Abstract
This paper reviews recent advances in non-invasive blood pressure monitoring and highlights the added value of a novel algorithm-based blood pressure sensor which uses machine-learning techniques to extract blood pressure values from the shape of the pulse waveform. We report results from preliminary studies on a range of patient populations and discuss the accuracy and limitations of this capacitive-based technology and its potential application in hospitals and communities.
Keywords: cNIBP, neonate, NICU, hypertension, hypotension, non-invasive blood pressure monitoring
1. Introduction
Blood pressure is a vital sign that is widely used in the diagnosis and treatment of many medical conditions. Blood pressure monitoring and management is an essential part of medical care, particularly in the treatment of chronic hypertension, critical care monitoring and trauma care.
According to the American Heart Association’s (AHA) 2017 Guidelines, 90% of US adults over the age of 65 may be hypertensive, thus leading to a large segment of the population that could benefit from blood pressure monitoring [1]. Blood pressure monitoring will be a vital part of care for virtually every one of the five million plus patients admitted annually to Intensive Care Units (ICUs) in the US [2,3]. Similarly, blood pressure monitoring will be a required part of care for virtually every one of the 130 million plus patients admitted annually to emergency departments (EDs) across the US [4,5].
The most commonly used methods for blood pressure (BP) monitoring generally involve either non-invasive inflatable cuff-based oscillometric or invasive arterial line manometric measurement.
Inflatable BP cuffs take intermittent measurements, typically limited in frequency to no more than one measurement every 5 min, due to patient discomfort and the risk of skin and nerve damage. Automated systems similar to those used for ambulatory BP monitoring (ABPM) may be used but units can be expensive. Most importantly, intermittent measurements may not be sufficient for critical care where the status of a patient can change from minute to minute.
Invasive arterial lines (IALs) are able to provide continuous BP measurements and are typically used in ICU settings as they can be difficult to correctly place and maintain and require highly skilled medical staff and equipment. IALs can be difficult to use where patient transport is required, and they carry risks of complications due to their invasive nature.
BP monitoring is vital to the care of critically ill patients since episodes of both hypertension and hypotension have been linked to greater mortality and unfavorable non-mortality outcomes [6,7,8,9,10,11,12]. Even short durations of hypotension may lead to traumatic brain injuries or complications due to a lack of perfusion [7]. However, it can be difficult to obtain accurate BP measurements, particularly from children and infants born prematurely who have fragile skin, extremely small blood vessels, and very low mean blood pressure values [13,14,15,16,17].
Frequent BP measurements are also essential in emergent or trauma care. Continuous monitoring is used primarily in critical care where better BP monitoring and faster responses to correct BP excursions can lead to better outcomes [6,18,19,20]. One study showed that patients with a single episode of prehospital hypotension were 12.4 times more likely to require immediate procedures than the normotensive controls and were also more likely to require additional procedures, ICU admission, and longer hospital stays [8]. Other studies showed that the integrated depth and duration of hypotensive episodes could be correlated to mortality [9] and non-mortality outcomes [10]. BP monitoring may also have a role in non-critical patients in whom changes in BP may provide earlier assessment of increased pain or agitation [21].
Outpatient BP management is important for many medical conditions. Stroke or cardiac events can be significantly reduced through tight BP control [22]. Mortality from uncontrolled hypertension doubles for every increase of 20 mmHg in systolic BP (SBP) [23]. The CDC estimates that only 25% of the 100 M hypertensive adults in the US adequately control their BP [24] and have set an aggressive target to raise that value to 80% by 2025 [25]. Hypertensive disorders of pregnancy are among the leading causes of maternal mortality and premature births [26,27].
The annual economic burden of hypertension has been estimated as $48.6 billion per year in the US [28]. In older adults, high blood pressure increases risk of many severe health concerns, including heart disease, congestive heart failure, ischemic stroke, and cerebral hemorrhage, vascular dementia and Alzheimer’s disease [29]. This problem persists in spite of the fact that multiple classes of low cost and effective drugs for reduction of hypertension have been available for decades. It seems that further progress in hypertension control requires improved understanding on the part of care providers and improved engagement on the part of the patient [30].
Currently, the AHA recommends that healthcare professionals confirm in-office diagnoses of high blood pressure with home self-measurement and/or 24-h ambulatory blood pressure monitoring (ABPM) [1]. By giving physicians a look at blood pressure during patients’ daily lives, ABPM more accurately diagnoses hypertension and better predicts associated risks [31,32,33,34]. In older adults, patient use of ABPM has even been associated with improved cognitive function [35]. ABPM is especially useful in diagnosing “white-coat hypertension” (when a patient has elevated blood pressure when tested at a medical facility) and “masked hypertension” (when a patient has normal blood pressure in a medical clinic, but elevated outside) [31,32,34,35]. Thus, ABPM is helpful in preventing both overtreatment of hypertension, leading to falls and other accidents, as well as undertreatment [36].
1.1. Current Standard of Care for Blood Pressure Monitoring
1.1.1. Invasive Arterial Catheters
Although cuff-based measurements are used today to guide most treatment decisions due to an abundance of literature and its ease of use, the “gold” standard for continuous BP monitoring is an invasive arterial line (IAL) [11,37,38]. This is a sensor that is inserted into the artery which directly measures changes in BP, generally after calibration with an inflatable cuff measurement. Approximately 8 million IALs are placed annually in the US [39] and in up to 2% of all births [40], but each placement carries the risk of serious complications such as infection, bleeding, clots and nerve damage [41]. IALs are generally used in ICUs due to the possibility of excessive bleeding and require maintenance to ensure patency. Costs can be high, over $750 per adult patient and over $2000 for neonates, due to the need for highly skilled medical staff and equipment [42].
While insertion of an IAL is considered essential for many critically ill patients and is generally benign for adults [12,37,38], it is more problematical for children who have smaller arteries and a greater tendency for vasospasm and must generally be sedated [11,12,15,41,43,44,45,46,47,48]. Neonates are particularly at risk due to their fragile skin [49]. Insertion of a pediatric arterial line requires the services of highly trained personnel and may require multiple attempts [12,15,43]. As many as half of pediatric arterial lines may be positioned non-optimally to such an extent that their accuracy may be insufficient to manage care [11]. Short-term and long-term complications such as obstruction of blood flow and possible tissue or organ damage from arterial thrombosis or vasoconstriction [15,41,43,44,47], infection [15,46], bleeding complications [47], and nerve damage [12] occur at a higher rate for children than adults, with younger, smaller children more likely to experience these issues [12,41,43,48]. Thrombosis has been reported in 24–66% of neonates with umbilical arterial lines in place [50]. In extreme cases, amputation may be required [51].
Despite the advantages to care management from continuous BP monitoring, the higher risk of complication leads many clinicians to avoid the use of IAL monitoring if possible, substituting intermittent cuff measurements or eschewing BP information entirely.
1.1.2. Inflatable Cuff Measurements
Inflatable cuffs measure blood pressure by inflating a cuff wrapped around a limb, determining the pressure required to obstruct blood flow through the arteries in that limb, gradually reducing the pressure in the cuff and determining the pressure at which blood flow is reestablished. The first pressure at which blood flow is stopped is the systolic blood pressure (SBP) and the value at which blood flow resumes is the diastolic blood pressure (DBP).
Cuff measurements can be time consuming if taken manually, and placement can be critical to the accuracy of the results. Frequent use of cuff devices is uncomfortable and carries risk of complications such as damage to the skin (e.g., petechiae, acute dermis capillary rupture, or skin necrosis) or peripheral nerves (e.g., compressive neuropathy, crush syndrome, or nerve ischemia) [52,53,54]. Cuffs are also less accurate at children’s lower BP values [55]. Most importantly, intermittent measurements may not be sufficient for critical care where the status of a patient can change within minutes.
There is strong demand for a safer, non-invasive alternative which could be used for the majority of critically ill patients who do not require frequent blood gas sampling. More frequent BP monitoring could become ubiquitous during the 0.5 million pediatric surgeries in the US each year [56] and ultimately could become routine in ICUs and step-down wards, particularly if the monitoring device is unobtrusive and does not interfere with the patient’s sleep or activity. This could impact the care for 400 thousand critically ill neonatal patients (10% [57] of 4 million live births [58]) and 3.5 million pediatric admissions in the US each year [56].
In the long-term, use of a comfortable, non-stressful, and easy-to-use BP monitor could spread to other applications as well. It potentially could be used to screen for some congenital heart defects such as patent ductus arteriosus, coarctation of the aorta, and aortic valve stenosis using BP and pulse waveform measurements at different body locations [59,60,61,62,63,64,65,66,67]. With a low price point, it could be used for routine BP measurements during office visits. It could also be used to assist in the diagnosis and care management of hypertensive patients, both children and adults, and the use of ambulatory BP monitoring for children is now encouraged by the American Academy of Pediatrics [68].
Home BP measurements improve BP control. Since use of arterial lines is limited to critical care facilities, multiple home BP measurements and/or 24-h ambulatory BP monitoring (ABPM) using automated brachial cuffs, are used to provide an indication of BP variation during patients’ daily lives. The AHA recommends using ABPM to confirm in-office diagnosis of hypertension, and it is especially useful in diagnosing “white-coat hypertension” (when a patient has high BP when tested by a medical professional) and “masked hypertension” (when a patient has normal BP in a medical clinic, but elevated BP outside the clinic) [1]. Thus, home BP measurement, particularly ABPM, is helpful in preventing both overtreatment of hypertension, leading to falls and other accidents, as well as undertreatment. It can also improve patient care through titrated treatment schedules for more precise BP control and has also been shown to be a useful tool in the diagnosis and prediction of gestational hypertension (GH) [69].
