Abstract
Pulmonary complications of cocaine among users are common. Manifestations include lung congestion, intra-alveolar edema, and diffuse alveolar hemorrhage (DAH). Direct cellular toxicity, eosinophilia, barotrauma, and vasoactive effects of cocaine are believed to induce DAH. We present a rare case of cocaine-associated focal alveolar hemorrhage mimicking malignancy on imaging. Initially contemplated biopsy was avoided based on rapid growth of concerning lung lesion, with subsequent near resolution on follow-up. This case illustrates the importance of epidemiologic and temporal multimodality correlation when evaluating indeterminate lung lesions.
KEY WORDS: Cocaine, fluorodeoxyglucose positron emission tomography/computed tomography, focal alveolar hemorrhage, lung injury, unnecessary biopsy
INTRODUCTION
Cocaine has vasoactive properties resulting in eosinophilia, cellular toxicity, and barotrauma with alveolar hemorrhage.[1] While cocaine-associated diffuse alveolar hemorrhage is relatively common, focal alveolar hemorrhage (FAH) is only reported in isolated cases without true numbers regarding prevalence.[2,3,4,5] Although FAH may mimic malignancy on imaging, this case illustrates the importance of follow-up and re-evaluation before taking biopsy for benign etiologies. Upon re-evaluation, rapid growth and eventual resolution of the lesion is apparent. In contrast, lung neoplasms naturally do not have such rapid size enlargement, with a reported mean tumor volume doubling time of 166 days.[6] Cessation of cocaine, oxygenation, and glucocorticoids remain the mainstay management of cocaine-induced alveolar hemorrhage.[4]
CASE REPORT
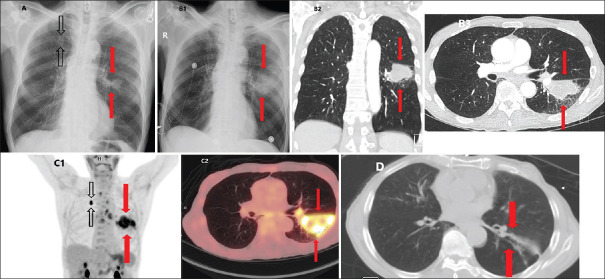
A 73-year-old man with long-standing history of smoking tobacco, cocaine, and cannabis presented with acute chest pain. Initial chest X-ray (CXR) showed two lung nodules up to 2.7 cm [Figure 1A, red arrows – left lower lobe (LLL), black arrows – right upper lobe (RUL)]. Subsequent CXR and chest computed tomography (CT) 4 days later showed the LLL nodule increase to 5 cm [Figure 1B1-3, red arrows]. Fluorodeoxyglucose positron emission tomography/CT (FDG PET/CT) performed showed an intensely avid (maximum standardized uptake value: 7.4) LLL lesion measuring 7 cm, with photopenic areas suggestive of necrosis [Figure 1C1 and 2, red arrows]. The RUL nodule was also intensely avid [Figure 1C1, black arrow]. Although biopsy was initially suggested by the PET reader, the tumor board determined that the lesion's rapid growth was inconsistent with malignancy. Indeed, the lesions nearly resolved with minimal residual fibrosis on chest CT approximately a month later [Figure 1d, red arrows]. Subsequent analysis determined that the presentation was most consistent with a rare cocaine-induced FAH.[7,8,9]
Figure 1.
