Abstract
Objective
This study investigated the consequences of Coronavirus Disease 2019 (COVID-19) pneumonia on lung function in the first 6 months after hospital discharge.
Methods
A prospective lung function assessment in SARS-CoV2 patients with COVID-19 pneumonia, hospitalized between March and April 2020, was conducted with spirometry measurements including lung volumes, mainly total lung capacity (TLC), lung diffusion capacity for carbon monoxide (DLCO) collected at 3 months after hospital discharge. Patients with restrictive ventilatory defect or impaired DLCO or both were re-evaluated at 6 months with global spirometry and chest HRCT scan.
Results
Among 40 consecutive patients, 19 (48%) had normal pulmonary functional tests (group A), and 21 (52%) showed residual lung function abnormalities at 3 months after hospital discharge (group B). In group B, 4 patients (19%) had only loss of lung volume as shown by TLC reduction (group 1), 13 patients (62%) had decreased both TLC and DLCO (group 2), and 4 patients (19%) had isolated reduction in DLCO (group 3). At 6-month follow-up in group 1, although all patients improved, only one normalized total lung capacity (TLC). In group 2, TLC and DLCO increased significantly (p < 0.01), but only 3 patients reached normal values. In group 3, DLCO improved for most patients, normalizing in 50% of them. At 6-months significant correlations between an internal-built chest HRCT scan severity score and TLC (r2 = 0.33; p < 0.01) and DLCO (r2 = 0.32; p < 0.01) were found.
Conclusions
Nearly 50% of patients recovered in the post-critical phase. Most of those with abnormal pulmonary function tests at 3 months improved subsequently, but only another 29% (6 out of 21) reached normal values at 6 months. These results indicate that lung function spontaneous recovery is faster at first and occurs more slowly thereafter, leaving more than one third (15 out of 40) of patients with abnormal lung function tests at 6 months.
Keywords: COVID-19, SARS-CoV2, Lung function, Pneumonia, Spirometry
1. Introduction
Severe Acute Corona Virus 2 Respiratory Syndrome (SARS-CoV2) is a serious complication of Coronavirus Disease 2019 (COVID-19), a pandemic infection that has affected more than 90 million people worldwide, causing the death of more than 1.2 million people since his first report in late December 2019 [1]. COVID-19 has caused a sudden and substantial increase in hospitalizations for pneumonia with respiratory failure and systemic impairment [2]. SARS-CoV-2 is transmitted mainly through droplets during speaking or cough or sneeze in close face-to-face contacts by asymptomatic, pre-symptomatic, and symptomatic carriers. It has been estimated that the overall proportion of asymptomatic and pre-symptomatic transmission can reach up to 62% [3], which provides a strong motivation for physical distancing. SARS-CoV-2 infection has a mean incubation period of 5.1–6.4 days and a median time from exposure to possible symptoms onset of 5 days [4]. Most subjects (97.5%) develop symptoms within 11.5 days [5].
Symptoms of COVID-19 can range from mild to severe, with sizeable but declared variable mortality rates of 2.3% in China, 7.2% in Italy, and 1.0% in South Korea [[6], [7], [8], [9]]. According to a Chinese study, about 81% of symptomatic adults and children with COVID-19 develop a mild to moderate flu-like illness with fever, malaise, dry cough, or dyspnea that resolves in about a week, and about 13% develops a more severe form of the disease [2], with pneumonia and hypoxemic respiratory failure. In most severe cases, the pulmonary disease can worsen into acute respiratory distress syndrome (ARDS), accompanied by a disproportionate response of inflammatory cytokines (cytokine storm) and multi-organ failure [[10], [11], [12], [13]], which require hospitalization in ICU. These cases are associated with high mortality, so careful monitoring of the patient is required to assess any disease progression and response to treatment.
Although overall hospital mortality from COVID-19 is about 15–20%, reaching up to 40% among patients needing admission to ICU, most of these patients luckily survive, but sometimes with several sequelae of disease in different systems [14].
Among COVID-19 future complications, the most frequent and dangerous are linked to pneumonia [2] with the possible development of pulmonary consolidation, scarring and/or interstitial fibrosis. At present, long-term pulmonary consequences have to be better defined due to the paucity of studies evaluating the respiratory consequences prospectively after COVID-19 pneumonia [15,16]. This study aimed to investigate the lung function changes in survivors of COVID pneumonia in the first six months after recovery and the correlations with chest HRCT scan.