BP monitoring is difficult to implement outside of a hospital; despite the utility of ABPM, it is not widely used. It is estimated that fewer than 1% of Medicare patients are prescribed ABPM to manage their BP [70]. Several factors prevent widespread use by providers and patients [31]. The primary barrier is patient compliance and a reluctance or inability to obtain measurements with sufficient frequency. Brachial cuff devices are cumbersome and can be uncomfortable, leading to incorrect or insufficient usage [71,72,73]. Measurements with ABPMs can be variable, inaccurate and inconvenient for patients. They also interfere with patients’ sleep and activities [31,32,35,73,74]. The devices are expensive–up to $3000 per unit–and may not be covered by health insurance [31,71]. Moreover, most patients cannot tolerate using an ABPM for more than 24 h and long-term usage can lead to tissue damage. For these reasons, traditional ABPM devices are less than ideal for long-term monitoring, such as monitoring women for hypertension throughout their pregnancy.
There is a clear need for a convenient, continuous, and cost-effective device that can enable widespread BP monitoring with minimal risk, training and effort. PyrAmes has developed a comfortable and easy-to-use wearable device for BP monitoring and management over long periods of time. It uses capacitive sensors to capture pulse waveform data which can provide more detailed information about the health of the cardiovascular system. It has been used successfully on neonates.
1.2. Current Alternatives in Blood Pressure Measurement
There have been many attempts to develop more convenient alternatives to address the limitations of current BP measurement techniques (Table 1). We separate them into cuff-based and cuffless non-invasive devices.
Table 1.
Comparison of non-invasive blood pressure monitoring technologies.
| Method | Measurement | Advantages | Disadvantages | Example Companies w/FDA/EU Clearance |
Example Companies w/Development Programs |
|
|---|---|---|---|---|---|---|
| Cuff | Finger, tabletop (volume clamp technology) | Continuous | Validated for adults, can be self-calibrated | Restricted mobility, bulky, expensive (>$15 K) | BMEYE, Finapres, ADI, Biopac, Edwards, CNAP |
|
| Finger, wearable (volume clamp technology) | Continuous | Validated for adults, can be self-calibrated | Expensive ($5 K), uncomfortable, restricts movement of hand, high power | Caretaker | ||
| Wrist | Intermittent | Validated for adults; established technology | Concerns about accuracy, bulky, high power | Omron, H2Care | ||
| Cuffless | PPG | Continuous | Wearable technology widely used for heart rate; low cost; can pick up signal almost anywhere on body | Noise from ambient light, skin color; uncomfortable; high power; periodic calibration | Aktiia, BioBeat, Aura | Apple, ASUS, Samsung, Sensifree, |
| PWV, PTT | Continuous | Widely studied technique; can use many combinations of sensor technology | Requires multiple sensors to determine pulse wave velocity (PWV) and pulse transit time (PTT), large training dataset, high power; many of the same issues as PPG | Sotera, Somnometics | Vital Insite, Quanttus, Scanadu, Blumio, Sibel |
|
| Tonometer | Continuous | Established technique; validated for adults | Requires calibration, expensive, uncomfortable, restricts movement | Tensys, HealthStat | ||
| Capacitance | Continuous | Demonstrated for neonates; highly detailed pulse waveforms; requires minimal contact with skin for less irritation; lower power | New technology | PyrAmes, Vena Vitals |
Abbreviations: FDA–US Food and Drug Administration; EU–European Union; PWV–Pulse wave velocity; PTT–Pulse transit time; PPG–Photoplethysmography.
1.2.1. Cuff-Based Devices
The most successful alternatives for monitoring adults are volume clamp devices worn on the fingers and calibrated with a standard inflatable cuff measurement [75,76,77]. These volume-clamp devices partially inflate the finger cuffs using a photoplethysmographic (PPG) absorbance as the metric for arterial expansion. The pressure required to minimize changes in arterial expansion and keep the blood volume constant during each pulse enables reconstruction of brachial BP after calibrating against a brachial oscillometric NIBP value. These devices may include proprietary algorithms to autocorrect vascular tone changes related to vasodilation, vasoconstriction, and use of vasoactive drugs.
Volume-clamp devices currently on the market include the Nexfin/ClearSight (Edwards Lifesciences, Irvine, CA, USA) [78], CNAP (CNSystems, Graz, Austria) [79], LiDCO Blood Pressure Module (LiDCO, London, England) and Finapres/Finometer (Finapres Medical Systems, Enschede, The Netherlands) systems [76]. These devices are bulky, uncomfortable, inconvenient, expensive, and not widely used. The Caretaker device (Caretaker Medical NA, Charlottesville, VA, USA) [80] is wearable but interferes with the use of the hand and is expensive ($5 K).
Wrist-worn inflatable cuffs (Heartguide (OMRON Healthcare, Kyoto, Japan) [81], H2-BP (Charmcare, Seoul, Korea) [82]) have the form-factor of a watch and do not interfere with the use of the hand. However, they provide only intermittent BP data. Moreover, frequent use of cuff devices can cause skin or nerve damage [52,53,54]. Importantly, like conventional cuff-based methods, these devices lose accuracy when mean arterial pressure (MAP) is <30 mmHg [83], a level very common in sick neonates. Some of these devices meet guidelines for accuracy cited by the US Food and Drug Administration (FDA) and the European Union (EU), i.e., a mean average error (MAE) or mean difference <±5 mmHg and standard deviation (sd) <8 mmHg [84,85].
1.2.2. Cuffless Devices
Cuffless devices have used mechanical and optical sensors to determine pulse transit time (PTT), pulse wave velocity (PWV), the pulse contour, or the acceleration pulse to extract BP values [86,87]. Some mechanical “cuffless” devices use arterial applanation tonometry to clamp an artery between a transducer and bone. Arterial applanation tonometry places a pressure transducer at a pulse point and gently compresses the underlying artery. The tonometer uses algorithms to estimate the arterial wall tension and quantify the arterial pulse. They generally require initialization or calibration with a cuff blood pressure measurement. Examples of this are the T-Line device (Tensys Medical, San Diego, CA, USA) [88,89] and the BPro (HealthStats, Singapore) which have received FDA clearance [90].
Smartphones use PPG, electrocardiographic signals, and acceleration sensors to measure BP [91]. The user can press a finger against the device attached to the smartphone and measure blood volume variations [92] although there are reservations about the accuracy of these applications [87]. The Health Monitor app (Samsung, Seoul, South Korea) tracks heart rate and blood pressure using pulse wave analysis in conjunction with their PPG heart rate monitors on their smartwatch. It measures the blood pressure change after calibration with a cuff measurement to determine blood pressure. The Samsung app received a European Union approved Conformity Marking (CE)-marking in December 2020 [93].
PPG-based devices illuminate the skin like a pulse oximeter and measure blood volume below the skin based on changes in absorbance. Unfortunately, PPG is prone to interference from ambient light, skin pigmentation, and hair. In addition, PPG requires significant skin pressure to reduce ambient light interference which can damage the fragile skin of neonates and elderly patients. Unlike IAL waveforms, PPG pulse waveforms generally lack detailed morphologic features which are critical in inferring BP using machine-learning methods.
Despite these downsides, there have been some successes with PPG. In a study of 86 participants that included 40% with high skin pigmentation and 20% with denser hair on the forearm, the Aktiia bracelet (Aktiia SA, Neuchâtel, Switzerland) was stated to meet the accuracy and precision required for ISO 81060-2:2013 for periods as long as a month [94]. In a study of 1075 subjects with both normal and high blood pressure levels, Biobeat (Biobeat, HaMerkaz, Israel) proved accurate and reliable in alignment with the same standard [95]. The U.S. FDA has granted a 510 K clearance for its patch and watch.
Another approach is to correlate BP to the PWV-the speed of a pressure pulse propagating along the arterial wall calculated from the PTT (the time between two pulse waves propagating during the same cardiac cycle from two separate arterial sites). Because of its simplicity, it is gaining popularity for tracking BP. Companies in this space (e.g., Vital Insite, Palo Alto, CA, USA, Quanttus, Mountain View, CA, USA, Scanadu, Sunnyvale, CA, USA, Blumio, San Francisco, CA, USA) have not yet found widespread commercial success. Many of these devices have struggled to accurately coordinate multiple sensors and require a large amount of training data to describe the wide variations of vasculature and demographics of the general populace.
Sensifree (Cupertino, CA, USA) is known to use Non-invasive Electromagnetic Sensing Technology (NEST), with results significantly more similar to arterial line data than a PPG sensor (p < 0.0001) [96]. They have algorithms to estimate and track systolic and diastolic pressure values from the mean arterial pressure and pulse morphology.
Similar to PyrAmes, Vena Vitals (Irvine, CA, USA) uses capacitive sensing to capture pulse waveform signals which are then processed by machine learning to derive BP values. Vena Vitals’ capacitive structures utilize wrinkled electrodes deposited on elastomeric substrates which historically have been difficult to manufacture in volume.
