Abstract
High salt levels are one of the significant and major limiting factors on crop yield and productivity. Out of the available attempts made against high salt levels, engineered nanoparticles (NPs) have been widely employed and considered as effective strategies in this regard. Of these NPs, titanium dioxide nanoparticles (TiO2 NPs) and selenium functionalized using chitosan nanoparticles (Cs–Se NPs) were applied for a quite number of plants, but their potential roles for alleviating the adverse effects of salinity on stevia remains unclear. Stevia (Stevia rebaudiana Bertoni) is one of the reputed medicinal plants due to their diterpenoid steviol glycosides (stevioside and rebaudioside A). For this reason, the current study was designed to investigate the potential of TiO2 NPs (0, 100 and 200 mg L−1) and Cs–Se NPs (0, 10 and 20 mg L−1) to alleviate salt stress (0, 50 and 100 mM NaCl) in stevia. The findings of the study revealed that salinity decreased the growth and photosynthetic traits but resulted in substantial cell damage through increasing H2O2 and MDA content, as well as electrolyte leakage (EL). However, the application of TiO2 NPs (100 mg L−1) and Cs–Se NPs (20 mg L−1) increased the growth, photosynthetic performance and activity of antioxidant enzymes, and decreased the contents of H2O2, MDA and EL under the saline conditions. In addition to the enhanced growth and physiological performance of the plant, the essential oil content was also increased with the treatments of TiO2 (100 mg L−1) and Cs–Se NPs (20 mg L−1). In addition, the tested NPs treatments increased the concentration of stevioside (in the non-saline condition and under salinity stress) and rebaudioside A (under the salinity conditions) in stevia plants. Overall, the current findings suggest that especially 100 mg L−1 TiO2 NPs and 20 mg L−1 Cs–Se could be considered as promising agents in combating high levels of salinity in the case of stevia.
Keywords: abiotic stress, antioxidant enzymes, essential oil, nanotechnology, oxidative stress, steviol glycosides
1. Introduction
Salt stress is one of the widely investigated abiotic stress factors because of its clearly reported adverse effects on plant growth and development, as manifested in crop yield loss [1,2,3,4]. Regarding osmotic adjustment, ion toxicity and oxidative stress, the negative effects of salt stress were reported for a quite number of plant species in recent years [5,6,7,8]. These reports suggest that most of the agronomically important crop species are not compatible with high salt levels, as uttered in a review by Parihar et al. [4]. Furthermore, the Food and Agriculture Organization (FAO) reports stated that salinity is a serious threat to over 6% of the world’s land [4] and the relevant reports anticipate that salinity will severely affect more than 50% of arable land by 2050 [9]. For this reason, the use of new methods and strategies to decrease the negative effects of salinity has gained great importance. Of the recent attempts undertaken, nanoscale materials were employed as a nanotechnological approach and accordingly exhibited protective roles for quite a number of crop plants, suggesting the better tolerance of plants against the harsh conditions [10,11].
Nanotechnology is the study and application of nanoscale particles with specific qualities and small diameters (1–100 nm); they are widely applied in various agriculture aspects, such as plant nutrition, plant protection and nanopesticides [12]. The relevant studies regarding functionalization, modification or newly conjugated structures of nanoparticles are some of the emerging and hot topics nowadays [10]. Out of the available nanomaterials, cerium dioxide (nCeO2) [13,14,15,16], magnetite (nFe3O4) [17,18,19], zinc oxide (nZnO) [20], silicon dioxide (nSiO2) [21], copper oxide (nCuO) [22], aluminum oxide (nAl2O3) [23] and carbon nanotubes [24] showed protective roles in plants under stress conditions. In the case of medicinal and aromatic plant species, the application of nanoparticles is one of the novel and wise strategies used to increase growth, yield and especially secondary metabolites in plants under salt stress [25]. Moreover, selenium functionalized by a chitosan nanocomposite (Cs–Se NPs) and titanium dioxide nanoparticles (TiO2 NPs) are some of the proven substantial molecules that are used to enhance the abiotic tolerance range of the crop via the activation of defense mechanisms [26,27]. Considering the relevant stress indicators, treatments of TiO2 NPs and Cs–Se NPs decreased MDA and H2O2 content and increased agronomic parameters, photosynthetic pigments content, chlorophyll fluorescence, soluble sugars, proline content and antioxidant enzymes activity in some plant species under salinity stress [25,27,28]. In addition to the enhanced crop productivity under salinity stress, total phenolic compounds and essential oil yield were also increased with TiO2 NPs and Cs–Se NPs treatments [25,27,29]. However, high TiO2 NP concentration decreased the growth parameters, photosynthetic pigments and antioxidant activity and increased MDA and H2O2 in the plant [25,28].
Stevia (Stevia rebaudiana Bertoni) belongs to the Asteraceae family and is a reputed medicinal plant, possessing diterpenoid steviol glycosides (i.a., stevioside and rebaudioside A), which are 300 times sweeter than sucrose. For this reason, stevia is known as sweet leaf or sugar leaf. Since the human body does not metabolize the glycosides of stevia, stevia contains zero calories. This property of stevia leads the plant to be assessed as a natural sweetener to control diabetes. In order to reveal the changes of these metabolites and other relevant biochemical responses of stevia against salt stress, a wide array of well-documented studies were undertaken [30,31,32,33,34,35]. However, to our best knowledge, no studies have hitherto investigated the effects of TiO2 NPs and Cs–Se NPs on stevia grown under salt stress. Corresponding to the enhancing and stimulating roles of elements and their application as nanoparticles at non-toxic concentrations, we hypothesized that the relevant nanoparticles will improve the antioxidant status, steviol glycosides and other physiological parameters of stevia; furthermore, these improvements will be manifested in the growth and development of stevia. Both nanoparticle types were previously tested [25,27] and produced promising results related to plant growth, the alleviation of salt stress and an increase in plant chemical constituents desired by humans. As deduced from the findings from the studies by Gohari et al. [25] and Sheikhalipour et al. [27], three concentrations of TiO2 NPs (0, 100 and 200 mg L−1) and Cs–Se NPs (0, 10 and 20 mg L−1) were assayed for the current study. Therefore, our research group set out to test the effects of these molecules on a new valuable plant species, namely, stevia, as part of a series of studies in this research area.
2. Results
2.1. Effect of Cs–Se NPs and TiO2 NPs on Plant Growth Parameters
Salinity significantly decreased the plant growth parameters, where higher salt levels decreased the shoot height by 40.83%, root height by 37.45%, shoot fresh weight by 62.67%, root fresh weight by 51.26%, shoot dry weight by 64.11% and root dry weight by 50.68% as compared with the non-saline condition (Table 1). However, the application of TiO2 NPs and Cs–Se NPs significantly increased the plant growth parameters under the saline conditions. The current findings revealed that the Cs–Se NPs (20 mg L−1) treatment positively affected the shoot height (17.80% and 18.94%), root height (9.82% and 13.32%), shoot fresh weight (16.26% and 16.43%), root fresh weight (7.16% and 11.52%), shoot dry weight (17.87% and 23.70%) and root dry weight (7.07% and 13.47%) under the 50 mM and 100 mM salt levels, respectively, in comparison with the non-NP-treated plants.
Table 1.
