Abstract
Aim
Describing acute respiratory distress syndrome patterns, therapeutics management, and outcomes of ICU COVID-19 patients and indentifying risk factors of 28-day mortality.
Methods
Prospective multicentre, cohort study conducted in 29 French ICUs. Baseline characteristics, comorbidities, adjunctive therapies, ventilatory support at ICU admission and survival data were collected.
Results
From March to July 2020, 966 patients were enrolled with a median age of 66 (interquartile range 58–73) years and a median SAPS II of 37 (29–48). During the first 24 h of ICU admission, COVID-19 patients received one of the following respiratory supports: mechanical ventilation for 559 (58%), standard oxygen therapy for 228 (24%) and high-flow nasal cannula (HFNC) for 179 (19%) patients. Overall, 721 (75%) patients were mechanically ventilated during their ICU stay. Prone positioning and neuromuscular blocking agents were used in 494 (51%) and 460 (48%) patients, respectively. Bacterial co-infections and ventilator-associated pneumonia were diagnosed in 79 (3%) and 411 (43%) patients, respectively. The overall 28-day mortality was 18%. Age, pre-existing comorbidities, severity of respiratory failure and the absence of antiviral therapy on admission were identified as independent predictors of 28-day outcome.
Conclusion
Severity of hypoxaemia on admission, older age (> 70 years), cardiovascular and renal comorbidities were associated with worse outcome in COVID-19 patients. Antiviral treatment on admission was identified as a protective factor for 28-day mortality. Ascertaining the outcomes of critically ill COVID-19 patients is crucial to optimise hospital and ICU resources and provide the appropriate intensity level of care.
Abbreviations: ICU, intensive care unit; LOS, length of stay; ARDS, acute respiratory distress syndrome; SAPS II, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; RRT, renal replacement therapy; ECMO, extra corporeal membrane oxygenation; IMV, invasive mechanical ventilation; NIV, non-invasive ventilation; HFNC, high-flow nasal cannula
Keywords: Viral pneumonia, Outcome, COVID-19, Management
1. Introduction
Since December 2019, a new agent, the SARS-CoV-2 coronavirus, has been spreading originally from the region of Wuhan in China, and rapidly overseas, causing an international outbreak of respiratory illnesses, designated as COVID-19 by the World Health Organization (WHO). In France, the first cases of COVID-19 have been reported at the end of January 2020. The increasing numbers of patients requiring intensive care urged local health and government officials to significantly increase ICU beds capacity to face COVID-19 patients [1].
While the outbreak has progressed, it appeared that SARS-Cov-2 was responsible for a very specific disease leading to a severe acute respiratory failure. Despite sharing a similar aetiology, COVID-19 patients may present quite different patterns from severely hypoxaemic patients to normally breathing hypoxaemic patients with or without associated hypercapnia and inconsistent response to prone position as an example [2], [3]. It is therefore difficult to identify which patients could benefit from one therapy to another. Currently, a variety of therapeutic strategies to manage COVID-19 patients in ICU have been suggested from supportive care alone to prescribing unproven medications. Apart from corticosteroids and tocilizumab, evidence from randomised clinical trials that potential therapies could significantly improve outcomes in patients suffering from severe COVID-19 is still needed [4], [5], [6]. Clinical features of hospitalised COVID-19 patients have been described in China, Europe and the United States [7], [8], [9], [10]. Although male gender, older age, comorbidities such as diabetes, immunosuppression and severe obesity appear as the most common risk factors of COVID-19 outcome worldwide, a great heterogeneity in COVID-19 features is reported amongst countries limiting potential extrapolation from other countries [11].
Accordingly, the primary objective was to perform a prospective, multicentre, observational study to provide a detailed description of the initial management of COVID-19 patients admitted to French ICUs. The secondary objective was to identify risk factors associated with 28-day mortality in a large cohort of ICU patients. These could promote an individualised therapeutic approach for COVID-19 patients during the current and potential future coronavirus-related outbreaks.
2. Methods
2.1. Study design and population
This study is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [12]. The AZUREA group, a French research network, conducted a prospective, observational, multicentre cohort study in 16 French university and 13 general hospitals. The study was approved by the “Comité de Protection des Personnes – Sud Méditerranée IV” (2020-A00797-32) for prospective (from the 2nd of April to the 3rd of July 2020) data collection and the Institutional Review Board of Nimes University Hospital for retrospective (from the 4th of March to the 1st of April) data collection, respectively. This study was registered in ClinicalTrials.gov on the 9th of April 2020, NCT04340466. According to French law, written informed consent was waived due to the non-interventional design of the study [13]. Patient or his/her surrogate decision-maker received an information letter prior to patient enrolment when possible.
All patients admitted to the intensive care unit for a diagnosis of probable or confirmed SARS-CoV-2 infection were enrolled into the study according to the predefined following criteria:
-
-
Age ≥ 18 years.
-
-
Patient presenting a confirmed SARS-CoV-2 infection (defined as positive result by reverse transcriptase polymerase chain reaction (RT-PCR) testing of a nasopharyngeal or lower respiratory tract swab) OR a probable SARS-CoV-2 infection (defined as a severe acute respiratory infection associated with inconclusive or unavailable RT-PCR testing) according to WHO guidance. This guidance was implemented locally with the adjunct of consistent COVID-19 CT scan imaging to classify SARS-CoV-2 infection as probable (https://www.who.int/publications-detail/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov)).
Were not included:
-
-
Patient presenting a severe acute respiratory syndrome with negative SARS-CoV-2 PCR and CT scan results.
-
-
Patient already enrolled in the present study.
-
-
Patient refusal to participate to the present study.
2.2. Data collection
For each included patient, the following data were recorded: demographic data (age, gender, weight, height), clinical data (admission diagnosis, comorbidities, Charlson score [14]), severity scores (SAPS II (Simplified Acute Physiology Score) [15], SOFA (Sequential Organ Failure Assessment) [16] scores) at ICU admission, and at day 7 and 14 for SOFA. Additionally, biological data (including serum creatinine concentration, lactate, ferritin, troponin, CRP, WBC count, haemoglobin, d-dimers, fibrinogen), infection data including clinical symptoms, antimicrobial therapy modalities (timing of initiation, dosing regimen, combination therapy), sedatives and respiratory support mode (invasive mechanical ventilation (IMV), non-invasive mechanical ventilation (NIV), oxygen mask, high-flow nasal cannula oxygen), adjunctive therapies, microbiological and imaging data (chest X-ray, thoracic CT scan, US exam) were collected. Moreover, complications (pulmonary embolism, acute kidney injury, cardiac arrhythmias, myocarditis, ventilator-associated pneumonia (VAP), liver failure) were recorded until hospital discharge or death. VAP was diagnosed based on French VAP/HAP guidelines and microbiological cultures [17]. Date of death was recorded and mortality at day 7, at ICU discharge and at day 28 as well as organ support requirement during 28-day follow-up were also reported.