2. Materials and Methods
2.1. PyrAmes’ Solution
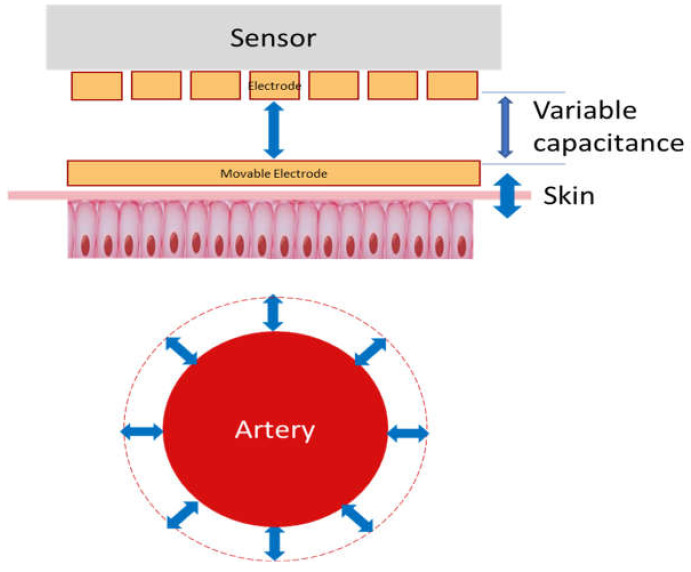
PyrAmes uses capacitive proximity sensing technology exclusively licensed from Stanford University to collect pulse waveform data which is used to derive continuous blood pressure measurements. The system consists of an array of sensors integrated into a soft foam band which is worn on the wrist or foot (Figure 1) which sends data wirelessly to a mobile device where continuous blood pressure values are displayed in real time. The sensor has been designed to be compatible with standard roll-to-roll processes for high volume manufacturing.
Figure 1.
BoppliTM device to monitor the blood pressure of infants in critical care.
2.2. How It Works
The PyrAmes device is based on a very sensitive relative displacement sensor that sits with light contact against the skin over a palpable pulse point. When the heart beats, it pushes a pulse of blood through the arteries which locally expand and contract as the pressure wavefront caused by this pulse travels through them. This causes the surface of the skin to be displaced slightly which, in turn, results in motion of a movable surface within the PyrAmes sensor (Figure 2).
Figure 2.
Schematic of operating principle of PyrAmes’ capacitive sensor technology.
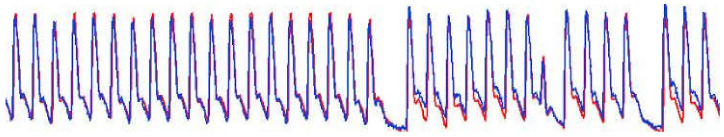
The change in distance between the movable surface and a fixed electrode in the PyrAmes sensor is measured as a change in capacitance. The capacitance changes with time in a manner which correlates to the blood pressure changes or pulse waveforms that are measured with an IAL (Figure 3). The capacitance signal from each array element is digitized with a capacitance-to-digital converter for wireless transmission to an Android mobile device via the Bluetooth Low Energy protocol.
Figure 3.
Comparison of normalized invasive arterial line data (red) taken simultaneously with normalized PyrAmes sensor data (blue).
An array of four independent sensing elements spanning an 8 × 15 mm2 area is used to ensure that it is easy to locate the device over a pulse point. The firmware on the electronics can determine the signal-to-noise from each element of the array and use this information to transmit only the best signal to the Android device to extend battery life.
An application on the Android device receives the sensor data and uses a Butterworth bandpass filter to remove noise and artifacts from the data. The cleaned data is evaluated for signal quality (QA score) with a convolutional neural network (CNN) that was trained on waveform data which had been visually graded against a rubric of quality values reflecting the similarity of the sensor signal to IAL data taken simultaneously.
Data with QA scores above a set threshold value are sent to another CNN trained on a combination of normalized arterial line data and sensor data to extract blood pressure values from the shape of the pulse waveform data. Data with QA scores below the threshold are discarded. Normalized waveform data are used to ensure that the model is trained from the shape and features of the waveform which makes the sensor less affected by environmental effects such as placement pressure, sensor positioning, skin temperature, and skin hair density. Since it is not optically based, it is insensitive to ambient light and skin color. However, like many other wearable devices, it is highly sensitive to motion, and motion-affected data is currently excluded through use of the QA score. Ultimately it is expected that some types of motion artifacts will be mitigated through signal processing and the use of reference sensors. Models for different use cases may require anchoring with demographic values such as age and weight, or with a recent cuff measurement.
The premise that blood pressure can be derived from pulse waveform data is well supported in the literature. Much effort has focused on identifying features in the waveform shape that can be correlated to specific blood pressure values and other hemodynamic parameters. For example, Baruch et al. correlated the ratio of the heights of the primary and secondary peaks seen in the waveforms for many individuals, particularly adult males, to the central systolic pressure [97]. They also correlated the time between the primary and tertiary peaks in milliseconds with diastolic pressure. Munir [98] and Donley [99] report similar analyses using different features of pulse waveforms. Secondary and tertiary peaks in the pulse waveform arise from reflections of the pressure wave when the pulse wavefront experiences a change in flow, such as at a branch point in the arteries. The timing and shape of these features are related to the geometry and mechanical properties of the arteries [100]. Stiffer arteries can cause more pronounced secondary peaks that appear closer in time to the primary peak, and thus different correlations may be needed to address the data for patients of different ages, medical conditions or lifestyles.
Clinical data for these studies were collected using protocols approved by the Institutional Review Boards at Stanford University and Aspire IRB. Details of the studies are provided below.
3. Results and Feasibility Studies
3.1. Proof of Concept
To demonstrate the feasibility of the PyrAmes sensor, an early prototype was used to collect data from a male subject, >89 years old, who exhibited a wide variation of blood pressure on a daily basis. Brachial blood pressure measurements were taken while the subject was seated with both feet flat on the ground with a Series 10 Model BP786 oscillatory cuff (OMRON Healthcare, Kyoto, Japan) or Model ABPM50 ambulatory blood pressure monitor (Contec, Hebei, China) at varying intervals over a period of 7 months. There were 1 to 3 sessions per day for a total of 412 blood pressure readings. With Omron measurements, three cuff measurements were taken 1 min apart on each arm at each session. With the Contec ABPM, measurements were taken every 15 min for up to 8 h each session. Sensor data was collected with an array of 4 sensors from the opposite arm at the radial pulse point throughout the session. An array of sensors is used to ensure that at least one sensor is located near the palpable pulse point to facilitate placement of the device. It was found that using multiple smaller sensors provided more detailed pulse waveform shapes than a single large sensor with the same areal coverage. The sensor array and electronics were held in place with a knitted elastic band. The data were streamed using Bluetooth 2.0 protocol to a Windows tablet and processed with a Labview executable application.
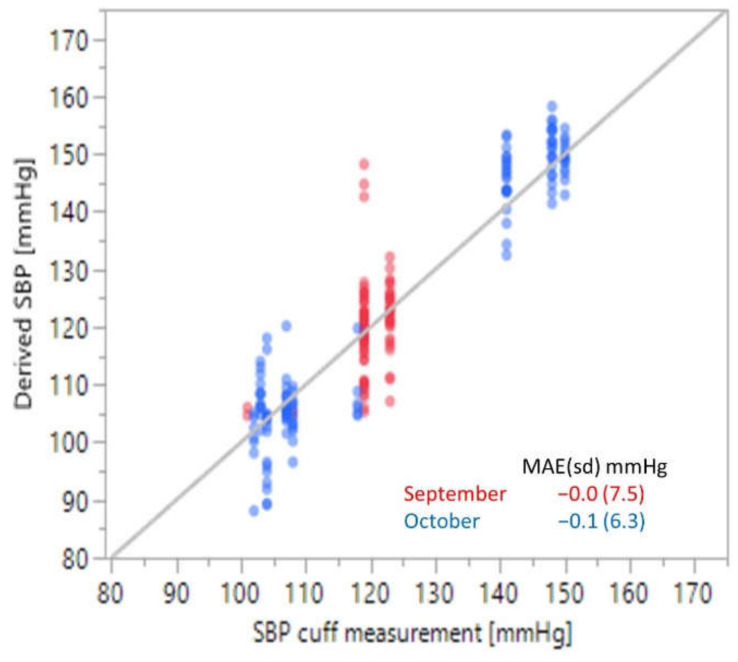
For analysis, the sensor data was processed post-collection with a 0.1 to 20 Hz 1st order Butterworth filter. The data were then divided into one-minute windows bracketing the time of each cuff measurement. Since there were 4 sensors in each array, there were four data windows for each cuff measurement which were tied to the same reference SBP value. 1162 data windows were randomly chosen from the data collected during the period from March through May and used to train a CNN to extract SBP values from the normalized sensor data. The remaining 255 data windows from the two-month period were used to test the model. 230 additional data windows taken in September and October were used to validate the CNN algorithm (BP-model-1) for accuracy with the results shown in Figure 4.
Figure 4.
Proof-of-concept results for a single person convolutional neural network algorithm trained on cuff data collected from March through May and then tested in September and October.
The mean average error (standard deviation) was −0.0 (7.5) mmHg in September and −0.1 (6.3) mmHg in October, well below the FDA guidelines of < ±5 (8) mmHg [84]. This suggests that the approach of using the pulse waveform data to derive blood pressure values is feasible and somewhat robust over time, potentially without additional calibration.