Effects of foliar application of Cs–Se NPs (0, 10 and 20 mg L−1) and TiO2 NPs (0, 100 and 200 mg L−1) on some growth and photosynthesis parameters of stevia (Stevia rebaudiana Bertoni) under different salt stress (0, 50 and 100 mM NaCl) treatments.
| NaCl (mM) | Treatments | Shoot Height (cm) |
Root Height cm) |
Shoot FW (g) |
Shoot DW (g) |
Root FW (g) |
Root DW (g) |
Chl a (mg g−1 FW) |
Chl b (mg g−1 FW) |
Total Chl (mg g−1 FW) |
Carotenoids (mg g−1 FW) | Fv/Fm | Pn (μmol m−2 s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Treatment | 46.72 ± 1.74 ab | 17.06 ± 0.40 a | 107.99 ± 4.50 a | 20.09 ± 0.67 a | 30.08 ± 0.17 a | 5.86 ± 0.04 a | 6.39 ± 0.18 a | 0.814 ± 0.012 a | 7.21 ± 0.18 a | 5.91 ± 0.12 a | 0.775 ± 0.010 b | 9.23 ± 0.04 c | |
| TiO2 NPs 100 mg L−1 | 46.93 ± 2.64 ab | 17.56 ± 0.21 a | 107.88 ± 6.12 a | 20.11 ± 0.94 a | 30.15 ± 0.23 a | 5.93 ± 0.03 a | 6.50 ± 0.21 a | 0.835 ± 0.011 a | 7.33 ± 0.22 a | 5.93 ± 0.05 a | 0.778 ± 0.006 b | 9.38 ± 0.01 ab | |
| 0 | TiO2 NPs200 mg L−1 | 48.11 ± 0.74 a | 17.23 ± 0.45 a | 107.75 ± 6.74 a | 20.12 ± 1.11 a | 30.16 ± 0.30 a | 5.92 ± 0.06 a | 6.44 ± 0.18 a | 0.819 ± 0.006 a | 7.26 ± 0.18 a | 5.89 ± 0.13 a | 0.772 ± 0.007 b | 9.32 ± 0.01 bc |
| Cs–Se NPs 10 mg L−1 | 46.80 ± 1.72 ab | 17.55 ± 0.32 a | 107.69 ± 3.69 a | 20.15 ± 0.91 a | 29.92 ± 1.57 a | 5.89 ± 0.28 a | 6.48 ± 0.29 a | 0.819 ± 0.003 a | 7.29 ± 0.29 a | 5.90 ± 0.09 a | 0.776 ± 0.003 b | 9.39 ± 0.06 ab | |
| Cs–Se NPs 20 mg L−1 | 47.81 ± 1.49 a | 17.65 ± 0.23 a | 108.37 ± 1.82 a | 20.30 ± 0.45 a | 30.97 ± 0.41 a | 6.12 ± 0.10 a | 6.59 ± 0.14 a | 0.836 ± 0.012 a | 7.43 ± 0.13 a | 5.97 ± 0.09 a | 0.789 ± 0.004 a | 9.48 ± 0.02 a | |
| No Treatment | 37.67 ± 1.57 e | 13.67 ± 0.23 c | 74.87 ± 3.44 d | 14.38 ± 0.41 c | 24.60 ± 0.41 c | 4.86 ± 0.08 c | 4.83 ± 0.09 e | 0.710 ± 0.008 d | 5.54 ± 0.08 d | 5.27 ± 0.05 d | 0.741 ± 0.000 e | 8.41 ± 0.03 g | |
| TiO2 NPs 100 mg L−1 | 44.29 ± 0.84 bcd | 15.01 ± 0.15 b | 84.31 ± 2.49 bc | 16.48 ± 0.26 b | 26.15 ± 0.21 bc | 5.18 ± 0.03 bc | 5.43 ± 0.08 bc | 0.739 ± 0.008 b | 6.17 ± 0.09 b | 5.67 ± 0.07 b | 0.752 ± 0.001 d | 8.78 ± 0.03 e | |
| 50 | TiO2 NPs 200 mg L−1 | 41.60 ± 1.94 d | 14.13 ± 0.25 c | 79.11 ± 0.77 cd | 14.43 ± 1.00 c | 25.49 ± 1.42 bc | 5.06 ± 0.29 bc | 5.08 ± 0.11 de | 0.732 ± 0.010 bc | 5.81 ± 0.12 c | 5.34 ± 0.05 cd | 0.754 ± 0.002 d | 8.58 ± 0.06 f |
| Cs–Se NPs 10 mg L−1 | 42.66 ± 1.56 cd | 14.12 ± 0.48 c | 81.60 ± 1.83 cd | 16.04 ± 0.50 bc | 25.16 ± 1.06 bc | 4.97 ± 0.24 bc | 5.19 ± 0.11 cd | 0.715 ± 0.004 cd | 5.90 ± 0.11 c | 5.44 ± 0.08 c | 0.758 ± 0.000 cd | 8.62 ± 0.03 f | |
| Cs–Se NPs 20 mg L−1 | 45.83 ± 1.17 abc | 15.16 ± 0.10 b | 89.41 ± 1.66 b | 17.51 ± 0.16 b | 26.50 ± 0.73 b | 5.23 ± 0.14 b | 5.50 ± 0.19 b | 0.730 ± 0.001 bcd | 6.23 ± 0.19 b | 5.65 ± 0.11 b | 0.767 ± 0.004 bc | 8.93 ± 0.04 d | |
| No Treatment | 27.64 ± 1.70 h | 10.67 ± 0.19 f | 40.31 ± 0.86 f | 7.21 ± 0.28 e | 14.66 ± 0.48 e | 2.89 ± 0.04 e | 3.41 ± 0.08 h | 0.623 ± 0.006 f | 4.04 ± 0.07 g | 4.61 ± 0.02 g | 0.655 ± 0.004 i | 7.16 ± 0.04 l | |
| TiO2 NPs 100 mg L−1 | 33.41 ± 0.14 fg | 12.03 ± 0.14 de | 48.42 ± 1.10 e | 9.48 ± 0.40 d | 16.41 ± 0.69 d | 3.25 ± 0.14 d | 3.82 ± 0.02 fg | 0.678 ± 0.001 e | 4.50 ± 0.02 ef | 4.96 ± 0.03 e | 0.717 ± 0.005 g | 7.67 ± 0.04 i | |
| 100 | TiO2 NPs200 mg L−1 | 30.45 ± 0.88 gh | 11.68 ± 0.05 e | 43.50 ± 0.57 ef | 8.76 ± 0.29 de | 15.75 ± 0.50 e | 3.13 ± 0.11 de | 3.62 ± 0.01 gh | 0.643 ± 0.014 f | 4.27 ± 0.02 fg | 4.76 ± 0.03 fg | 0.686 ± 0.005 h | 7.42 ± 0.03 k |
| Cs–Se NPs 10 mg L−1 | 30.68 ± 0.60 gh | 12.19 ± 0.09 de | 42.80 ± 0.42 ef | 8.73 ± 0.52 de | 15.92 ± 0.20 de | 3.19 ± 0.04 de | 3.75 ± 0.03 g | 0.668 ± 0.013 e | 4.42 ± 0.04 f | 4.79 ± 0.03 f | 0.665 ± 0.004 i | 7.53 ± 0.05 j | |
| Cs–Se NPs 20 mg L−1 | 34.10 ± 0.14 f | 12.31 ± 0.08 d | 48.24 ± 0.86 e | 9.45 ± 0.74 d | 16.57 ± 0.61 d | 3.34 ± 0.10 d | 4.03 ± 0.05 f | 0.675 ± 0.013 e | 4.70 ± 0.04 e | 5.05 ± 0.03 e | 0.730 ± 0.006 f | 7.78 ± 0.05 h |
Data are the average of 3 repetitions ± standard error. Different letters in a column indicate significantly different values according to Duncan’s post hoc analysis at p < 0.05.
Out of the applied TiO2 NP levels, TiO2 NPs (100 mg L−1) significantly improved the shoot height (14.94% and 17.27%), root height (8.92% and 11.30%), shoot fresh weight (11.19% and 16.74%), root fresh weight (5.92% and 10.66%), shoot dry weight (12.74% and 23.94%) and root dry weight (6.17% and 11.07%) under the 50 mM and 100 mM salt levels, respectively, in comparison with the non-NP-treated plants (Table 1).
2.2. Effect of Cs–Se NPs and TiO2 NPs on Photosynthetic Pigments, Net Photosynthetic Rate (Pn) and Maximum Quantum Efficiency of Photosystem II (Fv/Fm)
Salinity significantly decreased chlorophyll a and b, total chlorophyll, carotenoid concentrations, Pn and Fv/Fm (Table 1). However, the TiO2 NPs and Cs–Se NPs exhibited positive effects on the photosynthetic pigments, Pn and Fv/Fm under salinity. The current findings showed that a 100 mM salinity concentration decreased chlorophyll a by 46.63%, chlorophyll b by 23.46%, total chlorophyll by 43.96%, carotenoid by 21.99%, Pn by 22.42% and Fv/Fm by 15.48% in comparison with the non-saline condition. Cs–Se NPs (20 mg L−1) increased content of chlorophyll a (12.18% and 15.38%), chlorophyll b (2.73% and 7.70%), total chlorophyll (11.07% and 14.04%), carotenoid (6.72% and 8.71%), Pn (5.82% and 7.96%) and Fv/Fm (3.38% and 10.27%) in comparison with non-treated plants under the 50 mM and 100 mM salt levels, respectively. Furthermore, TiO2 NPs (100 mg L−1) significantly enhanced the concentration of chlorophyll a (11.04% and 10.73%), chlorophyll b (3.92% and 8.11%), total chlorophyll (10.21% and 10.22%), carotenoid (7.05% and 7.05%), Pn (4.21% and 6.64%) and Fv/Fm (1.46% and 8.64%) parameters in comparison with non-treated plants under the 50 mM and 100 mM salt levels, respectively (Table 1).
