Abstract
Necrotizing enterocolitis (NEC) is an acute inflammatory disease that unforeseeably develops in very low birth weight premature infants. NEC is characterized by impairment of the intestinal barrier resulting in intestinal necrosis and multisystem organ failure. Animal models of NEC have contributed significantly to a better understanding of the underlying molecular mechanisms of the disease and facilitated the exploration of potential new therapeutic strategies. Here, we provide a detailed protocol that recapitulates some of the main histological and transcriptional features of human NEC in newborn mice.
Keywords: Necrotizing enterocolitis, Neonatal mice, Mucosal barrier, Intestinal inflammation, Enteric bacteria
1. Introduction
Postnatal microbial ontogenesis, which is primarily shaped by key factors such as the gestational age, delivery route and feeding modes has significant consequences on the host immune system early in life as well as adulthood [1, 2]. In the context of prematurity, early microbial colonization of the immature intestine is often associated with the development of a devastating pathology referred to as necrotizing enterocolitis (NEC) [3]. NEC, a deadly disease most commonly affecting very low birth weight (< 1500 g) premature infants, is typically characterized by a plethora of clinical manifestations including feeding intolerance, abdominal distention, excessive gut inflammation and massive destruction of the epithelial barrier [4]. Although the etiology and the pathogenesis of NEC is not well understood, evidence suggests that formula feeding increases the risk of NEC and breast milk plays a protective role [5-7]. We have previously shown that the protection conferred by breast milk is mediated by different components of the milk including epidermal growth factor and the human milk oligosaccharide 2′-fucosyllactose [6, 7]. In addition to the type of enteral feeds received, it is widely accepted that the profile of microbial communities in the intestine of premature babies is a central factor involved in the initiation of NEC. Indeed, several studies have shown that the development of NEC is concomitant with a dysbiosis characterized by a predominance of Proteobacteria [2]. It is worth noting that delivery mode, diet and antibiotic administration can profoundly influence the configuration of the intestinal microbial population in premature infants, hence potentially contributing to the development of NEC [2, 8]. In addition to the aforementioned factors, intestinal hypoxic stress seems to play a critical role in triggering NEC as intestinal ischemia has been shown to be a prerequisite to the induction of the disease [9, 10]. Strikingly, it was previously demonstrated that 2′-fucosyllactose contained in breast milk reduces the severity of NEC through the restoration of intestinal perfusion [7].
Despite the substantial advances made in recent years in our understanding of the pathogenesis of NEC, several obscure facets of the disease need to be elucidated to develop an effective therapeutic strategy. Toward that end, modeling the disease in laboratory animals emerged as the method of choice to unravel the molecular mechanisms involved in NEC pathogenesis. Several investigators have developed different protocols to mimic human disease in multiple animal species [11, 12]. The use of genetically modified mice has proven to be a powerful tool in dissecting different signaling pathways implicated in the development of NEC. The majority of the current mouse models of NEC are based on subjecting neonatal pups to a combination of formula feeds, hypoxia, cold stress, lipopolysaccharide (LPS) and bacterial challenge as elegantly reviewed in [11, 12]. Using a different approach, Zhang et al. developed another model of NEC by combining chemical Paneth cell depletion and Klebsiella pneumoniae challenge [13]. Recently, Ginzel and colleagues showed that feeding 3 day old pups with formula containing dextran sodium sulfate results in NEC-like disease affecting the small intestine and the colon [14]. Similarly, another study showed that neonatal mice subjected to the haptenic agent 2,4,6-trinitrobenzene sulfonic acid (TNBS) display intestinal lesions comparable with those observed in humans with NEC [15, 16]. Interestingly, a gene analysis revealed that this model recapitulates the gene signature of the disease in humans [15].
In the current chapter, we will describe a mouse model of NEC based on the use of hypoxic stress and administration of infant formula supplemented with LPS—the ligand for the innate immune receptor toll-like receptor 4 as well as enteric bacteria obtained from a patient with the most severe form of NEC, NEC totalis [17].
2. Materials
All animal experiments were performed in accordance with the institutional regulations with Washington University Institutional Animal Care and Use Committee (IACUC). C57BL/6 mice were bred and maintained in a Specific-Pathogen-Free animal facility at Washington University School of Medicine in St. Louis.