2. Materials
2.1. Patients
In this prospective observational follow-up study, we enrolled the first 40 consecutive patients with COVID-19 pneumonia admitted between March and April 2020 in two general hospitals in the province of Brescia (Italy) who agreed to be followed subsequently to check their lung function.
All patients were adult, had laboratory-confirmed SARS-CoV-2 infection by real-time reverse transcription-polymerase chain reaction (RT-PCR) and pulmonary involvement confirmed by Chest X-ray or CT. Patients with a history of known obstructive, restrictive or mixed ventilatory defects caused by previous respiratory diseases were excluded from the study.
2.2. Methods
Three months after hospital discharge, patients underwent physical examination and pulmonary function tests. Recorded parameters were slow and forced vital capacity (VC and FVC), forced expiratory volume at the first second of maximal expiration (FEV1), and FEV1/VC % ratio, lung volumes, by inert gas dilution technique using Helium closed-circuit multi-breaths method, including functional residual capacity (FRC) and residual volume (RV) and total lung capacity (TLC). Lung diffusion capacity for CO (DLCO) and its main determinants: alveolar volume (VA) and transfer rate for CO (KCO) by single breath technique, were then measured. DLCO and KCO were adjusted for haemoglobin (BIOMEDIN Instruments, Padua, Italy). In the presence of FEV1/VC % ratio > LLN, the restriction was considered mild with TLC <80% pred. and moderate with TLC <60% pred. The reduction of lung diffusion capacity was considered mild with DLCO <80% pred. and moderate with DLCO <60% pred.
Based on pulmonary function tests at first examination (about 3 months after hospital discharge), patients were subdivided into two groups: group A without lung function abnormalities (according to predicted values) and group B with restrictive ventilatory defect alone that is reduced TLC with FEV1/VC% ratio > LLN and normal DLCO, or decreased DLCO or both. At six months of follow-up, only patients of group B underwent pulmonary function tests again and chest HRCT scan to assess the trend of lung function abnormalities and their correlations with lesions detected by lung imaging. We considered in each interstitial lung thickening, ground-glass opacities and consolidations/fibrotic outcomes, present as a single or multiple lesion. We made a chest HRCT scan score, build for internal purposes, to quantify the overall severity of lung involvement by assigning the following scores: interstitial thickening: 1 point, ground-glass opacities: 2 points, consolidations/fibrotic outcomes: 3 points. Single parameter scores are doubled when the specific lesion was bilateral. The total score is reached by summing the three components (range of possible total scores was between 0 and 12).
2.3. Statistics
The Chi-square test for categorical variables verified the difference between groups. Continuous variables were compared using the Student's t-test for paired data. Data were expressed as mean ± standard deviation, and categorical variables were recorded as frequencies and percentages. Statistical significance was taken as p < 0.05.
3. Results
Data were collected from the first consecutive 40 patients who were previously hospitalized in general wards, 14 of whom were transferred after a period of ICU treatment. The anthropometric and clinical characteristics of the patients are shown in Table 1 . They were classified according to the WHO Covid-19 criteria as suffering from mild (8 patients), moderate (10 patients), severe (8 patients), and critical (14 patients) illness (see Table 1) [17]. Among 40 patients, 19 (47.5%) had normal pulmonary function tests (PFT) (Group A), and 21 (52.5%) had a restrictive ventilatory defect or decreased DLCO or both (Group B). Patients in group A did not exhibit clinically or functionally relevant alterations. However, they had no previous pulmonary function tests, and we cannot say if the respiratory functional parameters observed at 3 months after hospital discharge worsened compared to their best while remaining within normal limits.
Table 1.