Computed tomography and fluorodeoxyglucose positron emission tomography modality imaging of cocaine-inducible focal alveolar hemorrhage reveals rapid doubling and resolution and exhibits a solid-like, hazy appearance and preserved underlying bronchial structures and vasculature. (A) Initial chest X-ray showing two lung nodules up to 2.7 cm (red arrows – left lower lobe, black arrows – right upper lobe. (B1) Subsequent chest X-ray illustrating left lower lobe nodule increase to 5 cm (red arrows). (B2) Subsequent coronal chest computed tomography illustrating left lower lobe nodule increase to 5 cm (red arrows). (B3) Subsequent axial chest computed tomography illustrating left lower lobe nodule increase to 5 cm (red arrows). (C1) Intensely avid, 7 cm lower lobe nodule lesion on coronal fluorodeoxyglucose positron emission tomography/computed tomography with photopenic areas suggestive of necrosis (red arrows) with additional intensely avid right upper lobe nodule (black arrows). (C2) Intensely avid, 7 cm lobe nodule lesion on axial fluorodeoxyglucose positron emission tomography/computed tomography with photopenic areas suggestive of necrosis (red arrows). (D) Resolved pulmonary lesion with minimal residual fibrosis on axial chest computed tomography 1 month after initial imaging (red arrows)
DISCUSSION
Cocaine-induced FAH may mimic benign and malignant conditions on imaging. In fact, there are several benign neoplasms that mimic lung malignancies on FDG PET-CT.[10,11,12,13,14,15,16] Pulmonary hamartoma is the most common benign neoplasm and is composed of mesenchymal tissues such as cartilage, fat, and connective tissues.[11,17] Hamartomas appear as a smooth, round, or lobulated mass on CT. However, in the absence of common features of fat and central calcification, it is difficult to discern pulmonary hamartoma from a round or lobulated primary lung malignancy.[13,18] Hamartomas also usually show low-grade uptake of FDG, appearing analogous to lung cancers with low metabolic rates like bronchoalveolar carcinoma.[12,19] Focal inflammatory and infectious conditions may further complicate a diagnosis of cocaine-induced FAH.[20,21,22,23,24,25,26] Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia similarly presents with necrotizing granulomatous inflammation, mimicking aggressive malignancy on FDG PET.[20] Tuberculosis (TB) is a chronic granulomatous infection caused by Mycobacterium tuberculosis. Similar to FAH, active TB presents with central necrosis and elevated FDG uptake on PET-CT, attributable to high macrophage count.[21,22] Fast-growing malignancy like small-cell lung cancer may also appear comparable to cocaine-induced FAH due to its highly rapid growth, with a mean doubling time of approximately 86 days.[6,27] By waiting and re-evaluating, unnecessary biopsy can be avoided in patients with cocaine-induced pulmonary injury.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We specially thank Dr. Pucar with the Yale School of Medicine and Dr. Keshavamurthy with the Augusta University Medical College of Georgia for providing original case images and guidance for this report.
REFERENCES
- 1.Murray RJ, Albin RJ, Mergner W, Criner GJ. Diffuse alveolar hemorrhage temporally related to cocaine smoking. Chest. 1988;93:427–9. doi: 10.1378/chest.93.2.427. [DOI] [PubMed] [Google Scholar]
- 2.Dushay KM, Evans SK, Ghimire S, Liu J. Cocaine-induced diffuse alveolar hemorrhage: A case report and review of the literature. R I Med J ( 2013;2016(99):34–6. [PubMed] [Google Scholar]
- 3.Underner M, Perriot J, Wallaert B, Peiffer G, Meurice JC, Jaafari N. Alveolar hemorrhage and cocaine use. Rev Mal Respir. 2018;35:134–48. doi: 10.1016/j.rmr.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Jiménez-Zarazúa O, López-García JA, Arce-Negrete LR, Vélez-Ramírez LN, Casimiro-Guzmán L, Mondragón JD. Alveolar hemorrhage associated with cocaine consumption. Heart Lung. 2018;47:525–30. doi: 10.1016/j.hrtlng.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Mégarbane B, Chevillard L. The large spectrum of pulmonary complications following illicit drug use: Features and mechanisms. Chem Biol Interact. 2013;206:444–51. doi: 10.1016/j.cbi.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Arai T, Kuroishi T, Saito Y, Kurita Y, Naruke T, Kaneko M Japanese Lung Cancer Screening Research Group. Tumor doubling time and prognosis in lung cancer patients: Evaluation from chest films and clinical follow-up study. Jpn J Clin Oncol. 1994;24:199–204. [PubMed] [Google Scholar]