ARDS was graded according to the Berlin Definition for patients receiving mechanical ventilation on ICU admission [18]. Mild ARDS was defined as a PaO2/FIO2 ratio of ≤ 300 mmHg to 200 mmHg with PEEP or continuous positive airway pressure of ≥ 5 H2O, moderate ARDS was defined as PaO2/FIO2 ratio of ≤ 200 mmHg to 100 mmHg with PEEP ≥ 5 H2O and severe ARDS defined as PaO2/FIO2 ≤ 100 mmHg with PEEP ≥ 5 cm H2O.
2.3. Data management
Data collection was performed by trained staff at each participating centre. Data were entered into a structured electronic password-protected and secured web-based case report form (eCRF). The eCRF was developed using the REDCap Data Management Platform designed to support data capture for research studies [19]. Data monitoring was handled by the coordinating Centre (Nîmes University Hospital, France). Outstanding queries regarding the completion of the CRF were undertaken with each participating centre when necessary to ensure accuracy of data.
2.4. Study outcomes
The primary outcome was all-cause mortality determined from patient medical chart at day 28. The secondary outcomes were ICU and hospital mortality, ICU and hospital length of stay (LOS), all-cause mortality at day 7 and requirement of organ support.
2.5. Statistical analysis
Simple descriptive statistics were used to characterise the study population; continuous data were summarised by median and interquartile range or median and (min; max), categorical data as n (%). Comparisons between survivors and non-survivor patients at 28 days were performed using Student’s t-test for quantitative variables, or the Mann–Whitney U test when the distribution of variables was non-Gaussian, and the Chi-square test for qualitative variables. We used a mixed logistic regression model with a centre-specific random intercept to assess relationships with mortality at day 28, considering the clustered structure of the data.
A primary analysis focused on patients’ characteristics at inclusion: age, gender, BMI (> 40 vs. < = 40), SOFA score without respiratory SOFA score component (< 2 vs. > = 2), chronic obstructive pulmonary disease, chronic heart failure, chronic kidney failure, cancer, arterial hypertension (with or without angiotensin-receptor blockers or ACE inhibitors treatment), and partial oxygen arterial blood pressure (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2 ratio).
A secondary analysis focused on care at admission with adjustment on characteristics at admission. Care parameters included in the model were: type of respiratory support, anticoagulants, antiviral therapy, hydroxychloroquine and corticosteroids.
Sensitivity analyses were made using generalised estimating equation (GEE) model with an exchangeable correlation matrix and Cox proportional hazards model with gamma frailty distribution. Statistical analyses were performed at the conventional two-tailed α level of 0.05 using SAS statistical software, version 9.4 (SAS Institute Inc).
3. Results
Between the 4th of March and the 3rd of July 2020, data from 966 patients admitted to 29 ICUs were analysed (Fig. 1 , study flow diagram). The distribution of included patients among the different participating hospitals is shown in Table S1 (Supplemental data). Baseline characteristics of the study cohort are presented in Table 1 . Among patients under mechanical ventilation on admission, 44 (8%) presented mild ARDS, 249 (47%) moderate ARDS and 224 (42%) severe ARDS. The main symptoms at ICU admission were fever (71%, n = 691), shortness of breath (69%, n = 666) and cough (58%, n = 565). Lymphopaenia, elevated d-dimer, fibrinogen and ferritin levels were the most frequent biological abnormalities observed at ICU admission. Most patients underwent CT scan (76%, n = 740) and/or PCR testing (98%, n = 944) for SARS-CoV-2 infection diagnosis (Table 2 ).
Fig. 1.
Flow Diagram.
Table 1.
Characteristics of COVID-19 patients admitted to ICU.
| Day 28 status |
||||
|---|---|---|---|---|
| Survivors (n = 793) | Non-survivors (n = 173) | All (n = 966) | p-value | |
| Age, years (n = 966) | 65 [57;72] | 70 [62;77] | 66 [58; 73] | < 0.0001 (a) |
| < 50 years | 95 (12%) | 7 (4%) | 102 (11%) | < 0.0001 (b) |
| 50–59 years | 157 (20%) | 25 (15%) | 182 (19%) | |
| 60–69 years | 278 (35%) | 47 (27%) | 325 (34%) | |
| 70–79 years | 218 (28%) | 61 (35%) | 279 (29%) | |
| > 80 years | 45 (6%) | 33 (19%) | 78 (8%) | |
| Sex, male (n = 966) | 593 (75%) | 127 (73%) | 720 (75%) | 0.7081 (b) |
| Weight, kg (n = 955) | 85 [74; 97] | 86 [73; 99] | 85 [74; 97] | 0.5119 (a) |