3.2. Ambulatory Adults
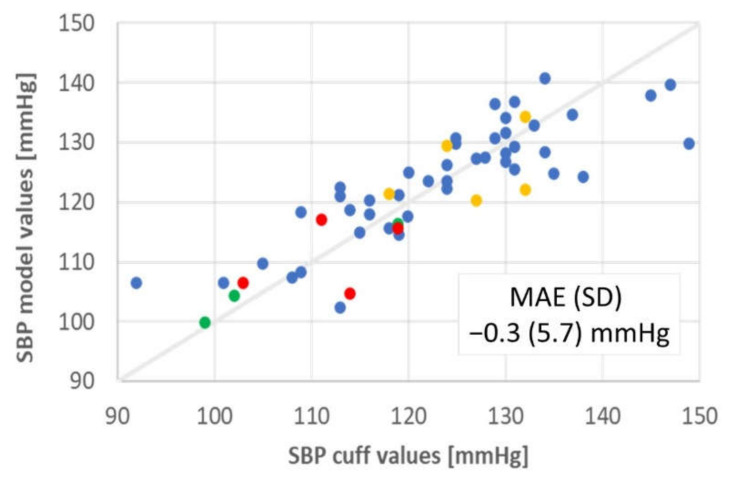
The concept was explored further in a collaboration with Drs. Vivek Bhalla and Tara Chang of the Stanford Hypertension Clinic and Dr. Sandra Tsai of the Stanford Cardiology Clinic (Study 1 in Table 2). Up to six cuff measurements were taken over the space of 10 min for each of 100 ambulatory individuals with an Omron Series 10 Model BP786 oscillatory cuff, three on each arm at the brachial position separated by 1-min intervals. PyrAmes sensor data was taken simultaneously on the opposite arm from the cuff using the same prototype as in the proof-of-concept demonstration. Subjects were seated with both arms elevated on the arm rests of the chair and with both feet flat on the ground. Usable sensor data was obtained from all 100 subjects although data was only obtained from a single arm for two individuals. Usability of the data was determined through a quality metric output by a CNN algorithm (QA-model-1). This model was trained on quality scores assigned upon visual inspection of 4-s samples of waveform data using a rubric that considered signal quality as well as similarity to typical pulse waveform shapes with quality scores ranging from 0 to 5. The one-minute interval bracketing each cuff measurement was divided into overlapping 4-s segments, each normalized using the minimum and maximum values of the waveform data in that segment. Segments with a quality above 2.5 were processed with a Butterworth filter (0.1 to 20 Hz, order 1), and used to train a second CNN (BP-model-2) to extract SBP from sensor data. The CNN algorithm was then tested without additional calibration with data from 4 adults where periodic cuff measurements were taken with a Contec ABPM50 for up to 24 h (Figure 5). The test group were all healthy individuals aged 48–68 years old and included three women and one man. The results meet the FDA guidelines for accuracy when the quality score of the sensor data is >4. However, less than 30% of the data met this criterion, particularly when the subject was moving.
Table 2.
Feasibility Studies.
| Features | Study #1: Ambulatory Adults |
Study #2a: Critically Ill Adults & Children |
Study #2b: NICU Infants |
|---|---|---|---|
| Study participants |
n = 104,
Age 21- > 89 y, 62% F |
n = 124, Age 4–87 y, 42% F |
n = 16,
Age 1–8 days, 33%F GA 25–40 w, Weight 0.7–3.6 kg |
| PyrAmes Data duration | ~10 min per subject (6 h total) ABPM subjects 4–24 h ea |
~5 h per patient (500 h) | ~10 h per patient (360 h) |
| Device | V2 Prototype (training data) Boppli Band (test data) |
V3 Prototype | Boppli Band |
| Sensor/Electronics size | Area of 4-sensor array: 6 × 10 mm2, Electronics: 2.1 × 6.3 cm, >30 g |
Area of 4-sensor array: 6 × 10 mm2, Electronics: 3.5 × 3.8 cm, >30 g |
Area of 4-sensor array: 15 × 7 mm2, Electronics: 2 × 2.5 cm, 12 g |
| PyrAmes device location | Wrist | Wrist and foot | |
| Source of training data | 3–100 cuff msmts per patient. | Stanford invasive arterial line (IAL) data (n = 899,
age 1 day ~53 year, Weight 0.83 Kg~114.8 kg, 45% F), Children’s National Hospital IAL data (n = 40, age 1–2 day, Weight 0.5 kg~4.7 kg, 40% F, GA 23–40 wk) (~9000 h total) |
|
|
Outcome: Meets FDA accuracy criteria? [84] MAE (sd) < ±5 (8) mmHg |
SBP: −0.3 (5.7) mmHg | SBP: −3 to 3 (>12) mmHg (no successful models) |
SBP: 2.8 (5.5) mmHg DBP: −0.2 (4.6) mmHg MAP: 0.1 (4.1) mmHg |
| Clinical collaborators | Drs. Vivek Bhalla, Tara Chang, Sandra Tsai | Drs. Anita Honkanen, Chandra Ramamoorthy, Archana Varma, Alexandria Joseph | Drs. William Rhine, Anoop Rao |
| Stanford study site | Cardiology & Hypertensive clinics | Intensive Care Unit (ICU), Pediatric ICU (PICU), Cardiovascular ICU (CVICU) | Neonatal (ICU), CVICU |
Abbreviations: M–Male; F–Female; GA–Gestational age; msmts–BP measurements; ABPM–Ambulatory Blood Pressure Monitor; ANN–Artificial neural network; ICU–Intensive Care Unit; PICU–Pediatric ICU; CVICU–Cardiovascular ICU; NICU–Neonatal ICU; SBP, DBP and MAP–Systolic, Diastolic and Mean arterial pressure; IAL–invasive arterial line.
Figure 5.
Results from convolutional neural network model trained with cuff data from Study 1 and tested with an ambulatory blood pressure monitor for 4 other ambulatory subjects (SBP values color coded by individual).
3.3. Critically Ill Patients
While the results from Study 1 were promising, it was clear that more data would be required to train robust algorithms which can meet the FDA guidelines for accuracy for a broad population of individuals. To that end, studies were initiated with critically ill patients who had IALs in place as part of their standard of care in collaboration with Drs. Anita Honkanen, Chandra Ramamoorthy, Archana Verma, and Alexandria Joseph (Study 2a in Table 2) and Drs. William Rhine and Anoop Rao (Study 2b in Table 2), all staff members of the Stanford University Medical Center (SUMC).
In Study 2a, 120 patients ranging from 4 to 87 years old with IALs already in place were recruited from the ICU, Pediatric ICU (PICU), and Cardiovascular ICU (CVICU) at SUMC. The IAL and the PyrAmes sensor were generally located contralaterally at the radial position. A few patients had the IAL at the brachial position. Some pediatric patients had the PyrAmes sensor placed at the dorsalis pedis pulse point when a radial location was unavailable. On average, about 6 h of data were collected per patient from each of 4 sensors in an array of sensors spanning an area of 6 × 10 mm2.
In Study 2b, 16 patients ranging from 1 to 8 days old with umbilical IALs already in place were recruited from the Stanford Neonatal ICU (NICU) and CVICU. Patients had gestational ages at birth of 25–40 weeks. They weighed 0.7–3.6 kg. The ratio of male to female patients was 11:5. Mean blood pressures and ranges were 54 (25–89), 66 (34–107), and 44 (17–81) mmHg for MAP, SBP, and DBP, respectively.
The PyrAmes sensor was placed at the radial (N = 13) and/or dorsalis pedis pulse point (N = 5). In some cases, more than one sensor location was used. Data collection ceased when the IAL was removed as part of standard care. On average, about 10 h of data were collected per patient from each of 4 sensors in an array of sensors spanning an area of 15 × 7 mm2. The sensor and electronics used in Study 2b were upgraded from Study 2a to improve the sensitivity of the device and to be more appropriately sized for infants.
Pulse waveform data from the IAL and PyrAmes sensor were processed with a Butterworth filter (0.1–20 Hz, order 2). The PyrAmes sensor data from Study 2a was upsampled from 67 Hz to 125 Hz to match the sampling frequency of IAL waveform data. Both IAL and sensor data in Study 2b were collected at a 125 Hz sampling rate.
The data were parsed into 4-sec windows centered around each peak and graded for quality with a CNN (QA-model-2). The training set for QA-model-2 consisted of 10,000 4-sec windows visually labeled for quality by 10 people against a rubric which compared the sensor data against the IAL data taken simultaneously. The sensor data were synchronized with IAL data taken simultaneously by determining a global lag time between the two time series through correlation of the highest quality windows of sensor data across the entire IAL time series. A local adjustment to the global lag time was then determined for each individual window to correct for missing data or other irregularities in data transmission.
The sensor windows were labeled with SBP, DBP, and MAP ground truth values derived from the corresponding IAL windows, as well as patient age and weight, and sensor and IAL QA scores. A random sampling of 5000 waveforms with a sensor QA score above 2.5 from each individual was used to train a CNN (BP-model-3) to extract blood pressure values from the sensor data. The training set was augmented with historical pediatric IAL data from SUMC (n = 899) and neonatal IAL data, courtesy of Children’s National Hospital (n = 40), which was processed with the same filters and graded with QA-model-2. Normalized IAL data with a QA score above 4 was used in the training set. The combined clinical and historical dataset was divided into 1-vs.-all cross validation sets to maximize the utility of the restricted amount of clinical data.
Early work indicated that our training set was insufficient to derive BP values for the entire age range of Study 2a that would meet the FDA guidelines for accuracy. While mean average errors were generally between −3 and +3 mmHg, standard deviations were routinely above 10 to 12 mmHg. Significantly more training data and more complex models would be needed to reduce the spread in the results. Thus BP-model-3 in Study 2b, a CNN based on TensorFlow [101], focused on neonatal data since this was a more homogeneous population for which we had comparatively more training data. This is also a more urgent unmet need since BP monitoring with IALs for this group is more difficult and poses greater risk due to the small size of neonatal arteries [12]. It is also difficult to obtain cuff measurements on a frequent basis since neonates are prone to pressure ulcers from the pressure of medical devices [102], and best practice is to avoid leaving cuffs in place for extended periods of time [103].
In Study 2b, blood pressure values were determined with BP-model-3 from the pulse waveform data collected by the PyrAmes sensor. It was loosely anchored with initial BP values and patient age and weight. Table 3 shows how this model clearly meets the FDA guidelines for accuracy.
Table 3.