- 7.de Almeida RR, Zanetti G, Souza AS, Jr, de Souza LS, Silva JL, Escuissato DL, et al. Cocaine-induced pulmonary changes: HRCT findings. J Bras Pneumol. 2015;41:323–30. doi: 10.1590/S1806-37132015000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mengar M, Gupta N, Chakrabarti S, Aggarwal V, Shah K, Ish P. Cocaine abuse induced diffuse alveolar haemorrhage: A rare entity. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1191. doi: 10.4081/monaldi.2020.1191. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida RR, de Souza LS, Mancano AD, Souza As, Jr, Irion KL, Nobre LF, et al. High-resolution computed tomographic findings of cocaine-induced pulmonary disease: A state of the art review. Lung. 2014;192:225–33. doi: 10.1007/s00408-013-9553-6. [DOI] [PubMed] [Google Scholar]
- 10.Wilson B, Becker A, Estes T, Keshavamurthy J, Pucar D. Adrenal hemangioma definite diagnosis on CT, MRI, and FDG PET in a patient with primary lung cancer. Clin Nucl Med. 2018;43:e192–4. doi: 10.1097/RLU.0000000000002069. [DOI] [PubMed] [Google Scholar]
- 11.Furuya K, Yasumori K, Takeo S, Sakino I, Uesugi N, Momosaki S, et al. Lung CT: Part 1, Mimickers of lung cancer – Spectrum of CT findings with pathologic correlation. AJR Am J Roentgenol. 2012;199:W454–63. doi: 10.2214/AJR.10.7262. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Song J, Shi J, Hu H, Wu Y, Yan J, et al. Slight uptake of (18) F-FDG on positron emission tomography in pulmonary hamartoma: A case report. Oncol Lett. 2015;10:430–2. doi: 10.3892/ol.2015.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Z, Qian F, Chen S, Yu G. Pulmonary hamartoma resembling multiple metastases: A case report. Oncol Lett. 2014;7:1885–8. doi: 10.3892/ol.2014.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavithran K, Manoj P, Vidhyadharan G, Shanmughasundaram P. Inflammatory myofibroblastic tumor of the lung: Unusual imaging findings. World J Nucl Med. 2013;12:126–8. doi: 10.4103/1450-1147.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobashi Y, Fukuda M, Nakata M, Irei T, Oka M. Inflammatory pseudotumor of the lung: Clinicopathological analysis in seven adult patients. Int J Clin Oncol. 2006;11:461–6. doi: 10.1007/s10147-006-0611-4. [DOI] [PubMed] [Google Scholar]
- 16.Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: The great mimicker. AJR Am J Roentgenol. 2012;198:W217–27. doi: 10.2214/AJR.11.7288. [DOI] [PubMed] [Google Scholar]
- 17.Bateson EM. An analysis of 155 solitary lung lesions illustrating the differential diagnosis of mixed tumours of the lung. Clin Radiol. 1965;16:51–65. doi: 10.1016/s0009-9260(65)80033-2. [DOI] [PubMed] [Google Scholar]
- 18.Siegelman SS, Khouri NF, Scott WW, Jr, Leo FP, Hamper UM, Fishman EK, et al. Pulmonary hamartoma: CT findings. Radiology. 1986;160:313–7. doi: 10.1148/radiology.160.2.3726106. [DOI] [PubMed] [Google Scholar]
- 19.Kim BT, Kim Y, Lee KS, Yoon SB, Cheon EM, Kwon OJ, et al. Localized form of bronchioloalveolar carcinoma: FDG PET findings. AJR Am J Roentgenol. 1998;170:935–9. doi: 10.2214/ajr.170.4.9530038. [DOI] [PubMed] [Google Scholar]
- 20.Hsu J, Jia L, Pucar D, Williams H, Keshavamurthy J. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia and granulomatous inflammation mimicking high-grade malignancy on FDG-PET/CT. Clin Nucl Med. 2017;42:47–9. doi: 10.1097/RLU.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 21.Nachiappan AC, Rahbar K, Shi X, Guy ES, Mortani Barbosa EJ, Jr, Shroff GS, et al. Pulmonary tuberculosis: Role of radiology in diagnosis and management. Radiographics. 2017;37:52–72. doi: 10.1148/rg.2017160032. [DOI] [PubMed] [Google Scholar]
- 22.Yang CM, Hsu CH, Lee CM, Wang FC. Intense uptake of [F-18]-fluoro-2 deoxy-D-glucose in active pulmonary tuberculosis. Ann Nucl Med. 2003;17:407–10. doi: 10.1007/BF03006610. [DOI] [PubMed] [Google Scholar]
- 23.Erdoğan Y, Özyürek BA, Özmen Ö, Yılmaz Demirci N, Duyar SŞ, Dadalı Y, et al. The Evaluation of FDG PET/CT scan findings in patients with organizing pneumonia mimicking lung cancer. Mol Imaging Radionucl Ther. 2015;24:60–5. doi: 10.4274/mirt.03016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang PS, Lee KS, Han J, Kim EA, Kim TS, Choo IW. Focal organizing pneumonia: CT and pathologic findings. J Korean Med Sci. 2001;16:573–8. doi: 10.3346/jkms.2001.16.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin HJ, Kim MS, Kho BG, Park HY, Kim T, Park C, et al. Delayed diagnosis of lung cancer due to misdiagnosis as worsening of sarcoidosis: a case report. BMC Pulm Med. 2020;20:71. doi: 10.1186/s12890-020-1105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun EJ, Lee HJ, Kang WJ, Kim KG, Goo JM, Park CM, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated (18) F-FDG PET/CT. Lung Cancer. 2009;65:180–6. doi: 10.1016/j.lungcan.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Harris K, Khachaturova I, Azab B, Maniatis T, Murukutla S, Chalhoub M, et al. Small cell lung cancer doubling time and its effect on clinical presentation: A concise review. Clin Med Insights Oncol. 2012;6:199–203. doi: 10.4137/CMO.S9633. [DOI] [PMC free article] [PubMed] [Google Scholar]