| Height, cm (n = 931) | 171 [165; 178] | 170 [165; 175] | 171 [165; 178] | 0.0110 (c) |
| Body Mass Index, kg.m−2 (n = 930) | 28.4 [25.2; 32.1] | 29.4 [26.0; 34.2] | 28.7 [25.2; 32.6] | 0.0523 (a) |
| BMI > 30, kg.m−2 | 305 (40%) | 74 (46%) | 379 (41%) | 0.1390 (b) |
| BMI > 40, kg.m−2 | 42 (6%) | 14 (9%) | 56 (6%) | 0.1167 (b) |
| Settings (n = 962) | 0.3533 (b) | |||
| Home or emergency department | 370 47%) | 83 (48%) | 453 (47%) | |
| Long term care facility | 6 (1%) | 2 (1%) | 8 (1%) | |
| Ward | 217 (28%) | 47 (27%) | 264 (27%) | |
| Transfer from another hospital | 114 (14%) | 30 (17%) | 144 (15%) | |
| Transfer from another ICU | 83 (11%) | 10 (6%) | 93 (10%) | |
| SAPS II score [15] (n = 957) | 36 [27; 46] | 45 [38; 60] | 37 [29; 48] | < 0.0001 (a) |
| SOFA score [16] (n = 960) | 4 [2; 7] | 7 [4; 9] | 4 [2; 8] | < 0.0001 (a) |
| Underlying conditions | ||||
| Arterial hypertension (n = 965) | 394 (50%) | 104 (61%) | 498 (52%) | 0.0103 (b) |
| Chronic cardiovascular disease (n = 965) | 439 (55%) | 118 (69%) | 557 (58%) | 0.0014 (b) |
| Diabetes (n = 966) | 240 (30%) | 57 (33%) | 297 (31%) | 0.4883 (b) |
| Ischaemic heart disease (n = 964) | 65 (8%) | 18 (11%) | 83 (9%) | 0.3246 (b) |
| Chronic heart failure (n = 960) | 25 (3%) | 18 (11%) | 43 (5%) | < 0.0001 (b) |
| Immunosuppression (n = 965) | 34 (4%) | 9 (5%) | 43 (4%) | 0.5861 (b) |
| COPD (n = 966) | 138 (17%) | 43 (25%) | 181 (19%) | 0.0228 (b) |
| Chronic kidney failure (n = 966) | 48 (6%) | 30 (17%) | 78 (8%) | < 0.0001 (b) |
| Cancer (n = 966) | 70 (9%) | 31 (18%) | 101 (10%) | 0.0004 (b) |
| Charlson score [14] (n = 966) | 1 [0; 2] | 2 [1; 4] | 1 [0; 2] | < 0.0001 (a) |
| Recent travel (n = 957) | 33 (4%) | 8 (5%) | 41 (4%) | 0.8072 (b) |
| Previous medications (n = 966) | ||||
| Use of angiotensin-receptor blockers | 137 (17%) | 41 (24%) | 178 (18%) | 0.0483 (b) |
| Use of ACE inhibitors | 159 (20%) | 41 (24%) | 200 (21%) | 0.2667 (b) |
| Anticoagulants | 53 (7%) | 25 (14%) | 78 (8%) | 0.0007 (b) |
| Antiplatelets | 162 (20%) | 47 (27%) | 209 (22%) | 0.0511 (b) |
For continuous variables mean ± standard deviation or median [interquartile-range] are given. For categorical variables, numbers (%) are given. BMI: body mass index. SOFA: Sequential Organ failure Assessment. SAPS: Simplified Acute Physiology Score. COPD: Chronic Obstructive Pulmonary Disease. ACE: Angiotensin Converting Enzyme. ICU: Intensive Care Unit.
(a) Wilcoxon test, (b) Chi2 test, (c) Student test.
Table 2.
Clinical, biological and radiological characteristics at ICU admission.
| Day 28 status |
||||
|---|---|---|---|---|
| Survivors (n = 793) | Non-survivors (n = 173) | All (n = 966) | p-value | |
| Number of days since symptoms onset (n = 961), days | 8 [6; 12] | 7 [3; 10] | 8 [6; 11] | < 0.0001 (a) |
| Symptoms at ICU admission (n = 966) | ||||
| Cough | 472 (60%) | 91 (53%) | 563 (58%) | 0.0945 (b) |
| Shortness of breath | 538 (68%) | 127 (73%) | 665 (69%) | 0.1520 (b) |
| Fever | 573 (72%) | 117 (68%) | 690 (71%) | 0.2222 (b) |
| Diarrhoea | 186 (23%) | 33 (19%) | 219 (23%) | 0.2125 (b) |
| Nausea | 20 (3%) | 3 (2%) | 23 (2%) | 0.7831 (d) |
| Asthenia | 51 (6%) | 8 (5%) | 59 (6%) | 0.3685 (b) |
| Anorexia | 14 (2%) | 4 (2%) | 18 (2%) | 0.5460 (d) |
| Weakness | 235 (30%) | 60 (35%) | 295 (31%) | 0.1915 (b) |
| Confusion | 31 (4%) | 13 (8%) | 44 (5%) | 0.0393 (b) |
| Headache | 94 (12%) | 13 (8%) | 107 (11%) | 0.0994 (b) |
| Myalgia | 171 (22%) | 23 (13%) | 194 (20%) | 0.0139 (b) |
| Anosmia | 71 (9%) | 13 (8%) | 84 (9%) | 0.5428 (b) |
| Ageusia | 40 (5%) | 6 (3%) | 46 (5%) | 0.3778 (b) |
| Vital signs | ||||
| Temperature, °C (n = 951) | 37.8 (±1.1) | 37.7 (±1.5) | 37.8 (±1.2) | 0.5668 (a) |
| SAP, mmHg (n = 955) | 128 (±27) | 125 (±32) | 128 (±28) | 0.0479 (a) |
| MAP, mmHg (n = 956) | 89 (±18) | 84 (±22) | 88 (±18) | 0.0019 (a) |
| Heart rate, beat/min (n = 957) | 89 (±20) | 93 (±24) | 89 (±21) | 0.0415 (a) |
| Laboratory tests | ||||
| Haemoglobin, g/dL (n = 950) | 12.7 [11.5; 14.0] | 11.9 [10.6; 13.4] | 12.6 [11.3; 13.9] | < 0.0001 (a) |
| WBC count, 103/mm3 (n = 948) | 8.2 [5.9; 10.9] | 8.3 [5.7; 11.7] | 8.2 [5.8; 11.1] | 0.4432 (a) |
| Neutrophil count, 103/mm3 (n = 830) | 6.5 [4.6; 9.0] | 6.3 [4.4; 9.6] | 6.5 [4.6; 9.2] | 0.8653 (a) |
| Lymphocyte count, 103/mm3 (n = 814) | 0.8 [0.6; 1.1] | 0.7 [0.5; 1.0] | 0.8 [0.5; 1.1] | 0.0083 (a) |
| Platelet count, 103/mm3 (n = 945) | 229 [172; 304] | 212 [145; 264] | 225 [169; 296] | 0.0002 (a) |
| Platelet/lymphocyte ratio (n = 811) | 283 [190; 425] | 272 [174; 453] | 283 [186; 428] | 0.8170 (a) |