2.3. Effect of Cs–Se NPs and TiO2 NPs on Proline Content and RWC
Salinity significantly increased the proline content and decreased the relative water content (RWC) (Figure 1). The findings of the current study revealed that severe salinity increased the proline content by 41.51% and decreased the RWC by 39.94% in comparison with the non-saline condition, whilst treatments of TiO2 NPs and Cs–Se NPs resulted in higher levels of proline content relative to the 50 and 100 mM NaCl stress alone. Moreover, TiO2 NPs and Cs–Se NPs increased the RWC in plants under salinity stress. Our results showed that the treatment with Cs–Se NPs (20 mg L−1) significantly increased the proline content (5.23% and 9.35%) and RWC (11.73% and 12.83%) in comparison with non-treated plants under the 50 and 100 mM salt levels, respectively. Furthermore, TiO2 NPs (100 mg L−1) significantly increased the proline content (7.27% and 8.40%) and RWC (10.60% and 10.33%) as compared with the non-treated plants under 50 and 100 mM salt levels, respectively (Figure 1A,B).
Figure 1.
Effect of different concentrations of Cs–Se NPs and TiO2 NPs on the proline content (A) and RWC (B) of stevia (Stevia rebaudiana Bertoni) leaf under salinity stress. Data are the average of 3 repetitions ± standard error. Different letters indicate significantly different values according to Duncan’s post hoc analysis at p < 0.05.
2.4. Effect of Cs–Se NPs and TiO2 NPs on Leaf Content of MDA and H2O2, as Well as Electrolyte Leakage
As expected, salinity caused substantial increases in leaf MDA, H2O2 and electrolyte leakage. The highest leaf MDA (5.41 nM mg−1 FW), H2O2 content (96.40 nM mg−1 FW) and electrolyte leakage (88.30%) were observed under the severe salinity condition (Figure 2). For the case regarding the enhanced agronomic performance and photosynthetic pigment concentration, both treatments of Cs–Se NPs and TiO2 NPs substantially affected the leaf contents of MDA and H2O2, as well as electrolyte leakage. Corresponding to the treatments, Cs–Se NPs (20 mg L−1) significantly decreased the content of MDA (38.52% and 22.18%) and H2O2 (9.34% and 10.38%), as well as electrolyte leakage (22.06% and 12.45%), in relation to the non-treated plants grown under the 50 mM and 100 mM salinity levels, respectively. Moreover, the TiO2 NPs (100 mg L−1) treatment significantly decreased the content of MDA (34.69% and 18.11%) and H2O2 (11.16% and 9.11%), as well as electrolyte leakage (14.25% and 9.66%), as compared with the non-treated plants under 50 mM and 100 mM levels of salinity (Figure 2A–C).
Figure 2.
Effect of different concentrations of Cs–Se NPs and TiO2 NPs on the content of MDA (A), H2O2 (B) and the electrolyte leakage (%) parameter (C) of stevia (Stevia rebaudiana Bertoni) leaves under salinity stress. Data are the average of 3 repetitions ± standard error. Different letters indicate significantly different values according to Duncan’s post hoc analysis at p < 0.05.
2.5. Effect of Cs–Se NPs and TiO2 NPs on Total Phenolics Content and DPPH Scavenging Activity
The application of Cs–Se NPs (20 mg L−1) significantly increased the total phenolics content (3.24% and 5.07%) and total antioxidant capacity (14.08% and 10.74%) as compared with the non-treated plants under the 50 mM and 100 mM levels of salinity (Figure 3). Similar to the Cs–Se NPs (20 mg L−1), the TiO2 NPs (100 mg L−1) treatments also significantly increased the total phenolics content (3.97% and 4.63%) and DPPH radical scavenging activity (9.85% and 7.30%) as compared with the non-treated plants under the 50 mM and 100 mM levels of salinity (Figure 3A,B).
Figure 3.
Effect of different concentrations of Cs–Se NPs and TiO2 NPs on the Total Phenol (A) and total antioxidant capacity (B) of stevia (Stevia rebaudiana Bertoni) leaves under salinity Stress. Data are the average of 3 repetitions ± standard error. Different letters indicate significantly different values according to Duncan’s post hoc analysis at p < 0.05.
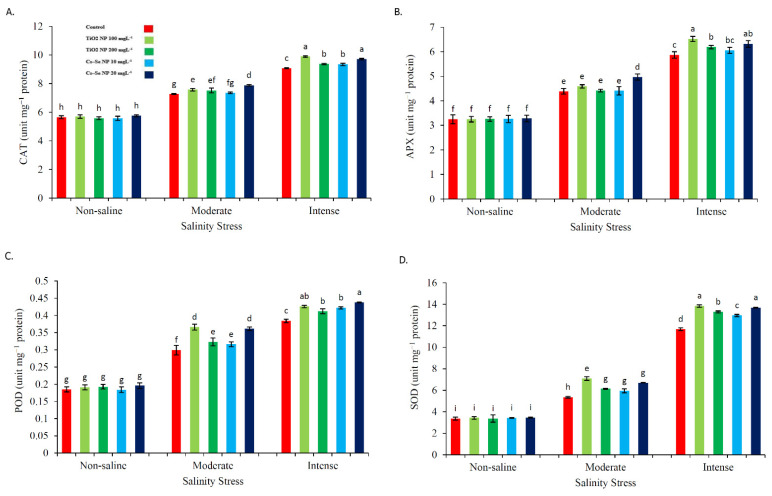
2.6. Effect of Cs–Se NPs and TiO2 NPs on Antioxidant Enzymes Activity
Antioxidant enzymes protect cells from oxidative damage by eliminating excess reactive oxygen species in plants. Current findings showed that the application of Cs–Se NPs (20 mg L−1) significantly increased the CAT (7.61% and 6.49%), APX (11.67% and 7.12%), POD (17.17% and 12.32%) and SOD (20.11% and 14.69%) activity in comparison with non-treated plants under the 50 mM and 100 mM salinity levels. Moreover, TiO2 NPs (100 mg L−1) significantly improved the CAT (3.83% and 8.19%), POD (18.30% and 9.85%) and SOD (24.50% and 15.67%) activity as compared with the non-treated plants under the 50 and 100 mM NaCl conditions, respectively. In addition, TiO2 NPs (100 mg L−1) significantly increased APX (9.96%) activity as compared with the non-treated plants under 100 mM NaCl (Figure 4A–D).
Figure 4.
Effect of different concentrations of Cs–Se NPs and TiO2 NPs on the CAT (A), APX (B), POD (C), and SOD (D) enzyme activities of stevia (Stevia rebaudiana Bertoni) leaves under salinity stress. Data are the average of 3 repetitions ± standard error. Different letters indicate significantly different values according to Duncan’s post hoc analysis at p < 0.05.
2.7. Effect of Cs–Se NPs and TiO2 NPs on Essential Oil Content, as Well as Stevioside and Rebaudioside A Content
Salinity and NP treatments significantly affected the essential oil content, as well as the stevioside and rebaudioside A contents (Figure 5). Increasing the salinity stress level led to an increase in essential oil content. The interactive effects of the major treatments on essential oil content were also found to be significant. Specifically, the findings revealed that Cs–Se NPs (20 mg L−1) increased the essential oil level the most (10.00% and 2.87%) as compared with the non-treated plants under the 50 mM and 100 mM levels of salinity, respectively. Moreover, TiO2 NPs (100 mg L−1) significantly improved the essential oil content (13.33% and 9.39%) as compared with the non-treated plants under the 50 mM and 100 mM saline conditions (Figure 5A). Regarding stevioside and rebaudioside A, the stevioside content increased with the severity of the stress, whilst rebaudioside A content increased up to 50 mM NaCl and then showed a decrease. In addition to the salt-stress-induced increases in stevioside content, Cs–Se NPs (20 mg L−1) treatments also significantly increased the stevioside content (18.38% and 15.59%) and rebaudioside A content (17.76% and 20.05%) as compared with the non-treated plants under the 50 mM and 100 mM saline conditions, respectively. Similar to the treatment of Cs–Se NPs, the application of TiO2 NPs (100 mg L−1) significantly increased the stevioside content (17.81% and 17.66%) and rebaudioside A content (14.61% and 18.18%) as compared with the non-treated plants under the 50 mM and 100 mM saline conditions, respectively (Figure 5B,C).