Anthropometric and clinical characteristics in all patients and divided into Group A (with normal PFT at 3 months after discharge from the hospital) and Group B (with altered PFT at 3 months after discharge from the hospital). Data are mean ± SD and percentages.
| All (n = 40) | Group A | (n = 19) | Group B | (n = 21) | p-value | |
|---|---|---|---|---|---|---|
| Total | 100% | 47.5% | 52.5% | A vs B | ||
| Age, years | 58.2 ± 10 | 57.3 ± 10.7 | 60.8 ± 7.8 | 0.242 | ||
| Gender | ||||||
| Men | 31 (77.5%) | 14 | 73.7% | 17 | 81.0% | |
| Women | 9 (22.5%) | 5 | 26.3% | 4 | 19.0% | |
| Weight, kg | 80.5 ± 14.6 | 79.4 ± 14.8 | 81.6 ± 14.9 | 0.649 | ||
| High, cm | 169.5 ± 10.4 | 168.5 ± 12.5 | 170.5 ± 8.3 | 0.550 | ||
| BMI (kg/m2) | 28.1 ± 4.9 | 28.0 ± 5.6 | 28.1 ± 4.5 | 0.986 | ||
| Comorbidities | ||||||
| Hypertension | 24 (60%) | 13 | 68.4% | 11 | 52.4% | >0.999 |
| Type II diabetes | 6 (15%) | 5 | 26.3% | 1 | 4.8% | 0.084 |
| Obesity (BMI>30) | 28 (70%) | 14 | 73.7% | 14 | 66.7% | 0.524 |
| Neoplasms | 4 (10%) | 3 | 15.8% | 1 | 4.8% | 0.331 |
| No and ex smokers | 36 (90%) | 17 | 89.5% | 19 | 90.5% | >0.999 |
| Symptoms at onset | ||||||
| Fever | 30 (75%) | 14 | 73.7% | 16 | 76.2% | >0.999 |
| Dyspnea | 25 (62.5) | 11 | 57.9% | 14 | 66.7% | 0.757 |
| Cough | 32 (80%) | 12 | 63.2% | 20 | 95.2% | < 0.05 |
| Gastro-enteric | 11 (27.5%) | 5 | 26.3% | 6 | 28.6% | >0.999 |
| Asthenia | 17 (42.5%) | 10 | 52.6% | 7 | 33.3% | 0.337 |
| Arthromyalgia | 6 (15%) | 4 | 21.1% | 2 | 9.5% | 0.398 |
| Anosmia/Dysgeusia | 9 (22.5%) | 6 | 31.6% | 3 | 14.3% | 0.265 |
| ARDS | 16 (40%) | 5 | 26.3% | 11 | 52.4% | 0.117 |
| WHO Severity Score | ||||||
| Mild illness | 8 (20%) | 8 | 42% | 0 | 0% | |
| Moderate illness | 5 (12.5%) | 1 | 5% | 4 | 19% | |
| Severe illness | 13 (32.5%) | 7 | 37% | 6 | 29% | |
| Critical illness | 14 (35%) | 3 | 16% | 11 | 52% | |
None of the patients showed an obvious obstructive ventilatory defect, FEV1/VC % ratio < LLN.
Comparing the two groups, significant differences were noted for the presence of cough (p < 0.05) and some methods of treatment during hospitalization (see Table 2 ). In particular, the adoption of oxygen therapy and mechanical ventilation was significantly more frequent in patients of group B (p < 0.001 and p < 0.03), reflecting greater severity of COVID-19 pneumonia in these patients. Subsequently, we identified 3 different scenarios dividing 21 patients of group B according to the decrease in lung volumes or DLCO or both. Four patients had only restrictive ventilatory defect (group 1), 13 subjects had a decrease in both lung volumes and DLCO (group 2), and 4 patients had an isolated reduction in DLCO (group 3).
Table 2.
Treatment features in group A (with normal PFR at 3 months after hospital discharge) and group B (with altered PFR at 3 months after discharge from the hospital).