Results of neonate BP-model-3 (N = 16).
| MAP | SBP | DBP | FDA Guidelines [84] | ||||
|---|---|---|---|---|---|---|---|
| MAE | sd | MAE | sd | MAE | sd | MAE | sd |
| 0.1 | 4.1 | 2.8 | 5.5 | −0.2 | 4.6 | <±5 | <8 mmHg |
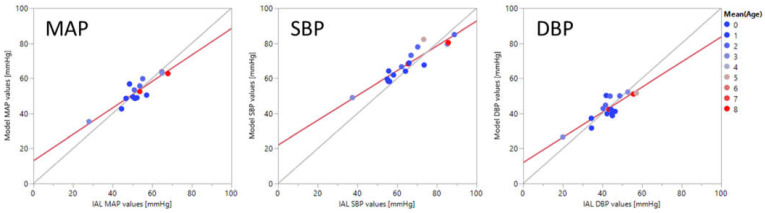
In Figure 6, mean average values of MAP, SBP, and DBP extracted with BP-model-3 are compared against corresponding blood pressure values measured simultaneously with an arterial catheter. Each point represents the average value for all the points taken for an individual. The color of the point indicates the age of the patient in days. The derived BP values are well correlated with the ground truth values, with correlation coefficients of 0.91, 0.93, and 0.85 for MAP, SBP, and DBP respectively.
Figure 6.
Plots of convolutional neural network model outputs vs. invasive arterial line ground truth values of mean arterial pressure (MAP), systolic (SBP), and diastolic (DBP) blood pressure values averaged over each individual. The points are color coded for the patients’ age in days.
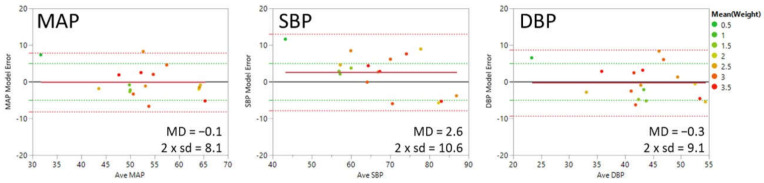
Figure 7 shows Bland-Altman plots for MAP, SBP, and DBP with the average of model and ground truth values for each individual on the x-axis and the average difference between model and ground truth values for each individual on the y-axis (model error or mean difference (MD)). The points are color-coded by the weight of the patient in kilograms. The solid red line shows the mean average error or bias. The green dotted lines indicate the FDA guidelines for the limit of acceptable error for the population of ±5 mmHg. The red dotted lines indicate the 95% confidence interval (1.96 × the standard deviation of the error in the measurement). The mean absolute error is 4.1, 5.5, and 4.7 mmHg for MAP, SBP, and DBP, respectively.
Figure 7.
Bland-Altman plots of BP-model-3 results for mean arterial (MAP), systolic (SBP), and diastolic (DBP) blood pressure values in mmHg averaged over each individual. The x-axis is the average of the model and reference blood pressure values. The y-axis is the difference between the model and reference values. The points are color coded for the patients’ weight in kilograms. The red solid line indicates the overall error of the model (Mean Difference (MD)). The dotted red lines indicate 1.96 × the standard deviation (sd). The green dotted lines indicate the MD limits for accuracy of ±5 mmHg per guidelines from the US Food and Drug Administration.
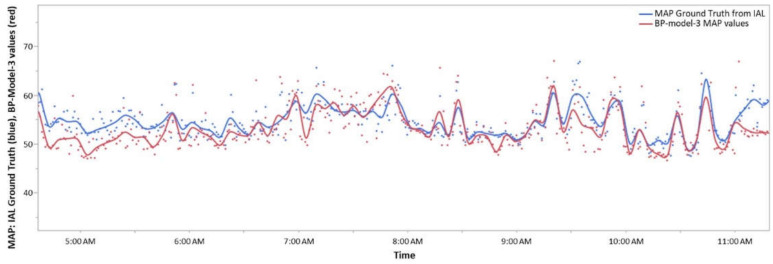
Figure 8 demonstrates how derived BP values (red curve) can track short term changes measured by the IAL (blue curve) over time. Data were collected for about six hours from an 8-day old infant boy (39 week 1 day gestational age at birth, 2.68 kg) with an umbilical arterial catheter in place. The Boppli sensor was placed on the left radial position. Both IAL and BP-model-3 values were averaged over 60 s intervals for this analysis. The curves are spline fits to the individual values with lambda = 1 × 10−6. Results for SBP and DBP exhibit similar correspondence to IAL data.
Figure 8.
Comparison of invasive arterial line (blue) and BP-model-3 (red) mean arterial pressure (MAP) values as a function of time. Each data point is averaged over a one-minute interval. The curves are a spline fit with lambda = 1 × 10−6.
4. Discussion
Continuous blood pressure monitoring is essential to the diagnosis and treatment of many medical conditions but the current standards of care, invasive arterial lines and inflatable cuffs, both have serious drawbacks which impede their use in many situations. There have been many attempts to develop non-invasive approaches which address the issues faced by the current standard of care which have not yet received widespread adoption due to cost or other considerations. We introduce in this article another non-invasive approach utilizing capacitive sensing technology which shows promise in addressing some of the issues of the current alternatives. High sensitivity and a soft, smooth form-factor enables use with the fragile skin of newborns and elderly patients, two vulnerable patient populations where use of either invasive arterial lines or cuffs can be problematic. User feedback from usability studies indicates that the PyrAmes device is comfortable and easy to use, addressing issues that lead to a lack of compliance in many self-monitoring situations. Non-invasive ease of use makes the technology attractive for emergent, trauma, and pre-hospital care.
In feasibility studies, we have demonstrated that capacitive sensing technology can collect pulse waveform data that can be used in conjunction with algorithms trained with machine learning to determine accurate blood pressure values which can be stable over long time periods without additional calibration. However, for models covering a wide population range, it appears that it will be difficult to obtain sufficient training data solely with cuff measurements, and we have found it necessary to augment our training sets with clinical data where sensor and arterial line data are taken simultaneously.
The bulk of this study focused on a somewhat homogeneous population of neonates under 8 days old with an umbilical arterial catheter in place. The training set was further augmented with historical arterial line data for neonates and older children. The pulse waveform collected with the capacitive sensing technology showed strong correlation with blood pressure values obtained through the arterial catheter. It was thus possible to use an unanchored model (without using initial measurements from other tools) to directly predict the blood pressure for this population. However, the current unanchored model predictions are less accurate than those from anchored models and do not meet the guidelines for standard deviation for all three BP parameters, SBP, DBP, and MAP, due to the limited clinical data. To fully meet the guidelines for accuracy for all three BP parameters, a model was developed to predict the blood pressure using pulse waveforms, anchored with an initial blood pressure value, as well as the age and weight of the patient.
Study Limitations
The studies described herein were executed to determine the feasibility of using PyrAmes’ sensor technology for several applications. Much work remains to develop products that are suitable for clinical use.
The proof-of-concept demonstration and Study 1 relied on a single oscillometric cuff device to collect data for training machine-learning algorithms to extract blood pressure values from the pulse waveform shape. It had not been specifically calibrated against a known standard, and it is possible that the blood pressure reference data were systematically biased by the error of this device. While this approach was sufficient for showing feasibility of the method, significantly more data which has been carefully calibrated will be required to train generalizable algorithms suitable for clinical use.
Study 2a was designed to meet this deficiency although it was found that the quantity and quality of data collected was still insufficient to develop an algorithm which was fully generalizable across a wide range of patient ages and conditions. Accordingly Study 2b focused on neonates, a limited patient population with substantial homogeneity. Algorithms were obtained which could meet accuracy targets with the use of an initial blood pressure value, but the data set was still too small to obtain an algorithm that fully met accuracy targets without a loose anchor to an external calibration value. In general, the standard deviation without calibration was higher than the guidelines issued by the FDA while the mean average error was within target. Based on the results to date, it is believed that with additional data, it will be possible to develop models to further reduce the spread of the derived results and do not require external calibration.
5. Future Work
With the collection of additional clinical data across a broader range of subjects, blood pressure values, and medical conditions, it is likely that the accuracy of an unanchored model which does not require external calibration can be further improved. Moreover, collection of longitudinal data for each individual will establish the connection between medical conditions and treatments and blood pressure values, which will ensure the temporal variation of blood pressure can be tracked accurately for a given individual. To further improve temporal monitoring, mechanisms and algorithms to improve data quality and mitigate motion artifacts will need to be developed, particularly for ambulatory applications.
For accurately predicting the blood pressure across multiple individuals, it is necessary to have a large sensor waveform database covering a diversified population. A diversified population, for example, can include a broader range of race and ethnicity and income across multiple sites critical for reducing medical artificial intelligence (AI) bias. Assessing the performance of the algorithms on different demographics across locations can reduce the risk of overfitting and assess disease prevalence [104]. Blood pressure monitoring can be deployed both at hospitals and communities to raise the representativeness of the populations sampled and thereby its accuracy. The PyrAmes sensor has already conducted feasibility studies of different population groups ranging from babies to seniors, healthy to critically ill patients. Inclusion of different races and ethnicities and varying income levels will further provide robust clinical outcomes valuable for widespread uses of blood pressure monitoring.
The widespread use of wearable blood pressure sensors in the near future can be a significant stride toward improving public health. Knowing one’s blood pressure easily and frequently is one of the best ways to prevent a multitude of major illnesses including heart attack, strokes, cerebral hemorrhage, cognitive impairment and risk factors associated with hypertension. The latest progress in this field, therefore, is not only important to advance science but also to encourage preventive healthcare for a larger number of people.
Acknowledgments
The authors are extremely grateful for the efforts and advice of our many collaborators including: Zhenan Bao, Amanda Nguyen, and Andrei Afanasiev for working with us on the initial development of this technology; Vivek Bhalla, Tara Chang, and Sandra Tsai, for their collaboration on Study 1; Anita Honkanen, Chandra Ramamoorthy, Archana Verma, Alexandra Joseph, Eric Helfenbein, and Olga Afanasiev for their collaboration on Study 2a; William Rhine, Ya’el Weiner, Elle Billman, and Fatima Eskandar-Afshari for their collaboration on Study 2b; and Lamia Soghier, Rathinaswamy Govindan, and Kevin Cleary, for the kind use of de-identified historical arterial line data from Children’s National Hospital.