Figure 5.
Effect of different concentrations of Cs–Se NPs and TiO2 NPs on the oil content (A), stevioside (B) and rebaudioside A (C) of stevia (Stevia rebaudiana Bertoni) leaves under salinity stress. Data are the average of 3 repetitions ± standard error. Different letters indicate significantly different values according to Duncan’s post hoc analysis at p < 0.05.
2.8. Heat Map Clustering and Principal Component Analysis of the Examined Parameters
Due to the large amounts of data about the examined parameters corresponding to the salt stress and NPs treatments, a heat map was constructed in order to clarify, visualize and correlate the relevant findings of the current study (Figure 6A). Based on the analysis results, the clustering revealed that different combinations of salt stress (S0: control, S1: 50 mM NaCl and S2: 100 mM NaCl) and NPs treatments (T1: TiO2 NPs 100 mg L−1, T2: TiO2 NPs 200 mg L−1, Se1: Cs–Se NPs 10 mg L−1 and Se2: Cs–Se NPs 20 mg L−1) were classified into two major groups. As part of the clustering, all salt stress groups were clearly classified. The first group was composed of the higher level of salt stress (S2: 100 mM NaCl), whilst the second group was composed of the control (S0) and the lower level of salt stress (S1: 50 mM NaCl). Interestingly, the scattering of the NPs was similar to the salt stress groups corresponding to the responses of stevia plants. Considering the examined parameters of the plant, two major clusters were also observed. The heat map clustering was supported by principal component analysis (PCA), with a high explained variance ratio of the components (accounting for 97.75% of the variability of the original data) (Figure 6B). As deduced from both the heat map and the PCA, the examined parameters were clearly sorted into two groups. The first group was regarded as the yield parameters and photosynthesis-related parameters, whilst the second group included stress-related physiological and biochemical parameters, as well as secondary metabolites.
Figure 6.
Heat map corresponding to the dependent and independent variables along with the treatments (A) and the principal component analysis regarding the observations (B) of salinity and TiO2 and Cs–Se nanoparticle applications on the examined traits in Stevia rebaudiana Bertoni (S0: 0 mM NaCl; S0Se1: 0 mM NaCl, 10 mg L−1 Cs–Se NPs; S0Se2: 0 mM NaCl, 20 mg L−1 Cs–Se NPs; S0T1: 0 mM NaCl, 100 mg L−1 TiO2 NPs; S0T2: 0 mM NaCl, 200 mg L−1 TiO2 NPs; S1: 50 mM NaCl; S1Se1: 50 mM NaCl, 10 mg L−1 Cs–Se NPs; S1Se2: 50 mM NaCl, 20 mg L−1 Cs–Se NPs; S1T1: 50 mM NaCl, 100 mg L−1 TiO2 NPs; S1T2: 50 mM NaCl, 200 mg L−1 TiO2 NPs; S2: 100 mM NaCl; S2Se1: 100 mM NaCl, 10 mg L−1 Cs–Se NPs; S2Se2: 100 mM NaCl, 20 mg L−1 Cs–Se NPs; S2T1: 100 mM NaCl, 100 mg L−1 TiO2 NPs; S2T2: 100 mM NaCl, 200 mg L−1 TiO2 NPs).
3. Discussion
In the present study, the agronomic and biochemical responses of stevia grown under salt stress and Cs–Se NPs and TiO2 NPs treatments were assessed. As previously reported for stevia, in particular [30,34,36], salinity stress reduces agronomic parameters, such as shoot and root length, shoot and root fresh weight and shoot and root dry weight, but the application of Cs–Se NPs (20 mg L−1) and TiO2 NPs (100 mg L−1) positively regulates the relevant parameters (shoot and root length, shoot and root fresh weight and shoot and root dry weight). Improvement in plant growth parameters might be explained by the NP-mediated enhancement in the performances of photosynthesis traits, such as chlorophyll a and b, total chlorophyll, carotenoid content, Pn and Fv/Fm under salinity conditions, as deduced from the present findings. Regarding TiO2 NPs, the affirmative acts of the relevant NPs were also noted for Moldavian balm [25] and broad bean [28], which were correlated with the contribution to the chlorophyll development and Rubisco activities [37]. As formerly reported by Yang et al. [37] and Frazier et al. [38], the upregulation of gene expression and activity of Rubisco enzyme was provided by TiO2 NPs treatments. Moreover, Tumburu et al. [39] reported that TiO2 NPs increased the expression of genes related to photosynthetic metabolism in leaves of the Arabidopsis thaliana plant. TiO2 NPs also increased photosynthesis parameters by increasing the light energy of the PSI absorbed by the chloroplast membrane to be transferred to PSII, the promotion of light energy conversion to electron energy and the electron transport and acceleration of water photolysis and oxygen evolution [40]. In addition to activation of the photosynthesis machinery of the plant, growth parameters are also positively correlated with the absorption of essential elements in the Cs–Se-NP- and TiO2-NP-treated plants under salinity conditions [27,41].
Considering Se NP applications, the current findings are consistent with the reports indicating that Se NPs significantly improved the growth and photosynthetic performances of strawberry [42] and tomato [43] plants under salinity stress conditions. As deduced from the former reports, TiO2 NPs and Cs–Se NPs have a positive regulatory role on the photosynthetic system and growth parameters.
Munns and Tester [44] reported that reduced water uptake due to increased osmolarity of soil solution is one of the earliest effects of salinity on plants. However, the water balance of the plants can be maintained by increasing their osmolyte (e.g., proline) levels. In addition to the maintained water status of the cells, Shamsul et al. [45] reported that the overaccumulation of proline might provide several benefits concerned with scavenging ROS to prevent a sustained oxidative burst and stabilizing membranes to prevent electrolyte leakage. Along with the treatments of Cs–Se NPs (20 mg L−1) and TiO2 NPs (100 mg L−1), notable increases were recorded for the proline content and RWC in stevia. As is well-known, salinity causes substantial decreases in the assimilation, accumulation and metabolism of nitrogen, which has a key role in the biosynthesis of proline [46]. However, Se NPs and TiO2 NPs treatments might elevate the proline content in plants through increasing the activity of nitrate reductase (a key enzyme of nitrogen assimilation) [29,37,47] and plant nitrogen status [48,49]. As in the case of enhanced growth parameters, current findings are also consistent with the reports indicating that the application of Cs–Se NPs [27], Se NPs [42] and TiO2 NPs [28] increased proline and RWC in bitter melon, strawberry and broad bean, respectively, under salinity stress conditions.
As is well reported for quite a number of plants, salinity stress causes excessive ROS production, which then attacks lipids, proteins, DNA and carbohydrates. Furthermore, an oxidative burst results in membrane lipid peroxidation (oxidative damages) and the production of MDA in plants [50]. In order to maintain their proper and sustainable development, plants have evolved two detoxification mechanisms, namely, enzymatic and non-enzymatic antioxidants defense mechanisms, in order to maintain ROS at safe levels. In this regard, CAT, SOD, POD and APX are some of the principal enzymatic antioxidants in plants [51], whilst phenolic compounds are one of the main non-enzymatic defense compounds [52]. As is well-documented in quite a number of studies, the total antioxidant capacity of plants is correlated with the phenolic contents available [53,54,55,56]. As in the current study, we observed that the application of Cs–Se NPs (20 mg L−1) and TiO2 NPs (100 mg L−1) significantly increased the total phenolics content; CAT, SOD, POD and APX activity; and total antioxidant capacity. The application of Se NPs (20 mg L−1) and TiO2 NPs (100 mg L−1) also significantly decreased H2O2, MDA content and EL in the stevia plants under salinity conditions. As previously reported for bitter melon under salinity stress, Cs–Se NPs increased the total phenols; CAT, SOD, POD and APX activity; and total antioxidant capacity; and decreased the H2O2 and MDA content, as well as the EL [27]. Zahedi et al. [42] reported that Se NPs improved POD and SOD activity and decreased H2O2 and MDA levels in strawberries under salinity conditions. Moreover, Gohari et al. [25] observed that the application of TiO2 NPs increased the CAT, SOD, GP (guaiacol peroxidase) and APX activity and decreased H2O2 content in Moldavian balm plant under salinity conditions. In addition, Abdel Latef et al. [28] reported that the application of TiO2 NPs increased SOD and APX activity and decreased MDA content in broad bean plants under saline conditions. Considering the relevant findings of detoxification elements examined as part of this study, the increases in activity of the enzymes and phenolic contents might explain the improved physiology and agronomic traits of stevia.