| Treatment | Group A | n = 19 | Group B | n = 21 | p-value |
|---|---|---|---|---|---|
| Oxygen | 11 | 57.9% | 21 | 100.0% | <0.001 |
| Antibiotics | 12 | 63.2% | 18 | 85.7% | 0.148 |
| Steroids | 11 | 57.9% | 16 | 76.2% | 0.314 |
| CPAP/NIV | 7 | 36.8% | 15 | 71.4% | 0.055 |
| Mechanical ventilation | 3 | 15.8% | 11 | 52.4% | <0.05 |
3.1. Group 1: patients (n = 4) with isolated lung volume decrease
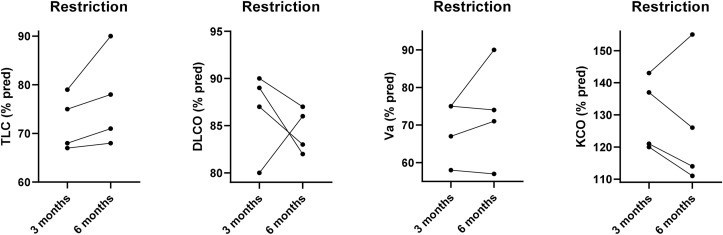
Four patients had low TLC and VA with a restrictive ventilatory defect, suggesting the presence of lung regions with reduced or absent ventilation. Their DLCO remained within normal limits because of the effective compensatory increase in KCO. In all these patients after 3 months, we observed a slight increase in TLC (% change = 4.7 ± 4.9; IC95%: 3.1–12.6), with decreasing KCO values (% change = −3.7 ± 10.6; IC95%: −20.7 – 13.2), thanks to the better perfusion in the previously poorly ventilated lung areas, while DLCO remained within normal limits (% change −2.0 ± 5.6; IC95%: −10.9 – 6.9). However, only one patient recovered completely, while the others showed at 6-month chest HRCT scan the presence of fibrotic consolidations in the absence of diffuse interstitial involvement (Fig. 1 ).
Fig. 1.
Total Lung Capacity (TLC), Lung Diffusion Capacity for CO (DLCO) and Alveolar Volume (VA) and coefficient transfer for CO (KCO) individual values (% pred.) at 3 and 6 months of follow-up in patients with an isolated baseline decrease of lung volumes (Restriction).
3.2. Group 2: patients (n = 13) with lung volume decrease and DLCO reduction
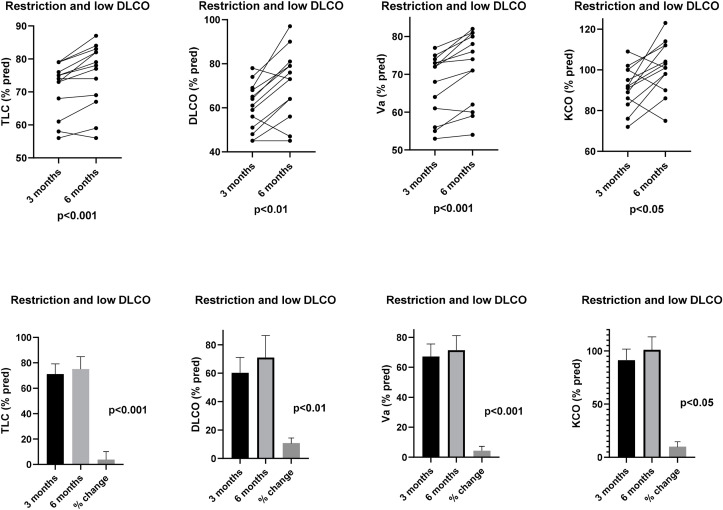
These patients had low TLC and VA, combined with decreased DLCO. Reduction in TLC and VA, as seen in Group 1, was likely due to lung regions with reduced or absent ventilation, but in addition, lung diffusion capacity was also compromised, suggesting the presence of interstitial involvement of other parts of the lungs, even if KCO was essentially within normal limits. Although in almost all patients either TLC (% change = 3.9 ± 3.1; IC95%: 2.1–5.8; p < 0.001), or KCO (% change = 10.0 ± 12.8; IC95%: 2.2–17.8; p < 0.05), significantly improved after 3 months, leading to a significant increase in DLCO (% change = 10.8 ± 9.9; IC95%: 4.8–16.9; p < 0.01), only 3 of them normalized completely their lung function parameters (Fig. 2 ). When low lung volumes and decreased lung diffusion capacity remained, chest HRCT scan at 6 months showed fibrotic areas associated with diffuse interstitial involvement.
Fig. 2.