Author Contributions
Conceptualization, X.Q.; methodology, X.Q. and A.R.; software, T.R., J.L., S.S. and W.L.; validation, A.R. and X.Q.; formal analysis, X.Q., J.L., T.R., S.S. and W.L.; investigation, X.Q., A.R., T.R. and W.L.; resources, X.Q., A.M. and W.L.; data curation, W.L., S.S., X.Q. and A.R.; writing—original draft preparation, X.Q., S.-M.C., S.S. and A.R.; writing—review and editing, A.M., J.L., T.R. and W.L.; visualization, X.Q. and A.M.; supervision, X.Q. and A.R.; project administration, X.Q. and A.R.; funding acquisition, X.Q. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded primarily by PyrAmes Inc., and in part by Stanford University and the National Institutes of Health|Small Business Innovation Research, grant number 1R43HD101175-01. The APC was funded by PyrAmes Inc.
Institutional Review Board Statement
The studies were conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Stanford University (protocol codes 38071, 3 August 2016; 38320, 9 December 2016; 45892, 23 September 2019; 47185, 9 June 2019) and by the Institutional Review Board of Aspire IRB (protocol code PyrAmes-2018-01, 11 September 2018).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study or their Legal Adult Representatives.
Data Availability Statement
Data presented in this document that were generated solely by PyrAmes are available from the corresponding author upon reasonable request. The data are not publicly available due to sensitive personal data that were collected during clinical studies on patients. Data generated or provided by Stanford University and Children’s National Hospital are restricted by the terms of contractual agreements and are available upon reasonable request with the permission of those organizations and if the requestor agrees to comply with the European General Data Protection Regulation and not to attempt to trace the origin of the data.
Conflicts of Interest
Xina Quan, Junjun Liu, Thomas Roxlo, Siddharth Siddharth, Weyland Leong and Arthur Muir are employees and shareholders of PyrAmes Inc. Anoop Rao and So-Min Cheong declare no conflict of interest. Stanford University and NIH had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muntner P., Carey R.M., Gidding S., Jones D.W., Taler S.J., Wright J.T., Jr., Whelton P.K. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. doi: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vranas K.C., Jopling J.K., Scott J.Y., Badawi O., Harhay M.O., Slatore C.G., Ramsey M.C., Breslow M.J., Milstein A.S., Kerlin M.P. The Association of ICU with Outcomes of Patients at Low Risk of Dying. Crit. Care Med. 2018;46:347. doi: 10.1097/CCM.0000000000002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpern N. Critical Care Statistics. [(accessed on 24 May 2021)]; Available online: https://www.sccm.org/Communications/Critical-Care-Statistics.
- 4.CDC National Center for Health Statistics. Emergency Department Visits. [(accessed on 24 May 2021)];2021 Available online: https://www.cdc.gov/nchs/fastats/emergency-department.htm.
- 5.Gabayan G.Z., Gould M.K., Weiss R.E., Derose S.F., Chiu V.Y., Sarkisian C.A. Emergency department vital signs and outcomes after discharge. Acad. Emerg. Med. 2017;24:846–854. doi: 10.1111/acem.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakaria E.R., Joseph B. Traumatic brain injury: An update. Surg. Res. Open J. 2017;4:e1–e5. doi: 10.17140/SROJ-4-e003. [DOI] [Google Scholar]
- 7.De Wall J. The ABCs of TBI. Evidence-based guidelines for adult traumatic brain injury care. J. Emerg. Med. Serv. 2010;35:54–61. doi: 10.1016/S0197-2510(10)70095-4. [DOI] [PubMed] [Google Scholar]
- 8.Seamon M.J., Feather C., Smith B.P., Kulp H., Gaughan J.P., Goldberg A.J. Just one drop: The significance of a single hypotensive blood pressure reading during trauma resuscitations. J. Trauma Acute Care Surg. 2010;1:1289–1295. doi: 10.1097/TA.0b013e3181db05dc. [DOI] [PubMed] [Google Scholar]
- 9.Spaite D.W., Hu C., Bobrow B.J., Chikani V., Barnhart B., Gaither J.B., Denninghoff K.R., Adelson P.D., Keim S.M., Viscusi C., et al. Association of out-of-hospital hypotension depth and duration with traumatic brain injury mortality. Ann. Emerg. Med. 2017;70:522–530. doi: 10.1016/j.annemergmed.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaite D.W., Hu C., Bobrow B.J., Chikani V., Sherrill D., Barnhart B., Gaither J.B., Denninghoff K.R., Viscusi C., Mullins T., et al. Mortality and prehospital blood pressure in patients with major traumatic brain injury: Implications for the hypotension threshold. JAMA Surg. 2017;152:360–368. doi: 10.1001/jamasurg.2016.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joffe R., Duff J., Guerra G.G., Pugh J., Joffe A.R. The accuracy of blood pressure measured by arterial line and non-invasive cuff in critically ill children. Crit. Care. 2016;20:1–9. doi: 10.1186/s13054-016-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuper N.J., de Graaff J.C., Hartman B.J., Verdaasdonk R.M., Kalkman C.J. Difficult arterial cannulation in children: Is a near-infrared vascular imaging system the answer? Br. J. Anaesth. 2012;109:420–426. doi: 10.1093/bja/aes193. [DOI] [PubMed] [Google Scholar]
- 13.Raising Children Network (Australia) Ltd. Your Premature Baby’s Appearance. [(accessed on 21 May 2021)]; Available online: https://raisingchildren.net.au/newborns/premature-babies/development/premature-appearance.
- 14.Aouad-Maroun M., Raphael C.K., Sayyid S.K., Farah F., Akl E. Ultrasound-guided arterial cannulation for paediatrics. Cochrane Database Syst. Rev. 2016;9:CD011364. doi: 10.1002/14651858.CD011364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler E., Kowald B., Suess H., Niehaus-Borquez B., Tausch B., Brecher A. Catheterization of the radial or brachial artery in neonates and infants. Pediatr. Anesth. 2005;15:677–682. doi: 10.1111/j.1460-9592.2004.01522.x. [DOI] [PubMed] [Google Scholar]
- 16.CDC High Blood Pressure. Facts About Hypertension. [(accessed on 25 May 2021)];2020 Available online: https://www.cdc.gov/bloodpressure/facts.htm.
- 17.Dempsey E.M., Al Hazzani F., Barrington K.J. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94:F241–F244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 18.Spaite D.W., Bobrow B.J., Stolz U., Sherrill D., Chikani V., Barnhart B., Sotelo M., Gaither J.B., Viscusi C., Adelson P.D., et al. Evaluation of the impact of implementing the emergency medical services traumatic brain injury guidelines in Arizona: The Excellence in Prehospital Injury Care (EPIC) study methodology. Acad. Emerg. Med. 2014;21:818–830. doi: 10.1111/acem.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carteron L., Taccone F.S., Oddo M. How to manage blood pressure after brain injury? Minerva Anestesiol. 2016;83:412–421. doi: 10.23736/S0375-9393.16.11696-7. [DOI] [PubMed] [Google Scholar]
- 20.Chesnut R.M. Secondary brain insults after head injury: Clinical perspectives. New Horiz. 1995;3:366–375. [PubMed] [Google Scholar]
- 21.Fortunato J. ((Northwestern University Feinberg School of Medicine, Lake Forest, IL, USA)). Personal communication. May 22, 2019.
- 22.Wright J.T., Jr., Williamson J.D., Whelton P.K., Snyder J.K., Sink K.M., Rocco M.V., Reboussin D.M., Rahman M., Oparil S., Lewis C.E., et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2013–2016 External Icon. US Department of Health and Human Services; Atlanta, GA, USA: 2019. [Google Scholar]
- 25.CDC Division for Heart Disease and Stroke Prevention National Hypertension Control Roundtable. [(accessed on 21 May 2021)];2020 Available online: https://www.cdc.gov/dhdsp/programs/hypertension-roundtable.htm.