| D-dimer, ng.mL−1 (n = 440) | 1570 [827; 3690] | 1480 [788; 3495] | 1560 [821; 3690] | 0.6687 (a) |
| Ferritin, (µg/L) (n = 211) | 1407 [843; 2407] | 1149 [409; 1976] | 1383 [738; 2389] | 0.0924 (a) |
| Fibrinogen, g.L−1 (n = 619) | 6.9 [5.9; 7.8] | 6.4 [5.2; 7.4] | 6.8 [5.8; 7.8] | 0.0008 (a) |
| Prothrombin, % (n = 844) | 85.0 [74.0; 96.0] | 80.5 [69.5; 91.5] | 84.5 [73.0; 95.0] | 0.0022 (a) |
| Procalcitonin, µg. L−1 (n = 568) | 0.5 [0.2; 1.3] | 0.7 [0.2; 4.0] | 0.5 [0.2; 1.6] | 0.0109 (a) |
| CRP, mg. L−1 (n = 753) | 157.7 [100.0; 233.0] | 143.4 [95.8; 235.4] | 154.9 [99.8; 234.0] | 0.6810 (a) |
| Arterial lactate, mmol. L−1 (n = 873) | 1.3 [1.0; 1.7] | 1.4 [1.0; 2.0] | 1.3 [1.0; 1.7] | 0.0070 (a) |
| Serum creatinine, µmol. L−1 (n = 947) | 74 [60; 96] | 93 [66; 142] | 77 [61; 103] | < 0.0001 (a) |
| Troponin Ic, ng.mL−1 (n = 353) | 3.4 [0.0; 15.0] | 13.0 [0.1; 81.5] | 4.0 [0.0; 18.1] | 0.0008 (a) |
| Troponin T, pg.mL−1 (n = 254) | 14 [8; 27] | 39 [24; 86] | 17 [10; 37] | < 0.0001 (a) |
| PaO2/FiO2, mmHg (n = 701) | 114 [83; 160] | 103 [77; 148] | 112 [81; 159] | 0.0456 (a) |
| PaO2, mmHg (n = 922) | 76 [65; 94] | 74 [63; 94] | 76 [64; 94] | 0.3592 (a) |
| PaCO2, mmHg (n = 922) | 37 [32; 42] | 38 [31; 46] | 37 [32; 43] | 0.3099 (a) |
| Radiological exams | ||||
| X-ray (n = 966) | 715 (90%) | 149 (86%) | 864 (89%) | 0.1175 (b) |
| CT-scan (n = 966) | 622 (78%) | 116 (67%) | 738 (76%) | 0.0014 (b) |
| US exam (n = 959) | 369 (47%) | 91 (53%) | 460 (48%) | 0.1522 (b) |
For continuous variables, mean ± standard deviation or median [interquartile-range] are given. For categorical variables, numbers (%) are given. SAP: systolic arterial pressure, WBC: white blood cells, CRP: C-reactive protein. PaO2: oxygen arterial pressure, PCO2: carbon dioxide arterial pressure, CT: computerised tomography, US: ultrasound.
(a) Wilcoxon test, (b) Chi² test, (d) Fisher’s exact test.
3.1. Microbiology
Microbiological samples were obtained from 963 (97%) patients with 96% of lower respiratory tract samples. For 857 (92%) patients, SARS-CoV-2 infections were proven by RT-PCR (Table 3 ). For 79 (3%) patients, bacterial co-infection was diagnosed, and during ICU stay, 342 (43%) patients developed ventilator-associated pneumonia (VAP).
Table 3.
Infection-related data from COVID-19 patients admitted to ICU (n = 966).
| Respiratory samples for SARS-CoV-2 Test (n = 931) | |
| BAL | 43 (5%) |
| Aspirates | 66 (7%) |
| Nasopharyngeal swab | 822 (88%) |
| SARS-CoV-2 Test (n = 944) | |
| RT-PCR | 926 (98%) |
| Rapid Diagnostic Testing | 3 (0.3%) |
| Unknown | 15 (2%) |
| SARS-CoV-2 Test result (n = 937) | |
| Positive | 857 (91%) |
| Negative | 80 (9%) |
| Microbiological tests on admission (n = 966) | 963 (99.5%) |
| Respiratory samples | 318 (33%) |
| Positive | 79 (3%) |
| Blood cultures | 465 (48%) |
| Positive | 32 (7%) |
| PCR Influenza A | 308 (32%) |
| Positive | 2 (0.6%) |
| PCR Influenza B | 303 (31%) |
| Positive | 3 (1%) |
| Pneumococcal urinary antigen | 425 (44%) |
| Positive | 8 (2%) |
| Legionella urinary antigen | 579 (60%) |
| Positive | 6 (1%) |
| Microbiological tests during ICU stay (n = 966) | 550 (57%) |
| Respiratory samples | 394 (41%) |
| PCR Pneumocystis jirovecii | 50 (5%) |
| Positive | 2 (4%) |
| Viral PCR | 267 (28%) |
| Positive HSV | 16 (6%) |
| Positive CMV | 3 (1%) |
| Positive HBV | 3 (1%) |
| Positive VZV | 2 (2%) |
| Galactomannan in BAL | 126 (13%) |
| Positive | 8 (6%) |
| Blood cultures | 156 (16%) |
| Positive | 69 (44%) |
BAL: bronchoalveolar lavage, RT-PCR: Reverse Transcriptase Polymerase Chain Reaction, HSV: Herpes Simplex Virus, CMV: cytomegalovirus, HBV: Hepatitis B virus, VZV: Varicella Zoster virus.
Results are given as numbers and percentages.
3.2. COVID-19 management
Respiratory, haemodynamic and therapeutic COVID-19 initial management are presented in Table 4 . More than half of the included patients received mechanical ventilation on ICU admission. For non-intubated patients on admission, median time to intubation and mechanical ventilation was 2 [1–3] days. Overall, 721 (75%) patients were mechanically ventilated during their ICU stay. Four hundred and ninety-four (51%) received prone positioning at a median time of 2 [1–5] days post admission in the ICU. Two-thirds of patients required vasopressor support. Antiviral treatment was prescribed in 242 (25%) patients with lopinavir/ritonavir being the most common used antiviral therapy (Table 4). Among adjunctive therapies, corticosteroids were administered to 212 (22%) patients and hydroxychloroquine to 289 (30%) patients.
Table 4.
COVID-19 management (n = 966).