Considering medicinal plants, the pharmaceutical activities or biological activities of these relevant plant species are dependent on the metabolites available [57,58,59]. In this regard, to increase or to keep the desired level of the medicinally important metabolites is one of the great interests of stevia researchers [30,32,60]. Out of the medicinal plants defined, stevia is one of the reputed medicinal plants used in the pharmaceutical industry, with it being well-known for its diabetes-controlling properties. Stevioside and rebaudioside A are the most important compounds of stevia. Steviol glycoside is a group of secondary metabolites that are derived from the mono-, di- and tetra-terpene biosynthetic pathways. The major steviol glycosides, such as stevioside and rebaudioside A, are non-caloric sweeteners that are used in many countries due to being sweeter than sucrose [61]. In this context, any exogenous treatments for stimulating the production of these metabolites might be of great interest to stevia growers. Along with the present study, we observed an increase in stevioside content but a decrease in rebaudioside A content under severe salt stress. Of the available reports with respect to the impacts of salt stress on stevioside and rebaudioside A content, Zeng et al. [30] showed that salt stress reduced the content of stevioside and rebaudioside A, whilst Cantabella et al. [32] reported that salinity increased rebaudioside A content in stevia plants. Moreover, osmoprotectant functions of those metabolites were reported under salt stress [32] and water stress [61]. In this study, we also recorded a substantial increase in stevioside content under severe saline conditions, which are consistent with the previous reports [32,61]. Regarding this study, the application of Cs–Se NPs (20 mg L−1) and TiO2 NPs (100 mg L−1) significantly increased the stevioside and rebaudioside A contents. In addition to these metabolites, the relevant NP treatments also augmented the essential oil content of stevia. In the report by Sheikhalipour et al. [27], Cs–Se NPs increased the essential oil content in bitter melon fruits under salinity conditions. Moreover, TiO2 NPs increased the essential oil content in Salvia officinalis [62], Mentha piperita [63] and Moldavian balm [25]. In addition, the application of Se NPs increased the synthesis of secondary metabolites through increases in the expression of biosynthesis pathway-related genes: Pal, 4CL, HCT, pAmt, Kas, Acl, Fat and AT3 in pepper [64], and Pal and 4CL in bitter melon plant [65]. The modulator roles of NPs on the pathways of plant secondary metabolites are not well-explained hitherto even though in this regard, Marslin et al. [66] postulated that the penetration and/or fixation of NPs on the cell surface and/or within cells causes elevated levels of ROS. Subsequently, cytoplasmic Ca2+, antioxidant system and mitogen-activated protein kinase (MAPK) cascades trigger transcriptional reprogramming of relevant genes involved in secondary metabolism.
4. Materials and Methods
4.1. Preparation of Titanium Dioxide Nanoparticles and Selenium Functionalized Using Chitosan Nanoparticles
The synthesis and characterization of the selenium functionalized using chitosan nanoparticles (Cs–Se NPs) and titanium dioxide nanoparticles (TiO2 NPs) used in this experiment are described in Sheikhalipour et al. [27] and Gohari et al. [25]. The synthesis was carried out in a nanochemistry laboratory, University of Maragheh, Iran. Regarding the characterization results, the sizes of the Cs–Se and TiO2 NPs were 60 nm and 25 nm, respectively.
4.2. Plant Material and Treatments
The experiment was conducted in the research greenhouses of the Faculty of Agriculture, Mohaghegh Ardabili University (46°16′ E, 37°23′ N, altitude 1485 m), as a factorial experiment using a random design. As a plant material, vegetatively propagated Stevia rebaudiana Bertoni cuttings with three fully-developed leaves were obtained from Pakanbazr Company, Isfahan, Iran. The seedlings were transferred to main pots (40 cm × 15 cm) containing coco peat and perlite (2:1, v/v) and uniformly irrigated with tap water each day for one week, then fertigated with half-strength Hoagland’s nutrient solution every 2 days until harvest. Thereafter, the plants were continuously watered with full-strength Hoagland’s nutrient solution supplemented with NaCl at concentrations of 0, 50 and 100 mM (non-saline conditions, moderate and intense stress) two weeks after being transferred to the main pots, which continued up to plant harvest (prolonged stress of approximately forty days after applying salt stress). To prevent the accumulation of salt in the culture medium, the culture medium was washed once a week with tap water. After two weeks from the beginning of salinity stress, the plants were sprayed with selenium nanoparticles (Cs–Se NPs) at concentrations of 0, 10 and 20 mg L−1 and anatase titanium dioxide nanoparticles (TiO2 NPs) at concentrations of 0, 100 and 200 mg L−1. Selenium nanoparticles (Cs–Se NPs) and titanium dioxide nanoparticles (TiO2 NPs) were applied once a week during the growth period (three times). All treatments were dispersed in deionized water (DIW) and then Tween 20 (Sigma-Aldrich Co, St. Louis, MO, USA) was added to the suspension and foliar application was performed. This experiment was performed in three repetitions and there were three plants in each repetition (9 plants for each treatment). The greenhouse temperatures of 26/19 ± 4 °C (day/night) and air relative humidity of ca. 80 ± 5% were maintained throughout the experiment under natural light and length of the day.
4.3. Plant Growth and Relative Water Content (RWC) in Leaves
Shoot and root height were recorded at the harvest stage. The fresh weight (FW) of the shoots and roots was recorded at harvest and the shoot and root dry weight (DW) was measured after samples were oven-dried (UFP800, Memmert, Büchenbach, Germany) at 70 °C for 72 h. The RWCs of leaves in treated and non-treated plants were determined using the method of Sairam and Srivastava [67].
4.4. Photosynthetic Pigments, Gas Exchange Capacity and Chlorophyll Fluorescence
Chlorophyll a (Chl a) and chlorophyll b (Chl b), total chlorophyll and carotenoids (Car) were extracted from fresh leaves (0.2 g) using 80% (v/v) acetone. After centrifugation (15,000× g for 5 min at 25 °C), the absorbance for each extract was spectrophotometrically recorded at 470, 646 and 663 nm (UV-1800 Shimadzu, Kyoto, Japan), and the concentration of photosynthetic pigments was determined using the following equations from Arnon [68]:
| Chl a = (12.47 × A663) − (3.62 × A645) | (1) |
| Chl b = (25.06 × A645) − (6.5 × A663) | (2) |
| Carotenoids = (1000 × A470) − (1.29 Chl a − 53.78 Chl b) | (3) |
Photosynthetic rates (Pn) of leaves at the second or third nodes of the plants were measured using an infrared (IR) gas analyzer (LI-6400T, Li-Cor Inc., Lincoln, NE, USA), with a red/blue light source (6400-02B) [69]. The chlorophyll fluorescence parameter (Fv/Fm) was measured using a DUAL-PAM-100 chlorophyll fluorometer (Heinz Walz, Efeltrich, Germany) after the adaption of stevia in the dark for 30 min. Chlorophyll fluorescence was determined on sunny days between 8:00 h and 9:00 h [70].
4.5. Proline, Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Contents and Electrolyte Leakage (EL)
The leaf proline content was quantified according to the method of Bates et al. [71]. Briefly, fresh leaves were homogenized in a 3% sulfosalicylic acid solution. Then, the homogenates were centrifuged at 15,000× g for 10 min at 4 °C. After centrifugation, 1 mL of the supernatant was placed in a tube and was allowed to react with 1 mL acid ninhydrin and 1 mL of glacial acetic acid. The relevant mixture was heated at 100 °C for 60 min. The reaction was terminated by placing the mixture on ice, then 2 mL of toluene was used for extracting the reaction mixture. The separation of the two phases was carried out after keeping the samples at room temperature for 30 min. Finally, the absorbances of the upper phase were read using a spectrophotometer at 520 nm and toluene was used as the blank.
Lipid peroxidation was measured using the amount of malondialdehyde (MDA) [72]. The samples of fresh leaf tissue (0.3 g) were ground in 20% trichloroacetic acid and centrifuged at 13,000 rpm for 15 min and 4 mL of 20% TCA were added to 1 mL of the supernatant. The mixtures were heated for 30 min in a hot water bath (95 °C) and were thereafter immediately cooled in an ice bath. Malondialdehyde content was determined at two wavelengths of 532 and 600 nm. To calculate the MDA concentration, a molar absorption coefficient of 155 mM−1 cm−1 was used.