Total Lung Capacity (TLC), Lung Diffusion Capacity for CO (DLCO) and Alveolar Volume (VA) and coefficient transfer for CO (KCO) individual values (% pred.) (upper panels) and their means and % changes (lower panels) at 3 and 6 months of follow-up in patients with baseline decrease of both lung diffusion capacity and lung volumes (Restriction and low DLCO).
3.3. Group 3: patients (n = 4) with reduced DLCO
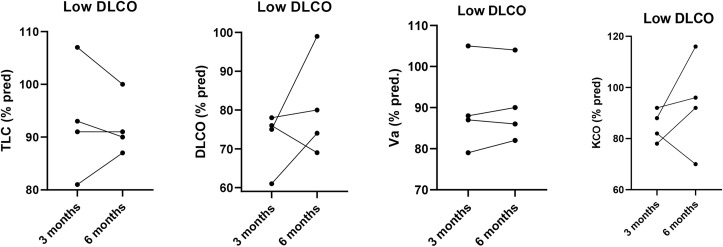
Four patients had reduced lung diffusion capacity without restrictive ventilatory defect. DLCO decrease was essentially linked to slightly reduced KCO. An improvement in KCO (% change 8.5 ± 16.8; IC95%: −18.3 – 35.3) and DLCO (% change 8.0 ± 13.4; IC95%: −13.4 – 29.4) was observed in most of these patients at 6 months of follow-up, but only two of them normalized DLCO after other 3 months (Fig. 3 ). On chest HRCT scan, a mild interstitial involvement of the lung without consolidations was found in those patients with persistently low DLCO.
Fig. 3.
Total Lung Capacity (TLC), Lung Diffusion Capacity for CO (DLCO) and Alveolar Volume (VA) and coefficient transfer for CO (KCO) individual values (% pred.) at 3 and 6 months of follow-up in patients with isolated baseline decrease in lung diffusion capacity (low DLCO).
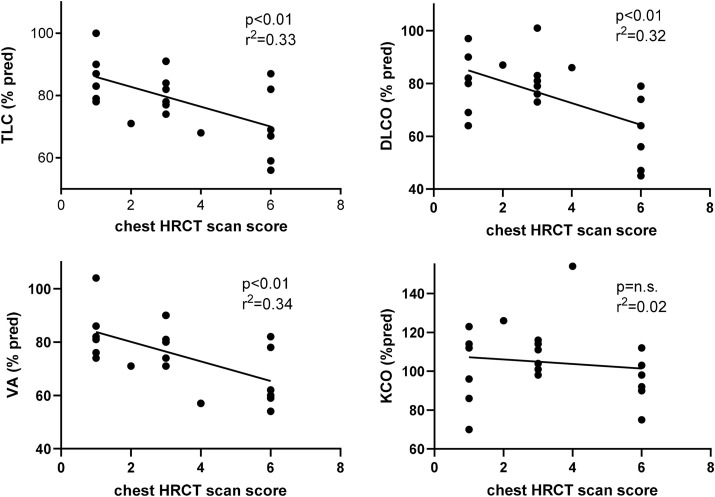
The correlations between TLC, DLCO, VA, and KCO and the chest HRCT scan severity score, as previously described, are shown in Fig. 4 at 6-month follow-up in patients of group B. The more severe and widespread the radiologic lesions, the greater the impairment of lung function, mainly in terms of DLCO (r [2] = 0.32; p < 0.01) and TLC (r [2] = 0.33; p < 0.01) reduction.
Fig. 4.
Correlations between chest HRCT scan score with Total Lung Capacity (TLC), Lung Diffusion Capacity for CO (DLCO) and Alveolar Volume (VA) and coefficient transfer for CO (KCO) in all patients of group B at 6 months after discharge from the hospital.
4. Discussion
Longitudinal monitoring of the lung function at 3 and 6 months can be very informative in the clinical course of the patients who suffered from COVID-19 pneumonia after their hospital discharge. The good news is that nearly 50% of them spontaneously recovered from a functional point of view at 3 months, even if it is not possible saying they had reached their previous personal best. In contrast, 3 different scenarios were observed in those who exhibited abnormal pulmonary function tests after 3 months, likely underlying different types of residual lung damage.
In order, the first can be found in those patients with residual pulmonary consolidations/ground grass opacities/limited fibrotic areas after pneumonia that reduce TLC and VA, being DLCO within normal limits, because the effective compensatory increase in KCO from the other parts of the lungs that are unaffected with preserved integrity of their alveolar-capillary membrane.