- 26.Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 27.Ghulmiyyah L., Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Center for Disease Control and Prevention . High Blood Pressure in The United States. Division for Heart Disease and Stroke Prevention; Washington, DC, USA: 2016. [(accessed on 8 June 2018)]. Available online: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_bloodpressure.htm. [Google Scholar]
- 29.Lionakis N., Mendrinos D., Sanidas E., Favatas G., Georgopoulou M. Hypertension in the elderly. World J. Cardiol. 2012;4:135–147. doi: 10.4330/wjc.v4.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jesus N.S.D., Nogueira A.D.R., Pachu C.O., Luiz R.R., Oliveira G.M.M.D. Blood pressure treatment adherence and control after participation in the ReHOT. Arq. Bras. Cardiol. 2016;107:437–445. doi: 10.5935/abc.20160165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kronish I.M., Kent S., Moise N., Shimbo D., Safford M.M., Kynerd R.E., O’Beirne R., Sullivan A., Muntner P. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J. Am. Soc. Hypertens. 2017;11:573–580. doi: 10.1016/j.jash.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellano P., Lurato J., Howell C., Edwards M. Top 5 Considerations for Implementing ABPM in Clinical Trials Med Device Online. [(accessed on 21 December 2019)];2019 Available online: https://www.ert.com/blog/top-5-considerations-for-implementing-abpm-in-clinical-trials/
- 33.Chung E., Chen G., Alexander B., Cannesson M. Non-invasive continuous blood pressure monitoring: A review of current applications. Front. Med. 2013;7:91–101. doi: 10.1007/s11684-013-0239-5. [DOI] [PubMed] [Google Scholar]
- 34.Pickering T.G., Shimbo D., Haas D. Ambulatory blood-pressure monitoring. N. Engl. J. Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 35.Banegas J.R., Ruilope L.M., de la Sierra A., Vinyoles E., Gorostidi M., de la Cruz J.J., Ruiz-Hurtado G., Segura J., Rodríguez-Artalejo F., Williams B. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N. Engl. J. Med. 2018;378:1509–1520. doi: 10.1056/NEJMoa1712231. [DOI] [PubMed] [Google Scholar]
- 36.Burr M.L., Dolan E., O’Brien E.W., O’Brien E.T., McCormack P. The value of ambulatory blood pressure in older adults: The Dublin outcome study. Age Ageing. 2008;37:201–206. doi: 10.1093/ageing/afm193. [DOI] [PubMed] [Google Scholar]
- 37.Weiner R.E., Yohannes-Tomicich J. Arterial Line Monitoring and Placement. In: Oropello J.M., Pastores S.M., Kvetan V., editors. Critical Care. McGraw-Hill; New York, NY, USA: 2016. [(accessed on 21 May 2021)]. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=1944§ionid=143522170. [Google Scholar]
- 38.Holt T., Davinia R., Withington E., Mitchell E. Which pressure to believe? A comparison of direct arterial with indirect blood pressure measurement techniques in the pediatric intensive care unit. Pediatric Crit. Care Med. Soc. Crit. Care Med. 2011;12:e391–e394. doi: 10.1097/PCC.0b013e3182230f43. [DOI] [PubMed] [Google Scholar]
- 39.Brzezinski M., Luisetti T., London M.J. Radial artery cannulation: A comprehensive review of recent anatomic and physiologic investigations. Anesth. Analg. 2009;109:1763–1781. doi: 10.1213/ANE.0b013e3181bbd416. [DOI] [PubMed] [Google Scholar]
- 40.McAdams R.M., Winter V.T., McCurnin D.C., Coalson J.J. Complications of umbilical artery catheterization in a model of extreme prematurity. J. Perinatol. 2009;29:685–692. doi: 10.1038/jp.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuttall G., Burckhardt J., Hadley A., Kane S., Kor D., Marienau M.S., Schroeder D.R., Handlogten K., Wilson G., Oliver W.C. Surgical and Patient Risk Factors for Severe Arterial Line Complications in Adults. Anesthesiology. 2016;124:590–597. doi: 10.1097/ALN.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 42.Froehler M.T., Chitale R., Magarik J.A., Fusco M.R. Comparison of a pressure-sensing sheath and radial arterial line for intraoperative blood pressure monitoring in neurointerventional procedures. J. Neurointerv. Surg. 2018;10:784–787. doi: 10.1136/neurintsurg-2018-013769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brotschi B., Hug M.I., Latal B., Neuhaus D., Buerki C., Kroiss S., Spoerri C., Albisetti M. Incidence and predictors of indwelling arterial catheter-related thrombosis in children. J. Thromb. Haemost. 2011;9:1157–1162. doi: 10.1111/j.1538-7836.2011.04271.x. [DOI] [PubMed] [Google Scholar]
- 44.Vasquez P., Burd A., Mehta R., Hiatt M., Hegyi T. Resolution of Peripheral Artery Catheter-induced Ischemic Injury Following Prolonged Treatment with Topical Nitroglycerin Ointment in a Newborn: A Case Report. J. Perinatol. 2003;23:348–350. doi: 10.1038/sj.jp.7210870. [DOI] [PubMed] [Google Scholar]
- 45.Gleich S.J., Wong A.V., Handlogten K.S., Thum D.E., Nemergut M.E. Major Short-term Complications of Arterial Cannulation for Monitoring in Children. Anesthesiology. 2021;134:26–34. doi: 10.1097/ALN.0000000000003594. [DOI] [PubMed] [Google Scholar]
- 46.Furfaro S., Gauthier M., Lacroix J., Nadeau D., Lafleur L., Mathews S. Arterial Catheter—Related Infections in Children A 1-Year Cohort Analysis: A 1-Year Cohort Analysis. Am. J. Dis. Child. 1991;145:1037–1042. doi: 10.1001/archpedi.1991.02160090089031. [DOI] [PubMed] [Google Scholar]
- 47.Veldman A., Nold M.F., Behnke I.M. Thrombosis in the critically ill neonate: Incidence, diagnosis, and management. Vasc. Health Risk Manag. 2008;4:1337–1348. doi: 10.2147/vhrm.s4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen M.M., Cameron C.B., Duncan P.G. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth. Analg. 1990;70:160–167. doi: 10.1213/00000539-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Blume-Peytavi U., Tan J., Tennstedt D., Boralevi F., Fabbrocini G., Torrelo A., Soares-Oliveira R., Haftek M., Rossi A.B., Thouvenin M.D., et al. Fragility of epidermis in newborns, children and adolescents. J. Eur. Acad. Dermatol. Venereol. 2016;30:3–56. doi: 10.1111/jdv.13636. [DOI] [PubMed] [Google Scholar]
- 50.Rizzi M., Goldenberg N., Bonduel M., Revel-Vilk S., Amankwah E., Albisetti M. Catheter-related arterial thrombosis in neonates and children: A systematic review. Thromb. Haemost. 2018;118:1058–1066. doi: 10.1055/s-0038-1642635. [DOI] [PubMed] [Google Scholar]
- 51.Mosalli R., Elbaz M., Paes B. Topical Nitroglycerine for Neonatal Arterial Associated Peripheral Ischemia following Cannulation: A Case Report and Comprehensive Literature Review. Case Rep. Pediatr. 2013;2013:1–7. doi: 10.1155/2013/608516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tareerath M., Wongyingsinn M. Comparison of the Incidences of Cuff-Related Trauma after Non-invasive Arterial Blood Pressure Measurement with and without Padding in Patients Undergoing Elective Surgery. J. Med. Assoc. Thail. 2018;101:1. [Google Scholar]
- 53.Jeon Y.S., Kim Y.S., Lee J.A., Seo K.H., In J.H. Rumpel-Leede phenomenon associated with noninvasive blood pressure monitoring-A case report. Korean J. Anesthesiol. 2010;59:203. doi: 10.4097/kjae.2010.59.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elmatite W., Mangla C., Upadhyay S., Yarmush J. Perioperative Automated Noninvasive Blood Pressure- (NIBP-) Related Peripheral Nerve Injuries: An Anesthetist’s Dilemma—A Case Report and Review of the Literature. Case Rep. Anesthesiol. 2020;2020:1–6. doi: 10.1155/2020/5653481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diprose G.K., Evans D.H., Archer L.N., Levene M.I. Dinamap fails to detect hypotension in very low birthweight infants. Arch. Dis. Child. 1986;61:771–773. doi: 10.1136/adc.61.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tzong K.Y., Han S., Roh A., Ing C. Epidemiology of pediatric surgical admissions in US children: Data from the HCUP kids inpatient database. J. Neurosurg. Anesthesiol. 2012;24:391–395. doi: 10.1097/ANA.0b013e31826a0345. [DOI] [PubMed] [Google Scholar]
- 57.Schulman J., Braun D., Lee H.C., Profit J., Duenas G., Bennett M.V., Dimand R.J., Jocson M., Gould J.B. Association between neonatal intensive care unit admission rates and illness acuity. JAMA Pediatrics. 2018;172:17–23. doi: 10.1001/jamapediatrics.2017.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.CDC National Center for Health Statistics Births and Natality. [(accessed on 24 May 2021)];2021 Available online: https://www.cdc.gov/nchs/fastats/births.htm.
- 59.Mohammadi A., Mehdizade A., Ghasemi-Rad M. Bilateral Tardus-Parvus Waveforms in a Patient With Aortic Coarctation. J. Ultrasound Med. 2011;30:1030–1031. doi: 10.7863/jum.2011.30.7.1030. [DOI] [PubMed] [Google Scholar]
- 60.Mayo Clinic Coarctation of the Aorta. [(accessed on 24 May 2021)]; Available online: https://www.mayoclinic.org/diseases-conditions/coarctation-of-the-aorta/diagnosis-treatment/drc-20352535.
- 61.Ahmed M. Aortic Stenosis a Tight Aortic Valve—A Comprehensive Patient Guide. [(accessed on 23 May 2021)];2016 Available online: https://myheart.net/articles/aortic-stenosis-a-tight-aortic-valve-a-comprehensive-patient-guide/
- 62.Kutty P.K., Krishnaswamy G. In: The Link: Pediatric History Taking and Physical Examination. Kutty P.K., Krishnaswamy G., editors. Jaypee Brothers; New Delhi, India: 2016. [Google Scholar]
- 63.Dhandare A. Cardiovascular System Examination. [(accessed on 23 May 2021)];2017 Available online: https://www.slideshare.net/AshishDhandare2/cardiovascular-system-examination-75965094.
- 64.Fang J., O’Gara P. The History and Physical Examination.An Evidence-Based Approach.2016. [(accessed on 23 May 2021)]; Available online: https://thoracickey.com/the-history-and-physical-examination/#s0060.
- 65.Gevers M., Van Der Mooren K., Stergiopulos N., Van Genderingen H.R., Lafeber H.N., Hack W.W., Westerhof N. Bisferiens peaks in the radial artery pressure wave during patent ductus arteriosus in newborn infants: Relationship with ascending aortic flow. Pediatric Res. 1996;40:163–168. doi: 10.1203/00006450-199607000-00028. [DOI] [PubMed] [Google Scholar]
- 66.Moses S. Arterial Pulse. [(accessed on 23 May 2021)];2021 Available online: https://fpnotebook.com/CV/Exam/ArtrlPls.htm.