For the quantification of hydrogen peroxide (H2O2), the method proposed by Alexieva et al. [73] was employed, where hydrogen peroxide was measured spectrophotometrically after reacting with KI. The reaction mixture consisted of 0.5 mL 0.1% trichloroacetic acid (TCA) leaf extract supernatant, 0.5 mL of 100 mM K-phosphate buffer and 2 mL reagent (1 MKI w/v in fresh double-distilled water). The blank probe consisted of 0.1% TCA in the absence of leaf extract. The reaction was developed for 1 h in darkness and the absorbance was measured at 390 nm. The amount of hydrogen peroxide was calculated using a standard curve prepared with known concentrations of H2O2.
Electrolyte leakage, as an indicator of stress damage, was determined according to Nanjo et al. [74]. Samples were kept in falcons comprising 10 mL of distilled water at room temperature (25 °C) and shook for 24 h at 120 rpm. The primary electrical conductivity of solution (EC1) was recorded. The electrolytes of the tissue were released by autoclaving the same samples at 100 °C for 2 h. Then, the solution was cooled at room temperature and the electrical conductivity of the solution (EC2) was registered. The relative electrolyte leakage EC1/EC2 × 100 was recorded.
4.6. Antioxidant Enzymes
Fresh leaf samples (0.5 g) were homogenized in 5 mL of 0.05 M phosphate buffer (1 mM EDTA, 1% PVP, pH 7.8) and the homogenates were centrifuged at 12,000× g for 20 min at 4 °C. The supernatants were collected and used for the determination of peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT) and superoxide dismutase (SOD) activity. The activity of POD was measured using the method of Hemeda and Klein [75]. To determine the peroxidase activity, a reaction mixture containing enzyme extract, 100 mM potassium phosphate buffer (pH 6.0), 5 µL of 10% (w/v) H2O2 and 16 mM guaiacol was used. The enzyme activity was expressed at 470 nm for 1 min as millimoles of produced tetraguaiacol per minute per milligram of soluble protein (U mg−1). The activity of CAT was measured using the method of Aebi [76]. The reaction mixture contained 25 mM phosphate buffer (pH 6.8) and 10 mM H2O2. The reduction at 240 nm was registered. The activity of SOD was measured using the method of Giannopolitis and Ries [77]. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction rate of NBT (nitro blue tetrazolium) at 560 nm. The activity of APX was measured using the method of Nakano and Asada [78]. To determine the ascorbate peroxidase activity, the reaction mixture contained enzyme extract, 50 mM phosphate buffer (pH 7.0), 0.5 mM AsA, 1 mM H2O2 and 0.1 mM EDTA. The reaction was started via the addition of H2O2 and the reduction in absorbance at 290 nm was recorded for 1 min.
4.7. Extraction and Quantification of Total Phenolics Content and Radical Scavenging Activity (DPPH)
The air-dried and powdered samples (0.5 g) were extracted using 3 mL 85% (w/w) methanol and then centrifuged at 12,000× g for 15 min. This methanolic extract was used to measure the total phenolic content and radical scavenging activity (DPPH). The total phenolic concentration was assayed used the Folin–Ciocalteu reagent, as described by Chun et al. [79]. For the total antioxidant capacity assay, the DPPH scavenging activities of the extracts were determined according to the method proposed by Suja et al. [80], which involved preparing a 0.1 mM solution of 2.2-diphenyl-1-picrylhydrazyl (DPPH) in absolute ethanol. The antioxidant activity (%) was determined using the following formula: A0 − A1/A0 × 100, where A0—the absorbance of the control and A1—the absorbance of the standard.
4.8. Stevioside, Rebaudioside A and Essential Oil Contents
The contents of essential oils (mL 100 g−1 FW) were measured in a Clevenger-type apparatus. A total of 0.1 g of powdered dried leaves were transferred to 15 mL tubes, 3 mL distilled water was added and the mixture was kept in a water bath for 30 min at 80 °C. The resultant solution was firstly centrifuged at 12,000× g for 5 min and the supernatant was recovered (this process was repeated three times). The volume of the final supernatant was diluted to exactly 10 mL using distilled water and filtered using a 0.45 m nylon filter attached to a syringe. Then, the quantifications of the stevioside and rebaudioside A were performed using HPLC (Unicam Crystal 200, Thermo Fisher, UK) with a diode array detector based on the method of Martins et al. [81]. The main part of the mobile phase consisted of acetonitrile (80% w/w) buffered to pH = 0.5 with 100 mL of 0.02 M glacial acetic acid and 200 mL of 0.1 M sodium hydroxide for every 500 mL of the total solvent. The injection volume of 5 mL and a constant flow rate of 0.7 mL/min were programmed to flow through the Agilent Zorbax column (250 mm × 9 mm × 4.6 mm, 51 min) in gradient mode varying from 10:90 to 90:10 v/v following the detection using UV at 210 nm.
4.9. Statistical Analysis
Data were analyzed by using SPSS 20.0 software and all data were statistically analyzed using Duncan’s multi-range test with p < 0.05 as the significant difference level. Moreover, a principal component analysis (PCA) was performed in order to discriminate the treatments of nanoparticles and salinity on the basis of agronomic traits, physiological attributes, enzymatic activities and the contents of stevioside and rebaudioside A (OriginLab Software). Furthermore, a heat map corresponding to the findings from the treatments was constructed for visualizing and relating the dependent and independent variables (ClustVis).
5. Conclusions
Nanotechnology is a new method for increasing plant tolerance against biotic and abiotic stresses. The results of this experiment showed that the application of TiO2 NPs and Cs–Se NPs increased the growth parameters by increasing photosynthetic parameters under salinity stress conditions. Moreover, the application of TiO2 NPs and Cs–Se NPs increased the proline content, which led to an increase in the RWC in the plants. Furthermore, the application of TiO2 NPs and Cs–Se NPs reduced the oxidative damage in the plants through increased enzymatic activity and non-enzymatic antioxidants compound content in stevia under salt stress conditions. Finally, the application of TiO2 NPs and Cs–Se NPs increased the essential oil content and concentration of stevioside (in non-saline and saline conditions) and rebaudioside A (under saline conditions) in stevia plants. Generally, the application of TiO2 NPs (100 mg L−1) and Cs–Se NPs (20 mg L−1) increased the measured traits of stevia more than TiO2 NPs (200 mg L−1) and Cs–Se NPs (10 mg L−1). Therefore, the use of TiO2 NPs (100 mg L−1) and Cs–Se NPs (20 mg L−1) might be regarded as a useful strategy for increasing growth, antioxidant activity, essential oil content and steviol glycosides concentration in stevia under saline conditions.
Acknowledgments
We would like to thank the University of Mohaghegh Ardabili, Ardabil, Iran, and the University of Agriculture in Krakow, Poland, for their kind support during this project.