| Day 28 status |
||||
|---|---|---|---|---|
| Alive at day 28 (n = 793) | Dead at day 28 (n = 173) | All (n = 966) | p-value | |
| Maximal respiratory support during the first 24 h in ICU (n = 966) | 0.0001 (b) | |||
| Standard oxygen therapy | 203 (26%) | 25 (15%) | 228 (24%) | |
| High-Flow Nasal Cannula | 156 (20%) | 23 (13%) | 179 (19%) | |
| Non-Invasive Ventilation | 25 (3%) | 8 (5%) | 33 (3%) | |
| Mechanical Ventilation | 434 (55%) | 125 (72%) | 559 (58%) | |
| Mechanical Ventilation mode (n = 545) | 0.3209 (d) | |||
| VAC | 403 (96%) | 123 (99%) | 526 (97%) | |
| BIPAP | 2 (0%) | 1 (1%) | 3 (1%) | |
| PSV | 5 (1%) | 0 (0%) | 5 (1%) | |
| APRV | 9 (2%) | 0 (0%) | 9 (2%) | |
| Tidal volume (mL) (n = 507) | 423 [241−658] | 418 [300−540] | 420 [241−658] | 0.1611 (a) |
| Respiratory rate (/min) (n = 528) | 22 [10−42] | 22 [12−35] | 22 [10−42] | 0.3712 (a) |
| PEEP (cmH20) (n = 535) | 12 [3–22] | 10 [2–20] | 12 [2–22] | 0.0683 (a) |
| FiO2 (%) (n = 543) | 80 [30−100] | 80 [40−100] | 80 [30−100] | 0.0427 (a) |
| Plateau pressure (cmH20) (n = 360) | 24 [10−40] | 25 [12−53] | 24 [10−53] | 0.0700 (a) |
| Intubation management (n = 489) | 0.9511 (b) | |||
| Video laryngoscopy | 156 (42%) | 52 (44%) | 208 (43%) | |
| Fiberoptic bronchoscopy | 37(10%) | 12 (10%) | 49 (10%) | |
| Direct Laryngoscopy | 177 (48%) | 55 (46%) | 232 (47%) | |
| Haemodynamic support | ||||
| Vasopressor support (n = 964) | 484 (61%) | 137 (79%) | 621 (64%) | < 0.0001 (b) |
| Inotropes (n = 963) | 41 (5%) | 29 (17%) | 70 (7%) | < 0.0001 (b) |
| Adjunctive therapies | ||||
| Antiviral therapy (n = 966) | 268 (34%) | 35 (20%) | 303 (31%) | 0.0005 (b) |
| Lopinavir/ritonavir | 213 (27%) | 29 (17%) | 242 (25%) | 0.0055 (b) |
| Remdesivir | 13 (2%) | 0 (0%) | 13 (1%) | 0.1410 (d) |
| Oseltamivir | 37 (5%) | 7 (4%) | 44 (5%) | 0.7232 (b) |
| Lamivudine | 1 (0%) | 0 (0%) | 1 (0%) | 1.0000 (d) |
| Nevirapine | 1 (0%) | 0 (0%) | 1 (0%) | 1.0000 (d) |
| Darunavir | 1 (0%) | 0 (0%) | 1 (0%) | 1.0000 (d) |
| Immunomodulatory agents (n = 966) | ||||
| Hydroxychloroquine | 236 (30%) | 53 (31%) | 289 (30%) | 0.8198 (b) |
| Corticosteroids | 175 (22%) | 37 (21%) | 212 (22%) | 0.8446 (b) |
| Tocilizumab | 12 (2%) | 1 (1%) | 13 (1%) | 0.4831 (d) |
| Interferon γ | 9 (1%) | 1 (1%) | 10 (1%) | 1.0000 (d) |
| Anti-interleukin 1 | 5 (1%) | 0 (0%) | 5 (1%) | 0.5922 (d) |
| Intravenous immunoglobulin | 2 (0%) | 1 (1%) | 3 (0%) | 0.4472 (d) |
| Antibiotics (n = 965) | 730 (92%) | 163 (94%) | 893 (92%) | 0.3530 (b) |
| Type of antibiotic therapy (n = 893) | 0.9372 (b) | |||
| Monotherapy | 141 (19%) | 33 (20%) | 174 (19%) | |
| Dual combination therapy | 481 (66%) | 105 (64%) | 586 (66%) | |
| Multiple combination therapy | 108 (15%) | 25 (15%) | 133 (15%) | |
| Anticoagulants (n = 963) | 703 (89%) | 152 (88%) | 855 (89%) | 0.8498 (b) |
| Anticoagulant dosing (n = 855) | 0.0189 (b) | |||
| Therapeutic dosing | 192 (27%) | 56 (37%) | 248 (29%) | |
| Prophylactic dosing | 511 (73%) | 96 (63%) | 607 (71%) | |
| Sedatives (n = 964) | 434 (55%) | 129 (75%) | 563 (58%) | < 0.0001 (b) |
| Neuromuscular blocking agents (n = 966) | 357 (45%) | 103 (60%) | 460 (48%) | 0.0004 (b) |
ICU: Intensive Care Unit. VAC: Volume Assist Control mode, BIPAP: Bi-level Positive Airway Pressures, APRV: Airway Pressure Release Ventilation, PSV: Pressure Support Ventilation. PEEP: Positive End Expiratory Pressure, VA: veno-arterial, VV: veno-venous. FIO2: inspired oxygen fraction.
For continuous variables mean ± standard deviation or median [min-max] are given. For categorical variables, numbers (%) are given.
(a) Wilcoxon test, (b) Chi2 test, (d) Fisher’s exact test.
3.3. Patient outcomes
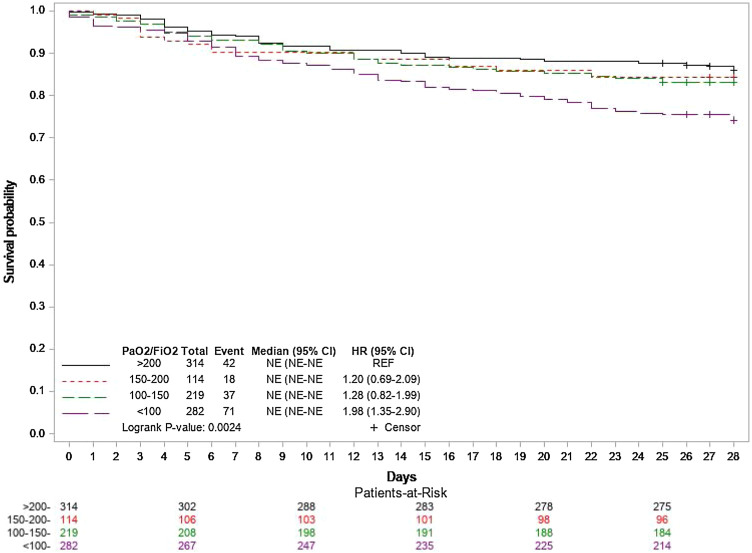
Overall 28-day mortality, ICU mortality and 7-day mortality were of 18% (173/966), 17% (166/966) and 8% (77/966), respectively. Twenty-eight-day mortality increased with the severity of hypoxaemia on admission (Fig. 2 ). Among deaths occurring in ICU, 78/166 (53%) were preceded by end-of-life decisions. The median (IQR) time from admission to death was 8 [4–16] days. Among the 793 patients alive at day 28, 250 (32%) patients were still hospitalised. Complications and organ support therapy are described in Table 5 . Median time to renal replacement therapy (RRT) and extra corporeal membrane oxygenation (ECMO) support were 5.5 [3–9] days and 5 [0–8] days post ICU admission. Multivariate analysis identified age, chronic kidney failure, chronic heart failure, SOFA score and PaO2/FiO2 at admission as independent risk factors of death at day 28 (Table 6 ). After adjustment on admission characteristics, antiviral therapy use was significantly associated with a lower risk of death at day 28. Sensitivity analyses confirmed these findings.