Isolette Air-Shields Vickers Model C100-200-2 Series 02 Infant Incubator set at 37 °C.
95% nitrogen, 5% oxygen compressed gas tank with a brass single-stage gas regulator connected to hypoxia chamber (Billups-Rothenberg Inc.) and oxygen analyzer (Maxtech Handi+).
Frozen glycerol stock of enteric bacteria obtained from an infant with severe surgical NEC (see Notes 1-3) with approval from the University of Pittsburgh Institutional Review Board.
1.9 French Single-Lumen Silicon Peripherally Inserted Central Catheter (Utah Medical Products).
NEC formula (prepare a 2:1 ratio of Abbott Nutrition, Similac Advance OptiGRO with Iron infant formula: Esbilac Puppy Milk replacer, see Note 4).
Autoclaved Luria-Bertani (LB) broth culture medium.
Lipopolysaccharide (LPS): Reconstitute LPS from Escherichia coli O127:B8 at 10 mg/mL with sterile Dulbecco’s phosphate buffered saline without CaCl2 and MgCl2 (PBS). Store at −20 °C.
Orbital shaker set at 150 r.p.m. and 37 °C.
15 mL culture tubes with dual position cap.
50 mL conicals.
1.7 mL microcentrifuge tubes.
Spectrophotometer with 1 cm cuvette.
Semimicro 1.7 mL cuvettes.
1 mL syringes.
Polystyrene 100 mm × 15 mm petri dish.
10% neutral buffered formalin.
RNAlater™ Solution (Invitrogen).
Dissection tools: Scissors, forceps, and dissecting pins.
Reusable 24-gauge gavage needle.
Macrosette processing/embedding cassettes.
Ethanol, isopropanol and chloroform.
Steel beads and TissueLyser LT (Qiagen).
TRIzol™ Reagent (Invitrogen).
Glycogen.
QuantiTec Reverse Transcription Kit (Qiagen).
IQ SYBR Green Supermix (Bio-Rad).
Plate Sealing Film.
Real-time PCR detection system.
Pedestal spectrophotometer (capable of measuring the concentration of nucleic acids).
3. Methods
3.1. Induction of Necrotizing Enterocolitis in Neonatal Mice
The day before the start of the model, take a frozen cryovial of enteric bacteria and gently scrape a small amount of the bacterial glycerol stock with a sterile pipette tip (see Notes 1 and 2).
Transfer the bacteria to a culture tube containing 2 mL of LB and grow the bacteria in an orbital shaker overnight (see Note 3).
The following day at 7 am, transfer 50 μL from the overnight bacterial culture to two culture flasks each containing 20 mL of LB.
Culture the inoculum for a minimum of 2 h in a shaking incubator set at 37 °C and 150 r.p.m.
In the animal facility, randomly assign 4-day-old C57BL/6 pups into two experimental groups. In the first group, the neonatal mice are kept with their mothers throughout the entire procedure (negative controls), while in the second group, the pups are separated from the dams, weighed, placed in a new cage and maintained in the infant incubator at 37 °C.
Measure the optical density of the 2 h culture at 600 nm using a spectrophotometer with a 1 cm cuvette. The OD600nm value should be around 0.6 (corresponding to the exponential phase of bacterial growth).
Once appropriate OD600nm is reached, transfer the bacteria to 50 mL conicals, centrifuge for 10 min at 3000 × g and discard the supernatant.
Resuspend the bacterial pellets in 1 mL PBS.
Inoculate 2 mL of bacteria into 20 mL of NEC formula.
Based on the average weight of the pups from that morning, add LPS (2.5 μg/g body weight) to the formula after vortexing the LPS for at least 5 min.
At 10 am, load a 1 mL syringe with the NEC formula and connect it to the PICC line in preparation for the oral gavage.