Author Contributions
Conceptualization, B.E. and G.G.; methodology, M.S. and G.G.; validation, M.S., G.G. and M.H.; formal analysis, M.S., H.F. and H.J.; data curation, M.S. and M.K.; writing—original draft preparation, M.S., M.H. and G.G.; writing—review and editing, G.G., M.H., M.K. and A.K.; supervision, B.E. and G.G.; project administration, B.E.; funding acquisition, B.E., G.G. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
We are thankful to the University of Mhaghighi for their kind support during this project. The authors also acknowledge the support from the University of Agriculture in Krakow, Poland, and the Ministry of Education and Science of the Republic of Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azarmi-Atajan F., Sayyari-Zohan M.H. Alleviation of salt stress in lettuce (Lactuca sativa L.) by plant growth-promoting rhizobacteria. J. Hortic. Postharvest Res. 2020;3:67–78. [Google Scholar]
- 2.El Moukhtari A., Cabassa-Hourton C., Farissi M., Savouré A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020;11:1127. doi: 10.3389/fpls.2020.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada A., Vetrano F., Miceli A. Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy. 2020;10:1523. doi: 10.3390/agronomy10101523. [DOI] [Google Scholar]
- 4.Parihar P., Singh S., Singh R., Singh V.P., Prasad S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- 5.Arif Y., Singh P., Siddiqui H., Bajguz A., Hayat S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020;156:64–77. doi: 10.1016/j.plaphy.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y.W., Kong X.W., Wang N., Wang T.T., Chen J., Shi Z.Q. Thymol confers tolerance to salt stress by activating anti-oxidative defense and modulating Na+ homeostasis in rice root. Ecotoxicol. Environ. Saf. 2020;188:109894. doi: 10.1016/j.ecoenv.2019.109894. [DOI] [PubMed] [Google Scholar]
- 7.Munns R., Passioura J.B., Colmer T.D., Byrt C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020;225:1091–1096. doi: 10.1111/nph.15862. [DOI] [PubMed] [Google Scholar]
- 8.Qian R., Ma X., Zhang X., Hu Q., Liu H., Zheng J. Effect of exogenous spermidine on osmotic adjustment, antioxidant enzymes activity, and gene expression of Gladiolus gandavensis seedlings under salt stress. J. Plant Growth Regul. 2020:1–15. doi: 10.1007/s00344-020-10198-x. [DOI] [Google Scholar]
- 9.Kibria M.G., Hoque M.A. A review on plant responses to soil salinity and amelioration strategies. Open J. Soil Sci. 2019;9:219–231. doi: 10.4236/ojss.2019.911013. [DOI] [Google Scholar]
- 10.Singh R.P., Handa R., Manchanda G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control Release. 2021;329:1234–1248. doi: 10.1016/j.jconrel.2020.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L., Lu L., Wang A., Zhang H., Huang M., Wu H., Xing B., Wang Z., Ji R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020;68:1935–1947. doi: 10.1021/acs.jafc.9b06615. [DOI] [PubMed] [Google Scholar]
- 12.Hawrylak-Nowak B., Hasanuzzaman M., Matraszek-Gawron R. Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In: Hasanuzzaman M., Fujita M., Oku H., Nahar K., Hawrylak-Nowak B., editors. Plant Nutrients and Abiotic Stress Tolerance. Springer; Singapore: 2018. pp. 269–295. [Google Scholar]
- 13.Wang Q., Ma X., Zhang W., Pei H., Chen Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics. 2012;4:1105–1112. doi: 10.1039/c2mt20149f. [DOI] [PubMed] [Google Scholar]
- 14.Rico C.M., Lee S.C., Rubenecia R., Mukherjee A., Hong J., Peralta-Videa J.R., Gardea-Torresdey J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.) J. Agric. Food Chem. 2014;62:9669–9675. doi: 10.1021/jf503526r. [DOI] [PubMed] [Google Scholar]
- 15.Andersen C.P., King G., Plocher M., Storm M., Pokhrel L.R., Johnson M.G., Rygiewicz P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016;35:2223–2229. doi: 10.1002/etc.3374. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi M.H.Z., Panahirad S., Navai A., Bahrami M.K., Kulak M., Gohari G. Cerium oxide nanoparticles (CeO2-NPs) improve growth parameters and antioxidant defense system in Moldavian balm (Dracocephalum moldavica L.) under salinity stress. Plant Stress. 2021;1:100006. doi: 10.1016/j.stress.2021.100006. [DOI] [Google Scholar]
- 17.Wang H., Kou X., Pei Z., Xiao J.Q., Shan X., Xing B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology. 2011;5:30–42. doi: 10.3109/17435390.2010.489206. [DOI] [PubMed] [Google Scholar]
- 18.Elfeky S.A., Mohammed M.A., Khater M.S., Osman Y.A., Elsherbini E. Effect of magnetite nano-fertilizer on growth and yield of Ocimum basilicum L. Int. J. Indig. Med. Plants. 2013;46:1286–1293. [Google Scholar]
- 19.Fahad, Balouch A., Agheem M.H., Memon S.A., Baloch A.R., Tunio A., Abdullah, Pato A.H., Jagirani M.S., Panah P., et al. Efficient mitigation of cadmium and lead toxicity in coriander plant utilizing magnetite (Fe3O4) nanofertilizer as growth regulator and antimicrobial agent. Int. J. Environ. Anal. Chem. 2020:1–12. doi: 10.1080/03067319.2020.1776861. [DOI] [Google Scholar]
- 20.Elsheery N.I., Helaly M.N., El-Hoseiny H.M., Alam-Eldein S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy. 2020;10:558. doi: 10.3390/agronomy10040558. [DOI] [Google Scholar]
- 21.Avestan S., Ghasemnezhad M., Esfahani M., Byrt C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy. 2019;9:246. doi: 10.3390/agronomy9050246. [DOI] [Google Scholar]
- 22.Liu J., Simms M., Song S., King R.S., Cobb G.P. Physiological effects of copper oxide nanoparticles and arsenic on the growth and life cycle of rice (Oryza sativa japonica ‘Koshihikari’) Environ. Sci. Technol. 2018;52:13728–13737. doi: 10.1021/acs.est.8b03731. [DOI] [PubMed] [Google Scholar]
- 23.Chahardoli A., Karimi N., Ma X., Qalekhani F. Effects of engineered aluminum and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-60841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gohari G., Safai F., Panahirad S., Akbari A., Rasouli F., Dadpour M.R., Fotopoulos V. Modified multiwall carbon nanotubes display either phytotoxic or growth promoting and stress protecting activity in Ocimum basilicum L. in a concentration-dependent manner. Chemosphere. 2020;249:126171. doi: 10.1016/j.chemosphere.2020.126171. [DOI] [PubMed] [Google Scholar]
- 25.Gohari G., Mohammadi A., Akbari A., Panahirad S., Dadpour M.R., Fotopoulos V., Kimura S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-57794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei Z., Mingyu S., Xiao W., Chao L., Chunxiang Q., Liang C., Hao H., Xiaoqing L., Fashui H. 2008. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 2008;121:69–79. doi: 10.1007/s12011-007-8028-0. [DOI] [PubMed] [Google Scholar]
- 27.Sheikhalipour M., Esmaielpour B., Behnamian M., Gohari G., Giglou M.T., Vachova P., Rastogi A., Brestic M., Skalicky M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials. 2021;11:684. doi: 10.3390/nano11030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel Latef A.A.H., Srivastava A.K., El-sadek M.S.A., Kordrostami M., Tran L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018;29:1065–1073. doi: 10.1002/ldr.2780. [DOI] [Google Scholar]
- 29.Khan M.N. Nano-titanium dioxide (nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill.) J. Plant Sci. 2016;11:1–11. doi: 10.3923/jps.2016.1.11. [DOI] [Google Scholar]
- 30.Zeng J., Chen A., Li D., Yi B., Wu W. Effects of salt stress on the growth, physiological responses, and glycoside contents of Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2013;61:5720–5726. doi: 10.1021/jf401237x. [DOI] [PubMed] [Google Scholar]
- 31.Reis M., Coelho L., Santos G., Kienle U., Beltrão J. Yield response of stevia (Stevia rebaudiana Bertoni) to the salinity of irrigation water. Agric. Water Manag. 2015;152:217–221. doi: 10.1016/j.agwat.2015.01.017. [DOI] [Google Scholar]
- 32.Cantabella D., Piqueras A., Acosta-Motos J.R., Bernal-Vicente A., Hernández J.A., Díaz-Vivancos P. Salt-tolerance mechanisms induced in Stevia rebaudiana Bertoni: Effects on mineral nutrition, antioxidative metabolism and steviol glycoside content. Plant Physiol. Biochem. 2017;115:484–496. doi: 10.1016/j.plaphy.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Fallah F., Nokhasi F., Ghaheri M., Kahrizi D., Agha A.B.A., Ghorbani T., Kazemi E., Ansarypour Z. Effect of salinity on gene expression, morphological and biochemical characteristics of Stevia rebaudiana Bertoni under in vitro conditions. Cell. Mol. Biol. 2017;63:102–106. doi: 10.14715/cmb/2017.63.7.17. [DOI] [PubMed] [Google Scholar]
- 34.Javed R., Gürel E. Salt stress by NaCl alters the physiology and biochemistry of tissue culture-grown Stevia rebaudiana Bertoni. Turk. J. Agric. For. 2019;115:484–496. [Google Scholar]