Fig. 2.
Kaplan-Meyer survival estimates during the 28 days following intensive care unit admission according to PaO2/FIO2 ratio in mmHg at admission.
PaO2: partial oxygen arterial blood pressure. FIO2: fraction of inspired oxygen
Table 5.
Clinical outcomes.
| Day-28 status |
||||
|---|---|---|---|---|
| Alive (n = 793) | Dead (n = 173) | All (n = 966) | p-value | |
| SOFA score at day 7 (n = 828) | 4 [2–7] | 8 [5–12] | 4 [2–8] | < 0.0001 (a) |
| SOFA score at day 14 (n = 686) | 3 [0–6] | 7 [5–10] | 3 [1–7] | < 0.0001 (a) |
| Overall complications (n = 966) | ||||
| Mechanical ventilation | 576 (73%) | 143 (83%) | 719 (74%) | 0.0062 (b) |
| Vasopressor support | 484 (61%) | 137 (79%) | 621 (64%) | < 0.0001 (b) |
| VAP/HAP | 342 (43%) | 69 (40%) | 411 (43%) | 0.4344 (b) |
| Myocarditis | 15 (2%) | 8 (5%) | 23 (2%) | 0.0488 (d) |
| Cardiac arrest | 18 (2%) | 28 (16%) | 46 (5%) | < 0.0001 (b) |
| Pulmonary embolism | 106 (13%) | 32 (19%) | 138 (14%) | 0.0806 (b) |
| AKI | 201 (25%) | 86 (50%) | 287 (30%) | < 0.0001 (b) |
| AKIN 1 score [32] (n = 287) | 63 (31%) | 7 (8%) | 70 (24%) | |
| AKIN 2 score [32] (n = 287) | 41 (20%) | 20 (24%) | 61 (21%) | |
| AKIN 3 score [32] (n = 287) | 97 (48%) | 58 (68%) | 155 (54%) | |
| RRT | 99 (13%) | 43 (25%) | 142 (15%) | 0.0001 (b) |
| RRT mode (n = 136) | 0.2344 (b) | |||
| CVVH | 19 (20%) | 14 (34%) | 33 (24%) | |
| CVVHD | 12 (13%) | 2 (5%) | 14 (10%) | |
| CVVHD Ci-Ca | 35 (37%) | 13 (32%) | 48 (35%) | |
| CVVHDF | 29 (31%) | 12 (29%) | 41 (30%) | |
| Duration to RRT (days) | 5 [3–10] | 6 [3–9] | 5 [3–9] | |
| ECMO (n = 966) | 43 (5%) | 20 (12%) | 63 (7%) | < 0.0031 (b) |
| Mode (n = 62) | ||||
| VA | 2 (5%) | 6 (30%) | 8 (13%) | |
| VV | 40 (95%) | 14 (70%) | 54 (87%) | |
| Duration to ECMO (days) (n = 63) | 5 [0−18] | 4 [0−15] | 5 [0−18] | 0.1088 (a) |
| Liver dysfunction | 46 (6%) | 15 (9%) | 61 (6%) | 0.1597 (b) |
| No complication | 263 (33%) | 19 (11%) | 282 (29%) | < 0.0001 (b) |
ICU: intensive care unit, LOS: length of stay, SOFA: Sequential Organ Failure Assessment, VAP/HAP: ventilator/healthcare-associated pneumonia, AKI: acute kidney injury, AKIN: acute kidney injury network, RRT: renal replacement therapy. CVVH: continuous veno-venous haemofiltration, CVVHD: continuous veno-venous haemodialysis, CVVHDF: continuous veno-venous haemodiafiltration. ECMO: Extra Corporeal Membrane Oxygenation. VA: venous-arterial. VV: Veno-venous.
For continuous variables median [interquartile-range] are given. For categorical variables, numbers (%) are given.
(a) Wilcoxon test, (b) Chi2 test, (d) Fisher’s exact test.
Table 6.
Independent risk factors associated with 28-day mortality.
| OR | 95% CI | p-value | |
|---|---|---|---|
| Model 1: Multivariate analyses of admission characteristics | |||
| Age | |||
| < 50 years | 1.0 | ref | _ |
| 50–59 years | 2.2 | [0.8–5.7] | 0.1126 |
| 60–69 years | 2.0 | [0.8–5] | 0.1423 |
| 70–79 years | 3.3 | [1.3–8.3] | 0.0113 |
| ≥ 80 years | 9.0 | [3.3–24.6] | < .0001 |
| Gender, female | 1.0 | [0.6–1.5] | 0.8533 |
| Body Mass Index > 40, kg.m−2 | 1.7 | [0.8–3.7] | 0.1793 |
| COPD | 1.3 | [0.8–2] | 0.2497 |
| Chronic heart failure | 2.4 | [1.1–5] | 0.0241 |
| Chronic renal failure | 2.1 | [1.2–3.8] | 0.0113 |
| Cancer | 1.6 | [0.9–2.7] | 0.0956 |
| Arterial hypertension | |||
| No | 1.0 | ref | – |
| Yes, with treatment by ACEI or ARB | 1.1 | [0.7–1.7] | 0.6448 |
| Yes, without treatment by ACEI or ARB | 0.9 | [0.5–1.5] | 0.6103 |
| SOFA score (without respiratory component) at inclusion | |||
| < 2 | 1.0 | ref | – |
| ≥ 2 | 2.4 | [1.6–3.7] | < .0001 |
| PaO2/FiO2 | |||
| > 200 mmHg | 1.0 | ref | – |
| 150−200 mmHg | 1.1 | [0.6–2.1] | 0.8012 |
| 100−150 mmHg | 1.1 | [0.7–1.9] | 0.6798 |
| < 100 mmHg | 1.8 | [1.1–2.9] | 0.0161 |
| Model 2: Multivariate analysis of care at admissiona | |||
|---|---|---|---|
| Respiratory support | |||
| Oxygen therapy or no ventilation | 1.0 | ref | – |
| High-Flow Nasal Cannula or Non-Invasive Ventilation | 0.6 | [0.2–1.5] | 0.2868 |
| Mechanical Ventilation | 1.6 | [0.7–3.4] | 0.2756 |
| Anticoagulants | |||
| No anticoagulants | 1.0 | ref | – |
| Prophylactic dosing | 0.7 | [0.4–1.4] | 0.3762 |
| Therapeutic dosing | 0.9 | [0.5–1.8] | 0.7503 |
| Antiviral therapy on admission | |||
| No antiviral therapy on admission | 1.0 | ref | |
| Antiviral therapy on admission | 0.5 | [0.3−0.9] | 0.0194 |
| Hydroxychloroquine on admission | 1.0 | [0.6–1.6] | 0.9414 |
| Corticosteroids on admission | 1.0 | [0.5–1.9] | 0.9000 |
OR were calculated using random effects logistic regression. The regression was based on 908 patients for 28-day mortality.