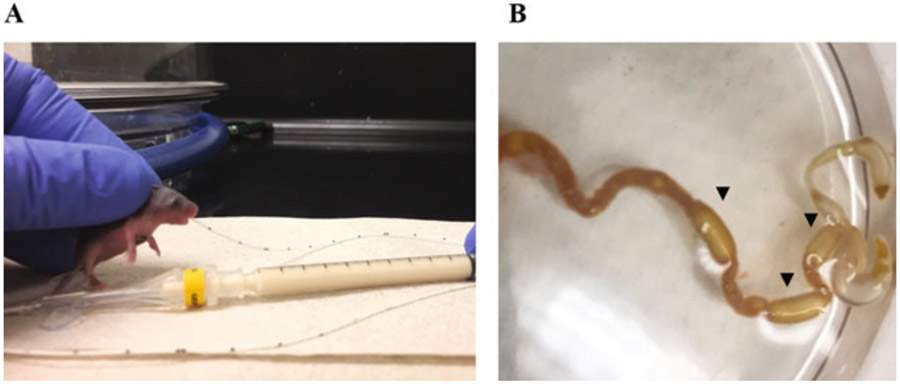
To perform the oral gavage, restrain the mouse by gently grasping the loose skin on the back of the neck. Introduce 1.5 cm of the PICC line into the esophagus through the oral cavity (Fig. 1a). Gavage slowly 80 μL of formula into the pup’s stomach. After removing the PICC line, make sure that the animal is breathing properly.
The feeding procedure is repeated at 1 pm, 4 pm, 7 pm, and 10 pm.
After 1 pm and 7 pm feeds, the pups are subjected to intermittent hypoxia. Pups are placed in a hypoxia chamber (95% nitrogen and 5% oxygen) for 10 min (see Note 5).
Return the pups to their cage and place them in the incubator.
Between feeds, the mice should be carefully monitored for apnea, cyanosis, and abdominal distension. Animals presenting these symptoms should be immediately euthanized consistent with IACUC policy.
At 3 pm, prepare bacterial culture for the next day as described in steps 1 and 2.
The following morning, repeat steps 3 and 4 before the 7 am feed.
At the 7 am feed on day 2 and 3, start by weighing the mice. Then feed them with 80 μL of formula (prepared the day before).
Repeat steps 6–17.
Fig. 1.
(a) Photograph of a 4-day-old mouse pup during an oral gavage feeding with a PICC line. (b) Photograph demonstrating the small intestine and colon of a pup subjected to the NEC protocol. A significant accumulation of gas is observed in the distal part of the gastrointestinal tract (arrowheads) associated with a thin and fragile bowel wall
3.2. Sacrificing Animals and Harvesting Tissue Samples
Early on day 4, after recording the weights, the pups are euthanized in accordance with IACUC policy.
Make a longitudinal incision along the midline of the abdominal cavity to uncover the intestines.
Carefully locate and remove the entire small intestine.
Place the small intestine in a petri dish filled with cold PBS.
Cut the terminal part of the ileum (approximately 2.5 cm from the cecum).
Cut a small piece of the terminal ileum and place it in RNAlater™ for transcriptional analysis (see Subheading 3.3).
Flush the remaining part of the terminal ileum with 10% formalin using a 24-gauge gavage needle (see Note 6).
Put tissue samples in histology processing/embedding cassettes and place them in a 10% formalin container.
After 24 h of fixing, the formalin is replaced by 70% ethanol.
Embed the intestinal specimens in paraffin by being careful to maintain them as flat as possible.
Cut tissue sections on a microtome (5 μm thick) and stain them with hematoxylin and eosin for histological assessment.
3.3. RNA Isolation, cDNA Synthesis, and Transcriptional Analysis of the Intestine
Add 1 mL of TRIzol™ to each tissue sample.
Add one steel bead per tube and homogenize the tissue using the Qiagen TissueLyser (7 min at 50 oscillations/s). Ensure that intestinal tissues have been completely homogenized.
Transfer the homogenized tissues to a 1.7 mL microcentrifuge tube and add 200 ul of chloroform to each tube.
Invert the tube 10 times and incubate at room temperature for 5 min.
Centrifuge the samples for 15 min at 19,350 × g.
The solution will separate into three layers. Transfer the top aqueous layer to a new 1.7 mL microcentrifuge tube and add successively 500 μL of isopropanol and 3 μL glycogen. Gently invert the tubes 10 times.