The second, characterized by the presence of both loss of lung volume and decrease of lung diffusion capacity, may pertain to patients with residual consolidations/ground grass opacities/limited fibrotic areas reducing TLC and VA who, without an adequate compensatory increase of KCO, have a decreased DLCO, suggesting the coexistence of diffuse interstitial disease.
The third, showing an isolated reduction of lung diffusion capacity with no relevant loss of lung volume, may occur in the presence of diffuse pulmonary interstitial involvement, without relevant residual consolidations, suggesting only persistent abnormalities of the alveolar-capillary membrane.
After other 3 months, although in all 3 groups there was a general trend towards lung function improvement, only 6 out of 21 (about 30%) of these patients spontaneously fully recovered, leaving 70% of them still with some functional abnormalities, generally of mild severity.
The findings of the chest HRCT scan performed only at 6 months well supported the insights deriving from the spirometry data analysis, showing that monitoring of these patients could be based essentially on adequate pulmonary function tests and so limiting the use of chest HRCT scan in patients who have persistent lung function abnormalities.
In two recently published studies concerning pulmonary function performed in patients after COVID-19 pneumonia at 30 days and 6 weeks after discharge from the hospital, respectively, DLCO and TLC emerged as the most frequently impaired parameters [15,16]. In fact, at 30 days from the hospital discharge on 57 patients, 53% had DLCO lower than 80% pred. and 12% had TLC lower than 80% pred., while at 6 weeks from the hospital discharge on 101 patients, 71% had DLCO lower than 80% pred. and 21% had TLC lower than 80% pred., suggesting a prevalent impairment of diffusion pathways because of abnormalities of the alveolar-capillary membrane due to interstitial alveolitis/fibrosis or reduction in capillary volume due to micro-thrombus formation or both. A lower prevalence of alveolar loss due to consolidations/ground-glass opacity or fibrotic/scarring outcomes or both were observed in these cohorts of patients after a relatively short follow-up.
After 3 months from the hospital discharge, our findings are consistent with these data showing abnormal lung diffusion capacity and a restrictive ventilatory defect or both as the most frequent functional sequelae in survivors from COVID-pneumonia. Actually, 43% of our patients (17 out of 40) had a mild-to-moderate reduction of DLCO, and 43% (17 out of 40) had a mild reduction of TLC, with percentages slightly decreasing after 6 months to 30% (12 out of 40) of the patients still suffering from a mild reduction of DLCO and 33% (13 out of 40) from a mild reduction of TLC. However, our patients showed a greater proportion of significant lung volume reduction and a lesser proportion of lung diffusion capacity impairment than those followed in the previous studies, perhaps because one-third of them had a critical COVID-19 related disease.
The limits of the study are low sample size and the absence of previous pulmonary function tests.
The study's strength is that all patients were assessed and followed by the same expert lab staff (technicians and physicians), using the same methods, procedures and instruments in only one centre.
Finally, we chose to use 80% predicted as a threshold to detect the presence of abnormality either for lung volumes or DLCO because this cut off value has been widely used as a limit to detect their clinically relevant reduction, although LLN would be statistically more adequate. We have also to say that the differences between these two methods (80% pred. vs LLN) to detect subjects with TLC and DLCO impairment are minimal. In our series, only 2 patients with mild DLCO reduction below 80% pred. in group 3 could be considered still in the normal range because marginally over the LLN.
5. Conclusion
In conclusion, pulmonary function tests are definitely useful to detect the presence and nature of the residual lung damage after COVID-19 related pneumonia and to monitor its natural course. Our results indicate that lung functions spontaneous recovery is faster at first and occurs more slowly thereafter, likely as a consequence of the different degree of severity of COVID-related pneumonia. Careful identification of patients who do not recover at 6 months (and perhaps even better at 3 months) might justify the implementation of therapeutic options to maximize the potential recovery of their pulmonary function.
Ethics approval and consent to participate
The study was performed in accordance with the Helsinki declaration and was approved by the local University-Hospital Ethic Committee. All participants signed written informed consent upon enrolling.
Funding
No Funding.