OR: odds ratio, CI: Confidence Interval, SOFA: Sequential Organ Failure Assessment. COPD: Chronic Obstructive Pulmonary Disease, ICU: Intensive Care Unit; SOFA: Sequential Organ failure Assessment; PaO2: Oxygen Arterial Pressure; PCO2: carbon dioxide arterial pressure; COPD: chronic obstructive pulmonary disease; ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker.
Bold values represent statistically significant values.
Model 2 was adjusted for characteristics at admission.
4. Discussion
4.1. Key findings
This large multicentre observational French cohort reports the initial management of 966 severe COVID-19 patients admitted to ICU over 4 months with complete data on 28-day outcome. The overall 28-day mortality was 18% with age, pre-existing comorbidities, severity of respiratory failure and the use of antiviral therapy as independent predictors of 28-day outcome. Initial management of COVID-19 patients consisted in IMV in 58%, in standard oxygen therapy in 53% and HFNC in 23% of patients on ICU admission. Prone positioning and neuromuscular blocking agents were used in half of patients. Bacterial co-infection rates were low (3%), whereas secondary pulmonary infections occurred in 43% patients.
4.2. Relationship with previous literature
The pandemic of COVID-19 has dramatically and rapidly challenged the global health care system in terms of hospital resources and patient care management. Reported rates of IMV may vary according to resources available among centres and experience. In this cohort, half of COVID-19 patients were intubated on admission ending to two-thirds of patients under IMV during their ICU stay in line with previous data from 4244 critically ill COVID-19 patients, showing a rate of 63% and 80% of patients mechanically ventilated on admission and during their ICU stay, respectively [20]. The rate of IMV was much higher (82–87%) in Italian and Spanish cohorts compared to reports from China (43%) [8], [9], [10]. The lack of experience in the treatment of patients with acute respiratory failure from a previously unknown viral agent and heterogeneity in recommendations might have had an effect on respiratory management of COVID-19 patients [21]. This may partially explain the differences observed in rates of mechanical ventilation in COVID-19 patients with similar median severity scores and similar severity of acute respiratory failure on admission. Although most of patients presented severe hypoxaemia on admission in the present cohort, intubation was not performed in half of cases. Some authors found a beneficial effect of early initial intubation after HFNC, whereas a recent meta-analysis suggested that timing of intubation might have no effect on critically ill COVID-19 patients’ outcome [22], [23]. Thus, the optimal timing for intubation in critically ill COVID-19 patients remains uncertain [24]. Performing unnecessary intubation in patients who may have improved without invasive MV can be detrimental, especially in medical resource-limited settings. A recently published cohort of 13 301 Brazilian critically ill patients found that non-invasive respiratory support was associated with improved outcome at day 60 but causal inference remains uncertain due to the observational nature of this study [25]. Additionally, early intubation itself may contribute to ventilator-associated pneumonia (VAP) risk in COVID-19 patients, and consequently had some negative impact on clinical outcomes. The high rate (43%) of VAP in our cohort is similar to the rate reported in the coVAPid study showing that ventilator-associated lower respiratory tract infections incidence was significantly higher in SARS-CoV-2 patients (36.1%), as compared to influenza patients (22.2%) or patients with no viral infection (16.5%) [26]. Several hypotheses have been formulated to explain the higher rate of secondary infections observed in critically ill COVID-19 patients, such as the use of immunosuppressive agents, the longer duration of mechanical ventilation and the severity of endothelial injury that may promote lung infection [26].
As previously reported, the most common comorbidities found in COVID-19 patients admitted to ICU were arterial hypertension, chronic cardiovascular disease, diabetes and obesity [8], [10], [27]. Among these comorbidities, chronic heart and kidney failures were associated with 28-day mortality in the present study. The 28-day mortality rate in the present cohort is lower than first published cohorts of critically ill COVID-19 patients with similar median severity score and median age on admission but in line with most recently published cohorts on the same study period [10], [25]. The mortality rates reported in the literature widely vary and could be potentially related to rationing of resources in overwhelmed ICUs, differences in respiratory and therapeutic interventions or cohorts reporting incomplete follow-up [28]. The lower mortality rate reported in our cohort could be partially explained by an increased use of corticosteroids compared to the COVID-ICU cohort [20]. Even though this factor was not associated with 28-day mortality in our cohort, the beneficial impact of corticosteroids in severe COVID-19 pneumonia has been demonstrated in a large randomised controlled trial and further confirmed in a meta-analysis [6], [29]. At the time of the present study data collection, benefits of corticosteroids were not clearly demonstrated.
Interestingly, after adjusting on patient characteristics on admission, receiving an antiviral treatment was an independent protective factor for 28-day mortality. At the time of enrolment in the study, lopinavir/ritonavir was the most common antiviral therapy used. However, lopinavir/ritonavir alone was not significantly associated with 28-day mortality when this variable was tested in the model. To date, no antiviral therapy has confirmed its efficiency in COVID-19 patients. Due to the observational nature of this study, some residual confounders may play a role in the association between antiviral treatment and outcome so that this association should be interpreted with caution. Finally, severe hypoxaemia (PaO2/FiO2 < 100) on admission and age > 70 years have been identified as prognostic factors in the present cohort. Elderly COVID-19 patients have much more severe disease and show poorer response to treatments than younger patients with reported 6-month mortality rate up to 72% [10], [20], [30], [31]. Consequently, the level of care intensity should be discussed in older patients with severe respiratory failure.
5. Clinical implications
This study provides large outcome data and detailed treatment strategies to establish risk stratification for COVID-19 patients on admission. Identifying prognostic factors on admission such as severity of hypoxaemia, older age, cardiovascular and renal comorbidities may allow the early identification of patients infected with SARS-CoV-2 who are at the highest risk of death to guide initial management and optimise resource allocation. Compared to the reported wave, current critically ill COVID-19 management has evolved with corticosteroids becoming a key component of therapeutic strategy as well as HFNC that was first considered cautiously. In future pandemics, taking into account patient medical conditions and severity of hypoxaemia will help to determine the best therapeutic approach and guide patient admission to appropriate care settings.
