;177:1‐10. [DOI] [PubMed] [Google Scholar]
- 61. Provost J, Papadacci C, Demene C, Gennisson J‐L, Tanter M, Pernot M. 3‐D ultrafast Doppler imaging applied to the noninvasive mapping of blood vessels in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2015;62:1467‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Y, Ma F, Sun J et al. Conventional and contrast‐enhanced ultrasound in evaluating hemophilia joint structure and predicting recurrent joint bleeding. Res Pract Thromb Haemost. 2019;3:263. [Google Scholar]
- 63. Doria AS, Kiss MHB, Lotito APN et al. Juvenile rheumatoid arthritis of the knee: evaluation with contrast‐enhanced color Doppler ultrasound. Pediatr Radiol. 2001;31:524‐531. [DOI] [PubMed] [Google Scholar]
- 64. Kidder W, Chang EY, M. Moran C, Rose SC, von Drygalski A. Persistent vascular remodeling and leakiness are important components of the pathobiology of re‐bleeding in hemophilic joints: two informative cases. Microcirculation. 2016;23:373‐378. [DOI] [PubMed] [Google Scholar]
- 65. Cooke EJ, Zhou JY, Wyseure T et al. Vascular permeability and remodelling coincide with inflammatory and reparative processes after joint bleeding in factor VIII‐deficient mice. Thromb Haemost. 2018;118:1036‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martinoli C, Di Minno MN, Pasta G, Tagliafico A. Hemosiderin detection with ultrasound: reality or myth? AJR Am J Roentgenol. 2016;206:W30. [DOI] [PubMed] [Google Scholar]
- 67. von Drygalski A, Moore RE, Nguyen S et al. Advanced hemophilic arthropathy: sensitivity of soft tissue discrimination with musculoskeletal ultrasound. J Ultrasound Med. 2018;37(8):1945‐1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Foppen W, Fischer K, van der Schaaf IC. Imaging of haemophilic arthropathy: awareness of pitfalls and need for standardization. Haemophilia. 2017;23:645‐647. [DOI] [PubMed] [Google Scholar]
- 69. Soliman M, Daruge P, Dertkigil SSJ et al. Imaging of haemophilic arthropathy in growing joints: pitfalls in ultrasound and MRI. Haemophilia. 2017;23:660‐672. [DOI] [PubMed] [Google Scholar]
- 70. Lawson W, Uy M, Strike K et al. Point of care ultrasound in haemophilia: building a strong foundation for clinical implementation. Haemophilia. 2017;23:648‐651. [DOI] [PubMed] [Google Scholar]
- 71. Nguyen S, Lu X, Ma Y, Du J, Chang E, Von Drygalski A. Musculoskeletal ultrasound for intra‐articular bleed detection: a highly sensitive imaging modality compared with conventional magnetic resonance imaging. J Thromb Haemost. 2018;16:490‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berro M, Elichiry M, Wasen K, Insagaray J, Rodriguez I. Use of ultrasound for evaluation of painful joint episodes perceived as haemarthrosis in adult patients with severe haemophilia. Haemophilia. 2018;24:e124‐e125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