- 35.Kurunc A., Aslan G.E., Karaca C., Tezcan A., Turgut K., Karhan M., Kaplan B. Effects of salt source and irrigation water salinity on growth, yield and quality parameters of Stevia rebaudiana Bertoni. Sci. Hortic. 2020;270:109458. doi: 10.1016/j.scienta.2020.109458. [DOI] [Google Scholar]
- 36.Shahverdi M.A., Omidi H., Tabatabaei S.J. Stevia (Stevia rebaudiana Bertoni) responses to NaCl stress: Growth, photosynthetic pigments, diterpene glycosides and ion content in root and shoot. J. Saudi Soc. Agric. Sci. 2019;18:355–360. doi: 10.1016/j.jssas.2017.12.001. [DOI] [Google Scholar]
- 37.Yang F., Hong F., You W., Liu C., Gao F., Wu C., Yang P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006;110:179–190. doi: 10.1385/BTER:110:2:179. [DOI] [PubMed] [Google Scholar]
- 38.Frazier T.P., Burklew C.E., Zhang B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum) Funct. Integr. Genom. 2014;14:75–83. doi: 10.1007/s10142-013-0341-4. [DOI] [PubMed] [Google Scholar]
- 39.Tumburu L., Andersen C.P., Rygiewicz P.T., Reichman J.R. Molecular and physiological responses to titanium dioxide and cerium oxide nanoparticles in Arabidopsis. Environ. Toxicol. Chem. 2017;36:71–82. doi: 10.1002/etc.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingyu S., Fashui H., Chao L. Effects of nano-anatase TiO2 on absorption, distribution of light, and photoreduction activities of chloroplast membrane of spinach. Biol. Trace Elem. Res. 2007;118:120–130. doi: 10.1007/s12011-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 41.Rahneshan Z., Nasibi F., Moghadam A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018;13:73–82. doi: 10.1080/17429145.2018.1424355. [DOI] [Google Scholar]
- 42.Zahedi S.M., Abdelrahman M., Hosseini M.S., Hoveizeh N.F., Tran L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019;253:246–258. doi: 10.1016/j.envpol.2019.04.078. [DOI] [PubMed] [Google Scholar]
- 43.Morales-Espinoza M.C., Cadenas-Pliego G., Pérez-Alvarez M., Hernández-Fuentes A.D., de la Fuente M.C., Benavides-Mendoza A., Valdés-Reyna J., Juárez-Maldonado A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl Stress. Molecules. 2019;24:3030. doi: 10.3390/molecules24173030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munns R., Tester M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 45.Shamsul H., Qaiser H., Alyemeni M.N., Wani A.S., Pichtel J., Aqil A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullrich W.R. Salinity and nitrogen nutrition. In: Läuchli A., Lüttge U., editors. Salinity: Environment–Plants–Molecules. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 229–248. [Google Scholar]
- 47.Sotoodehnia-Korani S., Iranbakhsh A., Ebadi M., Majd A., Ardebili Z.O. Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum; an in vitro study. Environ. Pollut. 2020;265:114727. doi: 10.1016/j.envpol.2020.114727. [DOI] [PubMed] [Google Scholar]
- 48.Zahedi S.M., Hosseini M.S., Meybodi N.D.H., da Silva J.A.T. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. S. Afr. J. Bot. 2019;124:350–358. doi: 10.1016/j.sajb.2019.05.019. [DOI] [Google Scholar]
- 49.Yuan S.J., Chen J.J., Lin Z.Q., Li W.W., Sheng G.P., Yu H.Q. Nitrate formation from atmospheric nitrogen and oxygen photocatalysed by nano-sized titanium dioxide. Nat. Commun. 2013;4:1–7. doi: 10.1038/ncomms3249. [DOI] [PubMed] [Google Scholar]
- 50.Tripathy B.C., Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 52.Racchi M.L. Antioxidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants. 2013;2:340–369. doi: 10.3390/antiox2040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taârit M.B., Msaada K., Hosni K., Marzouk B. Fatty acids, phenolic changes and antioxidant activity of clary sage (Salvia sclarea L.) rosette leaves grown under saline conditions. Ind. Crops Prod. 2012;38:58–63. doi: 10.1016/j.indcrop.2012.01.002. [DOI] [Google Scholar]
- 54.Lim J.H., Park K.J., Kim B.K., Jeong J.W., Kim H.J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012;135:1065–1070. doi: 10.1016/j.foodchem.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 55.Wong C.C., Li H.B., Cheng K.W., Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- 56.Hichem H., Mounir D. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crops Prod. 2009;30:144–151. doi: 10.1016/j.indcrop.2009.03.003. [DOI] [Google Scholar]
- 57.Hussein R.A., El-Anssary A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019;1:13. [Google Scholar]
- 58.Wang M., Lamers R.J.A., Korthout H.A., van Nesselrooij J.H., Witkamp R.F., van der Heijden R., Voshol P.J., Havekes L.M., Verpoorte R., van der Greef J. Metabolomics in the context of systems biology: Bridging traditional Chinese medicine and molecular pharmacology. Phytother. Res. 2005;19:173–182. doi: 10.1002/ptr.1624. [DOI] [PubMed] [Google Scholar]
- 59.Verpoorte R. Exploration of nature’s chemodiversity: The role of secondary metabolites as leads in drug development. Drug Discov. 1998;3:232–238. doi: 10.1016/S1359-6446(97)01167-7. [DOI] [Google Scholar]
- 60.Ghaheri M., Kahrizi D., Bahrami G., Mohammadi-Motlagh H.R. Study of gene expression and steviol glycosides accumulation in Stevia rebaudiana Bertoni under various mannitol concentrations. Mol. Biol. Rep. 2019;46:7–16. doi: 10.1007/s11033-018-4250-4. [DOI] [PubMed] [Google Scholar]
- 61.Ceunen S., Geuns J.M. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013;76:1201–1228. doi: 10.1021/np400203b. [DOI] [PubMed] [Google Scholar]
- 62.Ghorbanpour M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant Physiol. 2015;20:249–256. doi: 10.1007/s40502-015-0170-7. [DOI] [Google Scholar]
- 63.Ahmad B., Shabbir A., Jaleel H., Khan M.M.A., Sadiq Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant Biol. 2018;13:6–15. doi: 10.1016/j.cpb.2018.04.002. [DOI] [Google Scholar]
- 64.Li D., Zhou C., Zhang J., An Q., Wu Y., Li J.Q., Pan C. Nanoselenium foliar applications enhance the nutrient quality of pepper by activating the capsaicinoid synthetic pathway. J. Agric. Food Chem. 2020;68:9888–9895. doi: 10.1021/acs.jafc.0c03044. [DOI] [PubMed] [Google Scholar]
- 65.Rajaee Behbahani S., Iranbakhsh A., Ebadi M., Majd A., Ardebili Z.O. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bitter melon (Momordica charantia); an in vitro experiment. PLoS ONE. 2020;15:e0235556. doi: 10.1371/journal.pone.0235556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marslin G., Sheeba C.J., Franklin G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017;8:832. doi: 10.3389/fpls.2017.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sairam R., Srivastava G. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat geno-types in response to long term salt stress. Plant Sci. 2002;162:897–904. doi: 10.1016/S0168-9452(02)00037-7. [DOI] [Google Scholar]
- 68.Arnon A. Method of extraction of chlorophyll in the plants. Agron. J. 1967;23:112–121. [Google Scholar]
- 69.Silva E.N., Ferreira-Silva S.L., de Vasconcelos Fontenele A., Ribeiro R.V., Viégas R.A., Silveira J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010;167:1157–1164. doi: 10.1016/j.jplph.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Maxwell K., Johnson G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 71.Bates L.S., Waldren R.P., Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 72.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 73.Alexieva V., Sergiev I., Mapelli S., Karanov E. The effect of drought and ultraviolet radiation on growth and stress marker in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- 74.Nanjo T., Kobayashi M., Yoshiba Y., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 1999;461:205–210. doi: 10.1016/S0014-5793(99)01451-9. [DOI] [PubMed] [Google Scholar]
- 75.Hemeda H.M., Klein B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990;55:184–185. doi: 10.1111/j.1365-2621.1990.tb06048.x. [DOI] [Google Scholar]
- 76.Aebi H. Catalase. Methods Enzym. Anal. 1983;59:309–314. [Google Scholar]
- 77.Giannopolitis C.N., Ries S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 79.Chun O.K., Kim D.O., Lee C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003;51:8067–8072. doi: 10.1021/jf034740d. [DOI] [PubMed] [Google Scholar]
- 80.Suja K., Jayalekshmy A., Arumughan C. Antioxidant activity of sesame cake extract. Food Chem. 2005;91:213–219. doi: 10.1016/j.foodchem.2003.09.001. [DOI] [Google Scholar]
- 81.Martins P.M., Thorat B.N., Lanchote A.D., Freitas L.A. Green extraction of glycosides from Stevia rebaudiana (Bert.) with low solvent consumption: A desirability approach. Resour. Effic. Technol. 2016;2:247–253. doi: 10.1016/j.reffit.2016.11.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.