6. Study limitations
This study has several limitations. First, due to the design of the study, the reasons determining therapeutic approaches (antiviral agents, corticosteroids) or adjunctive therapies (prone position) used were not analysable and the ventilatory strategy may not be representative of clinical practice in non-pandemic circumstances. Second, due to the critical moment of the pandemic and the limited resources to conduct research at that time, some variables have missing data and 400 critically ill COVID-19 patients admitted to participating ICUs could not be included and may differ from the study cohort in terms of outcome. However, our multivariable model included 908 (94%) patients of the cohort, which is higher than previously reported [20]. Third, as the participating ICUs were exclusively located in different French regions, these results may not be extrapolated to other countries. Still, this cohort provides an interesting national overview of the initial management of COVID-19 patients.
7. Conclusion
Severity of hypoxaemia, older age (> 70 years), cardiovascular and renal comorbidities are prognostic factors for COVID-19 patients. Identifying the determinants of outcomes of critically ill COVID-19 patients is crucial to optimise hospital and ICU resources and provide the appropriate intensity level of care.
Ethics approval and consent to participate
The study was approved by the “Comité de Protection des Personnes – Sud Méditerranée IV” (2020-A00797-32).
According to French law, written informed consent was waived due to the non-interventional design of the study. Patient or his/her surrogate decision-maker received an information letter prior to patient enrollment when possible.
Disclosure of interest
The authors declare that they have no competing interests.
Funding
No funding.
Authors' contributions
CR and BA contributed to study design, data collection, data analysis and drafting the manuscript. LM and JYL contributed to study design, data collection and data interpretation. OC, OAA, LT, MG, CH, PGG, EN, PA, TC, JB, GB, LF, MF, AO, OJB, MOF, ML, YAT, JP, PYC, PA, MDM, EH, MB, JMJ, JC, MDD and NM contributed to data collection and data analysis. SL contributed to study coordination and data monitoring. MM and TM contributed to study design, data analysis and statistical analysis. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Lucie Vettoretti, Sandrine Brisset, Arnaud Romoli, Audrey Ambert, Loubna Elotmani, Sophie Lloret, Mathieu Expert, and Christophe Masseguin for their help in data collection and supervision of the study conduct.
The authors acknowledge support from the SFAR research network.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.accpm.2021.100931.
Appendix A. Investigators list
Names of the individual members of the FRENCH CORONA study collaborators to be searchable through their individual PubMed records:
Nadiejda Antier, Bruno Langevin (CH Alès).
Nadège Ngapmen, Habibou Mahamenrabiou (CH Château Thierry).
Antoine Dewitte (CHU Bordeaux).
Eline Bonnardel (CHU Bordeaux).
Pauline Ponsin, Emma Forsans, Astrée Swiech, Mathieu Pissot, Elisabeth Falzone (Hia Percy, Clamart).
Elsa Josefowicz, Julie Bellet, Benoit Graffin (CHU Lille).
Fabrice Thiolliere (Hospices Civiles de Lyon, Hôpital Lyon Sud).
Thomas Rimmelé, Elodie Brie (Hospices Civiles de Lyon, Hôpital Edouard Herriot).
Marc Gainnier (CHU La Timone Marseille, Aix Marseille University).
Laurent Zieleskiewicz (CHU Hôpital Nord Marseille).
Olivier Barbot, Aziz Akouz (CH Perpignan).
Diane Léna, Arnaud Causeret (Institut Arnault Tzanck, Saint Laurent du Var).
Hélène Charbonneau, Benoît Richard (Clinique Pasteur Toulouse).
Olivier Desebbe (Clinique de la Sauvegarde, Lyon).
Nicolas Herzog, Christophe Giacardi (HIA Clermont Tonnerre, Brest).
Pauline Ponsin (Percy Military Teaching Hospital, Burn centre).
Emma Forsans (Percy Military Teaching Hospital, Department of Anaesthesiology).
Sebastien Pili-Floury (CHU Besançon).
Thien-Nga Chamaraux-Tran (Hôpitaux Universitaires de Strasbourg, Hôpital de Hautepierre).
Appendix B. Supplementary data
The following is Supplementary data to this article:
References
- 1.Aziz S., Arabi Y.M., Alhazzani W., Evans L., Citerio G., Fischkoff K. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46(7):1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerin C., Albert R.K., Beitler J., Gattinoni L., Jaber S., Marini J.J. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group WHOREAfC-TW, Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrando C., Suarez-Sipmann F., Mellado-Artigas R., Hernandez M., Gea A., Arruti E. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie J., Wu W., Li S., Hu Y., Hu M., Li J. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020;46(10):1863–1872. doi: 10.1007/s00134-020-06211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 13.Toulouse E., Lafont B., Granier S., McGurk G., Bazin J.E. French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med. 2020;39:883–885. doi: 10.1016/j.accpm.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Le Gall J.R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 16.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 17.Leone M., Bouadma L., Bouhemad B., Brissaud O., Dauger S., Gibot S. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med. 2018;37(1):83–98. doi: 10.1016/j.accpm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Network C-IGobotR, the C-ICUI Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q., Shen J., Chen L., Li S., Zhang W., Jiang C. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. 2020;89(6):1092–1098. doi: 10.1097/TA.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papoutsi E., Giannakoulis V.G., Xourgia E., Routsi C., Kotanidou A., Siempos I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25(1):121. doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y.H., Choi K.J., Choi S.H., Lee S.Y., Kim K.C., Kim E.J. Clinical significance of timing of intubation in critically ill patients with COVID-19: a multi-center retrospective study. J Clin Med. 2020;9(9) doi: 10.3390/jcm9092847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtz P., Bastos L.S.L., Dantas L.F., Zampieri F.G., Soares M., Hamacher S. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47:538–548. doi: 10.1007/s00134-021-06388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouze A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47(2):188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Network C-IGobotR, the C-ICUI Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2020;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quah P., Li A., Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Mao B., Liang S., Yang J.W., Lu H.W., Chai Y.H. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillon A., Laurent E., Godillon L., Kimmoun A., Grammatico-Guillon L. Long-term mortality of elderly patients after intensive care unit admission for COVID-19. Intensive Care Med. 2021;47:710–712. doi: 10.1007/s00134-021-06399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta R.L., Kellum J.A., Shah S.V., Molitoris B.A., Ronco C., Warnock D.G. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.