Allow the samples to incubate for 20 min at −20 °C.
Centrifuge the samples for 15 min at 19,350 × g.
Remove the supernatant and add 0.5 mL of 75% ethanol.
Spin the samples for 5 min at 19,350 × g.
Remove supernatant and allow the pellet to dry for at least 15 min.
Add 50 μL of RNase free water, measure the RNA concentration using a pedestal spectrophotometer and store at −80 °C.
Use QuantiTec Reverse Transcription Kit to synthesize the cDNA following the manufacturer’s instructions.
Real-time PCR (qPCR) is performed using IQ SYBR Green Supermix according to the manufacturer’s instructions. The primer sets used in the reaction mix are respectively.
Il1b Forward primer: AGTGTGGATCCCAAGCAATACCC.
Il1b Reverse primer: TGTCCTGACCACTGTTGTTTCCCA.
Cxcl2 Forward primer: TCCAGAGCTTGAGTGTGACG.
Cxcl2 Reverse primer: CTTCCGTTGAGGGACAGCAG.
RegIIIg Forward primer: GTACCCTGTCAAGAGCCTCA.
RegIIIg Reverse primer: TGTGGGGAGAATGTTCCCT.
RPLO Forward primer: GGCGACCTGGAAGTCCAACT.
RPLO Reverse primer: CCATCAGCACCACAGCCTTC.
The qPCR reaction is carried out using a real-time PCR detection system under the following conditions: an initial denaturation step at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 10 s, primer annealing at 55 °C for 10 s, and primer extension at 72 °C for 30 s. A melting curve can be added to the amplification protocol in order to ensure the absence of primer dimer formation.
3.4. Assessment of NEC-Like Pathologic Intestinal Injury
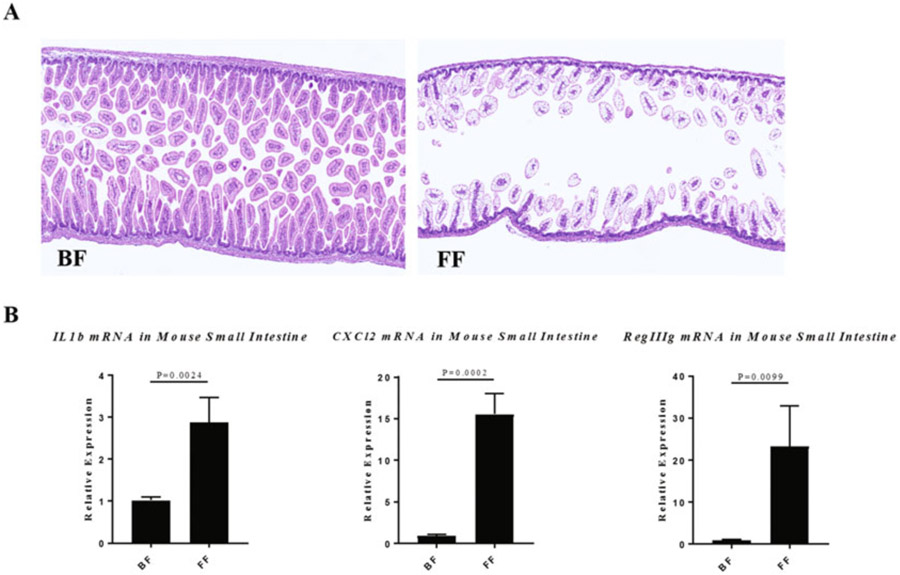
The protocol described above induces a NEC-like intestinal injury and can result in approximately 30–50% mortality in C57BL/6 Mice (see Notes 7 and 8). At autopsy, the pups subjected to the NEC model show a large accumulation of gas in the distal part of the gastrointestinal tract (see Fig. 1b). The presence of gas is usually associated with a thin and transparent intestinal wall sometimes resulting in an intestinal perforation. Just as in patients with NEC, microscopically as shown in Fig. 2a, the terminal part of the ileum displays mild to complete destruction of the intestinal mucosa. Depending on the disease severity, the ileum is scattered with patchy lesions ranging from a mild destruction of the apical part of the villi to significant transmural necrosis with a complete loss of the intestinal architecture. These histological changes are associated with a significant upregulation of inflammatory factors and antimicrobial peptides such as Il1b, Cxcl2 and Reg3g when compared to dam fed controls (Fig. 2b). These histological and transcriptional patterns are also observed in the intestine of human infants with NEC [18, 19].