- 67.Giese E.A., O’Connor F.G., Brennan F.H., Jr., Depenbrock P.J., Oriscello R.G. The Athlete Preparticipation Evaluation: Cardiovascular Assessment. Am. Fam. Physician. 2007;75:1008–1014. [PubMed] [Google Scholar]
- 68.Flynn J.T., Kaelber D.C., Baker-Smith C.M., Blowey D., Carroll A.E., Daniels S.R., de Ferranti S.D., Dionne J.M., Falkner B., Flinn S.K., et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140 doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 69.Ilic A., Ilic D., Papović J., Stojsic S., Milovancev A., Grkovic D., Stojsic-Milosavljevic A., Redzek-Mudrinic T., Bjelica A., Rankov O., et al. Non-Dipping Patten of Blood Pressure and Gestational Hypertension. Blood Press. Bench Bed. 2018 doi: 10.5772/intechopen.77018. [DOI] [Google Scholar]
- 70.Desai R., Park H., Dietrich E.A., Smith S.M. Trends in ambulatory blood pressure monitoring use for confirmation or monitoring of hypertension and resistant hypertension among the commercially insured in the U.S., 2008–2017. Int. J. Cardiol. Hypertens. 2020;6:100033. doi: 10.1016/j.ijchy.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taira D., Sentell T., Albright C., Lansidell D., Nakagawa K., Seto T., Stevens J.M. Insights in Public Health: Ambulatory Blood Pressure Monitoring: Underuse in Clinical Practice in Hawaii. J. Med. Public Health. 2017;76:314–317. [PMC free article] [PubMed] [Google Scholar]
- 72.Ghuman N., Campbell P., White W.B. Role of ambulatory and home blood pressure recording in clinical practice. Curr. Cardiol. Rep. 2009;11:414–421. doi: 10.1007/s11886-009-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olejnikov V.E., Eliseeva I.V., Tomashevskaya Y.A., Borisova N.A., Fadeeva S.S. The Efficacy Of Antihypertensive Therapy In Elderly Patients And Treatment Compliance Analysis. Ration. Pharm. Cardiol. 2014;10:391–396. doi: 10.20996/1819-6446-2014-10-4-391-396. [DOI] [Google Scholar]
- 74.Setia S., Subramaniam K., Teo B.W., Tay J.C. Ambulatory and home blood pressure monitoring: Gaps between clinical guidelines and clinical practice in Singapore. Int. J. Gen. Med. 2017;10:189–197. doi: 10.2147/IJGM.S138789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.BIOPAC Systems. [(accessed on 8 June 2021)]; Available online: https://www.biopac.com/
- 76.Finapres Medical Systems: Enschede. [(accessed on 8 June 2021)]; Available online: https://www.finapres.com/
- 77.ADIntruments: Dunedin. [(accessed on 8 June 2021)]; Available online: https://www.adinstruments.com/
- 78.Edwards Lifesciences Corporation: Irvine. [(accessed on 8 June 2021)]; Available online: https://www.edwards.com/
- 79.CNAP: Graz. [(accessed on 8 June 2021)]; Available online: https://www.cnsystems.com/
- 80.Caretaker Medical: Charlottesville. [(accessed on 8 June 2021)]; Available online: https://caretakermedical.net/
- 81.Hodsden S. Omron Introduces Smartwatch-size Blood Pressure Monitor. Med Device Online. [(accessed on 8 June 2018)];2019 Available online: https://www.meddeviceonline.com/doc/omron-introduces-smartwatch-size-blood-pressure-monitor-0001.
- 82.H2CARE. [(accessed on 8 June 2021)]; Available online: http://charmcare.com/
- 83.Dionne J.M., Bremner S.A., Baygani S.K., Batton B., Ergenekon E., Bhatt-Mehta V., Dempsey E., Kluckow M., Koplowitz L.P., Apele-Freimane D., et al. Method of blood pressure measurement in neonates and infants: A systematic review and analysis. J. Pediatrics. 2020;221:23–31. doi: 10.1016/j.jpeds.2020.02.072. [DOI] [PubMed] [Google Scholar]
- 84.Association for the Advancement of Medical Instrumentation . American National Standard. Manual, Electronic or Automated Sphygmomanometers ANSI/AAMI SP10-2002. 3330 Washington Boulevard. AAMI; Arlington, VA, USA: 2003. [Google Scholar]
- 85.ISO 81060-2:2019 . Non-Invasive Sphygmomanometers—Part 2: Clinical Investigation Of In-termittent Automated Measurement Type. ISO; Washington, DC, USA: 2019. [Google Scholar]
- 86.Tamura T. Cuffless Blood Pressure Monitors: Principles, Standards and Approval for Medical Use. IEICE Trans. Commun. 2021;104:580–586. doi: 10.1587/transcom.2020HMI0002. [DOI] [Google Scholar]
- 87.Bruining N., Caiani E., Chronaki C., Guzik P., van der Velde E. Acquisition and analysis of cardiovascular signals on smartphones: Potential, pitfalls and perspectives: By the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur. J. Prev. Cardiol. 2014;21(Suppl. 2):4–13. doi: 10.1177/2047487314552604. [DOI] [PubMed] [Google Scholar]
- 88.Matthys K., Verdonck P. Development and modelling of arterial applanation tonometry: A review. Technol. Health Care. 2002;10:65–76. doi: 10.3233/THC-2002-10107. [DOI] [PubMed] [Google Scholar]
- 89.Tensys Medical Inc.: San Diego, CA, United States. [(accessed on 8 June 2021)]; Available online: https://www.dicardiology.com/company/tensys-medical-inc.
- 90.Bard D.M., Joseph J.I., Van Helmond N. Cuff-Less Methods for Blood Pressure Telemonitoring. Front. Cardiovasc. Med. 2019;6:40. doi: 10.3389/fcvm.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stojanova A., Koceski S., Koceska N. Continuous Blood Pressure Monitoring as a Basis for Ambient Assisted Living (AAL)—Review of Methodologies and Devices. J. Med. Syst. 2019;43:24. doi: 10.1007/s10916-018-1138-8. [DOI] [PubMed] [Google Scholar]
- 92.Pandit J.A., Lores E., Batlle D. Cuffless Blood Pressure Monitoring: Promises and Challenges. Clin. J. Am. Soc. Nephrol. 2020;15:1531–1538. doi: 10.2215/CJN.03680320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samsung Samsung Expands Vital Blood Pressure and Electrocardiogram Tracking to Galaxy Watch3 and Galaxy Watch Active2 in 31 More Countries. [(accessed on 25 May 2021)];2021 Available online: https://news.samsung.com/global/samsung-expands-vital-blood-pressure-and-electrocardiogram-tracking-to-galaxy-watch3-and-galaxy-watch-active2-in-31-more-countries.
- 94.Vybornova A., Polychronopoulou E., Wurzner-Ghajarzadeh A., Fallet S., Sola J., Wuerzner G. Blood pressure from the optical Aktiia Bracelet: A 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press. Monit. 2021;30 doi: 10.1097/MBP.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nachman D., Gepner Y., Goldstein N., Kabakov E., Ben Ishay A., Littman R., Azmon Y., Jaffe E., Eisenkraft A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-73172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sensifree A New Standard of Care in Blood Pressure Monitoring. [(accessed on 25 May 2021)]; Available online: https://sensifree.com.
- 97.Baruch M.C., Kalantari K., Gerdt D.W., Adkins C.M. Validation of the pulse decomposition analysis algorithm using central arterial blood pressure. Biomed. Eng. Online. 2014;13:1–19. doi: 10.1186/1475-925X-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munir S., Guilcher A., Kamalesh T., Clapp B., Redwood S., Marber M., Chowienczyk P. Peripheral Augmentation Index Defines the Relationship Between Central and Peripheral Pulse Pressure. Hypertension. 2008;51:112–118. doi: 10.1161/HYPERTENSIONAHA.107.096016. [DOI] [PubMed] [Google Scholar]
- 99.Donley D.A., Fournier S.B., Reger B.L., DeVallance E., Bonner D.E., Olfert I.M., Frisbee J.C., Chantler P.D. Aerobic Exercise Training Reduces Arterial Stiffness in Metabolic Syndrome. J. Appl. Physiol. 2014;116:1396–1404. doi: 10.1152/japplphysiol.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee B.-K. Computational fluid dynamics in cardiovascular disease. Korean Circ. J. 2011;41:423. doi: 10.4070/kcj.2011.41.8.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.TensorFlow Developers. Zenodo; Geneva, Switzerland: 2021. Version v1.14.0. [Google Scholar]
- 102.Visscher M., Taylor T. Pressure ulcers in the hospitalized neonate: Rates and risk factors. Sci. Rep. 2014;4:1–6. doi: 10.1038/srep07429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seattle Children’s Hospital Improving Neonatal Skin Care. [(accessed on 26 May 2021)]; Available online: https://www.seattlechildrens.org/globalassets/documents/healthcare-professionals/neonatal-briefs/neonatal-skin-care.pdf.
- 104.Wu E., Wu K., Daneshjou R., Ouyang D., Ho D.E., Zou J. How medical AI devices are evaluated: Limitations and recommendations from an analysis of FDA approvals. Nat. Med. 2021;27:582–584. doi: 10.1038/s41591-021-01312-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this document that were generated solely by PyrAmes are available from the corresponding author upon reasonable request. The data are not publicly available due to sensitive personal data that were collected during clinical studies on patients. Data generated or provided by Stanford University and Children’s National Hospital are restricted by the terms of contractual agreements and are available upon reasonable request with the permission of those organizations and if the requestor agrees to comply with the European General Data Protection Regulation and not to attempt to trace the origin of the data.