CRediT authorship contribution statement
Nicla Orzes: Study design, Data curation, Data collection, Formal analysis, Data analysis, Interpretation of results, Writing – original draft, Initial draft, Review of the manuscript for intellectual content. Laura Pini: Review of the manuscript for intellectual content. Guido Levi: Formal analysis, Data analysis, Review of the manuscript for intellectual content. Silvia Uccelli: Review of the manuscript for intellectual content. Francesca Cettolo: Review of the manuscript for intellectual content. Claudio Tantucci: Study design, Interpretation of results, Review of the manuscript for intellectual content.
Declaration of competing interest
NO reports no conflicts of interest in this work; LP reports no conflicts of interest in this work; GL reports no conflicts of interest in this work; SU reports no conflicts of interest in this work; FC reports no conflicts of interest in this work; CT reports no conflicts of interest in this work.
Acknowledgements
The Authors acknowledge Mr. M. Guerini, Lab. technician, for his invaluable technical support.
References
- 1.World Health Organization 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-themission-briefing-on-covid-19 12-march.
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., et al. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020 Apr;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Pre-symptomatic transmission of SARS-CoV-2 - Singapore, jan 23–mar 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention [Zhonghua Liu Xing Bing Xue Za Zhi] vol. 41. 2020. pp. 145–151. (The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) in China). Feb 10. 2. [DOI] [PubMed] [Google Scholar]
- 7.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. J. Am. Med. Assoc. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. Apr 14. [DOI] [PubMed] [Google Scholar]
- 8.Shojaee S., Pourhoseingholi M.A., Ashtari S., Vahedian-Azimi A., Asadzadeh-Aghdaei H., Zali M.R. Predicting the mortality due to Covid-19 by the next month for Italy, Iran and South Korea; a simulation study. Gastroenterol Hepatol Bed Bench. 2020;13(2):177–179. Spring. [PMC free article] [PubMed] [Google Scholar]
- 9.Caminati M., Vultaggio A., Matucci A., Senna G., Almerigogna F., Bagnasco D., Chieco-Bianchi F., Cosini F., Girelli D., Guarnieri G., Menzella F., Micheletto C., Olivieri O., Passalacqua G., Pini L., Rossi O., Vianello A., Vivarelli E., Crisafulli E. Asthma in a large COVID-19 cohort: prevalence, features, and determinants of COVID-19 disease severity. Respir. Med. 2021 Jan;176:106261. doi: 10.1016/j.rmed.2020.106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B., Zhang H.Y., Sun W., Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020 Jun;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffler E., Detoraki A., Contoli M., Papi A., Paoletti G., Malipiero G., Brussino L., Crimi C., Morrone D., Padovani M., Guida G., Gerli A.G., Centanni S., Senna G., Paggiaro P., Blasi F., Canonica G.W., SANI Working Group COVID-19 in Severe Asthma Network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2021 Mar;76(3):887–892. doi: 10.1111/all.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffler E., Detoraki A., Contoli M., Papi A., Paoletti G., Malipiero G., Brussino L., Crimi C., Morrone D., Padovani M., Guida G., Gerli A.G., Centanni S., Senna G., Paggiaro P., Blasi F., Canonica G.W., SANI Working Group Reply to: kow CS et al. Are severe asthma patients at higher risk of developing severe outcomes from COVID-19? Allergy. 2021 Mar;76(3):961–962. doi: 10.1111/all.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng F., Liao C., Fan Q.H., Chen H.B., Zhao X.G., Xie Z.G., Li X.L., Chen C.X., Lu X.X., Liu Z.S., Lu W., Chen C.B., Jiao R., et al. Clinical characteristics of children with coronavirus disease 2019 in hubei, China. Curr Med Sci. 2020 Apr;40(2):275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson S., Hirsch J.S., Narashimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Sar-van der Brugge S., Talman S., Boonman-de Winter L., de Mol M., Hoefman E., van Etten R.W., De Backer I.C. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir. Med. 2020;176:106272. doi: 10.1016/j.rmed.2020.106272. Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y., Tan C., Wu J., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;(21):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/3321296/WHO-2019-CoV-clinical-2020.5-eng.pdf;Table 2;13-15..