Fig. 2.
(a) Representative histology of the terminal ileum from a neonatal mouse that was either dam fed (breast fed, BF) or subjected to the NEC protocol (formula fed, FF). (b) mRNA gene expression of Il1b, Cxcl2, and Reg3g in the ileum of breast-fed (BF) pups compared to NEC (FF). Statistical significance was computed by the two-tailed Student’s t-test
Acknowledgments
MG is supported by grants K08DK101608, R03DK111473, and R01DK118568 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17-79, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, and the Department of Pediatrics at Washington University School of Medicine, St. Louis. Belgacem Mihi, Wyatt E. Lanik, and Qingqing Gong contributed equally to this work.
Footnotes
The enteric bacterial stock was generated by overnight culture of resected intestinal tissue from an infant with NEC totalis. The overnight culture was centrifuged, resuspended in 50% glycerol and stored at −80 °C.
Avoiding excessive freezing–thawing of the bacterial stock is important.
A control tube with 3 mL of sterile LB should be cultured overnight in parallel to ensure the absence of bacterial contamination.
Do not use old stocks of puppy milk and infant formula.
If the pups present with signs of apnea while in the hypoxia chamber, gently shake the hypoxia chamber to stimulate their breathing reflexes. If the apnea persists after the hypoxia treatment, stimulate the breathing reflex by squeezing the terminal part of the pup’s tail.
Animals with NEC-like intestinal disease have very fragile intestines. Therefore, the intestine has to be slowly flushed in order to avoid causing further damage to the tissue.
It is essential to check the integrity of the esophagus and the absence of formula in the lungs during the autopsy. Animals with a ruptured esophagus and milk in the respiratory tract should be excluded from the study and considered as technical deaths.
The mortality of this protocol can be higher than 50% in some susceptible mouse strains when subjected to the NEC protocol. A lower LPS concentration or bacterial challenge can reduce the severity of the disease.
References
- 1.Tanaka M, Nakayama J (2017) Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 66:515–522 [DOI] [PubMed] [Google Scholar]
- 2.Warner BB, Tarr PI (2016) Necrotizing enterocolitis and preterm infant gut bacteria. Semin Fetal Neonatal Med 21:394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihi B, Good M (2019) Impact of toll-like receptor 4 signaling in necrotizing enterocolitis: the state of the science. Clin Perinatol 46:145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364:255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Good M, Sodhi CP, Hackam DJ (2014) Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol 10:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Good M, Sodhi CP, Egan CE et al. (2015) Breast milk protects against the development of necrotizing enterocolitis through inhibition of toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 8:1166–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good M, Sodhi CP, Yamaguchi Y et al. (2016) The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr 116:1175–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning NL, Prince JM (2018) Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol Med 24:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins DJ, Besner GE (2013) The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg 22:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazji I, Sodhi CP, Lee EK et al. (2013) Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A 110(23):9451–9456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodhi C, Richardson W, Gribar S et al. (2008) The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech 1:94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulistyo A, Rahman A, Biouss G et al. (2018) Animal models of necrotizing enterocolitis: review of the literature and state of the art. Innov Surg Sci 3:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Sherman MP, Prince LS et al. (2012) Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech 5:522–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginzel M, Feng X, Kuebler JF et al. (2017) Dextran sodium sulfate (DSS) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS One 12:e0182732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MohanKumar K, Namachivayam K, Cheng F et al. (2017) Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr Res 81:99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MohanKumar K, Kaza N, Jagadeeswaran R et al. (2012) Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol 303:G93–G102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Good M, Sodhi CP, Ozolek JA et al. (2014) Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 306:G1021–G1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballance WA, Dahms BB, Shenker N et al. (1990) Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr 117:S6–S13 [DOI] [PubMed] [Google Scholar]
- 19.MohanKumar K, Namachivayam K, Ho TT et al. (2017) Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin Perinatol 41:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]